Abstract
Power-to-Methane as one part of Power-to-Gas has been recognized globally as one of the key elements for the transition towards a sustainable energy system. While plants that produce methane catalytically have been in operation for a long time, biological methanation has just reached industrial pilot scale and near-term commercial application. The growing importance of the biological method is reflected by an increasing number of scientific articles describing novel approaches to improve this technology. However, these studies are difficult to compare because they lack a coherent nomenclature. In this article, we present a comprehensive set of parameters allowing the characterization and comparison of various biological methanation processes. To identify relevant parameters needed for a proper description of this technology, we summarized existing literature and defined system boundaries for Power-to-Methane process steps. On this basis, we derive system parameters providing information on the methanation system, its performance, the biology and cost aspects. As a result, three different standards are provided as a blueprint matrix for use in academia and industry applicable to both, biological and catalytic methanation. Hence, this review attempts to set the standards for a comprehensive description of biological and chemical methanation processes.
1. Introduction
Already today “human activities are estimated to have caused approximately one degree centigrade of global warming above pre-industrial levels (…). Global warming is likely to reach 1.5 °C between 2030 and 2052 if it continues to increase at the current rate” [1]. Facing this threat, several nations have committed in the Paris Agreement [2] to keep the rise of global temperatures this century well below two degree centigrade above pre-industrial level and to make efforts to limit the temperature increase even further to 1.5 °C. First steps to lay down hard rules for adherence on this agreement were taken at the 24th Conference of the Parties to the United Nations Framework Convention on Climate Change in Katowice/Poland in late 2018 [3]. The ambitious goals can only be reached, if the greenhouse gas emissions of the energy sector will be dramatically reduced by more than 70% until 2050 compared to the levels of 2015 [4]. At the moment, 70% of the total greenhouse gas emissions, i.e., more than 34 gigatons of carbon dioxide equivalents, can be traced back to the energy sector [5], with 42% originating from power and heat generation, 24% from the transport sector and 19% from industrial processes [6]. The required reduction of carbon dioxide emissions is only achievable by a change in consumption patterns, increasing energy efficiency and a massive deployment of renewable energy forms such as wind, solar and hydro power to replace fossil generation. However, energy generation from renewable sources, especially wind and solar, is fluctuating and intermittent and therefore technologies for long term and large capacity electricity storage are required to store energy during times of overproduction and to provide energy in the case of shortage [7,8]. Furthermore, the transportation of renewable energy resources on a global scale requires electricity storage with high energy density and the use of existing transport infrastructures. Regions with an excess on renewable energies will have to contribute to the green energy demand of the world’s megacities and industrialized regions with energy carriers originating from Power-to-Gas or Power-to-Liquids.
Power-to-Gas, also called PtG, is an essential technology to convert the energy sector into a renewable system which can provide the required long-term storage capacity and reduce carbon footprints by gradually substituting fossil feedstocks with renewable gas [9,10]. The technology uses renewable electric power to produce hydrogen by electrolysis (Power-to-Hydrogen), which then can be further converted into methane with carbon dioxide from an external source in the methanation step (Power-to-Methane). Both gases can be injected into the existing natural gas grid, which offers a storage capacity, e.g., for Germany and Denmark, that exceeds the energy consumption needs of several months [11,12]. While the volumes of hydrogen that can be injected into the natural gas grid are limited due to regulatory and technical reasons [13,14], methane, also known as synthetic natural gas (SNG), can be injected basically without limitation for storage. Alternatively, the produced methane can be used as compressed natural gas (CNG) motor fuel and in any well-established natural gas facility including distribution through the gas network [8]. By this, PtG promotes not only the transformation of the electrical power system from fossil to renewable energy sources but can also help to transform the heat and gas as well as the transportation sectors. This approach is notably demonstrated by press releases, study reports and presentations from gas grid operators and their partners who have identified the renewable synthetic gas as the future for a fossil-free gas grid [15,16,17,18].
Today, two processes exist for the conversion of hydrogen and carbon dioxide to methane, namely thermochemical or catalytic methanation and biological methanation [19,20]. Thermochemical methanation, also known as Sabatier-process, utilizes metal catalysts like e.g., Ni/Al2O3 to catalyze the methanation reaction [21,22,23,24]. The process operates at high temperatures between 200 and 550 °C depending on the optimal activity of the catalyst and pressures up to 100 bar because the methanation is thermodynamically more favorable at high operation pressure [24,25]. Due to the reactor design and the catalyst, catalytic methanation is characterized by high space-time yields and high methane selectivity. However, a major restriction for the chemical methanation is the requirement of high reactant gas purities because of the sensitivity of the metal catalyst towards contaminants such as hydrogen sulfide [20,22,24,26]. Biological methanation uses biological catalysts i.e., methanogenic microorganisms to catalyze the methanation reaction [27,28,29]. As a consequence, reactors work normally at temperatures between 37 and 65 °C and pressures from one to 15 bars to meet the optimal growth conditions of these microorganisms. In addition, methanogens are more robust towards fluctuations in reactant gas supply and impurities such as hydrogen sulfide than metal catalysts. The key limitation of the biological process is the low hydrogen gas-to-liquid mass transfer especially at 65 °C, which leads to lower space-time yields and the requirement of bigger reactor dimensions. To overcome this challenge, more and more research groups as well as industrial entities work on the reactor optimization and the plethora of literature published on different biological methanation reactor types, plants and experimental setups is showing progress and potentials of the technology for national and international applications [25,27,30].
Although many data are available, there is no common basis for comparison of biological methanation systems, because reports differ in their definitions of system parameters and boundaries. In order to highlight the benefits of certain system configurations for biological methanation and to potentially compare them to chemical methanation systems, a comprehensive set of parameters is desired.
In this work, we evaluated current literature on biological methanation to determine a set of system parameters and characteristic variables for all reactor types used for this technology. We focus on two-step processes and do not take bioelectronic systems and in-situ methanation into account which are described in detail by Geppert et al. [31] and Graf et al. [32]. A definition of system boundaries of the different PtG configurations is provided together with mass and energy balances. The parameters presented in this paper should be applicable to both industrial and academic projects and therefore provide a solid basis for the characterization and techno-economical comparison of biological and chemical methanation systems.
2. Methodology and Motivation
To meet the objective of standardization and to allow better comparability, classification and quantification of efficiencies in various biological CO2-Methanation systems, the present work defines system boundaries and develops performance parameters which are applicable to chemical methanation systems as well. The design of this study is based on a review of existing literature on biological CO2-Methanation and the summary of parameters used for the characterization of this process. The results were discussed by all authors, representing not only academia and industry but also a German gas distribution system operator (Westnetz GmbH, Düsseldorf, Germany) and an association for guideline preparation (DVGW – German Technical and Scientific Association for Gas and Water, Karlsruhe, Germany). Subsequently, system boundaries and the most common parameters and units were identified, defined and adopted to close gaps in the existing set of parameters. As a result of this process, a robust framework as recommended standard was developed and is presented in this article.
Systems and processes for biological CO2-Methanation are various and can differ significantly in their components (e.g., the reactor type), operating modes (e.g., batch or continuous) or the biocatalyst applied. In current literature, a confusing variety in nomenclature of parameters and units, reference on standard conditions and given information is present. This fact becomes apparent in Table 1 which summarizes indications and units used to describe the methane production rate of trickle-bed reactor (TBR) systems. Most authors use ‘methane productivity’ [33,34,35] or the German equivalent ‘Methanbildungsrate’ [36,37,38] but nine other indications were found as well [33,35,36,39,40,41,42,43,44]. The heterogeneity of units for this parameter is even bigger: in total, 25 different units were identified for the same parameter with some authors using more than one unit within the same manuscript.

Table 1.
Variety in indications and units for the methane production rate in current literature on CO2-methanation in trickle-bed reactors. Minor differences in spelling or case sensitivities reflect inaccuracies of indications and units in literature. A summary of indications and units of other relevant parameters for CO2-methanation is given in Table A1 in the Appendix A.
The inconsistency in indication is similar for other parameters like ‘methane concentration’ (10 different indications and three different units), ‘reactant gas composition’ (12 indications, 10 units), ‘loading rate’ (nine indications, eight units), ‘hydrogen/carbon dioxide conversion rate’ (six indications, three units), ‘gas flow rate’ (eight indications, seven units), ‘recirculation rate of the trickle medium’ (eight indications, eight units), ‘methane yield’ (six indications, three units), ‘gas retention time’ (three indications, one unit), ‘hydraulic retention time’ (three indications, one unit) or the ‘reactor volume’ (eight indications, five units).
Just for basic parameters and units there are no major differences. Some authors provide temperature in ‘degree centigrade’ [33,34,36,37,38,42,43,44,45,46] and others in ‘Kelvin’ [35,40]. Regarding pressure, it is not always clear if absolute or relative pressure is given [33,36,37,38,45] and some authors only state their values as ‘ambient’ [43,44,46] or ‘atmospheric’[35,40] (see Table A1 in the Appendix A).
Regarding other reactor types like continuous stirred tank reactors (CSTR), bubble column reactors (BCR) or membrane reactors (MR) the complexity and inconsistency of parameters and units are assumed to further increase.
3. Power-to-Gas—Definitions and System Boundaries
Power-to-Gas is a general term for the technology of converting renewable electrical power into chemical energy in the form of flammable gases [9,55,56] and comprises of two concepts: Power-to-Hydrogen (also Power-to-Gas-Hydrogen, PtG-H2) [47,57,58] and Power-to-Methane (Power-to-Gas-Methane, PtG-CH4) [9].
For a standardization of the biological CO2-Methanation as one step of PtG-CH4, it is necessary to define the system boundaries not only of this process but also for the entire technology (Figure 1 and Table 2). The system boundaries and components of both PtG-H2 and PtG-CH4 were set in accordance with Sterner and Stadler [9] and are summarized briefly herein. For further reading, especially on the principles and necessity of both PtG-concepts, the authors refer to the following reviews (e.g., [20,45,59]). To facilitate the comparison of academic and industrial projects, we distinguish two system boundaries for the methanation step (called methanation system herein): (i) the ‘CO2-Methanation reactor’, summarizing all components of the reactor (yellow box, Figure 1; Section 3.1.1) and (ii) the ‘CO2-Methanation process’, extending the ‘CO2-Methanation reactor’ boundary by necessary peripherals as up- and downstream gas or water treatment and other balance of plant (green box, Figure 1; Section 3.1.2).
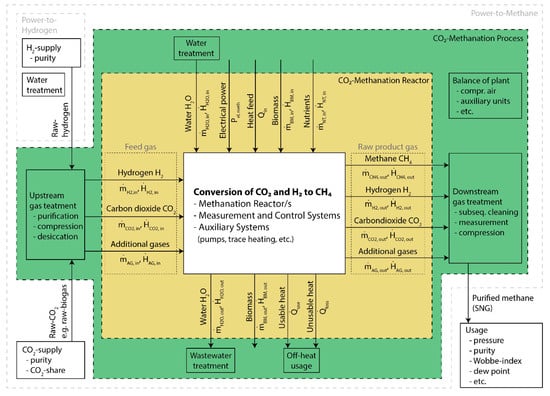
Figure 1.
Boundaries and mass and energy streams of the systems CO2-Methanation reactor (yellow), CO2-Methanation process (green), Power-to-Hydrogen and Power-to-Methane.

Table 2.
Boundaries and components of the systems ‘CO2-Methanation reactor’, ‘CO2-Methanation process’, ‘Power-to-Hydrogen’ and ‘Power-to-Methane’. Background colors of the first two systems refer to the system boundary colors given in Figure 1.
