Variation of Petrophysical Properties and Adsorption Capacity in Different Rank Coals: An Experimental Study of Coals from the Junggar, Ordos and Qinshui Basins in China
Abstract
:1. Introduction
2. Samples and Experiments
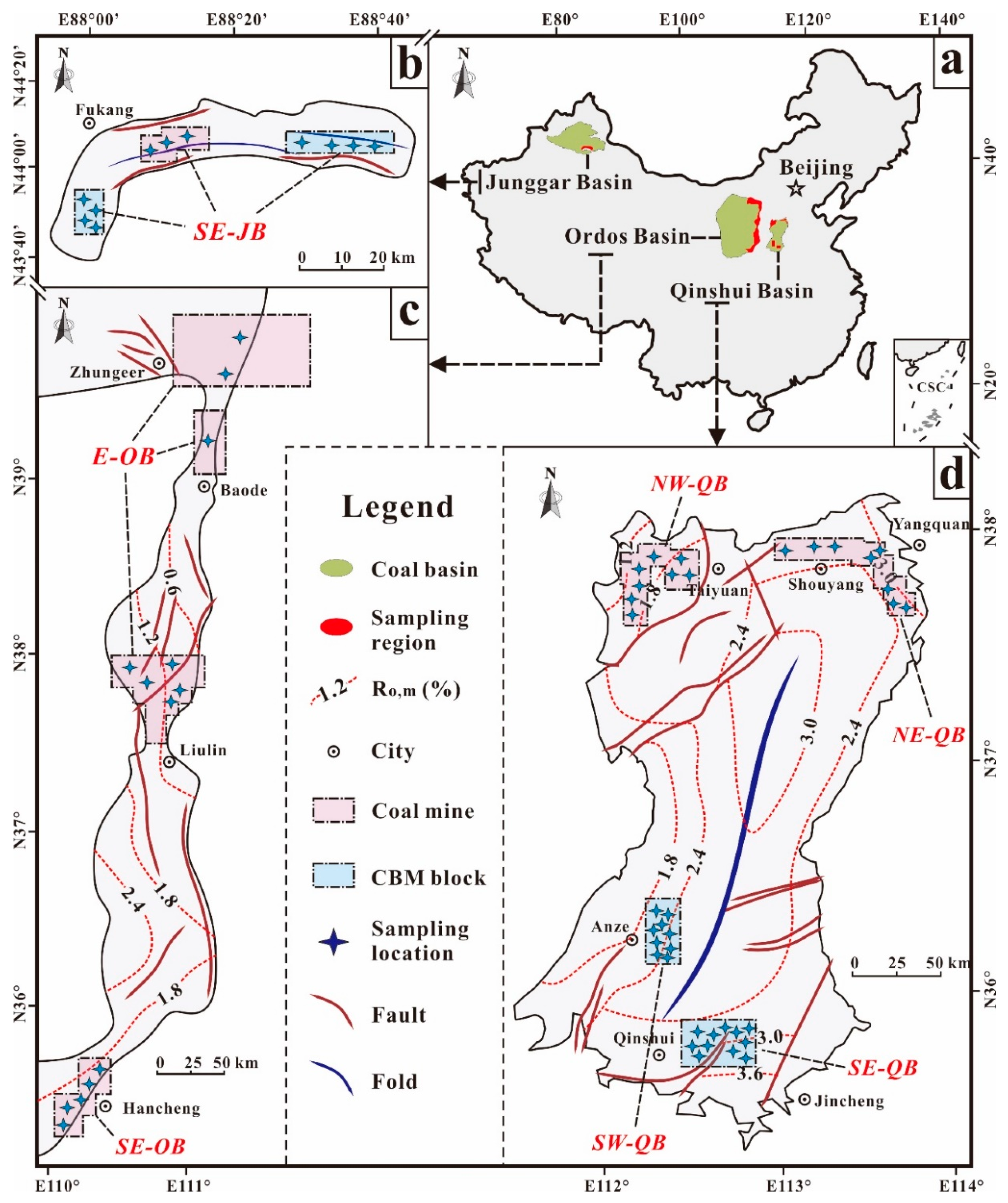
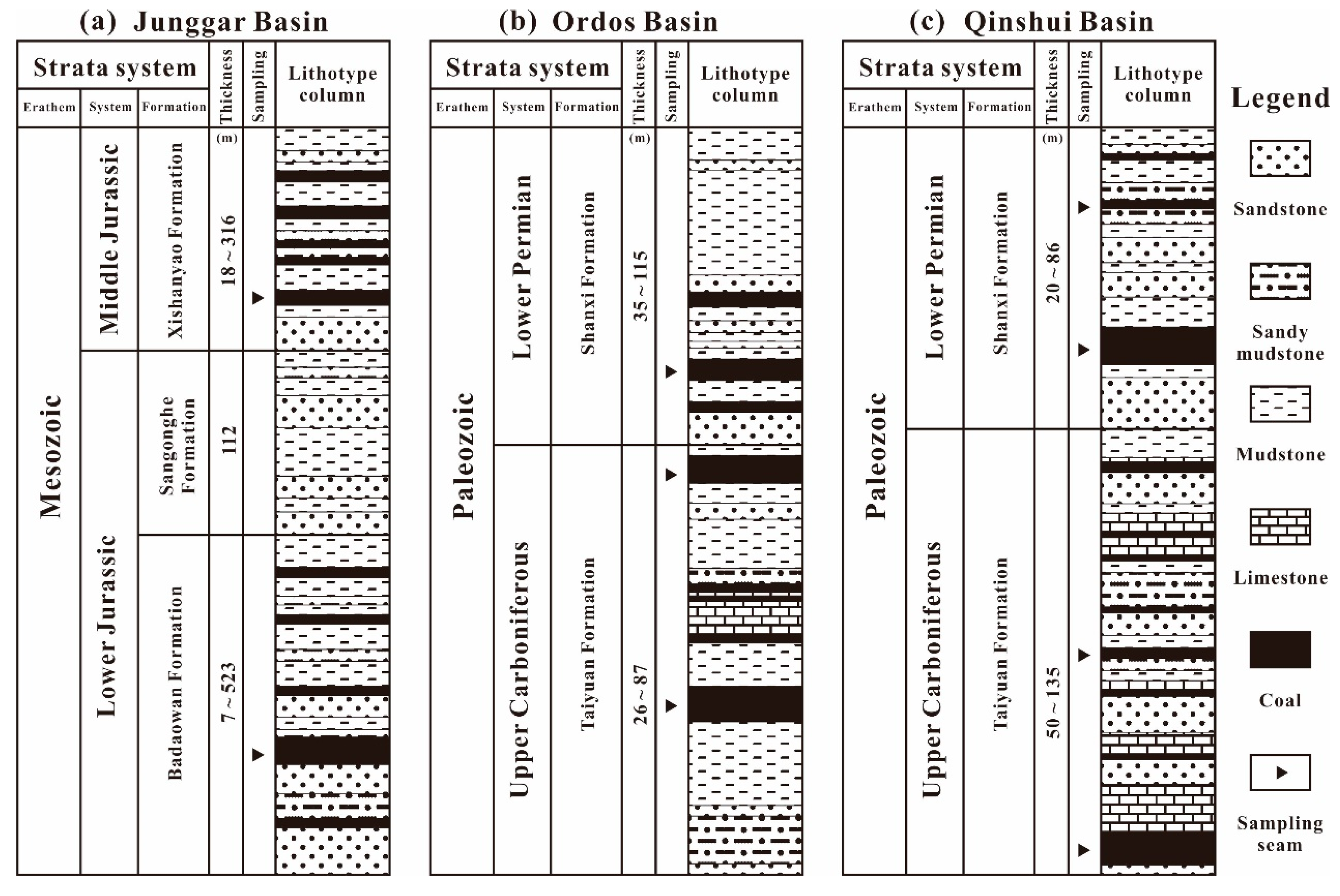
2.1. Sampling Areas
2.2. Methodology
3. Results
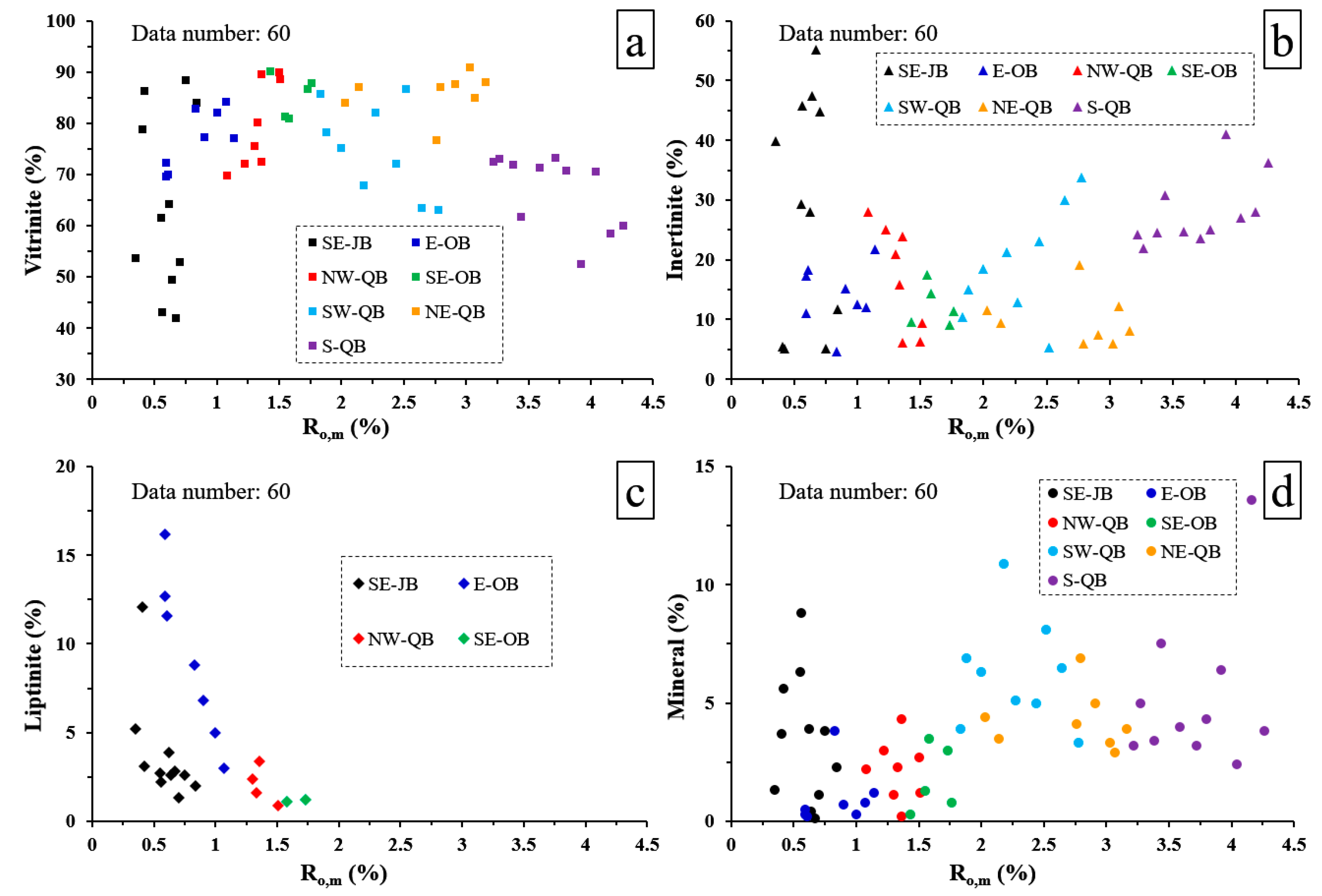
3.1. Coal Compositions
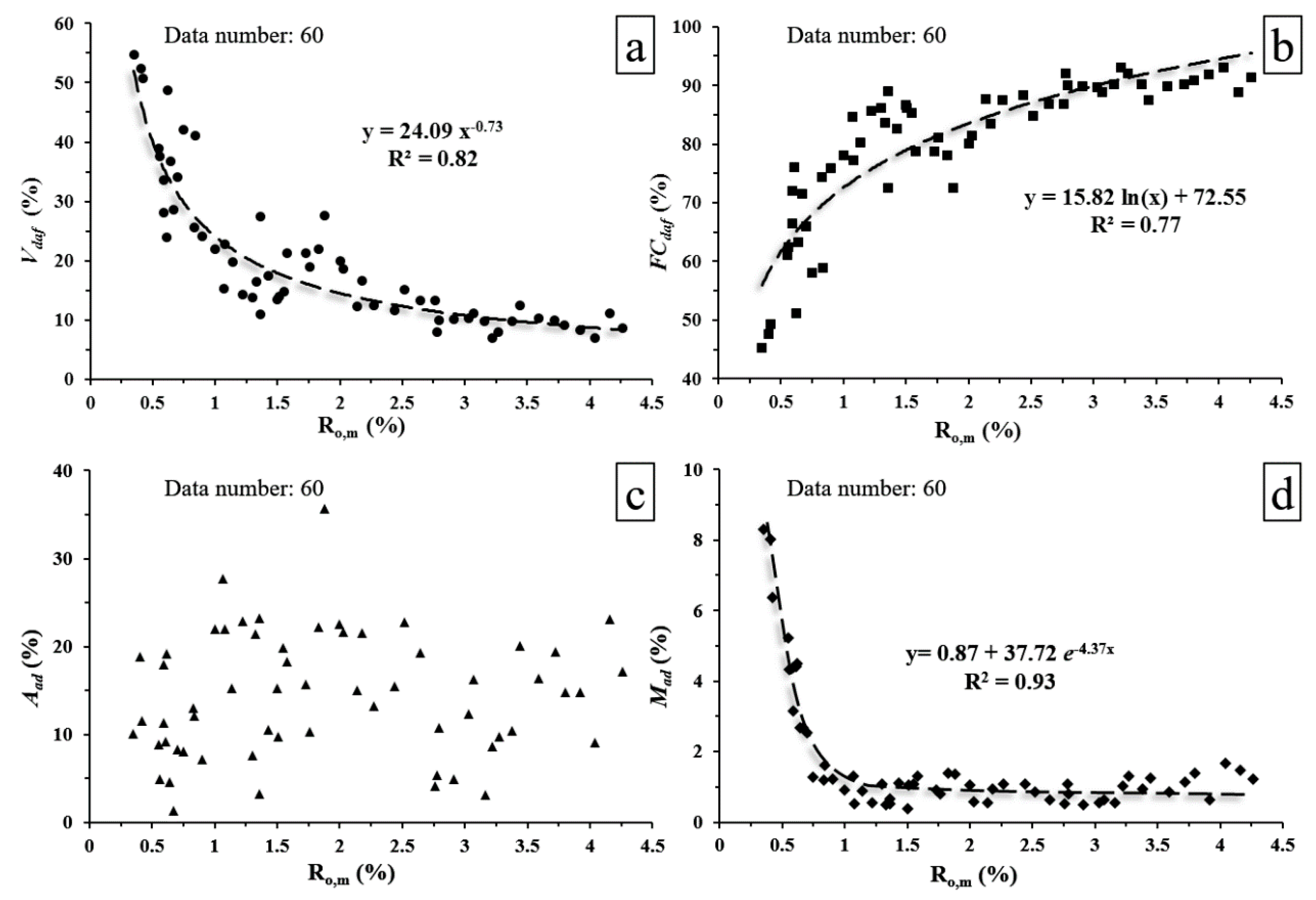
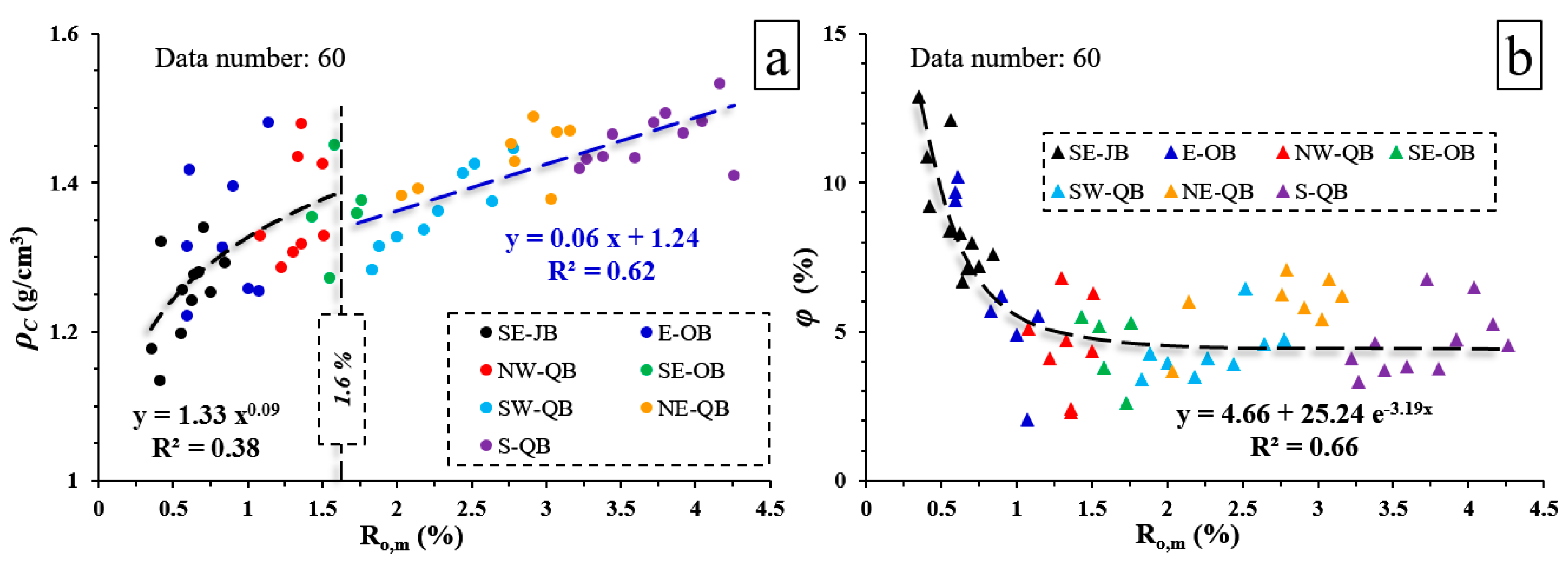
3.2. Coal Physical Properties
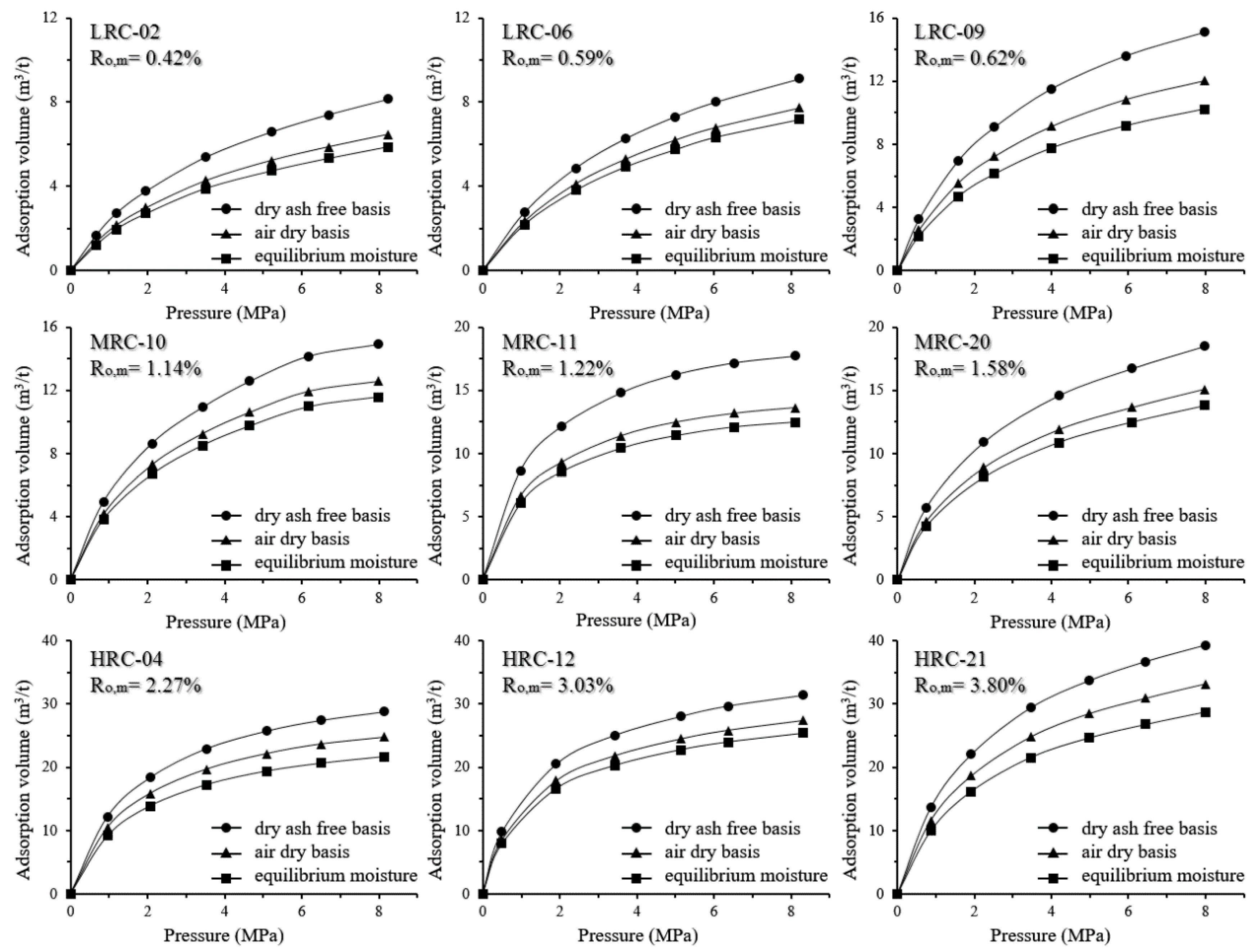
3.3. CH4 Isothermal Adsorption Capacity
