Organic Matter Pore Characterization of the Wufeng-Longmaxi Shales from the Fuling Gas Field, Sichuan Basin: Evidence from Organic Matter Isolation and Low-Pressure CO2 and N2 Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. OM Isolation
2.2.2. TOC and XRD
2.2.3. FE-SEM
2.2.4. LPGA
3. Results
3.1. Organic Geochemistry and Mineralogy
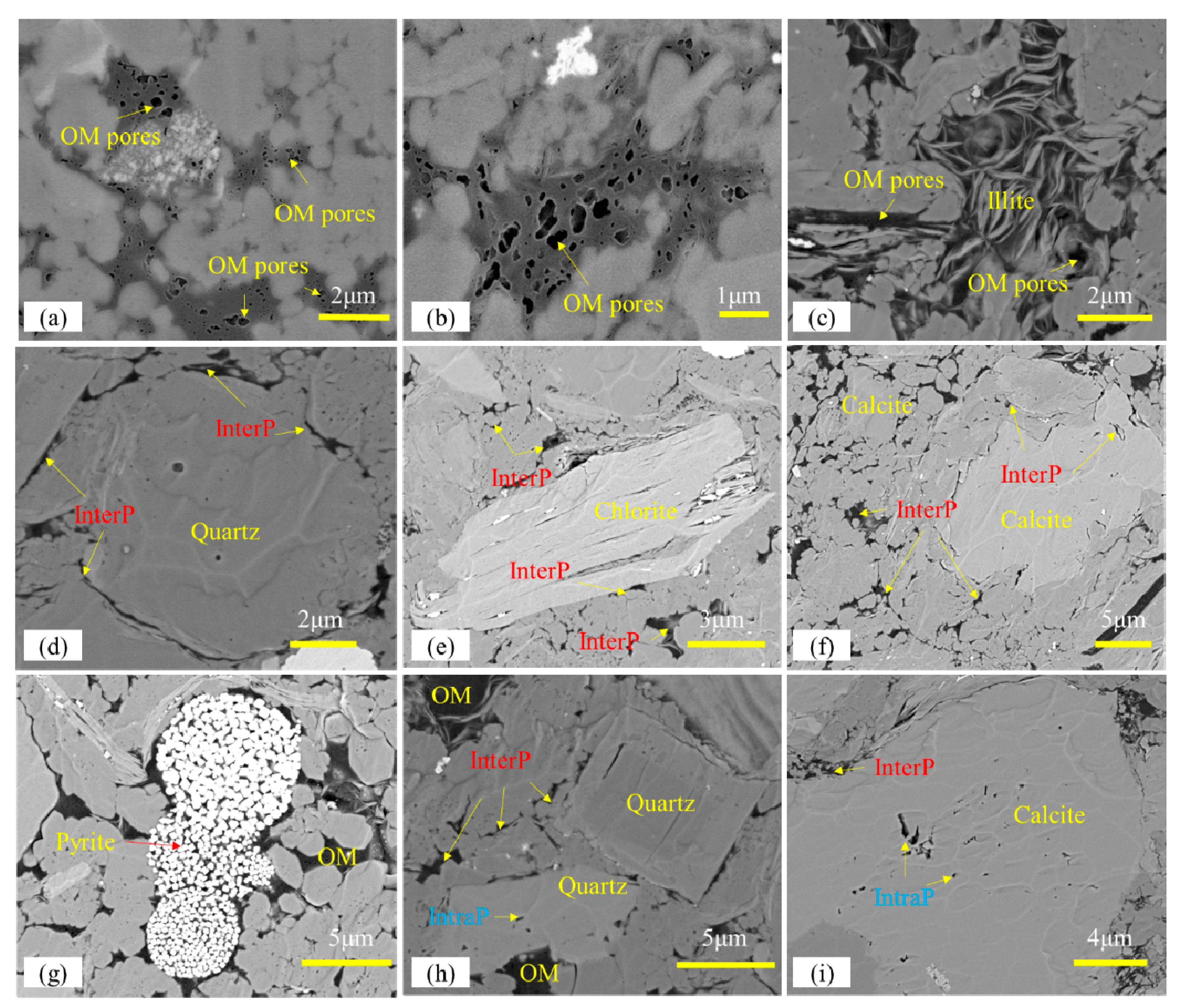
3.2. FE-SEM Imaging
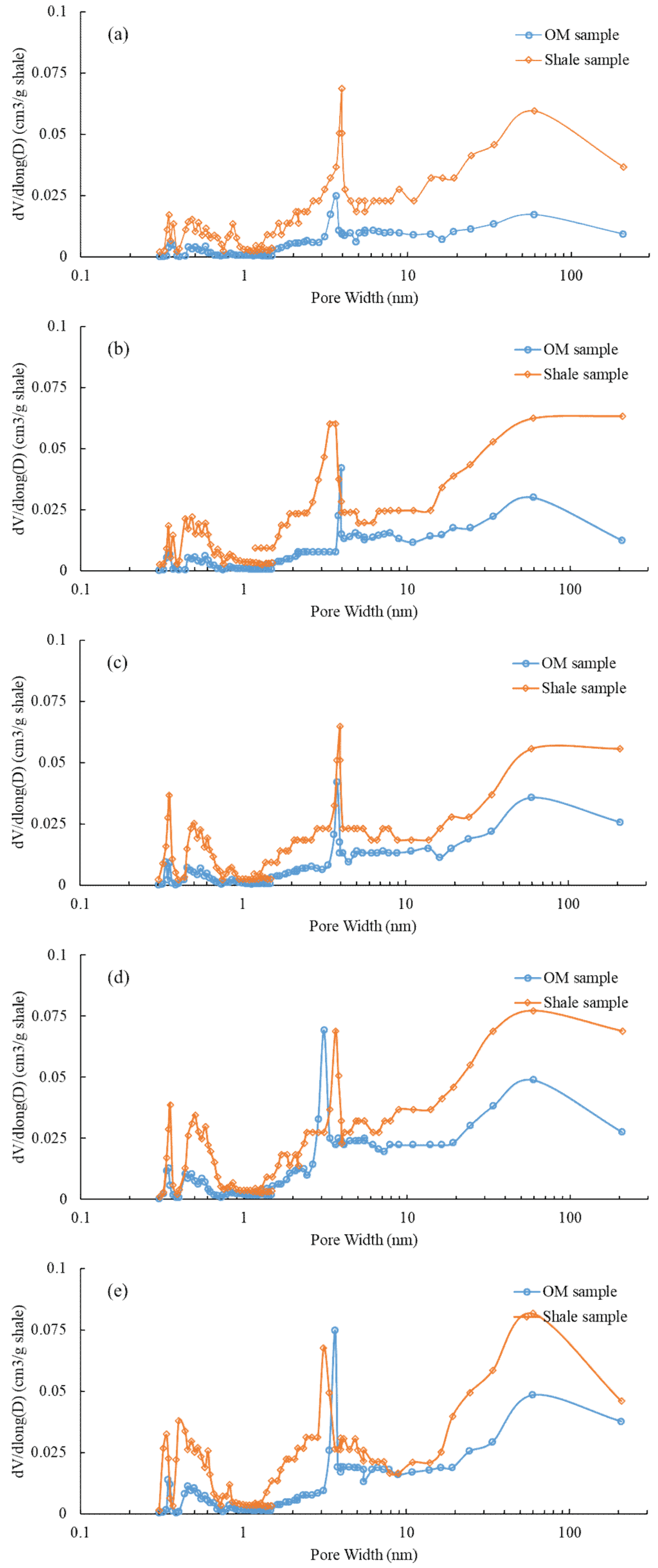
3.3. LPGA (CO2 and N2)
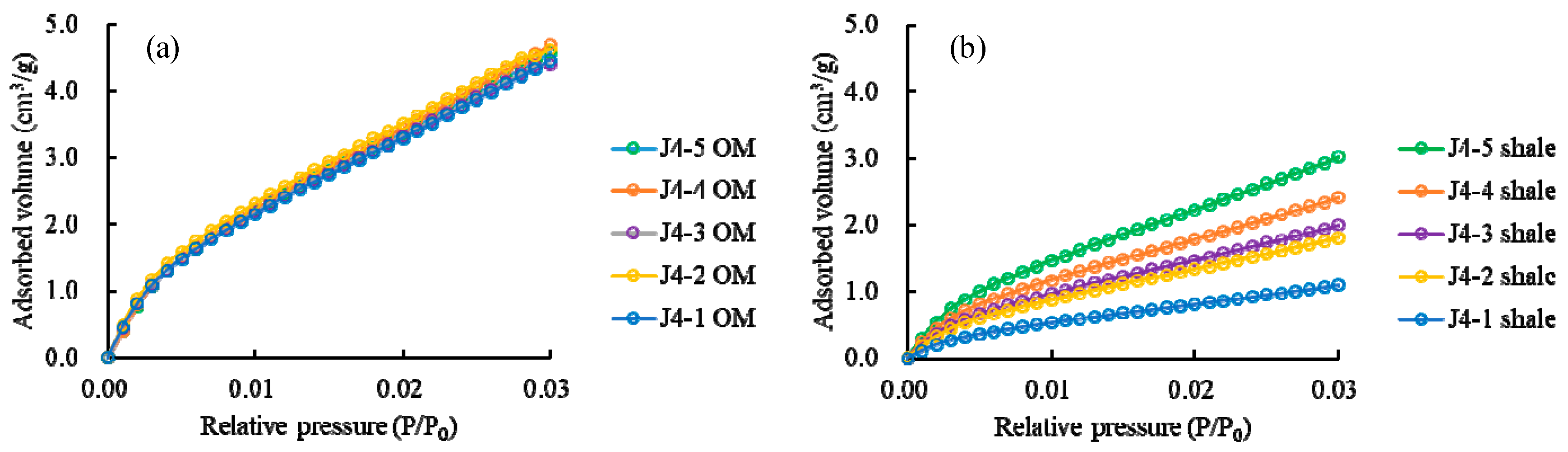
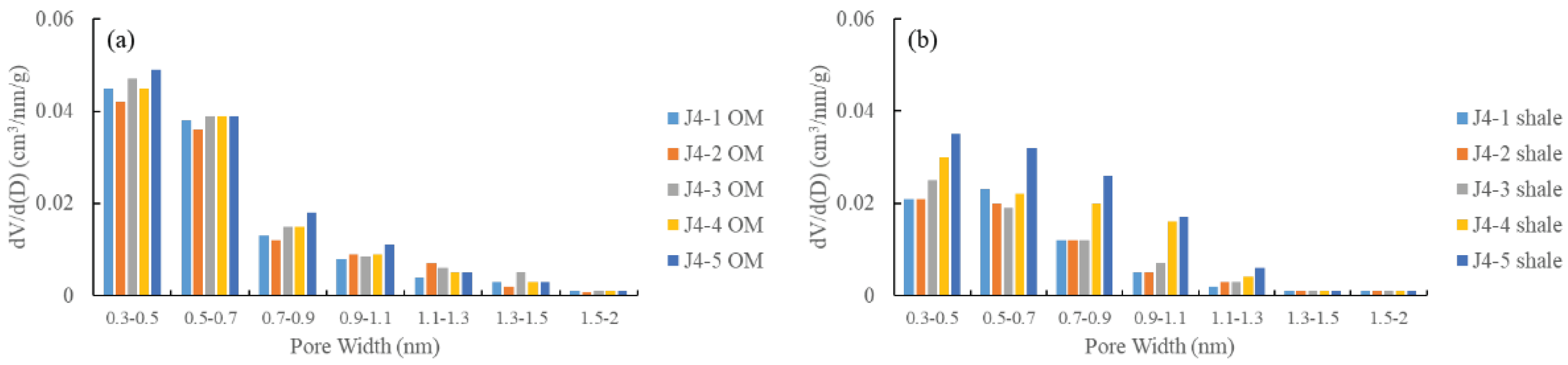
3.3.1. Low-Pressure CO2 Adsorption
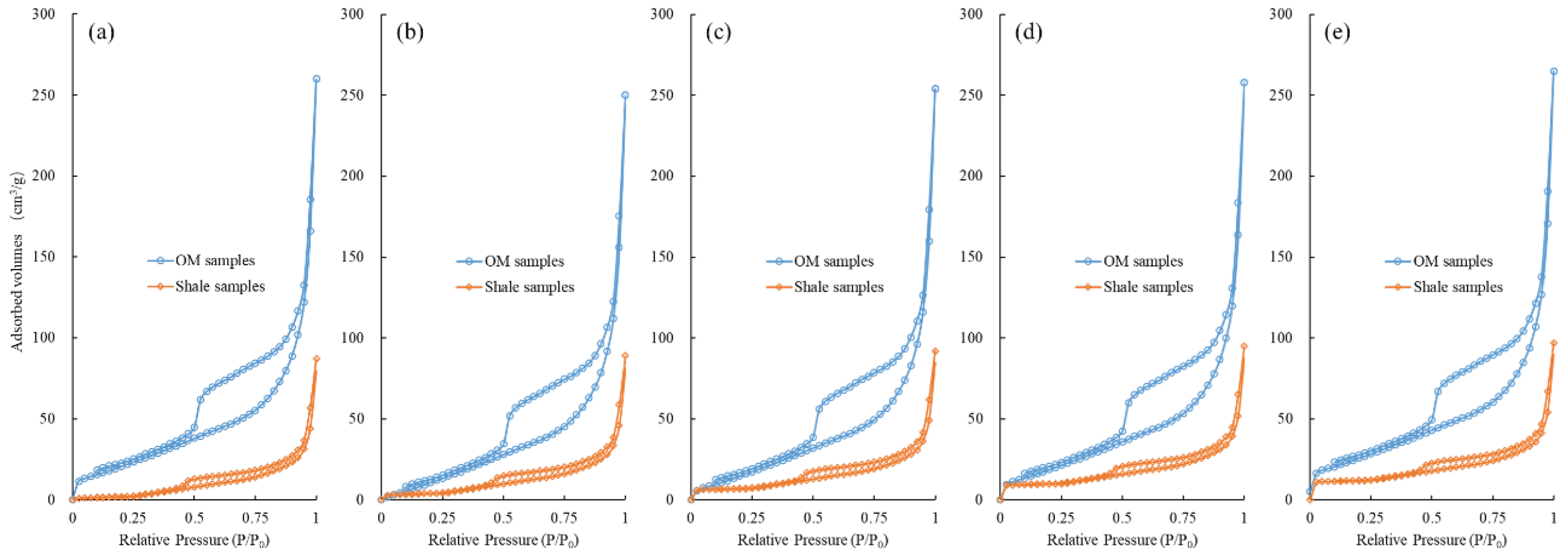
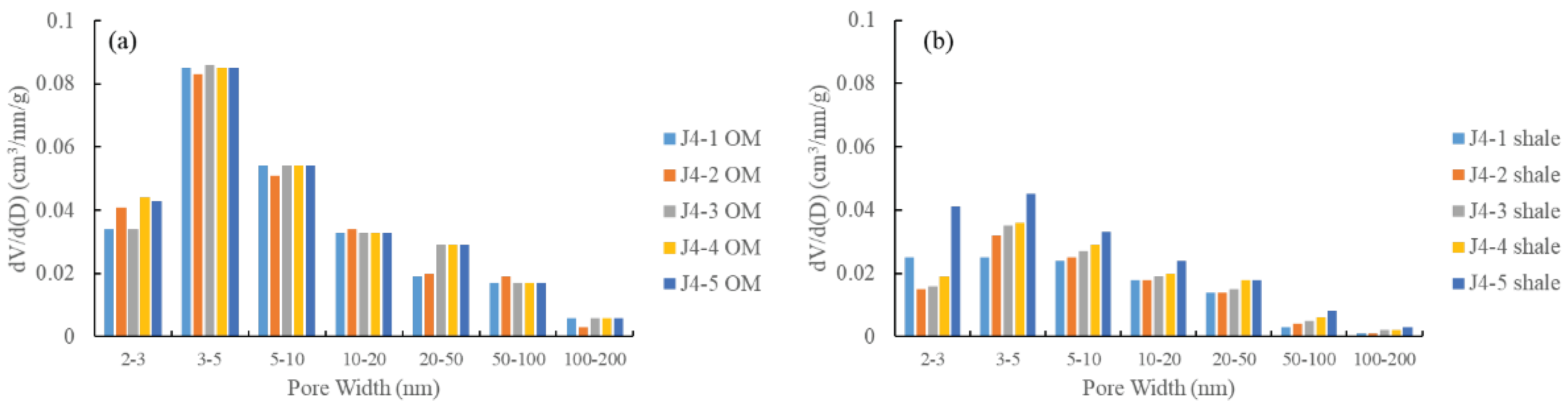
3.3.2. Low-Pressure N2 Physisorption
4. Discussion
4.1. Comparison of PSDs between Isolated OM and Bulk Shales
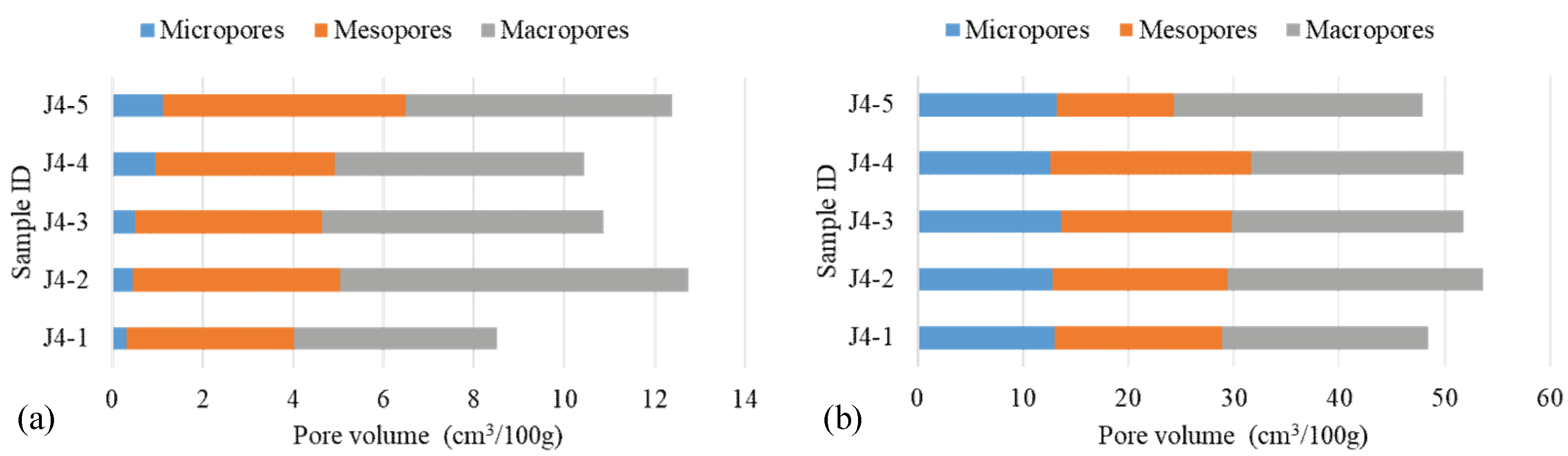
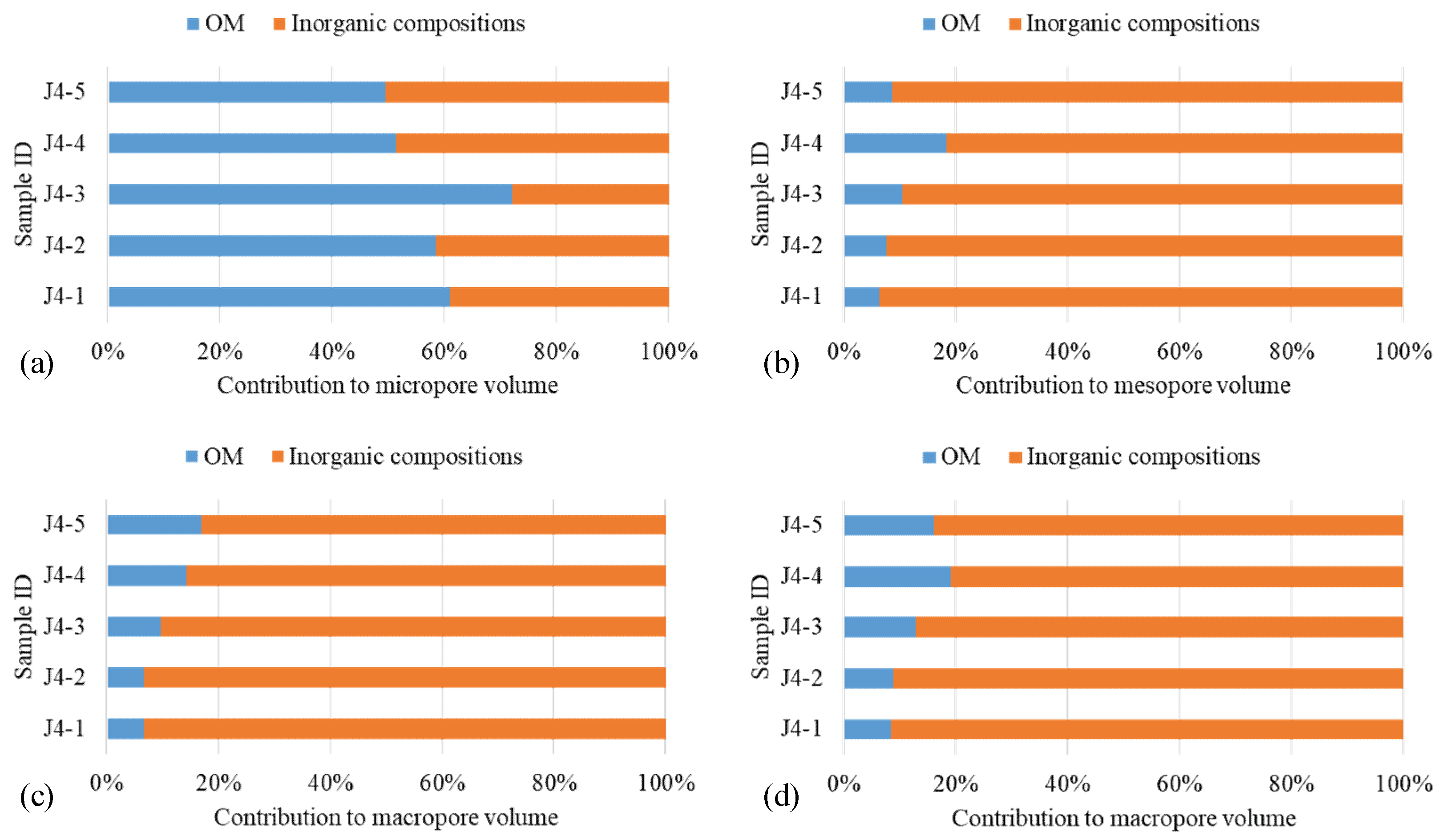
4.2. Contributions of OM Pore Volumes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ross, D.; Bustin, R. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Loucks, R.; Reed, R.; Ruppel, S.; Hammes, U. Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. AAPG Bull. 2012, 96, 1071–1098. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Solano, N.; Bustin, R.M.; Bustin, A.M.M.; Chalmers, G.R.L.; He, L.; Melnichenko, Y.B.; Radlinski, A.P.; Blach, T.P. Pore structure characterization of North American shale gas reservoirs: Using USANS/SANS, gas adsorption, and mercury intrusion. Fuel. 2013, 103, 606–616. [Google Scholar] [CrossRef]
- Sing, K.S.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewsha, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl.Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Wu, K.L.; Chen, Z.X.; Li, X.F. Real gas transport through nanopores of varying cross-section type and shape in shale gas reservoirs. Chem. Eng. J. 2015, 281, 813–825. [Google Scholar] [CrossRef]
- Wu, K.L.; Chen, Z.X.; Li, X.F.; Xu, J.Z.; Li, J.; Wang, K. Flow behavior of gas confined in nanoporous shale at high pressure: Real gas effect. Fuel 2017, 205, 173–183. [Google Scholar] [CrossRef]
- Collell, J.; Galliero, G.; Vermorel, R.; Ungerer, P.; Yiannourakou, M.; Montel, F. Transport of multicomponent hydrocarbon mixtures in shale organic matter by molecular simulations. J. Phys. Chem. C 2015, 119, 22587–22595. [Google Scholar] [CrossRef]