3.1. Methanation System
The methanation system is the core process of PtG-CH4 and describes the chemical or biological conversion of carbon dioxide and hydrogen into methane and water according to Equation (1) or (2). For biological methanation, Equation (2) is valid, since the main part of the water is in liquid phase (gaseous water in the products occurs at about two percent depending on pressure):
For this reason, biological CO2-methanation releases more heat than chemical, where the product water leaves the reactor as vapor. Nevertheless, losses within the system boundary ‘CO2-Methanation reactor’ which are related to the lower heating value, as more common in power engineering, are equal for biological and chemical methanation and therefore comparable and have to be calculated with Equation (1).
Our study revealed that some authors use the term ‘biological hydrogen methanation’ (BHM) to define the biologically-driven conversion as opposite to the catalytic-chemical process [30,60]. However, this term implies that hydrogen is converted to methane but the conversion of one element into another, namely hydrogen (H) into carbon (C), is not possible from a chemical point of view. In the case of (biological) methanation, molecular hydrogen acts as electron donor/reducing agent that is oxidized to water. Carbon dioxide on the other hand is reduced to methane (Equation (1)). Consequently, the name of this process should be ‘biological CO2-Methanation’ and equals the definition of ‘CO2-Methanation reactor’ provided herein in a biological system. Analogous, the term ‘chemical CO2-Methanation’ is defined as the chemical conversion of carbon dioxide into methane within the ‘CO2-Methanation reactor’ boundary.
3.1.1. CO2-Methanation Reactor
The ‘CO2-Methanation reactor’ boundary, is the innermost part of the methanation system and includes (i) the methanation reactor, (ii) the measurement control system of the reactor and (iii) all potentially required components for operation of the reactor, such as pumps, heating, cooling or stirring. For our standardization approach of the biological CO2-Methanation given in Section 4, we refer to all relevant parameters of the biological conversion into methane within the ‘CO2-Methanation reactor’ boundary.
All relevant mass and energy streams crossing the ‘CO2-Methanation reactor’ system boundary are shown in Figure 1. The derived mass and energy balances for this system are given exemplarily in Equations (3) to (5) and can be deduced for all other system boundaries. In case additional energy flows enter or exit the process, they have to be considered in the equations:
The ‘CO2-Methanation reactor’ neither includes process steps for pre- and post-treatment of the feed and product gas, nor water and wastewater management, treatment and heating or cooling required for any peripheral processes. Since gas pre-treatment is not considered within this system boundary, every reactant is assumed to be already supplied at a purity tolerated by the methanation system. Therefore the ‘CO2-Methanation reactor’ system definition is generally valid for the comparison of the methanation performance of any biologically or chemically catalyzed methanation system. Both from academia and industry. However, the disadvantage of this system definition is that the tolerance of systems towards impurities in the feed gases, which is one of the major advantages of biological vs. chemical methanation, is not revealed. As a consequence, reactant and product gases have to be specified separately (see Section 4.3.5).
3.1.2. CO2-Methanation Process
Although the ‘CO2-Methanation reactor’ system definition has the advantage to be generally applicable to any methanation system, it might be of limited use when comparing industrial systems. Industrial systems normally are obliged to produce gas with a certain quality and reliability, which depends on the application (e.g., grid injection or biogas upgrading) and potentially requires additional treatment of feed and product gases. To overcome this limitation, we defined the ‘CO2-Methanation process’ system boundary as an extension of the ‘CO2-Methanation reactor’ system boundary (Figure 1), including the necessary peripherals. Here, potential water and wastewater treatment steps, as well as pre- and post-treatment of feed and product gases such as compression, drying or cleaning steps are considered. Since the product gas treatment will depend on the anticipated use (purity requirements, etc.), the definition of the ‘CO2-Methanation process’ system boundary does not allow for a general comparison of methanation systems, but for a comparison of systems used for similar and specific applications.
3.2. Power-to-Hydrogen
The ‘Power-to-Hydrogen’ concept depicts the splitting of water into hydrogen and oxygen via an electrolyzer (Equation (6)) using renewable electric power. There are different electrolyzer technologies and the topic has been reviewed in detail [9,61,62]:
The hydrogen can be used in chemical and industrial processes and for transport applications. Injection into the natural gas grid is also possible in limited quantities (see Section 4.2) to make it available for the power sector as well as for heating or industrial applications. Further conversion of hydrogen into methane or other chemicals is not part of the ‘Power-to-Hydrogen’ system boundary.
3.3. Power-to-Methane
The Power-to-Methane system is the combination of the Power-to-Hydrogen and the CO2-Methanation process systems including carbon dioxide supply. It describes the conversion of renewable power into natural gas substitutes namely methane or SNG, according to Equation (7) which combines Equations (2) and (6). Theoretically, hydrogen can be derived from other processes than PtG-H2. The methane can be injected and stored in the existing gas network and gas storages if it reaches the quality required by local regulations (see Section 4.2 or e.g., DVGW G260(A) [63], SVGW G13 [64], ÖVGW G31 [65]). Alternatively, it can be used as CNG motor fuel, or it can easily be utilized in all other well-established natural gas facilities. Grid injection, storage or other gas use is not included within the system boundary of ‘Power-to-Methane’:
4. System Parameters and Characteristic Variables
The following section lists our recommended standards and regulations for the description of the system boundary ‘CO2-Methanation reactor’ including: (i) system-related, (ii) performance-relevant, (iii) microbiology-based parameters and (iv) those important for cost calculation. Although the authors focus on biological CO2-Methanation, all parameters proposed herein should be applicable to chemical systems as well. In Table A2 in the Appendix A as well as in the Supplementary Materials of this paper, the set of parameters and units is proposed as a blueprint for plant description. Here, we distinguish three quality levels:
- (1)
- A-Standard: detailed scientific data and background information
- (2)
- B-Standard: basic information including economic aspects
- (3)
- C-Standard: basic information on most important parameters
4.1. Standard Conditions
For a better comparability of gas processes and specifically to eliminate variability in the calculation of gas volumes due to pressure and temperature effects, gas volumes are usually reported at standard conditions. However, for natural gas and natural gas substitutes, several definitions for standard conditions exist, which differ in temperature and pressure (Table 3) and therefore lead to diverging results. In some papers, it is ambiguous to which standard the authors refer to.

Table 3.
Existing definitions of standard conditions.
The standard DIN 1343 (1990-01-00) “explains the terms reference conditions, normal conditions and normal volume and specifies values for normal temperature, normal pressure and molar normal volume of an ideal gas” [66].
The objective of the international standard DIN EN ISO 13443 is “to specify the standard reference conditions of temperature, pressure and humidity to be used for measurements and calculations carried out on natural gases, natural-gas substitutes and similar fluids” [67] in compliance with ISO 5024 [68] which standardizes measurement and reference conditions for petroleum liquids and gases. It refers to other standardization literature as ISO 6976 (Natural gas-calculation of calorific values, density, relative density and Wobbe-index from composition, [69]) for detailed explanation of the parameters. The international standard ISO 2533 [70] defines a standard atmosphere on which calculations can be based.
For the characterization of biological CO2-Methanation systems, we recommend using the norm condition as consistently specified in DIN 1343 and DIN EN ISO 13443 as 1.01325 bar and 0 °C. This standard is also used by the German Technical and Scientific Association for Gas and Water [63]. Consequently, all parameters given in scientific publications should refer to this pressure and temperature for better comparability of different methanation systems or varying operating parameters.
According to the standard ISO 13443 ([67], comment 5), reference conditions should not be part of the unit (e.g., not Nm³ or ) but of the symbol. Therefore, units in this paper e.g., for gas volumes are given e.g., as m³ also if norm cubic meters are meant.
4.2. Feed-in Relevant Standards and Regulations
Feed-in restrictions are an important topic for methanation process applications, since strict limits for gas impurities like hydrogen and carbon dioxide in the gas network would result in downstream methane enrichment of the methanation plant to fulfill the requirements due to technical specifications in infrastructure. However, European standards for gas quality are quite inhomogeneous as listed in Table 4. One important reason might be that there are different approaches to setting gas quality specifications across the European Union. In some countries, parts of gas quality restrictions (and hence the specifications) relate to the safety and protection of the general public and have become enshrined in national safety legislation. In that regard, relevant technical reasons are underground storages, CNG steel vehicle tanks, gas engines, gas turbines and gas burners in the domestic sector. For example, CNG steel tanks limit the hydrogen content to two percent, if the tensile strength exceeds 950 MPa [71]. In addition to that, inhomogeneous standards translate into necessary adjustments of reactor systems in different European countries.

Table 4.
Regulative standards and regulations on natural gas quality and SNG in different countries with a focus on Europe.
Projects for harmonization have been carried out, such as the Common Business Practice 2005-001/02 on “Harmonisation of Gas Qualities” from EASEE-gas [72] or the activity on gas quality harmonization by the European Commission and its related legislation [73].
For example, in Switzerland (SVGW G13), limits for hydrogen and carbon dioxide can be defined via the methane content required. In Denmark, the threshold for hydrogen in the grid indirectly results from the Wobbe-index required.
4.3. System Related Parameters
4.3.1. Reactor and Plant Type
For a comprehensive description of the reactor and plant type used for biological CO2-Methanation, the following parameters should be specified:
- Reactor type (TBR, CSTR, BCR, MB, etc.; explanations are given below)
- Mode of operation (e.g., batch/fed-batch/continuous/semi-continuous) and
- Required plant components, according to the ‘CO2-Methanation process’ boundary definition,
- Potential specific characteristics of the plant/process concept e.g., co-current/counter-current mode, flow chart, etc.
The key limitation of the biological process is the slow hydrogen gas-to-liquid mass transfer, leading to low space-time yields and vice versa requiring larger reactor dimensions than for chemical methanation. The problem can be highlighted by the following Equation (8) of gas-to-liquid mass transfer of various gaseous species i (gas phase mass transfer is neglected):
As can be seen, the mass transfer issue can be optimized by either increasing the mass transfer coefficient kL, the specific surface area Aspec or the concentration gradient between the phases. kL can effectively be increased by intensifying intermixing of the liquid phase e.g., by stirring or by higher flow velocities in the liquid phase. The specific gas-liquid surface area Aspec can be increased by e.g., adding a packing material into the reactor or by adapting hydrodynamics within the reactor to favor e.g., smaller bubbles or droplets. The concentration gradient between the phases can be increased by operating the system at higher pressures.
Due to this, various types of biological methanation systems based on different reactor systems are currently under investigation. The spectrum of applied systems ranges from conventional continuous stirred tank reactors (CSTR), trickle-bed reactor (TBR) systems to bubble column reactors (BCR) which are illustrated insee Figure 2. Mixed types, e.g., flooded fixed bed systems or stirred bubble column systems are also present in the field. Most recently the use of membrane reactors (MR) is considered for biological methanation. Table 5 gives an overview of the characteristics of the different standard configurations.
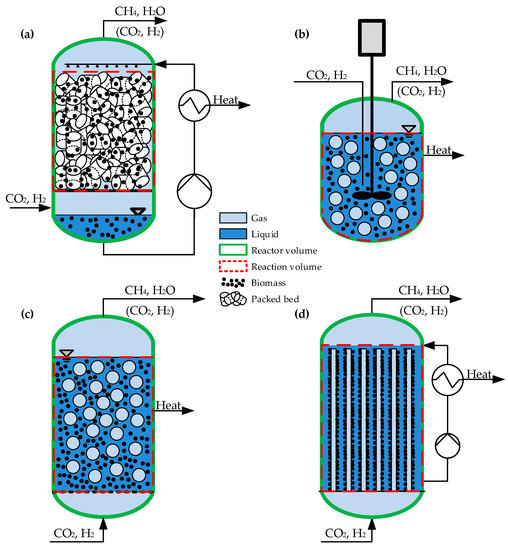
Figure 2.