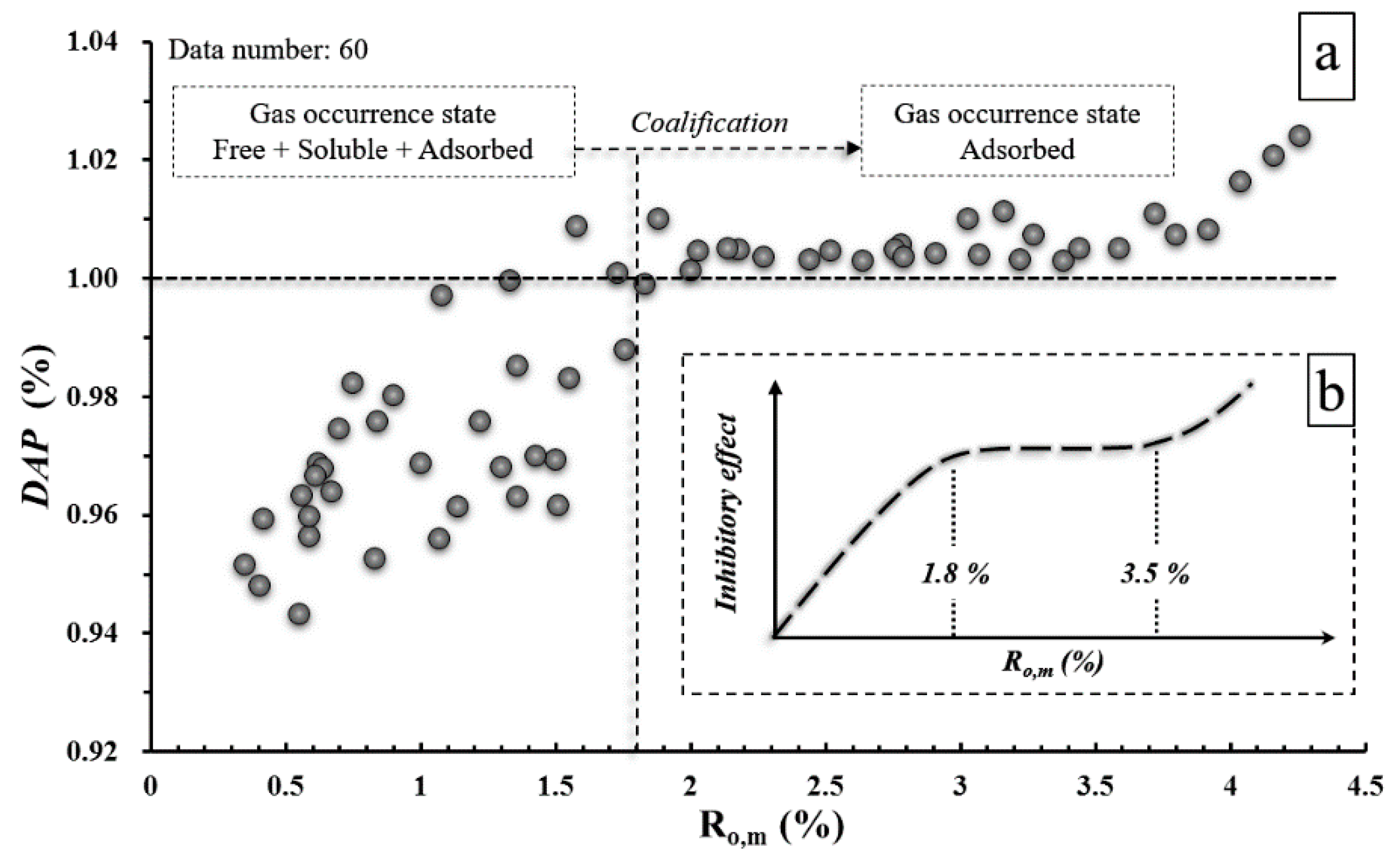
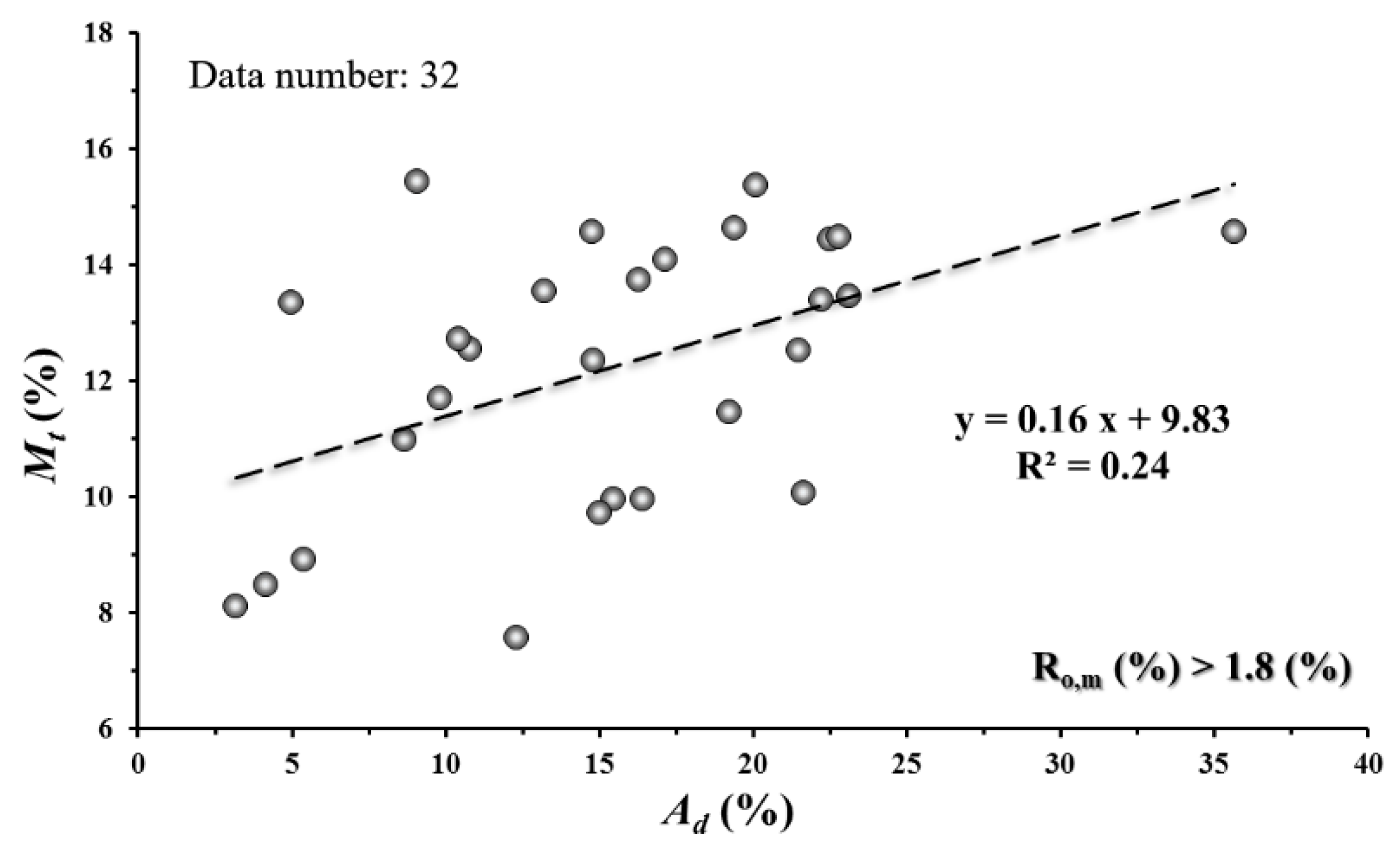
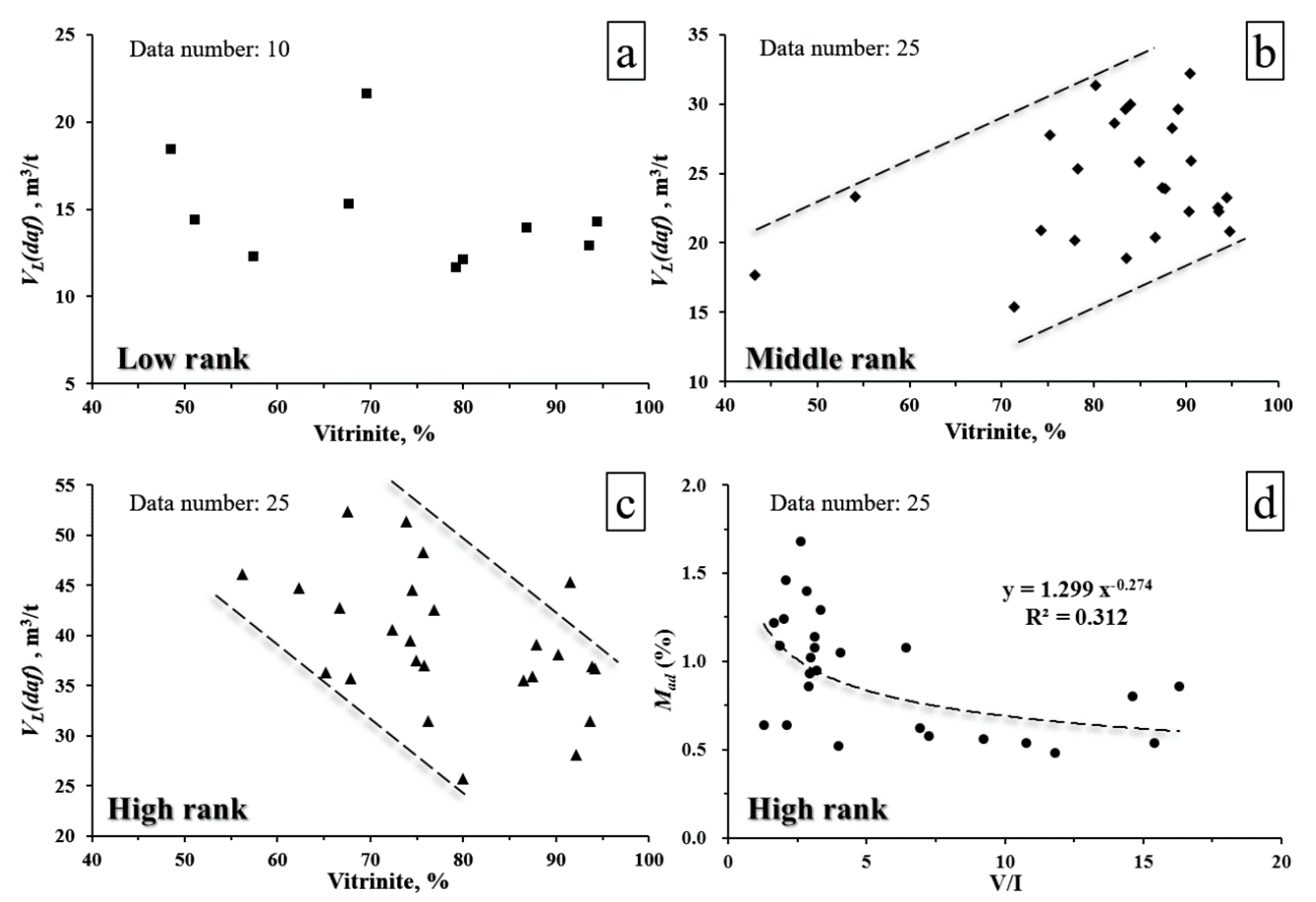
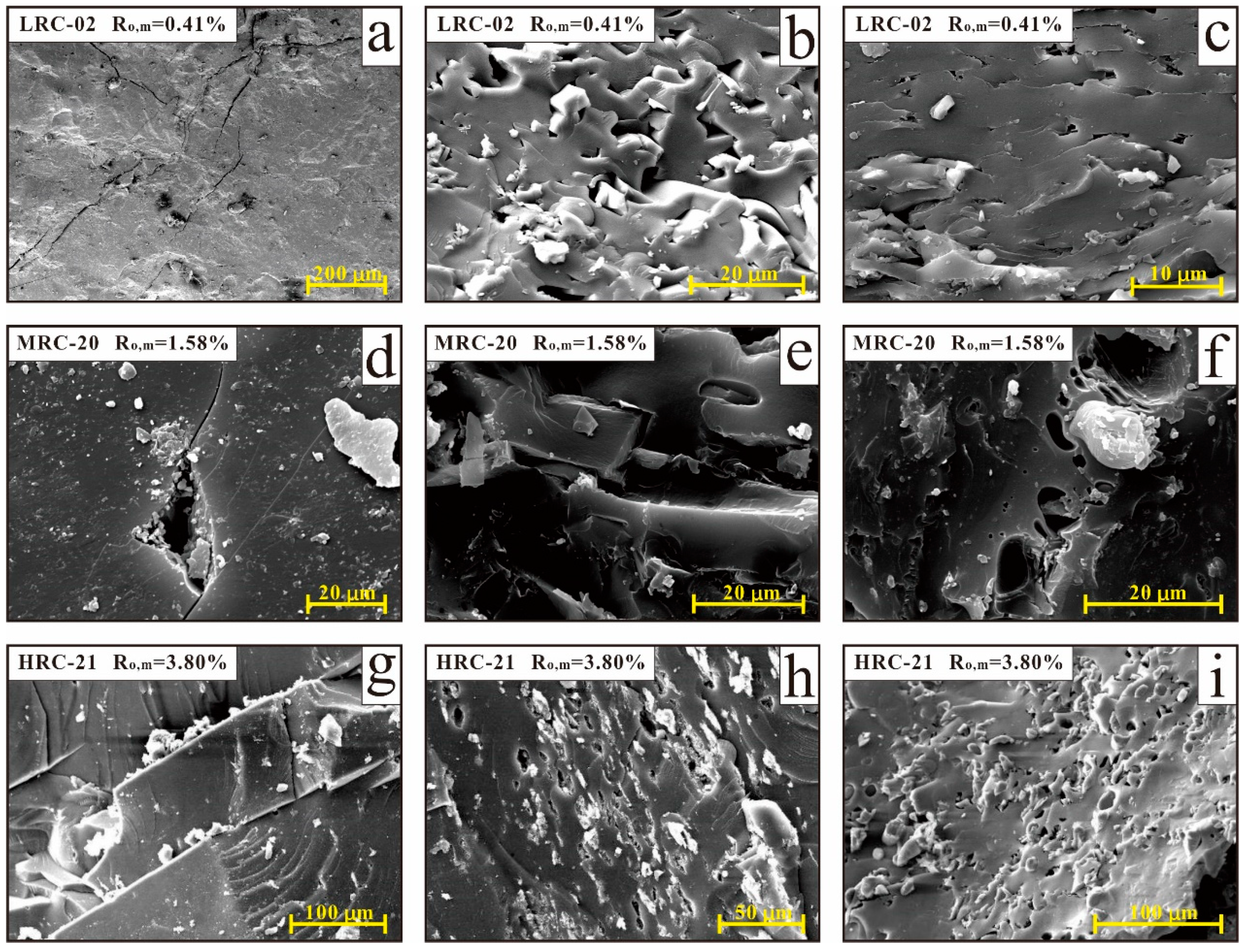
4. Discussion
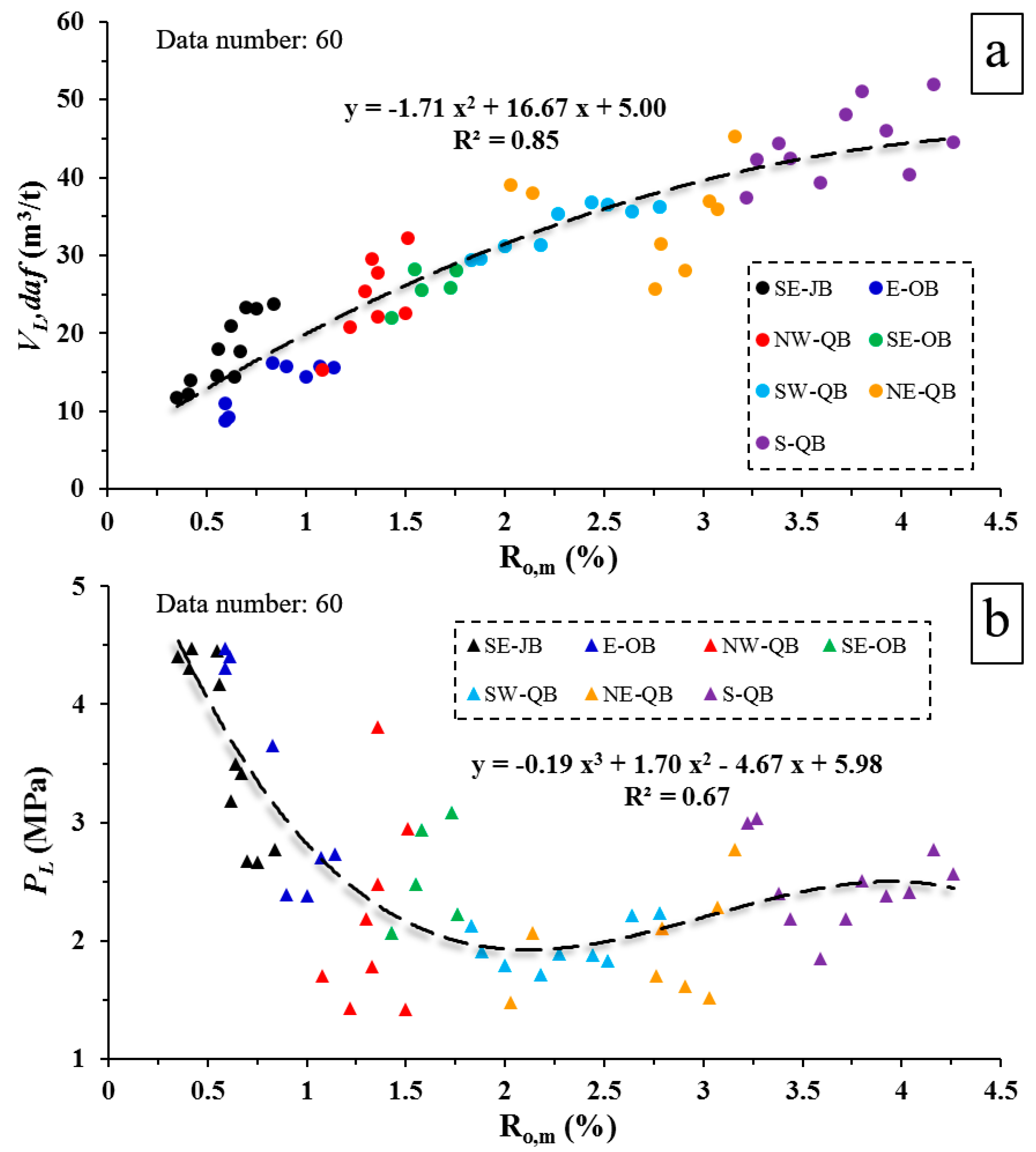
4.1. Correlation between Coal Rank (Ro,m) and Langmuir Constants (VL and PL)
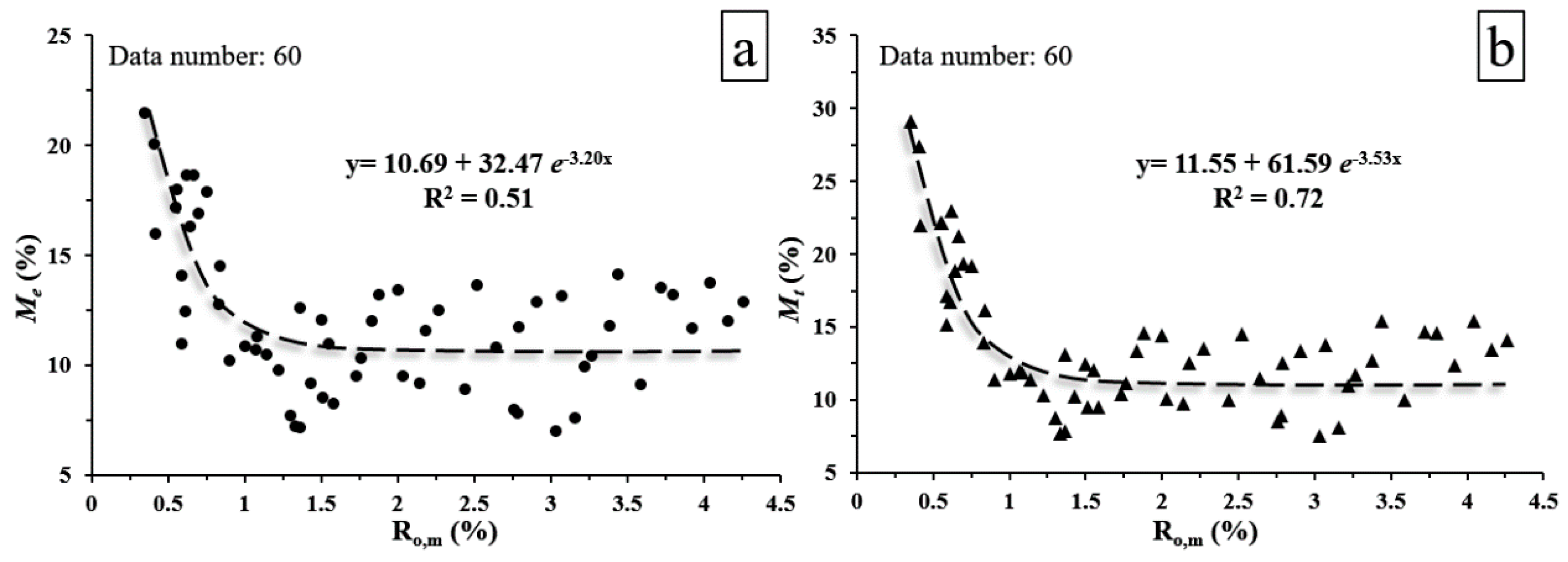
4.2. Quantitative Influence of Moisture on CH4 Adsorption
4.3. Relation between Maceral and CH4 Adsorption
4.4. Relation between Pore Structure and Coal Adsorption Capacity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Area | Sample No. | Ro,m (%) | Maceral