- Lee, T.; Bocquet, L.; Coasne, B. Activated desorption at heterogeneous interfaces and long-time kinetics of hydrocarbon recovery from nanoporous media. Nat. Commun. 2016, 7, 11890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javadpour, F.; McClure, M.; Naraghi, M.E. Slip-corrected liquid permeability and its effect on hydraulic fracturing and fluid loss in shale. Fuel 2015, 160, 549–559. [Google Scholar] [CrossRef]
- Naraghi, M.E.; Javadpour, F. A stochastic permeability model for the shale-gas systems. Int. J. Coal Geol. 2015, 140, 111–124. [Google Scholar] [CrossRef]
- Naraghi, M.E.; Javadpour, F.; Ko, L.T. An object-based shale permeability model: Non-Darcy gas flow, sorption, and surface diffusion effects. Transp. Porous Med. 2018, 125, 23–39. [Google Scholar] [CrossRef]
- Berthonneau, J.; Obliger, A.; Valdenaire, P.L.; Grauby, O.; Ferry, D.; Chaudanson, D. Mesoscale structure, mechanics, and transport properties of source rocks’ organic pore networks. Proc. Nat. Acad. Sci. 2018, 115, 12365–12370. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, genesis, and distribution of nanometer-scale pores in siliceous mudstones of the Mississippian Barnett Shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, Z.; Ji, W.; Zhang, C.; Yuan, Y.; Chen, L. Heterogeneity of intergranular, intraparticle and organic pores in Longmaxi shale in Sichuan basin, south China: Evidence from SEM digital images and fractal and multifractal geometries. Mar. Pet. Geol. 2016, 72, 122–138. [Google Scholar] [CrossRef]
- Milliken, K.L.; Esch, W.L.; Reed, R.M.; Zhang, T. Grain assemblages and strong diagenetic overprinting in siliceous mudrocks, Barnett Shale (Mississippian), Fort Worth Basin, Texas, U.S.A. AAPG Bull. 2012, 96, 1553–1578. [Google Scholar] [CrossRef]
- Milliken, K.L.; Rudnicki, M.; Awwiller, D.N.; Zhang, T. Organic matter-hosted pore system, Marcellus Formation (Devonian), Pennsylvania. AAPG Bull. 2013, 97, 177–200. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R.M.; Power, I.M. Characterization of gas shale pore systems by porosimetry, pycnometry, surface area, and field emission scanning electron microscopy/transmission electron microscopy image analyses: Examples from the Barnett, Woodford, Haynesville, Marcellus, and Doig units. AAPG Bull. 2012, 96, 1099–1119. [Google Scholar]
- Jiang, F.J.; Chen, J.; Xu, Z.; Wang, Z.F.; Hu, T.; Chen, D. Organic matter pore characterization in lacustrine shales with variable maturity using nanometer-scale resolution X-ray computed tomography. Energ. Fuel. 2017, 31, 2669–2680. [Google Scholar] [CrossRef]