Schematic flow diagram of a trickle-bed reactor TBR (a), a continuous stirred tank reactor CSTR (b), a bubble column reactor BCR (c) and a membrane reactor MR (d) for biological methanation. Reactor volume VR (green), liquid volume VL (blue), gas volume VG (light blue), reaction volume (red), biomass and packing/reaction volume VP is shown.

Table 5.
Overview of the mass transport potential and the energy consumption related to the mass transport in the trickle-bed reactor (TBR), the stirred tank reactor (CSTR), the bubble column reactor (BCR) and the membrane reactor (MR) [81,82,83,84,85,86].
The concepts differ in the phase ratio of gas/liquid/solids inside the reactor system. In a TBR, the gas phase is the continuous phase and the volume of the packing material (solid, neglecting the volume of the microorganisms) is less than 10%. Thereby the microorganisms can either are immobilized on the packing material or can be suspended in the circulating liquid phase. In a CSTR and BCR, the liquid phase is the continuous phase and the microorganisms are suspended in the liquid. The gas phase is dispersed in the liquid in form of bubbles and the gas hold-up is lower compared to a TBR. In a MR, the microorganisms are immobilized in a biofilm outside of the cylindrical membranes which separate the liquid (outside) and the gas phase (inside). Thus, the gas (H2, CO2) diffuses through the membrane into the biofilm and the liquid stays bubble-free.
The effective surface area aeff is higher in a CSTR and a BCR due to the dispersal of the gas phase into small bubbles. Nevertheless, the specific power input for dispersing the gas phase is highest for a CSTR followed by a BCR. The energy demand of a TBR is considerably lower due to the fact that the liquid only has to be pumped to the top of the column but no additional energy has to be spent for dispersing the liquid into droplets. Since a MR is only in focus of academic research nowadays, the energy demand is unknown.
A relevant question for the evaluation of the different reaction systems is the calculation basis in terms of reactor volume VR for the methane production rate (MPR, see Section 4.4.6). In general, the MPR can be calculated based on the volume of the liquid VL, the active volume of e.g., the liquid/gas bubbles volume in case of BCR or the packing volume VP in case of the TBR. Nevertheless, it is also useful to consider the total volume of the reactor, as the pressure vessel volume itself is critical in terms of financial aspects.
Besides the data given in Table 5, the choice of reactor concept also influences the backmixing behavior of the phases and hereby the effective methane production rate. While both phases are perfectly back-mixed within a CSTR, a TBR features plug flow behavior in both phases. Furthermore, TBRs offer an additional degree of freedom by the choice of operating in co- or counter-current operation mode. BCRs and MRs feature mixed flow behavior. BCRs can be perfectly back-mixed in both phases or feature back-mixing in liquid and plug flow in gas phase. MRs usually feature plug flow behavior in the gas phase and backmixing on the liquid side.
4.3.2. Reactor-, Gas-, Reaction- and Liquid Volume, h/d-Ratio, Packing Volume
Different parameters were identified to characterize the volume of the methanation reactor. The usual definition of the actual ‘reactor volume’ (VR in m³) is the sum of the volume of all sections within the reactor, including e.g., head space, sump, liquid and internal components. The volume in pipes is usually negligible and not considered in the calculation of the reactor volume. If the vessel is cylindrical, VR is defined by ‘reactor height’ (hR in m) and ‘reactor diameter’ (dR in m) from which the ‘h/d-ratio’ can be calculated.
The ‘liquid volume’ (VL in m³) comprises only the liquid present within the reactor during operation including volume of suspended biomass and solids. For the specific application of trickle-bed reactors, additionally the suspended biomass and ‘packing volume’ (VP in m³) can be given. It is the volume of the packing zone and, if cylindrical, can be calculated by the ‘packing height’ (hP in m) and ‘packing diameter’ (dP in m). The ‘gas volume’ (VG in m³) contains total volume of gaseous phase within the reactor volume VR. The ‘reaction volume’ (VReaction in m³) is the volume in which the reaction itself occurs. A mapping of the different volumes is illustrated in Figure 2.
4.3.3. Plant Capacity, Size and Footprint
The size specification of the methanation plant (not including the electrolyzer) should be provided by the following parameters:
- Reactor volume VR (see Section 4.3.2)
- Nominal capacity of the methanation given by:
- ⚬
- Methane production rate in nominal point (MPR) in m³/h and related to the lower heating value MPRLHV in kW/m³ (see Section 4.4.6)
- ⚬
- The lower heating value (LHV) of the methane produced (in kW or MW). The LHVs of potential further product gas components n are not considered here but can be summed up as .
- The footprint of the methanation plant, both, absolute FPMeth in square meters (m2) and as specific area value fpMeth, where the area is normalized by the nominal capacity of the methanation plant referred to the lower heating value of the methane produced (m2/kW)
- The volume specific power input calculated as in Wh/m³
- Nominal capacity of the entire plant PN (connected electric power of the electrolyzer and methanation including all peripheral systems in kW or MW)
4.3.4. Plant Operating States
This section defines the most common operating states of biological CO2-Methanation plants. It is recommended to use this terminology and to indicate the parameters specific to individual states. ‘Temperature’ T in °C, ‘pressure’ p in bar and ‘methane production rate’ (see Section 4.4.6) should always be provided for each operating state.
- In the ‘nominal operating state’, the plant is operating at its nominal capacity (PN,Meth).
- When the plant is operated in a ‘partial load operation state’, the load should be provided as percent of the nominal operating capacity.
- In ‘intermittent operation state’ (i.e., load following), the plant is operated in accordance to the availability of (renewable) electric power for hydrogen generation which is the essential idea behind the Power-to-Gas concepts. For this operating mode there is no specific parameter definition, but the conditions of operation should be provided, i.e., frequency and order of magnitude of load changes.
- ‘Hot standby’ (HSB) is defined as the operation state that is chosen during short breaks of operation with the possibility to change quickly back to one of the aforementioned operation states. Therefore, temperature and pressure settings in warm standby are expected to be as at nominal operating state, but the reactant gas feed is stopped.
- The plant is put in ‘cold standby’ (CSB) during longer shut down of operation. In this mode, most components are not in operation to save energy. Accordingly, the temperature is allowed to decrease to a critical value where heating is only switched on to avoid damage to the process e.g., due to freezing of liquids. System pressure and gas composition can be different from operating conditions.
- ‘Shut down’ (SD) describes the state of no operation. Only components to ensure plant safety like gas detection or fire detection are in operation.
4.3.5. Reactant and Product Gas Specification
For the feed gases hydrogen and carbon dioxide as well as for the product gas, specifications listed in Table 6 should be provided. Methane content in the product gas should always be given as maximum mole fraction and as mole fraction at nominal point in percent.

Table 6.
Recommendation on feed and product gas specifications to indicate.
The mole fraction for different gases i is defined in Equation (9) for ideal gases. The unit of the mole fraction y is percent by volume or amount of substance and must be given in % and explicitly not as e.g., vol.-%:
4.4. Performance Parameters
4.4.1. Gas Hourly Space Velocity
The gas hourly space velocity (GHSV) is the ratio between the gas inflow and the reactor volume. To distinguish between the operation with pure carbon dioxide and gases where carbon dioxide is only a fraction of the carbon feed gas (e.g., with biogas), we define two different space velocities. One being related to the entire reactant gas mixture (GHSVt, Equation (10)) with an total gas inflow in m³/h. The other only to the hydrogen supplied to the methanation plant (GHSVH2, Equation (11)). It is important to mention that in contrast to the calculation of the gas retention time (Section 4.4.2), the volumetric flows are provided at normal conditions in (and not or Nm3/h) [22,67,87]:
4.4.2. Gas Retention Time
The gas retention time provides information on the average time the reactant gases remain in the reactor assuming plug-flow (i.e., no back mixing). For both the continuous trickle-bed reactor and the CSTR, the gas retention time is defined by the superficial gas velocity at reactor pressure and the reactor height hR with the cross-sectional area without reactor internals as follows in Equation (12):
The retention time is calculated by the reactant gas flow or as the mean retention time calculated by the average gas velocity [88]. As the mean retention time depends on the conversion rate, we recommend doing the calculation with the total gas flow. So is specified as the total gas flow (hydrogen, carbon dioxide and other gases if present, e.g., methane or nitrogen) entering the system. With the methanation reaction progressing, however the gas volumes and volumetric flows are reduced by a factor of five (Equation (1)) and therefore, vG,in and can have different values at different parts of the reactor. Therefore in Equation (13) we propose a second gas retention time which refers to the average gas flow between and :
4.4.3. Hydraulic Retention Time and Liquid Recirculation
In biological CO2-Methanation processes, usually there is also an exchange of the reactor liquid. This can be due to the requirement of (i) removing water formed by the methanation reaction (Equation (1)), (ii) a continuous addition of liquid process feeds to maintain the process (see Section 4.5.5 for more information) or (iii) the requirement of liquid recirculation due to the reactor concept e.g., with trickle-bed reactors. Therefore, analogously to , the hydraulic retention time should be specified:
with the liquid recirculation :
4.4.4. Gas Conversion Rate
The gas conversion rate X can be defined for both feed gases and describes the amount of hydrogen and carbon dioxide consumed for the generation of methane (and biomass). The conversion rate can be specified based on volumetric, mass and molar flows (Equations (16) and (17)):
4.4.5. Methane Yield
The absolute methane yield provides information on how many normal cubic meters of gas are required for the generation of one normal cubic meter of methane. We define two methane yields. The first and preferred one is related to hydrogen because it gives information about the power converted:
Due to the stoichiometry of the reaction (Equation (1)), this yield can never be higher than 0.25 (Equation (18)). The second yield refers to carbon dioxide (Equation (19)). In continuous plant operation, this value often contains errors due to high solubility of the carbon dioxide. It can reach a maximum value of 1 or (100%):
In order to report the yield as value between zero and 100%, the relative methane yield is defined by the methane yield relative to the theoretical maximum of 0.25 (Equation (20)):
4.4.6. Methane Production Rate
Due to the specific characteristics of reactors used for biological methanation, there are several definitions of the methane production rate (Table 1). We suggest to always indicate two of them: at first the (Equation (21)) reporting the methane produced in the system in norm cubic meter per hour normalized to the reactor volume. It provides information on the productivity of the plant:
Second, which indicates the reactor power given by the energy of the produced methane by lower heating value in proportion to the reactor volume VR (Equation (22)):
Other common definitions are the absolute methane production rate MPR which indicates the total amount of methane produced in the system in nominal point as m3/h (Equation (23)):
Or the methane production rate normalized to only the liquid volume contained in the reactor (MPRL, Equation (24)) which rather provides information on the productivity of the biocatalyst of biological CO2-Methanation:
An additional definition is to relate the methane produced to the packing of the trickle-bed reactor, but is neglected here, since it would be very specific to this reactor type and the other definitions for the methane production rate already provide comparable information on the methane productivity of the system.
4.4.7. Methane Production Dynamics or Load Change Rates
The methane production dynamics describe the reaction time of the system towards varying loads. Specifically, the following information should be provided:
- Start-up time from any operating state to partial and full load (partial loads should be given as percent of the nominal operating capacity, see Section 4.3.4).