Content | Proximate Analysis | Adsorption Capacity | PL MPa | Me (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V (%) | I (%) | L (%) | Vdaf (%) | Mad (%) | Aad (%) | VL(me) m3/t | VL(ad) m3/t | |||||
| SE-JB | LRC-01 | 0.35 | 53.63 | 39.85 | 5.19 | 54.81 | 8.31 | 9.22 | 9.01 | 10.15 | 4.41 | 21.47 |
| SE-JB | LRC-02 | 0.41 | 78.80 | 5.40 | 12.10 | 52.41 | 8.01 | 17.36 | 8.75 | 9.64 | 4.31 | 20.07 |
| SE-JB | LRC-03 | 0.42 | 86.20 | 5.10 | 3.10 | 50.78 | 6.38 | 10.87 | 10.58 | 11.82 | 4.48 | 15.99 |
| SE-JB | LRC-04 | 0.55 | 61.61 | 29.39 | 2.70 | 38.96 | 5.21 | 8.36 | 11.95 | 13.25 | 4.46 | 17.18 |
| SE-JB | LRC-05 | 0.56 | 43.17 | 45.84 | 2.20 | 37.58 | 4.32 | 4.72 | 14.42 | 16.78 | 4.17 | 18.00 |
| SE-JB | LRC-09 | 0.62 | 64.17 | 28.06 | 3.86 | 48.83 | 4.49 | 18.32 | 14.15 | 16.68 | 3.18 | 18.67 |
| SE-JB | LRC-10 | 0.64 | 49.50 | 47.50 | 2.60 | 36.71 | 2.67 | 4.44 | 11.72 | 13.38 | 3.50 | 16.30 |
| SE-JB | MRC-01 | 0.67 | 42.00 | 55.10 | 2.80 | 28.56 | 2.68 | 1.28 | 14.50 | 17.00 | 3.42 | 18.67 |
| SE-JB | MRC-02 | 0.70 | 52.80 | 44.80 | 1.30 | 34.11 | 2.54 | 8.13 | 17.74 | 20.83 | 2.67 | 16.89 |
| SE-JB | MRC-03 | 0.75 | 88.40 | 5.20 | 2.60 | 42.01 | 1.28 | 7.94 | 17.66 | 21.12 | 2.66 | 17.89 |