- Mastalerz, M.; He, L.; Melnichenko, Y.B.; Rupp, J.A. Porosity of coal and shale: Insights from gas adsorption and SANS/USANS techniques. Energ. Fuel. 2012, 26, 5109–5120. [Google Scholar] [CrossRef]
- Sun, M.; Yu, B.; Hu, Q.; Zhang, Y.; Li, B.; Yang, R.; Melnichenkoe, Y.B.; Cheng, G. Pore characteristics of Longmaxi shale gas reservoir in the Northwest of Guizhou, China: Investigations using small-angle neutron scattering (SANS), helium pycnometry, and gas sorption isotherm. Int. J. Coal Geol. 2017, 171, 61–68. [Google Scholar] [CrossRef]
- Wang, G.; Ju, Y.; Yan, Z.; Li, Q. Pore structure characteristics of coal-bearing shale using fluid invasion methods: A case study in the Huainan–Huaibei Coalfield in China. Mar. Pet. Geol. 2015, 62, 1–13. [Google Scholar] [CrossRef]
- Labani, M.M.; Rezaee, R.; Saeedi, A.; Al Hinai, A. Evaluation of pore size spectrum of gas shale reservoirs using low pressure nitrogen adsorption, gas expansion and mercury porosimetry: A case study from the Perth and Canning Basins, Western Australia. J. Petrol. Sci. Eng. 2013, 112, 7–16. [Google Scholar] [CrossRef]
- Tian, H.; Pan, L.; Xiao, X.M.; Wilkins, R.W.T.; Meng, Z.P.; Huang, B.J. A preliminary study on the pore characterization of Lower Silurian black shales in the Chuandong Thrust Fold Belt, southwestern China using low pressure N2 adsorption and FE-SEM methods. Mar. Pet. Geol. 2013, 48, 8–19. [Google Scholar] [CrossRef]
- Sigal, R.F. Pore-size distributions for organic-shale-reservoir rocks from nuclear magnetic resonance spectra combined with adsorption measurements. SPE J. 2015, 20, 1–7. [Google Scholar] [CrossRef]
- Fleury, M.; Romero-Sarmiento, M. Characterization of shales using T1–T2 NMR maps. J. Petrol. Sci. Eng. 2016, 137, 55–62. [Google Scholar] [CrossRef]
- Meng, M.; Ge, H.; Ji, W.; Shen, Y.; Shuai, S. Monitor the process of shale spontaneous imbibition in co-current and counter-current displacing gas by using low field nuclear magnetic resonance method. J. Nat. Gas. Sci. Eng. 2015, 27, 336–345. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, S.; Li, J.; Chen, C.; Xue, H.; Zhang, J. Petrophysical characterization of oil-bearing shales by low-field nuclear magnetic resonance (NMR). Mar. Petrol. Geol. 2018, 9, 775–785. [Google Scholar] [CrossRef]
- Li, A.; Ding, W.; Jiu, K.; Wang, Z.; Wang, R.; He, J. Investigation of the pore structures and fractal characteristics of marine shale reservoirs using NMR experiments and image analyses: A case study of the Lower Cambrian Niutitang Formation in northern Guizhou Province, South China. Mar. Pet. Geol. 2018, 89, 530–540. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, Z. Fractal characterization of pores in shales using NMR: A case study from the Lower Cambrian Niutitang Formation in the Middle Yangtze Platform, Southwest China. J. Nat. Gas. Sci. Eng. 2016, 35, 860–872. [Google Scholar] [CrossRef]