- In case of load following operation, no specific states as for the nominal operating are specified. But the reaction times should be specified together with a description of the expected load variation dynamics.
Load change rate from shut down to nominal operating point LCRSD-N is defined as the nominal plant capacity PN in kW or MW divided by the startup time from shut down to nominal operating state (Equation (25)):
Load change rate from hot standby to nominal operating point LCRHSB-N is defined as the difference ΔPHSB-N in capacity between nominal operating point PN and power consumption in hot standby Pel,HSB divided by the startup time from hot standby to nominal operating state ΔtHSB-N (Equation (26)):
Load change rate from cold standby to nominal operating point LCRCSB-N is defined as the difference ΔPCSB-N in capacity between nominal operating point PN and power consumption in cold standby Pel,CSB divided by the startup time from cold standby to nominal operating state ΔtCSB-N (Equation (27)):
Load change rate from partial load to nominal operating state LCRxN-N is defined as die capacity difference ΔPxN-N between power consumption in partial load PxN and nominal power consumption PN divided by the time of load change ΔtxN-N from PxN to PN (Equation (28)):
With x = 0–0.99 as partial load coefficient indicating zero to 99 % of nominal power consumption.
4.4.8. Methanation Efficiency
The energetic efficiency of the biological CO2-Methanation process is determined by methane output and input according to the previously introduced system boundaries (Section 3.1). We do not consider the process components for the supply of gases (hydrogen, carbon dioxide and pressurized air) and units for a potentially required pre- and post-treatment of water and gases for the definition of the efficiency of the biological CO2-Methanation.
As previously introduced in Section 3.1.1, the energy content of the gas flows is calculated by multiplying the mass flows with the enthalpy flow of each gas (Equations (3) and (4)). The energy content of further mass flows (such as additional gases, water, nutrients and biomass) is not considered in the efficiency calculation (Equation (29)):
As temperature and pressure of the gas volume flows are not considered, the efficiency calculation can be simplified by using lower heating values (LHVs) instead of the enthalpy flows (Equation (30)):
For a better comparability to the gas-sector, we recommend using lower (LHV) and not upper heating values (UHV).
4.4.9. Specific Power Demand
For each operation state, the specific power demand should be reported. The specific power demand for biological methanation (Equation (31)) is defined as the ratio of electrical power supplied to the methanation unit Pel,meth and the volumetric flow of converted methane . Equation (31) is valid for both cases: for methane influx e.g., at biogas upgrade applications () and for the case that only hydrogen and carbon dioxide are fed to the system ():
Additionally, the specific power demand during hot standby (HSB), cold standby (CSB) and shut down (SD) of the methanation plant should be specified as average power. This should be done as absolute energy demand EBM,CSB, EBM,WSB, EBM,SD (in kW) and as specific power demand eBM,CSB, eBM,HSB, eBM,SD in relation to the nominal power of the methanation plant PN (in ) with n = CSB, HSB, SD (Equation (32)):
4.4.10. System Availability
The annual system availability should be given in operating hours top per year (h/a) and in percent (Equation (33)). Operating hours per year top are affected by periods of regular repair, maintenance and deployment planning. Information should be provided about how many hours the system can be operated continuously at the nominal point (full load hours per year) and how much maintenance time tmaint is required (how often the system requires a maintenance and how long the system is not operating during this timeframe):
4.4.11. Gas and Liquid Hold-up, Effective Surface Area
The gas hold-up describes the amount of gaseous phase VG related to the reaction volume VReaction according to Equation (34):
The liquid hold-up contains the liquid volume (including the volume of suspended microorganisms and solids) related to the reaction volume VReaction (Equation (35)):
The effective surface area for gas-liquid mass transfer aeff is defined as the relation between the specific surface Aspec in m3/m3 within the reaction volume referred to the reaction volume VReaction itself (Equation (36)):
Specific surface Aspec for TBR, is the specific surface of the packing. For CSTR and BCR it is the total surface of dispersed gas bubbles and for MR, it is the total active membrane surface.
4.5. Microbiology-Based Parameters
Although methanogens are the core component of biological CO2-Methanation, literature often attaches too little importance to this ‘component’ of the reactor. Ongoing research projects and industrial pilot plants either work with pure cultures of methanogens [28,89] or use e.g., sludge from wastewater treatment plants [44,46,90] for inoculation. In both cases, data provided on the inoculum and the methanogenic strains are rather inconsistent and/or incomplete. Consequently, without considering biocatalytic parameters, a neutral comparison of the performance of different methanation systems and reactor types can be hardly given. To overcome these difficulties, authors of biological CO2-Methanation processes should provide detailed information about the organisms used in each project following the microbiology-based parameters proposed in this chapter. This includes the type and origin of the inoculum, the species or genus/genera of methanogenic archaea, the biocatalyst concentration and the nutrients required for growth and methanation. In addition, further information e.g., hydraulic retention time for nutrient feeding, stirring of the liquid (in case of CSTRs) and recirculation (in case of trickle-bed reactors) should be provided.
4.5.1. Methanogenic Archaea
All microorganisms that produce methane as main product during metabolism belong to the domain of Archaea, established by Carl Woese in 1990 [91]. Methanogens are still erroneously called Bacteria in many scientific studies, which should be avoided, and the correct term Archaea should be used instead. In biological CO2-Methanation, the methanogenic archaea catalyze the conversion of carbon dioxide and hydrogen to methane. This process called hydrogenotrophic methanogenesis is the last step of degradation of complex organic compounds in oxygen-free environments like wetlands, rice paddies, the digestive tract of ruminants or humans but also on hydrothermal vents or at black smokers in the deep sea [29,92]. Accordingly, methanogens are very divers, not only in morphology and growth requirements, but also taxonomically.
4.5.2. Type and Origin of the Inoculum
To better understand the background of the different projects in biological CO2-Methanation, we aim to encourage researchers to state whether they inoculate their reactor with pure cultures or with organic material like sludge or slurry from biogas or wastewater treatment plants. In the latter case, the name and location of the plant including the date of collection should be provided together with the inoculation ratio, the number of methanogens in the inoculum in % of all and the most abundant genera/species in the inoculum. In case of pure cultures, the correct name of the species should be given (see Section 4.5.3 for more details), the designation of the strain, and where the culture originates from (e.g., culture collection including strain number or lab that provided the strain). In addition, if the organism is a type strain, this has to be indicated by superscription of the letter T behind the name of the strain and the culture collection number (e.g., Methanothermobacter thermoautotrophicus, strain deltaHT, DSM 1053T). When own isolates are used, the origin of the sample and the enrichment/isolation procedure should be mentioned. Same standards should be applied when a mixture of defined strains is utilized.
4.5.3. Nomenclature of Species/Genera of Methanogenic Archaea
All names of species and higher taxonomic ranks (genus, family) should be given in accordance with the ‘List of Prokaryotic Names with Standing in Nomenclature’ (LPSN) [93,94,95,96]. This includes writing all names of validly published species, genera, families and orders in italics. For names included in the category Candidatus, researchers should follow the instructions given by the LPSN. Investigators should be aware that, in case of methanogenic archaea, classification changed fundamentally in the early 2000s [97,98]. This resulted in the renaming of several organisms, e.g., Methanothermobacter thermoautotrophicus instead of Methanobacterium thermoautotrophicum or Methanothermococcus lithotrophicus instead of Methanococcus lithotrophicus. Unfortunately, the old and by now incorrect names are still found in literature confusing operators and researchers not familiar with taxonomy.
4.5.4. Biocatalyst Concentration
In biological CO2-Methanation, the biocatalyst concentration refers to the cell density or amount of the methanogenic archaea in the reactor. For planktonic cells suspended in the liquid volume VL of the reactor, the cell density should be given as cells per milliliter (cells/ml) determined by e.g., cell counting or as grams dry weight of biomass per milliliter (gDW/ml).
For biofilms present in trickle-bed or fixed bed reactors, the packing material from different zones of the reactor should be analyzed by e.g., scanning electron or fluorescent microscopy to analyze the number of adherent cells. Weighing of material from the reactor seems to be inapplicable because, for pure cultures, the weight of the cells is neglible compared to the material and for sludge, the weight of non-degraded organic material and non-methanogenic cells is much higher than that of the biocatalyst. In case of projects working with organic material like sludge or slurry, the number of methanogens (in percent of all organisms) and/or the most abundant genera/species, optimally for both, the inoculum and the mixture in the established reactor system should be determined.
4.5.5. Nutrients and other Supplements
The performance of biological methanation systems relies to a large extent on the fitness and viability of the biocatalyst, i.e., the methanogenic microorganisms. Therefore, the conditions in the reactor should be optimal for the methanogens in terms of nutrients, temperature and pH-value. These parameters should be the minimum provided for each project working on biological CO2-Methanation.
The nutrient solution in which the microorganisms are cultivated, also called the growth medium, has to provide a matrix as found in the natural habitat of the methanogenic archaea to allow optimal growth and performance. Studies on biological CO2-Methanation should therefore always state the compounds that are present in the medium used, together with their individual concentrations. Generally, those components are all essential elements present in living cells, namely carbon C, hydrogen H, oxygen O, nitrogen N, sulfur S, phosphor P and trace elements like nickel Ni, iron Fe, selenium Se, or cobalt Co. In biological CO2-Methanation processes, H and C are provided by the reactant gases hydrogen and carbon dioxide. If the organisms can grow autotrophically, meaning that they use the carbon dioxide not only for energy generation but also for synthesis of organic cell material, no other carbon source is needed in the medium. In case of reactors inoculated with slurry or sludge, other microorganisms present in the organic material can also produce hydrogen, carbon dioxide or other carbon compounds that can be used by the methanogens. All the further elements besides H and C are usually provided in form of inorganic salts or organic compounds. Exemplarily, sodium sulfide (Na2S) is often used as sulfur source and additionally serves as reducing agent.
Foam formation due to high cell densities of methanogenic archaea in the liquid volume VL at high gas throughputs can cause plugging of gas and condensate pipelines or pumps and thereby damage the reactor and downstream equipment. To prevent foam-formation, antifoam agents (AFA) like oils, fatty acids or esters can be added to the reactor (see Vardar-Sukan [99] for detailed information about AFA and their mode of action). Similar to nutrients, authors should state which AFA they use, its concentration and how (dissolved in e.g., the medium or as additional solution) and how often it is given to the process.
4.5.6. Reactor-related Parameters
Since the conversion of carbon dioxide and hydrogen to methane results in the formation of water (Equation (1)), nutrients are diluted during the biological CO2-Methanation process and have to be added to the reactor either continuously or periodically in all operating states with reactant gas feed. Consequently, it should be provided which nutrients (like medium or trace elements) are fed together with their concentration and their hydraulic retention time (see Section 4.4.3).
In addition, parameters specific for the reactor type should be provided. In case of CSTR, this comprises the rotation speed and energy input of the stirrer, since the methane production rate depends on the solution of the reactant gases in the liquid, which correlates with the stirring velocity. For trickle-bed reactors, the trickling liquid circulation rate has to be given as part of the hydraulic retention time (see Section 4.4.3). Also, reactor temperature at nominal point TR,N, nominal pH-value and reactor pressure at nominal point pR,N schould be mentioned here.
4.6. Cost Parameters
In reality, the demand for biological CO2-Methanation systems will be mostly determined by their profitability. Therefore, one of the goals of this article is to define several parameters that allow a comparison of different systems from an economical point of view.