| SE-JB | MRC-05 | 0.84 | 84.00 | 11.70 | 2.00 | 41.13 | 1.62 | 11.88 | 18.01 | 20.66 | 2.77 | 14.53 |
| E-OB | LRC-06 | 0.59 | 72.30 | 11.00 | 16.20 | 33.57 | 4.38 | 10.87 | 11.00 | 11.82 | 4.48 | 10.99 |
| E-OB | LRC-07 | 0.59 | 69.60 | 17.40 | 12.70 | 28.04 | 3.15 | 17.36 | 8.85 | 9.64 | 4.31 | 14.07 |
| E-OB | LRC-08 | 0.61 | 69.90 | 18.30 | 11.60 | 23.87 | 4.41 | 8.82 | 9.30 | 10.15 | 4.41 | 12.47 |
| E-OB | MRC-04 | 0.83 | 82.80 | 4.60 | 8.80 | 25.60 | 1.20 | 12.84 | 16.21 | 17.89 | 3.65 | 12.77 |
| E-OB | MRC-06 | 0.90 | 77.30 | 15.20 | 6.80 | 24.04 | 1.23 | 7.12 | 15.72 | 17.28 | 2.39 | 10.19 |
| E-OB | MRC-07 | 1.00 | 82.10 | 12.60 | 5.00 | 21.87 | 0.91 | 21.78 | 14.37 | 15.74 | 2.38 | 10.87 |
| E-OB | MRC-08 | 1.07 | 84.10 | 12.10 | 3.00 | 15.31 | 1.29 | 27.32 | 15.76 | 17.12 | 2.70 | 10.70 |
| E-OB | MRC-10 | 1.14 | 77.00 | 21.80 | 19.70 | 0.89 | 15.16 | 15.58 | 16.95 | 2.73 | 10.47 | |
| NW-QB | MRC-12 | 1.30 | 75.50 | 21.00 | 2.40 | 13.79 | 1.07 | 7.56 | 21.63 | 23.17 | 2.18 | 7.71 |
| NW-QB | MRC-09 | 1.08 | 69.80 | 28.00 | 22.70 | 0.52 | 21.93 | 10.63 | 11.95 | 1.70 | 11.33 | |
| NW-QB | MRC-11 | 1.22 | 72.00 | 25.00 | 14.34 | 0.54 | 22.75 | 14.70 | 16.03 | 1.43 | 9.77 | |