- Wang, Y.; Pu, J.; Wang, L.H.; Wang, J.Q.; Jiang, Z.; Song, F.Y. Characterization of typical 3D pore networks of Jiulaodong formation shale using nano-transmission X-ray microscopy. Fuel 2016, 170, 84–91. [Google Scholar] [CrossRef]
- Tang, X.L.; Jiang, Z.X.; Jiang, S.; Li, Z. Heterogeneous nanoporosity of the Silurian Longmaxi Formation shale gas reservoir in the Sichuan Basin using the QEMSCAN, FIB-SEM, and nano-CT methods. Mar. Petrol. Geol. 2016, 78, 99–109. [Google Scholar] [CrossRef]
- Wang, P.F.; Jiang, Z.X.; Chen, L.; Yin, L.S.; Li, Z.; Zhang, C.; Tang, X.L.; Wang, G.Z. Pore structure characterization for the Longmaxi and Niutitang shales in the Upper Yangtze Platform, South China: Evidence from focused ion beam-He ion microscopy, nano-computerized tomography and gas adsorption analysis. Mar. Petrol. Geol. 2016, 77, 1323–1337. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.F.; Wang, Q.; Zhang, R.; Krooss, B.M. Pore structure characteristics of lower Silurian shales in the southern Sichuan Basin, China: Insights to pore development and gas storage mechanism. Int. J. Coal Geol. 2016, 156, 12–24. [Google Scholar] [CrossRef]
- Shao, X.; Pang, X.; Li, Q.; Wang, P.; Di, C.; Shen, W. Pore structure and fractal characteristics of organic-rich shales: A case study of the lower Silurian Longmaxi shales in the Sichuan basin, SW china. Mar. Pet. Geol. 2017, 80, 192–202. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Tang, X.H.; Cheng, L.J.; Li, Z.; Zhang, Y.Y.; Bai, Y.Q. Characterization and origin of the Silurian Wufeng-Longmaxi Formation shale multiscale heterogeneity in southeastern Sichuan Basin, China. Interpretation 2015, 3, 61–74. [Google Scholar] [CrossRef]
- Yang, R.; He, S.; Yi, J.; Hu, Q.H. Nano-scale pore structure and fractal dimension of organic-rich Wufeng-Longmaxi shale from Jiaoshiba area, Sichuan Basin: Investigations using FE-SEM, gas adsorption and helium pycnometry. Mar. Petrol. Geol. 2016, 70, 27–45. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Z.X.; Liu, K.Y.; Tan, J.; Gao, F.L.; Wang, P.F. Pore structure characterization for organic-rich Lower Silurian shale in the Upper Yangtze Platform, South China: A possible mechanism for pore development. J. Nat. Gas. Sci. Eng. 2017, 46, 1–15. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Wang, Q.; Liu, H.Q. Pore structure of Cambrian shales from the Sichuan Basin in China and implications to gas storage. Mar. Petrol. Geol. 2016, 70, 14–26. [Google Scholar] [CrossRef]