We suggest reporting two different cost parameters for biological CO2-Methanation systems: the first is related to the capital expenditures (CAPEX) and should be provided as cost per power contained per normal cubic meter product gas at the nominal point (related to the lower heating value LHV) in €/kW. The other regards the operational expenditures (OPEX) and should be given in €/kWh also related to nominal point of operation and LHV.
For definition of the cost parameters of the methanation system, those components within the system boundaries of the ‘CO2-Methanation process’ unit (as described in Section 3.1.2) should be considered. For cost calculation, all costs listed in Table 7 should be considered as part of the methanation unit.

Table 7.
CAPEX and OPEX cost parameters for the biological CO2-Methanation process.
The following points should be considered as already present on site and not regarded as part of the costs of the methanation process:
- Building works on site or construction site preparation (e.g., foundations, fences Specification of required connections and weight of the methanation unit by provider)
- Water supply and sewage hook-up on site (definition of water quality, delivery pressure, amounts and connection size specified by supplier)
- Gas connections for methane available on site (definition of gas amounts, pressure and size of the injection station by provider)
- Connection to hydrogen and carbon dioxide source available on site
- Heating management: heating and cooling water circuit available on site
- Pressurized air available on site
- Flare available
- All project development costs (e.g., environmental impact assessments, noise insulation/protection certificates, architectural surveys, landscape planning, permits, fire protection, feasability studies, property/land costs, etc.)
5. Conclusions
Biological CO2-Methanation as part of PtG is a promising technology to store energy from renewable sources on a global scale. However, many challenges have to be faced to be competitively viable with chemical CO2-Methanation processes or other PtG or Power-to-Liquids applications. One of the main challenges at this moment of time is the inconsistent use of parameters and units for description of different biological CO2-Methanation approaches. Hence, this paper recommends a new set of parameters and system boundary definitions to unify and standardize data given in upcoming publications on biological CO2-Methanation. The definitions set herein are summarized in a parameter matrix presenting three standard levels that range from a detailed scientific description in standard A to basic information in standard C. The presented nomenclature and most of the calculation standards are applicable to chemical CO2-Methanation and partly also to one-step processes like microbial electrochemical cells. Project managers and authors are highly encouraged to use this matrix to facilitate performance comparisons between different biological CO2-Methanation technologies and bioreactor principles, all defined by various CO2 sources, regulatory requirements for gas grid injection or other local and application-specific requirements. Additionally, our standardization approach is an essential prerequisite in further application and improvement of the technology, in particular for upscaling and for comparing efficiencies and costs. By this, our standard will help to highlight advantages of a certain biological or chemical CO2-Methanation project and will be of special interest for commercial clients to attract and obtain funding or financiers.
The need for a standardization is strongly supported by the fact, that the first authors of this work initiated the formation of the VDI 4635 guideline committee which aims to ensure a consistent and reliable comparison of PtG projects in the future. The definitions reported in this paper are the starting point for normalization and comparability of biological and chemical CO2-Methanation and will be extended also to other Power-to-X applications like Power-to-Methane, Power-to-Liquids and Power-to-Chemicals.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1073/12/9/1670/s1: Table A1 as excel- and csv-file for easy fill in.
Author Contributions
Conceptualization, M.T., M.H., and T.W.; data curation, M.T., T.W., M.H. and A.B.; investigation, M.T., A.B. and T.W.; methodology, M.T., M.H. and T.W.; validation, M.S., J.K.; resources, M.S., J.K., H.H., D.S., R.B. and D.H.; writing—original draft preparation, M.T., M.H. and T.W.; writing—review and editing, M.T., M.H., A.B., T.W., F.M., T.T., M.N., F.H., J.G., M.K., C.S., F.O., D.H., M.S. and J.K.; visualization, M.T., F.M.; supervision, M.S., J.K., A.B., R.B., F.O., M.N. and D.H.; project administration, M.S., M.T.; funding acquisition, M.S., J.K. and A.B.
Funding
This research was funded by the German Federal Ministry of Economic Affairs and Energy, grant number 03ET6125.
Acknowledgments
We acknowledge support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding programme Open Access Publishing.
Conflicts of Interest
The authors declare no conflict of interest. Also, there is no conflict with the upcoming guideline VDI 4635 Power-to-X.
Nomenclature
| Acronym | Meaning | Units |
| A | System availability | % |
| AFA | Antifoam agents | - |
| AG | Additional gases | - |
| Al2O3 | Aluminium oxide | - |
| Cross-sectional area of the reactor | m3 | |
| Specific surface area | m3/m3 | |
| BCR | Bubble column reactor | - |
| BM | Biomass | - |
| CAPEX | Capital expenditures | €/kW |
| CH4 | Methane | - |
| Concentration of i in gas phase | mol/m3 | |
| Concentration of i in liquid phase | mol/m3 | |
| CNG | Compressed natural gas | - |
| CO2 | Carbon dioxide | - |
| Co | Cobalt | - |
| COS | Carbonyl sulfide | y |
| CSB | Cold standby | - |
| CSTR | Continuously stirred tank reactor | - |
| Reactor diameter | m | |
| Packing diameter | m | |
| DVGW | German Technical and Scientific Association for Gas and Water | - |
| Absolute energy demand of biological CO2-Methanation | kWh/d | |
| Specific power demand biol. CO2-Methanation | ||
| Specific power demand biol. CO2-Meth. during cold standby | ||
| Specific power demand biol. CO2-Meth. during hot standby | ||
| Specific power demand biol. CO2-Meth. during shut-down | ||
| Gas hold-up | - | |
| Liquid hold-up | - | |
| ηtotal | Methanation efficiency | - |
| Fe | Iron | - |
| Absolute footprint of methanation plant | m3 | |
| Footprint of methanation plant (specific area value) | m3/kW | |
| Gas hourly space velocity related to hydrogen supplied | h−1 | |
| Gas hourly space velocity related to entire reactant gas mixture | h−1 | |
| Dimensionless Henry-coefficient | - | |
| Reactor height | m | |
| Packing height | m | |
| H2 | Hydrogen | - |
| H2O | Water | - |
| H2S | Hydrogen sulfide | - |
| HSB | Hot stand-by | - |
| kL,i | Liquid mass transfer coefficient | m/s |
| Load change rate from shut down to nominal operating point | kW/s | |
| Load change rate from hot standby to nominal operating point | kW/s | |
| Load change rate from cold standby to nominal operating point | kW/s | |
| Load change rate from partial load x to nominal operating point | kW/s | |
| LHV | Lower heating value | kW or MW |
| LPSN | List of Prokaryotic Names with Standing in Nomenclature | - |
| Mass flow rate | kg s−1 | |
| MPR | Methane production rate (referred to volume) | |
| Methane production rate normalized to reactor volume | h−1 | |
| Methane production rate normalized to liquid volume | h−1 | |
| Methane production rate (referred to energy/LHV) | kW/m3 | |
| MR | Membrane reactor | - |
| N | Nitrogen | - |
| Material/molar flow rate | mol s−1 | |
| Ni | Nickel | - |
| NT | Nutrients | - |
| O2 | Oxygen | - |
| OPEX | Operational expenditure | €/kWh |
| P | Phosphor | - |
| Total nominal capacity | kW or MW | |
| Nominal capacity of methanation (gas capacity rel. to LHV) | ||
| p | (Process) Pressure | bar(a) |
| Reactor pressure at nominal point | bar(a) | |
| Volume specific power input | Wh/m3 | |
| pH | pH-Value | - |
| PtG | Power-to-Gas | - |
| PtG-CH4 | Power-to-Methane, Power-to-Gas-Methane | - |
| PtG-H2 | Power-to-Hydrogen, Power-to-Gas-Hydrogen | - |
| Liquid recirculation rate | m3/h | |
| Ratio of H2/CO2 used in the methanation process | %/% | |
| S | Sulphur | - |
| SD | Shut-down | - |
| Se | Selenium | - |
| SNG | Synthetic natural gas | - |
| STP | Standard Temperature and Pressure | - |
| - | System availability | h/a |
| tmaint | Maintenance time | h/a |
| Operation time | h/a | |
| T | (Process) Temperature | °C |
| TBR | Trickle-bed reactor | - |
| Reactor temperature at nominal point | °C | |
| Gas retention time | s | |
| Gas retention time referred to average gas flows | s | |
| Hydraulic retention time | s | |
| UHV | Upper heating value | kW or MW |
| Volumetric flow rate | m3/h | |
| Total gas inflow rate | m3/h | |
| Gas velocity | m/s | |
| Gas volume | m3 | |
| Liquid volume | m3 | |
| Packing volume | m3 | |
| Reactor volume | m3 | |
| Reaction volume | m3 | |
| Carbon dioxide conversion rate | - | |
| Hydrogen conversion rate | - | |
| Absolute methane yield related to hydrogen | - | |
| Absolute methane yield related to carbon dioxide | - | |
| Relative methane yield | - | |
| Maximum methane mole fraction | % | |
| Nominal methane mole fraction | % | |
| Hydrogen mole fraction | % | |
| Carbon dioxide mole fraction | % |
Appendix A
In Table A1, we propose a blueprint containing all standardized parameters and units described in this article, which we recommend for project description. This blueprint contains three levels of detail: the ‘C-Standard’ only provides basic information on the most important parameters. The ‘B-Standard’ presents an extended version of C and additionally includes economic aspects. The ‘A-Standard’ contains all parameters presented in this article and is meant to provide detailed scientific data and background information.

Table A1.
Variety in indications and units in current literature on CO2-methanation in trickle-bed reactors (extension of Table 1). Minor differences in spelling or case sensitivities reflect inaccuracies of indications and units in literature.
Table A1.
Variety in indications and units in current literature on CO2-methanation in trickle-bed reactors (extension of Table 1). Minor differences in spelling or case sensitivities reflect inaccuracies of indications and units in literature.
| Parameters | Indications from Literature | Units from Literature |
|---|---|---|
| Process temperature | K/°C [35,40] | |
| °C [33,34,36,38,42,43,44,45,46] | ||
| Process pressure | ambient [43,44,46]; atm [35,40] | |
| bar/mbar [33,36,38,45] | ||
| Methane concentration | [36,37,43,44] CH4 at outlet [33] CH4 concentrations [33] End concentrationT [38] Gas components in outlet gas [34] Methane concentration(T) [36,43,44] Methane content of the exit gas [42]; (in/of the product gasT) [37,38] | % [33,34,37,38,42,44,45,46] vol% [43] Vol.-% [36] vol.% [47]2 vol-% [54]2 |
| Reactant gas composition | Feeding gas mixture [48] Gas (inlet) composition [38,40] H2/CO2 ratio [33,38]; H2/CO2 ratio [33]; RatioT H2:CO2 [37] H2-CO2-relationship [44] H2/CO2 (gas) mixture [41,43] Loading rateT C/H [45] Mixed gas feed rate [42] v/v CO2/H2 volumetric shareT [37] | [43]; [38,42]; [41] [33] mole percent CO2, mole percent H2 [35] Nl/h [45] v/v [37,42]; %v/v [41] % vol. [39] |
| Loading rate | bR [36]; H2 [43] | [33] |
| Gas feedT [37]; Hydrogen feed rate [34] | [36] | |
| G (gas flow rate) [35] | (T) [43,44] | |
| H2 loading rate [33,43] | [37] | |
| LRH2 [33,44]; Loading rateT C/H [45] | [34] | |
| OLR (Organic loading rate) [43] | mL/h [35]; Nl/h [45] | |
| Injection rate [50]1 | [54]2 | |
| Volumetric gas loading rate [50]1 | ml/min [50]2 | |
| Hydrogen conversion rate | H2 consumption [35]; H2 conversion [33,34] H2-degradation rate [43] Removal efficiency [39] Utilisation efficiency of H2 [48] Conversion rate [50]1 | % [33,34,36,39,42,43,48] mmol/h [35]; mmol/hr [40] |
| Carbon dioxide conversion rate | analogous to Hydrogen conversion rate | analogous to Hydrogen conversion rate |
| Flow Rate (Gas) | Flow rate [43] G [40]; qin [43] Gas flow rate [35,40]; Inflow rate [41] Hydrogen feed rate [34]; Gas feedT [37] Loading rateT C/H [45] | l/h [42]; L/h [35]; Nl/h [45] ml/min [34,41] mL/hr [40]; mL/h [40] [43] |
| (Specific) Recirculation rate of the trickle medium | Liquid recycle rate [35] Medium feed rate [42] Nutrient solution circulation [100] Qfl [36]; QRV [44] Recirculation (rate) [34,43] SprinklingT [36] | m3/s [33] mL/min [35,100]; l/min [38]; ml/h [42]; ml/72 h [34] m3/(m3·d) [36] (T) [44] [43] |
| Methane yield | CH4 yield on H2 [40]; Methane yield(T) [36,43]; CH4 yield [54]1 Y [36,43]; YCH4 [42]; Y(x/CH4) [47]1 Yield [35] Growth product yield [47]1 | % [40,42] mol/mol [35] [36,43] [47]2 [54]2 |
| Gas retention time | (Average) retention time [33,43,44] [39,43,44] Gas retention time (GRT) [39,48,50]1 | h [33,34,39,44] |
| Hydraulic retention time | (Calcul. average) retention time [34,39] τ [39] | h [34,39,44] |
| Reactor volume3 | Fixed-bed volume [43]; Packed volume [100]; Volume of packed support [42]; VSV [44]; Trickle-bedT [37] Volume [33,44] Working volumeT [37] | mL [40]; l [34,36,38,41,43,44,45]; L [37,46] cm3 [35,42]; m3 [33,100] |
Indices: Indication (1) or unit (2) found in other literature than on trickle-bed systems (not comprehensive); 3 vague, which volume is meant (packing volume, net reactor volume, liquid volume, etc.). Terms from German references were translated into English language (Index T) or allotted to the appropriate English term (Index (T)).

Table A2.
Parameter matrix blueprint and standard classification. ‘A-Standard’: detailed scientific information, ‘B-Standard’: basic and economic information, ‘C-Standard’: basic information provided. x parameter has to be provided in the respective standard; - parameter not needed to fulfill the respective standard.
Table A2.
Parameter matrix blueprint and standard classification. ‘A-Standard’: detailed scientific information, ‘B-Standard’: basic and economic information, ‘C-Standard’: basic information provided. x parameter has to be provided in the respective standard; - parameter not needed to fulfill the respective standard.
| Parameter | Unit | Standard | |||
|---|---|---|---|---|---|
| A | B | C | |||
| System-related Parameters | |||||
| Reactor and Plant Type | |||||
| Reactor type | - | x | x | x | |
| Required plant components (according to ‘CO2-Methanation process’ boundary) | - | x | |||
| Mode of operation | - | x | |||
| Plant/process characteristics | - | x | |||
| Reactor and Liquid Volume, h/d-Ratio, Packing Volume and Surface, Hold-up | |||||
| Reactor volume () | m3 | x | x | x | |
| Reactor height () | m | x | |||
| Reactor diameter () | m | x | |||
| h/d ratio (h/d) | - | x | |||
| Liquid volume () | m3 | x | |||
| Packing volume () | m3 | x | |||
| Packing height () | m | x | |||
| Packing diameter () | m | x | |||
| Gas volume ( | m3 | x | |||
| Reaction volume ( | m3 | x | |||
| Liquid hold-up ( | - | x | |||
| Gas hold-up () | - | x | |||
| Effective surface for gas-liquid mass transfer () | m−1 | x | |||
| Plant Size | |||||
| Nominal capacity of methanation () | kW or MW | x | x | ||
| Methanation plant footprint () | m3 | x | |||
| Normalized methanation plant footprint ( | m3/kW | x | |||
| Volume specific power input ( | Wh/m3 | x | |||
| Nominal plant capacity () | kW or MW | x | x | x | |
| Operating States (indicate at least T, p, MPR for each state) | |||||
| Nominal operating state (TN; pN; MPRN) | °C; bar(a); h−1 | x | |||
| Partial load operating state (TPL; pPL; MPRPL) | °C; bar(a); h−1 | x | |||
| Intermittent operating state (TIM; pIM; MPRIM) | °C; bar(a); h−1 | x | |||
| Hot standby (HSB) | °C; bar(a); h−1 | x | |||
| Cold standby (CSB) | °C; bar(a); h−1 | x | |||
| Shut down (SD) | °C; bar(a); h−1 | x | |||
| Reactant and Product Gas Specification | |||||
| Reactant gas mole fraction (, , ) | % | x | |||
| Maximum methane purity () | % | x | x | x | |
| Nominal methane purity () | % | x | x | x | |
| Product gas mole fraction (, ) | % | x | |||
| Contamination levels of oxygen, hydrogen sulfide, ammonia tolerated by the system | ppm or ppb | x | |||
| Thresholds of components tolerated by the methanation process (only feed gas) | - | x | |||
| Moisture content/humidity (only product gas) | x | ||||
| Performance Parameters | |||||
| Gas hourly space velocity related to entire reactant gas mixture () | h−1 | x | x | ||
| Gas hourly space velocity related to hydrogen supplied () | h−1 | x | x | ||
| Gas retention time () | s | x | x | ||
| Gas retention time () | s | x | |||
| Hydraulic retention time () | s | x | |||
| Liquid recirculation () | m3/h | x | |||
| Carbon dioxide gas conversion rate () | - | x | |||
| Hydrogen gas conversion rate () | - | x | |||
| Absolute methane yield related to hydrogen () | x | ||||
| Absolute methane yield rel. to carbon dioxide () | x | ||||
| Relative methane yield () | - | x | |||
| Methane production rate normalized to reactor volume () | h−1 | x | x | x | |
| Methane production rate relating to lower heating value of methane produced () | x | x | x | ||
| Absolute Methane production rate (MPR) | x | ||||
| Methane production rate normalized to liquid volume (MPRL) | h−1 | x | |||
| Load change rate from shut down to nominal operating point () | kW/s | x | x | ||
| Load change rate from hot standby to nominal operating point () | kW/s | x | |||
| Load change rate from cold standby to nominal operating point () | kW/s | x | |||
| Load change rate from partial load x to nominal operating point () | kW/s | x | |||
| Methanation efficiency () | % | x | x | ||
| Specific power demand () | x | ||||
| Specific power demand during hot standby () | x | ||||
| Specific power demand during cold standby () | x | ||||
| Specific power demand during shut-down () | x | ||||
| Absolute energy demand biological CO2-Methanation (EBM) | kWh/d | x | |||
| System availability top and A | h/a and % | x | |||
| Maintenance time | h/a | x | |||
| Microbiology-based Parameters | |||||
| Type and Origin of the Inoculum | |||||
| Information, whether pure/defined mixed cultures or organic material (like slurry, etc.) is used* | pc, dmc, om* | x | x | x | |
| For pure/defined mixed cultures (pc/dmc) | |||||
| Name(s) of species | - | x | x | ||
| Strain designation (incl. type strain or not) | - | x | |||
| Culture origin (culture collection no. or providing lab) | - | x | x | ||
| If own strain(s): origin of sample and enrichment procedure | - | x | |||
| For organic material (om) like sludge or slurry | |||||
| Sampling site (name and location of plant incl. date of collection) | - | x | x | ||
| Inoculation ratio | - | x | |||
| Number of methanogens in inoculum | % of all organisms | x | x | ||
| Most abundant genera/species in inoculum | gen./spec. name | x | |||
| Biocatalyst Concentration | |||||
| Cell density | cells/ml or gDW/ml | x | x | ||
| Amount of adherent cells (if biofilm) | - | x | x | ||
| Number of methanogens during operation (if sludge or slurry as inoculum) | % of all organisms | x | x | ||
| Most abundant genera/species in established reactor system (if sludge or slurry as inoculum) | - | x | |||
| Nutrients and Supplements | |||||
| Media composition | - | x | x | ||
| Additives at operation start (like reducing agent, buffers, etc.) | - | x | x | ||
| Additives during operation (like reducing agents, AFA, etc.) | - | x | |||
| Reactor-related Parameters | |||||
| Reactor/process temperature nominal () | °C | x | x | x | |
| pH-value | - | x | x | ||
| Reactor/process pressure () | bar(a) | x | x | x | |
| Rotation speed (ωST) and energy input (EST) of the stirrer (for CSTR) | rpm and kWh/d | x | |||
| Cost Parameters | |||||
| Capital expenditures (CAPEX) | €/kW | x | x | ||
| Operational expenditures (OPEX) | €/kWh | x | x | ||
| Contact Information | |||||
| Contact person biology (name, affiliation, email, phone) | x | x | x | ||
| Contact person engineering (name, affiliation, email, phone) | x | x | x | ||
* pc: pure culture, dmc: defined mixed culture, om: organic material.
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R. Summary for Policymakers. In Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Conference of the Parties. Paris Agreement. 2015. Available online: https://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf (accessed on 16 April 2019).
- United Nations Framework Convention on Climate Change (UNFCCC). 24th Conference of the Parties: COP24. Available online: https://cop24.gov.pl/ (accessed on 18 December 2018).
- IRENA. Global Energy Transformation. A Roadmap to 2050. 2018. Available online: https://www.irena.org//media/Files/IRENA/Agency/Publication/2018/Apr/IRENA_Report_GET_2018.pdf (accessed on 16 April 2019).
- WRI, (n.d.). Höhe der Weltweiten Treibhausgasemissionen Nach Quellgruppe im Jahr 2014 (in Millionen Tonnen CO2-Äquivalent). Available online: https://de.statista.com/statistik/daten/studie/311844/umfrage/globale-treibhausgasemissionen-nach-quellgruppe/ (accessed on 6 September 2018).
- IEA, (n.d.). Verteilung der Energiebedingten CO2-Emissionen Weltweit Nach Sektor im Jahr 2015. Available online: https://de.statista.com/statistik/daten/studie/167957/umfrage/verteilung-der-co-emissionen-weltweit-nach-bereich/ (accessed on 16 April 2019).