- Rexer, T.F.; Mathia, E.J.; Aplin, A.C.; Thomas, K.M. High-pressure methane adsorption and characterization of pores in Posidonia shales and isolated kerogens. Energy Fuels 2014, 28, 2886–2901. [Google Scholar] [CrossRef]
- Ji, W.M.; Song, Y.; Rui, Z.; Meng, M.M.; Huang, H.X. Pore characterization of isolated organic matter from high matured gas shale reservoir. Int. J. Coal. Geol. 2017, 174, 31–40. [Google Scholar] [CrossRef]
- Han, H.; Pang, P.; Li, Z.L.; Shi, P.T.; Guo, C.; Liu, Y.; Chen, S.J.; Lu, J.G.; Gao, Y. Controls of organic and inorganic compositions on pore structure of lacustrine shales of Chang 7 member from Triassic Yanchang Formation in the Ordos Basin, China. Mar. Petrol. Geol. 2019, 100, 270–284. [Google Scholar] [CrossRef]
- Ji, W.M.; Song, Y.; Jiang, Z.X.; Chen, L.; Li, Z.; Yang, X.; Meng, M.M. Estimation of marine shale methane adsorption capacity based on experimental investigations of Lower Silurian Longmaxi formation in the Upper Yangtze Platform, south China. Mar. Pet. Geol. 2015, 68, 94–106. [Google Scholar] [CrossRef]
- Jarvie, D.M.; Hill, R.J.; Ruble, T.E.; Pollastro, R.M. Unconventional shale-gas systems: The Mississippian Barnett Shale of northcentral Texas as one model for thermogenic shale-gas assessment. AAPG Bull. 2007, 91, 475–499. [Google Scholar] [CrossRef]
- Zhang, T.W.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R.S. Effect of organicmatter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Gasparik, M.; Bertier, P.; Gensterblum, Y.; Ghanizadeh, A.; Krooss, B.M.; Littke, R. Geological controls on the methane storage capacity in organic-rich shales. Int. J. Coal. Geol. 2014, 123, 34–51. [Google Scholar] [CrossRef]
- Jiang, F.J.; Chen, D.; Wang, Z.F.; Xu, Z.Y.; Chen, J.; Liu, L. Pore characteristic analysis of a lacustrine shale: A case study in the Ordos Basin, NW China. Mar. Petrol. Geol. 2016, 73, 554–571. [Google Scholar] [CrossRef]
- Curtis, M.E.; Ambrose, R.J.; Sondergeld, C.H. Structural Characterization of Gas Shales on the Micro-and Nano-Scales. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, Alberta, Canada, 19–21 October 2010. [Google Scholar]
- Sondergeld, C.H.; Ambrose, R.J.; Rai, C.S.; Moncrieff, J. Microstructural studies of gas shales. SPE J. 2010, 2, 23–25. [Google Scholar]