- Blanco, H.; Faaij, A. A review at the role of storage in energy systems with a focus on Power to Gas and long-term storage. Renew. Sustain. Energy Rev. 2018, 81, 1049–1086. [Google Scholar] [CrossRef]
- Götz, P.; Huneke, F.; Lenck, T.; Linkenheil, C.P. Minimaler Bedarf an Langfristiger Flexibilität im Stromsystem bis 2050: Studienerweiterung; Greenpeace Energy: Berlin, Germany, 2016. [Google Scholar]
- Energiespeicher. Bedarf, Technologien, Integration, 2nd ed.; Sterner, M., Stadler, I., Eds.; Springer: Berlin, Germany, 2017; ISBN 978-3-662-48892-8. [Google Scholar]
- Thema, M.; Sterner, M.; Lenck, T.; Götz, P. Necessity and Impact of Power-to-gas on Energy Transition in Germany. Energy Procedia 2016, 99, 392–400. [Google Scholar] [CrossRef]
- Sedlacek. Untertage-Erdgasspeicherung in Deutschland: Underground Gas Storage in Germany. Erdgasspeicherung. Erdöl Erdgas Kohle 2016, 132, 409–417. [Google Scholar]
- Angenent, L.T.; Usack, J.G.; Xu, J.; Hafenbradl, D.; Posmanik, R.; Tester, J.W. Integrating electrochemical, biological, physical, and thermochemical process units to expand the applicability of anaerobic digestion. Bioresour. Technol. 2018, 247, 1085–1094. [Google Scholar] [CrossRef]
- Vereinigung der Fernleitungsnetzbetreiber Gas e.V. Netzentwicklungsplan Gas 2012–2014. FNBGas. Available online: https://www.fnb-gas.de/files/2015-01-28_nep_gas_2014.pdf (accessed on 10 April 2019).
- Newton, J. Power-to-Gas and Methanation-Pathways to a ‘Hydrogen Economy’. In Proceedings of the 14th Annual Apgtf Workshop, London, UK, 12–13 March 2014. [Google Scholar]
- Wiede, T.; Land, A. Sektorenkopplung: Amprion und Open Grid Europe Geben Power-to-Gas in Deutschland einen Schub; Amprion GmbH, Open Grid Europe: Berlin, Germany, 2018. [Google Scholar]
- Northseawindpowerhub.eu. Power to Gas: North Sea Wind Power Hub. Available online: https://northseawindpowerhub.eu/ (accessed on 16 April 2019).
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Utilization of surplus electricity from wind power for dynamic biogas upgrading: Northern Germany case study. Biomass Bioenergy 2014, 66, 126–132. [Google Scholar] [CrossRef]
- Gas for Climate-A path to 2050. Role of Renewable Gas and the Existing Gas Infrastructure in 2050. Available online: https://gasforclimate2050.eu/news (accessed on 16 April 2019).
- Bär, K.; Mörs, F.; Götz, M.; Graf, F. Vergleich der biologischen und katalytischen Methanisierung für den Einsatz bei PtG-Konzepten. Gwf-Gas 2015, 156, 466–473. [Google Scholar]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F.-Z. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Neubert, M.; Treiber, P.; Krier, C.; Hackel, M.; Hellriegel, T.; Dillig, M.; Karl, J. Influence of hydrocarbons and thiophene on catalytic fixed bed methanation. Fuel 2017, 207, 253–261. [Google Scholar] [CrossRef]
- Neubert, M.; Hauser, A.; Pourhossein, B.; Dillig, M.; Karl, J. Experimental evaluation of a heat pipe cooled structured reactor as part of a two-stage catalytic methanation process in power-to-gas applications. Appl. Energy 2018, 229, 289–298. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation-From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Götz, M.; McDaniel Koch, A.; Graf, F. State of the Art and Perspectives of CO2 Methanation Process Concepts for Power-to-Gas Applications. Conference Paper. 2014. Available online: https://www.researchgate.net/publication/273139805_State_of_the_Art_and_Perspectives_of_CO2_Methanation_Process_Concepts_for_Power-to-Gas_Applications (accessed on 16 April 2019).
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported Catalysts for CO2 Methanation: A Review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Martin, M.R.; Fornero, J.J.; Stark, R.; Mets, L.; Angenent, L.T. A Single-Culture Bioprocess of Methanothermobacter thermautotrophicus to upgrade Digester Biogas by CO2-to-CH4 Conversion with H2. Archaea 2013, 2013, 157529. [Google Scholar] [CrossRef]
- Enzmann, F.; Mayer, F.; Rother, M.; Holtmann, D. Methanogens: Biochemical background and biotechnological applications: Mini-Review. AMB Express 2018, 8, 1. [Google Scholar] [CrossRef]
- Lecker, B.; Illi, L.; Lemmer, A.; Oechsner, H. Biological hydrogen methanation-A Review. Bioresour. Technol. 2017, 245, 1220–1228. [Google Scholar] [CrossRef]
- Geppert, F.; Liu, D.; van Eerten-Jansen, M.; Weidner, E.; Buisman, C.; ter Heijne, A. Bioelectrochemical Power-to-Gas: State of the Art and Future Perspectives: Review. Focus on Bioelectrochemistry. Trends Biotechnol. 2016, 34, 879–894. [Google Scholar] [CrossRef]
- Graf, F.; Krajete, A.; Schmack, U. Techno-Ökonomische Studie zur Biologischen Methanisierung bei Power-to-Gas-Konzepten. Abschlussbericht. 2014. Available online: http://www.dvgw-innovation.web33.dvgw-sc.de/fileadmin/dvgw/angebote/forschung/innovation/pdf/g3_01_13.pdf (accessed on 16 April 2019).
- Rachbauer, L.; Voitl, G.; Bochmann, G.; Fuchs, W. Biological biogas upgrading capacity of a hydrogenotrophic community in a trickle-bed reactor. Appl. Energy 2016, 180, 483–490. [Google Scholar] [CrossRef]
- Alitalo, A.; Niskanen, M.; Aura, E. Biocatalytic methanation of hydrogen and carbon dioxide in a fixed bed bioreactor. Bioresour. Technol. 2015, 196, 600–605. [Google Scholar] [CrossRef]
- Kimmel, D.E.; Klasson, K.T.; Clausen, E.C.; Gaddy, J.L. Performance of trickle-bed bioreactors for converting synthesis gas to methane. Appl. Biochem. Biotechnol. 1991, 28–29, 457–469. [Google Scholar] [CrossRef]
- Burkhardt, M.; Tietze, M.; Jordan, I.; Behrens, J. Biologische Methanisierung im Rieselbettverfahren: Untersuchung im Technikumsmaßstab und scale-up. In Biologische Methanisierung. 2. Fachforum Biologische Methanisierung, Regensburg, Germany, 25 October 2016; Ostbayerisches Technologie-Transfer-Institut e.V., Ed.; OTTI: Regensburg, Germany, 2016; pp. 31–41. [Google Scholar]
- Strübing, D.; Huber, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. Biologische Methanisierung in Rieselbettreaktoren durch Mischbiozönosen unter Thermophilen Bedingungen. In Biologische Methanisierung. 2. Fachforum Biologische Methanisierung, Regensburg, Germany, 25 October 2016; Ostbayerisches Technologie-Transfer-Institut e.V., Ed.; OTTI: Regensburg, Germany, 2016; pp. 43–53. [Google Scholar]
- Dröge, S.; Kreubel, J.; König, H. Biologische Methanisierung im Rieselstrom-Reaktor unter thermophilen Bedingungen. In Biologische Methanisierung. 1. Fachforum Biologische Methanisierung, Regensburg, Germany, 11 November 2015; Ostbayerisches Technologie-Transfer-Institut e.V., Ed.; OTTI: Regensburg, Germany, 2015; pp. 47–57. [Google Scholar]
- Dupnock, T.L.; Deshusses, M.A. High-Performance Biogas Upgrading Using a Biotrickling Filter and Hydrogenotrophic Methanogens. Appl. Biochem. Biotechnol. 2017, 183, 488–502. [Google Scholar] [CrossRef]
- Klasson, K.T.; Cowger, J.P.; Ko, C.W.; Vega, J.L.; Clausen, E.C.; Gaddy, J.L. Methane production from synthesis gas using a mixed culture of R. rubrum, M. barkeri, and M. formicicum. Appl. Biochem. Biotechnol. 1990, 24–25, 317–328. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Lu, J.; Maekawa, T. Continuous methane fermentation and the production of vitamin B12 in a fixed-bed reactor packed with loofah. Bioresour. Technol. 2004, 92, 285–290. [Google Scholar] [CrossRef]
- Jee, H.S.; Nishio, N.; Nagai, S. Continuous CH4 Production from H2 and CO2 by Methanobacterium thermoautotrophicum in a fixed-bed reactor. J. Ferment. Technol. 1988, 66, 235–238. [Google Scholar] [CrossRef]
- Burkhardt, M.; Busch, G. Methanation of hydrogen and carbon dioxide. Appl. Energy 2013, 111, 74–79. [Google Scholar] [CrossRef]
- Burkhardt, M.; Koschack, T.; Busch, G. Biocatalytic methanation of hydrogen and carbon dioxide in an anaerobic three-phase system. Bioresour. Technol. 2015, 178, 330–333. [Google Scholar] [CrossRef]
- Ullrich, T. Einsatz der Biologischen Methanisierung für Power-to-Gas-Konzepte: Hochdruckmethanisierung von H2; Universität Hohenheim, Ostbayerisches Technologie-Transfer-Institut e.V. (OTTI): Regensburg, Germany, 2016. [Google Scholar]
- Strübing, D.; Huber, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. High performance biological methanation in a thermophilic anaerobic trickle bed reactor. Bioresour. Technol. 2017, 245, 1176–1183. [Google Scholar] [CrossRef]
- Bernacchi, S.; Krajete, A.; Herwig, C. Experimental workflow for developing a feed forward strategy to control biomass growth and exploit maximum specific methane productivity of Methanothermobacter marburgensis in a biological methane production process (BMPP). AIMS Microbiol. 2016, 2, 262–277. [Google Scholar] [CrossRef]
- Porté, H.; Kougias, P.G.; Alfaro, N.; Treu, L.; Campanaro, S.; Angelidaki, I. Process performance and microbial community structure in thermophilic trickling biofilter reactors for biogas upgrading. Sci. Total Environ. 2019, 655, 529–538. [Google Scholar] [CrossRef]
- Szuhaj, M.; Acs, N.; Tengolics, R.; Bodor, A.; Rakhely, G.; Kovacs, K.L.; Bagi, Z. Conversion of H2 and CO2 to CH4 and acetate in fed-batch biogas reactors by mixed biogas community: A novel route for the power-to-gas concept. Biotechnol. Biofuels 2016, 9, 102. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, J.H.; Chang, W.S.; Pak, D. Biological conversion of CO2 to CH4 using hydrogenotrophic methanogen in a fixed bed reactor. J. Chem. Technol. Biotechnol. 2012, 87, 844–847. [Google Scholar] [CrossRef]
- Savvas, S.; Donnelly, J.; Patterson, T.; Chong, Z.S.; Esteves, S.R. Biological methanation of CO2 in a novel biofilm plug-flow reactor: A high rate and low parasitic energy process. Appl. Energy 2017, 202, 238–247. [Google Scholar] [CrossRef]
- Biologische Methanisierung; Ostbayerisches Technologie-Transfer-Institut e.V. (OTTI), Ed.; 1. Fachforum Biologische Methanisierung, Regensburg, Sorat Hotel, 11 November 2015; OTTI: Regensburg, Germany, 2015. [Google Scholar]
- Seifert, A.H.; Rittmann, S.; Herwig, C. Analysis of process related factors to increase volumetric productivity and quality of biomethane with Methanothermobacter marburgensis. Appl. Energy 2014, 132, 155–162. [Google Scholar] [CrossRef]
- Laborde, H.P. Comparison of Different Reactor Configurations for Ex-Situ Biological Biogas Upgrading. Masterarbeit; Linköping University: Linköping, Sweden, 2016. [Google Scholar]
- Friedl, M.; Meier, B.; Ruoss, F.; Schmidlin, L. Thermodynamik von Power-to-Gas; Hochschule für Technik Rapperswil: Rapperswil, Switzerland, 2016. [Google Scholar]
- Sterner, M. Bioenergy and Renewable Power Methane in Integrated 100% Renewable Energy Systems. Limiting Global Warming by Transforming Energy Systems. Ph.D. Thesis, University of Kassel, Kassel, Germany, 2009. [Google Scholar]
- Hou, P.; Enevoldsen, P.; Eichman, J.; Hu, W.; Jacobson, M.Z.; Chen, Z. Optimizing investments in coupled offshore wind -electrolytic hydrogen storage systems in Denmark. J. Power Sources 2017, 359, 186–197. [Google Scholar] [CrossRef]
- Kopp, M.; Coleman, D.; Stiller, C.; Scheffer, K.; Aichinger, J.; Scheppat, B. Energiepark Mainz: Technical and economic analysis of the worldwide largest Power-to-Gas plant with PEM electrolysis. Int. J. Hydrogen Energy 2017, 42, 13311–13320. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Biotechnol. 2015, 14, 727–759. [Google Scholar] [CrossRef]
- Lemmer, A.; Ullrich, T. Effect of Different Operating Temperatures on the Biological Hydrogen Methanation in Trickle Bed Reactors. Energies 2018, 11, 1344. [Google Scholar] [CrossRef]
- Buttler, A.; Spliethoff, H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- DVGW Deutscher Verein des Gas- und Wasserfaches e. V. DVGW G 260 (A) Gasbeschaffenheit; Wirtschafts- und Verlagsgesellschaft Gas und Wasser mbH: Bonn, Germany, 2013. [Google Scholar]
- Swiss Gas and Water Industry Association (SVGW). Richtlinie für die Einspeisung von Erneuerbaren Gasen. SVGW G 13, 2016 (G13). Available online: https://epaper.svgw.ch/Epaper/Render/Download/?editionId=279ae750-a781-e711-80d8-001dd8b729e1 (accessed on 16 April 2019).