- Curtis, M.E.; Cardott, B.J.; Sondergeld, C.H.; Rai, C.S. Development of organic porosity in the Woodford shale with increasing thermal maturity. Int. J. Coal. Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Ma, Y.; Zhong, N.N.; Li, D.H.; Pan, Z.J.; Liu, K.Y. Organic matter/clay mineral intergranular pores in the Lower Cambrian Lujiaping Shale in the north-eastern part of the upper Yangtze area, China: A possible microscopic mechanism for gas preservation. Int. J. Coal Geol. 2015, 137, 38–54. [Google Scholar] [CrossRef]
- Chalmers, G.R.L.; Bustin, R.M. A multidisciplinary approach in determining the maceral (kerogen type) and mineralogical composition of Upper Cretaceous Eagle Ford Formation: Impact on pore development and pore size distribution. Int. J. Coal Geol. 2017, 171, 93–110. [Google Scholar] [CrossRef]
- Sun, M.D.; Yu, B.S.; Hu, Q.H.; Yang, R.; Zhang, Y.F.; Li, B. Pore connectivity and tracer migration of typical shales in south China. Fuel 2017, 203, 32–46. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Wang, Y.; Kang, W.; Chen, S.B. Comparison of organic matter occurrence and organic nanopore structure within marine and terrestrial shale. J. Nat. Gas. Sci. Eng. 2016, 32, 356–363. [Google Scholar] [CrossRef]
- Liang, C.; Jiang, Z.X.; Yang, Y.T.; Wei, X.J. Shale lithofacies and reservoir space of the Wufeng–Longmaxi formation, Sichuan Basin, China. Pet. Explor. Dev. 2012, 39, 736–743. [Google Scholar] [CrossRef]
- Han, H.; Cao, Y.; Chen, S.; Lu, J.G.; Huang, C.X.; Zhu, H.H. Influence of particle size on gas-adsorption experiments of shales: An example from a Longmaxi Shale sample from the Sichuan Basin, China. Fuel 2016, 186, 750–757. [Google Scholar] [CrossRef]
- Wei, M.M.; Xiong, Y.Q.; Zhang, L.; Li, J.H.; Peng, P.A. The effect of sample particle size on the determination of pore structure parameters in shales. Int. J. Coal Geol. 2016, 163, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Ufer, K.; Stanjek, H.; Roth, G.; Dohrmann, R.; Kleeberg, R.; Kaufhold, S. Quantitative phase analysis of bentonites by the Rietveld method. Clays Clay Miner. 2008, 56, 272–282. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Mastalerz, M.; Schimmelmann, A.; Drobniak, A.; Chen, Y.Y. Porosity of Devonian and Mississippian New Albany Shale across a maturation gradient: Insights from organic petrology, gas adsorption, and mercury intrusion. AAPG Bull. 2013, 97, 1621–1643. [Google Scholar] [CrossRef]