- Österreichische Vereinigung für das Gas- und Wasserfach (ÖVGW). National Gas in Austria-Gas Quality; Österreichische Vereinigung für das Gas- und Wasserfach (ÖVGW): Wien, Austria, 2001; (ÖVGW G 31). [Google Scholar]
- Deutsches Institut für Normung e.V. Referenzzustand, Normzustand, Normvolumen; Begriffe und Werte. Reference conditions, Normal Conditions, Normal Volume; Concepts and Values; Beuth Verlag GmbH: Berlin, Germany, 1990. [Google Scholar]
- Deutsches Institut für Normung e.V. DIN EN ISO 13443 Natural Gas-Standard Reference Conditions. Erdgas-Standardbezugsbedingungen; Beuth Verlag GmbH: Berlin, Germany, 2007. [Google Scholar]
- International Organization for Standardization. Petroleum Liquids and Liquefied Petroleum Gases-Measurement-Standard Reference Conditions, 75.180.30; ISO 5024; International Organization for Standardization: Geneva, Switzerland, 1999. [Google Scholar]
- DIN Deutsches Institut für Normung e.V. Natural Gas-Calculation of Calorific Values, Density, Relative Density and Wobbe Indices from Composition; Beuth Verlag GmbH: Berlin, Germany, 2016; 75.060 (DIN EN ISO 6976). [Google Scholar]
- Deutsches Institut für Normung e.V. Standard Atmosphere. Normatmosphäre; Beuth Verlag GmbH: Berlin, Germany, 1979; 551.51 (DIN ISO 2533). [Google Scholar]
- Altfeld, K.; Pinchbeck, D. Admissible hydrogen concentrations in natural gas systems: There are proposals to inject hydrogen (H2) from renewable sources in the natural gas network. This measure would allow the very large transport and storage capacities of the existing infrastructure, particularly high-pres- sure pipelines, to be used for indirect electricity transport and storage. Gas Energy 2013, 2013, 1–12, Reprint. [Google Scholar]
- European Association for the Streamlining of Energy Exchange-gas (EASEE-gas). Harmonization of Natural Gas Quality. Common Business Practice. 2005-001/02. 2008. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwjuoo311O3fAhVRKFAKHdNaDRQQFjAAegQICRAC&url=https%3A%2F%2Feasee-gas.eu%2Fdownload_file%2FDownloadFile%2F4%2Fcbp-2005-001-02-harmonisation-of-natural-gas-quality&usg=AOvVaw0najV_hNvzYHY6HmfdWbbh (accessed on 15 January 2019).
- European Commission. Gas Quality Harmonisation. 2019. Available online: https://ec.europa.eu/energy/en/topics/markets-and-consumers/wholesale-market/gas-quality-harmonisation (accessed on 15 January 2019).
- Gikopoulos, C. Einspeisung von Biogas in ein Erdgasnetz. Qualitätserfordernisse aus der Sicht eines Gasnetzbetreibers. Gas&Wärme Energie Steiermark. Available online: http://www.lev.at/Download/Int_Biogas_Ex_StT1/Biogaseinspeisung_Gikopoulos.pdf (accessed on 16 April 2019).
- Bryant, N.; Paxton, A. Gas Quality Harmonisation Cost Benefit Analysis. Final report. 2012. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/2012_gas_quality_harmonisation_cost_benefit_analysis_.pdf (accessed on 15 January 2019).
- Van Renssen, S.; Edwardes-Evans, H. Power in Europe: French prompt surges into 2017. S&P Glob. Platts 2017, 5–8. Available online: http://www.itm-power.com/wp-content/uploads/2017/01/platts-itm-article-Januart-2016.pdf (accessed on 26 April 2019).
- Dansk Gasteknisk Center a/s. Kontrolmanual til Måling af Biogas og Bionaturgas. Naturgasselskabernes Kontrolmanual til Måling af Biogas og Bionaturgas. Kontrolmanual 3. Ugdave. 2015. Available online: https://www.dgc.dk/sites/default/files/filer/publikationer/M02_kontrolmanual_bionaturgas_0.pdf (accessed on 29 January 2019).
- Bekendtgørelse om gasreglementets afsnit C-12, bestemmelser om gaskvaliteter. BEK nr 1264 af 14/12/2012. 2012. Available online: https://www.retsinformation.dk/Forms/R0710.aspx?id=144715 (accessed on 16 April 2019).
- DVGW Deutscher Verein des Gas- und Wasserfaches e. V. DVGW G 262 (A) Nutzung von Gasen aus regenerativen Quellen in der öffentlichen Gasversorgung; Wirtschafts- und Verlagsgesellschaft Gas und Wasser mbH: Bonn, Germany, 2011; G 262 (A). [Google Scholar]
- Berger, R. Development of Business Cases for Fuel Cells and Hydrogen Applications for Regions and Cities. Hydrogen Injection into the Natural Gas Grid; Roland Berger: Munich, Germany, 2017. [Google Scholar]
- Maćkowiak, J. Modellierung des flüssigkeitsseitigen Stoffüberganges in Kolonnen mit klassischen und gitterförmigen Füllkörpern. Chemie Ingenieur Technik 2008, 80, 57–77. [Google Scholar] [CrossRef]
- Chemical Reactors for Gas-Liquid Systems, 1st ed.; Kaštánek, F., Sharp, D.H., Eds.; Ellis Horwood: New York, NY, USA, 1993. [Google Scholar]
- Baerns, M.; Hofmann, H.; Renken, A. Chemische Reaktionstechnik. Lehrbuch der Technischen Chemie-Band 1. Mit 41 Tabellen, 3rd ed.; Thieme: Stuttgart, Germany, 1999; ISBN 313687501X. [Google Scholar]
- Wanner, O.; Debus, O.; Reichert, P. Modelling the spatial distribution and dynamics of a xylene-degrading microbial population in a membrane-bound biofilm. Water Sci. Technol. 1994, 29, 243–251. [Google Scholar] [CrossRef]
- Shen, Y.; Brown, R.; Wen, Z. Syngas fermentation of Clostridium carboxidivoran P7 in a hollow fiber membrane biofilm reactor: Evaluating the mass transfer coefficient and ethanol production performance. Biochem. Eng. J. 2014, 85, 21–29. [Google Scholar] [CrossRef]
- Lee, K.-C.; Rittmann, B.E. A novel hollow-fibre membrane biofilm reactor for autohydrogenotrophic denitrification of drinking water. Water Sci. Technol. 2000, 41, 219–226. [Google Scholar] [CrossRef]
- Biegger, P.; Medved, A.R.; Lehner, M.; Ebner, H.M.; Friedacher, A. Methanisierung im Umfeld von “Power to Gas”. In Kurzfassungsband zum 14. Symposium Energieinnovation, Graz; Verlag der Technischen Universität: Graz, Austria, 2016. [Google Scholar]
- Hertwig, K.; Martens, L.; Hamel, C. Chemische Verfahrenstechnik. Berechnung, Auslegung und Betrieb Chemischer Reaktoren, 3rd ed.; De Gruyter Inc.: Berlin, Germany; Boston, MA, USA, 2018; ISBN 9783110500998. [Google Scholar]
- Electrochaea.dk ApS. Power-to-Gas via Biological Catalysis (P2G-BioCat). Available online: http://biocat-project.com (accessed on 14 December 2018).
- Burkhardt, M.; Buschmann-Kosel, J.; Tietze, M.; Hilse, H. Biologische Methanisierung im Rieselbettverfahren: Leistungs- und Flexibilitätsnachweis im Technikumsmaßstab. Energie Wasser Praxis 2018, 8, 42–45. [Google Scholar]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef]
- Hedderich, R.; Whitman, W.B. Physiology and Biochemistry of the Methane-Producing Archaea. In The Prokaryotes, 4th ed.; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F.L., Eds.; Springer: Berlin, Germany, 2013–2014; pp. 635–662. ISBN 978-3-642-30140-7.
- Euzéby, J.P. List of Bacterial Names with Standing in Nomenclature: A folder available on the Internet. Int. J. Syst. Bacteriol. 1997, 47, 590–592. [Google Scholar] [CrossRef]
- Parte, A.C. LPSN-list of prokaryotic names with standing in nomenclature. Nucleic Acids Res. 2014, 42, D613–D616. [Google Scholar] [CrossRef]
- Parte, A.C. LPSN-List of Prokaryotic names with Standing in Nomenclature (bacterio.net), 20 years on. Int. J. Syst. Evol. Microbiol. 2018, 68, 1825–1829. [Google Scholar] [CrossRef]
- Bacterio.net. List of Procariotic Names with Standing in Nomenclature: LPSN. Available online: http://www.bacterio.net/ (accessed on 14 December 2018).
- Wasserfallen, A.; Nölling, J.; Pfister, P.; Reeve, J.; Conway de Macario, E. Phylogenetic analysis of 18 thermophilic Methanobacterium isolates supports the proposals to create a new genus, Methanothermobacter gen. nov., and to reclassify several isolates in three species, Methanothermobacter thermautotrophicus comb. nov., Methanothermobacter wolfeii comb. nov., and Methanothermobacter marburgensis sp. nov. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 1, 43–53. [Google Scholar] [CrossRef]
- Validation of publication of new names and new combinations previously effectively published outside the IJSEM. Int. J. Syst. Evol. Microbiol. 2002, 52, 685–690. [CrossRef]
- Vardar-Sukan, F. Foaming: Consequences, prevention and destruction. Biotechnol. Adv. 1998, 16, 913–948. [Google Scholar] [CrossRef]
- Rachbauer, L.; Beyer, R.; Bochmann, G.; Fuchs, W. Characteristics of adapted hydrogenotrophic community during biomethanation. Sci. Total Environ. 2017, 595, 912–919. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).