- Schieber, J. Common themes in the formation and preservation of intrinsic porosity in shales and mudstones: Illustrated with examples from across the Phanerozoic. SPE J. 2010, 2, 23–25. [Google Scholar]
- Pommer, M.; Milliken, K. Pore types and pore-size distributions across thermal maturity, Eagle Ford Formation, southern Texas. AAPG Bull. 2015, 99, 1713–1744. [Google Scholar] [CrossRef]
- Wang, G.; Ju, Y.; Han, K. Early Paleozoic shale properties and gas potential evaluation in Xiuwu basin, western lower Yangtze platform. J. Nat. Gas. Sci. Eng. 2015, 22, 489–497. [Google Scholar] [CrossRef]
- Slatt, R.M.; O’Brien, N.R. Pore types in the Barnett and Woodford gas shales: Contribution to understanding gas storage and migration pathways in fine-grained rocks. AAPG Bull. 2011, 95, 2017–2030. [Google Scholar] [CrossRef]
- Kuila, U.; McCarty, D.K.; Derkowski, A.; Fischer, T.B.; Topór, T.; Prasad, M. Nano-scale texture and porosity of organic matter and clay minerals in organic-rich mudrocks. Fuel 2014, 135, 359–373. [Google Scholar] [CrossRef] [Green Version]
| Sample ID | Depth (m) | TOC (%) | Ro (%) | Quartz (%) | Feldspar (%) | Calcite (%) | Dolomite (%) | Clay mineral (%) | Pyrite (%) |
|---|---|---|---|---|---|---|---|---|---|
| J4-1 | 2282.5 | 1.5 | 2.49 | 35.6 | 10.5 | 7.1 | 1.3 | 41.1 | 3.1 |
| J4-2 | 2354.6 | 2.1 | 2.52 | 37.3 | 13.6 | 5.4 | 1.2 | 37.6 | 2.3 |
| J4-3 | 2368.1 | 2.7 | 2.53 | 41.2 | 8.8 | 3.2 | 0.5 | 38.3 | 3.3 |
| J4-4 | 2386.4 | 3.2 | 2.55 | 39.2 | 11.2 | 5.1 | 1.3 | 38.4 | 3.2 |
| J4-5 | 2408.4 | 4.2 | 2.6 | 51.1 | 7.1 | 0.1 | 0.1 | 38.5 | 2.1 |
| Sample ID | Bulk Shales | Isolated OM | |||
|---|---|---|---|---|---|
| DFT Surface Area (m2/g) | DFT Pore Volume (cm3/100g shale) | DFT Surface Area (m2/g) | DFT Pore Volume (cm3/100g) | DFT Pore Volume (cm3/100g shale) | |
| J4-1 | 12.68 | 0.32 | 32.61 | 13.0 | 0.19 |
| J4-2 | 15.92 | 0.46 | 31.13 | 12.8 | 0.26 |
| J4-3 | 16.88 | 0.51 | 33.34 | 13.6 | 0.36 |
| J4-4 | 24.12 | 0.95 | 30.98 | 12.6 | 0.40 |
| J4-5 | 28.95 | 1.12 | 34.85 | 13.2 | 0.55 |
| Sample ID | Bulk Shales | Isolated OM | |||||
|---|---|---|---|---|---|---|---|
| BET Surface Area (m2/g) | BJH Pore volume (cm3/100 g) | Average Pore Size (nm) | BET Surface Area (m2/g) | BJH Pore Volume (cm3/100 g) | BJH Pore Volume (cm3/100 g shale) | Average Pore Size (nm) | |
| J4-1 | 27.21 | 8.2 | 9.16 | 86.23 | 35.43 | 0.53 | 17.78 |
| J4-2 | 28.20 | 12.29 | 11.44 | 83.28 | 40.80 | 0.86 | 19.72 |
| J4-3 | 29.33 | 10.35 | 9.59 | 80.21 | 38.13 | 1.03 | 19.75 |
| J4-4 | 27.24 | 9.48 | 11.48 | 75.87 | 39.17 | 1.25 | 22.02 |
| J4-5 | 32.34 | 11.27 | 14.15 | 70.22 | 34.67 | 1.46 | 21.72 |
| Sample ID | Bulk Shales (cm3/100 g) | Isolated OM (cm3/100 g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Micropores | Mesopores | Macropores | Total | Micropores | Mesopores | Macropores | Total | |
| J4-1 | 0.32 | 3.70 | 4.50 | 8.52 | 13.04 | 15.83 | 19.60 | 48.43 |
| J4-2 | 0.46 | 4.58 | 7.71 | 12.75 | 12.80 | 16.57 | 24.23 | 53.60 |
| J4-3 | 0.51 | 4.14 | 6.21 | 10.86 | 13.62 | 16.17 | 21.96 | 51.73 |
| J4-4 | 0.95 | 3.98 | 5.50 | 10.43 | 12.61 | 19.05 | 20.12 | 51.77 |
| J4-5 | 1.12 | 5.38 | 5.89 | 12.39 | 13.21 | 11.11 | 23.56 | 47.87 |
| Sample ID | Contributions to the Total Volume of Shales | |||||||
|---|---|---|---|---|---|---|---|---|
| OM | Inorganic Compositions | |||||||
| Micropores (%) | Mesopores (%) | Macropores (%) | Total (%) | Micropores (%) | Mesopores (%) | Macropores (%) | Total (%) | |
| J4-1 | 60.9 | 6.4 | 6.5 | 8.5 | 39.1 | 93.6 | 93.5 | 91.5 |
| J4-2 | 58.4 | 7.6 | 6.6 | 8.8 | 41.6 | 92.4 | 93.4 | 91.2 |
| J4-3 | 72.0 | 10.5 | 9.5 | 12.9 | 28.0 | 89.5 | 90.5 | 87.1 |
| J4-4 | 51.3 | 18.5 | 14.2 | 19.2 | 48.7 | 81.5 | 85.8 | 80.8 |
| J4-5 | 49.5 | 8.7 | 16.8 | 16.2 | 50.5 | 91.3 | 83.2 | 83.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Jiang, Z.; Yu, H.; Liang, Z. Organic Matter Pore Characterization of the Wufeng-Longmaxi Shales from the Fuling Gas Field, Sichuan Basin: Evidence from Organic Matter Isolation and Low-Pressure CO2 and N2 Adsorption. Energies 2019, 12, 1207. https://doi.org/10.3390/en12071207
Li Z, Jiang Z, Yu H, Liang Z. Organic Matter Pore Characterization of the Wufeng-Longmaxi Shales from the Fuling Gas Field, Sichuan Basin: Evidence from Organic Matter Isolation and Low-Pressure CO2 and N2 Adsorption. Energies. 2019; 12(7):1207. https://doi.org/10.3390/en12071207
Chicago/Turabian StyleLi, Zhuo, Zhenxue Jiang, Hailong Yu, and Zhikai Liang. 2019. "Organic Matter Pore Characterization of the Wufeng-Longmaxi Shales from the Fuling Gas Field, Sichuan Basin: Evidence from Organic Matter Isolation and Low-Pressure CO2 and N2 Adsorption" Energies 12, no. 7: 1207. https://doi.org/10.3390/en12071207
APA StyleLi, Z., Jiang, Z., Yu, H., & Liang, Z. (2019). Organic Matter Pore Characterization of the Wufeng-Longmaxi Shales from the Fuling Gas Field, Sichuan Basin: Evidence from Organic Matter Isolation and Low-Pressure CO2 and N2 Adsorption. Energies, 12(7), 1207. https://doi.org/10.3390/en12071207