| NW-QB | MRC-13 | 1.33 | 80.20 | 15.90 | 1.60 | 16.41 | 0.50 | 21.30 | 21.48 | 23.15 | 1.78 | 7.22 |
| NW-QB | MRC-14 | 1.36 | 89.50 | 6.20 | 27.43 | 0.67 | 23.09 | 15.83 | 16.94 | 2.48 | 7.17 | |
| NW-QB | MRC-15 | 1.36 | 72.50 | 23.90 | 3.40 | 10.96 | 0.51 | 3.22 | 23.82 | 26.73 | 3.81 | 12.63 |
| NW-QB | MRC-17 | 1.50 | 90.00 | 6.30 | 13.39 | 0.38 | 15.25 | 17.10 | 19.03 | 1.42 | 12.08 | |
| NW-QB | MRC-18 | 1.51 | 88.50 | 9.40 | 0.90 | 13.92 | 1.04 | 9.65 | 26.64 | 28.76 | 2.95 | 8.51 |
| SE-OB | MRC-16 | 1.43 | 90.10 | 9.60 | 17.45 | 1.11 | 10.43 | 18.14 | 19.67 | 2.06 | 9.18 | |
| SE-OB | MRC-19 | 1.55 | 81.20 | 17.50 | 14.72 | 1.09 | 19.63 | 20.37 | 22.67 | 2.48 | 10.97 | |
| SE-OB | MRC-20 | 1.58 | 81.00 | 14.40 | 1.10 | 21.31 | 1.29 | 18.09 | 19.03 | 20.82 | 2.94 | 8.25 |
| SE-OB | MRC-21 | 1.73 | 86.70 | 9.10 | 1.20 | 21.30 | 0.92 | 15.53 | 19.56 | 21.62 | 3.08 | 9.49 |
| SE-OB | MRC-22 | 1.76 | 87.80 | 11.40 | 18.92 | 0.80 | 10.27 | 22.63 | 25.10 | 2.22 | 10.34 | |
| SW-QB | MRC-23 | 1.83 | 85.63 | 10.47 | 21.86 | 1.38 | 21.90 | 19.98 | 22.70 | 2.12 | 12.04 | |
| SW-QB | MRC-24 | 1.88 | 78.14 | 14.96 | 27.53 | 1.36 | 35.18 | 16.38 | 19.03 | 1.91 | 13.23 | |
| SW-QB | MRC-25 | 2.00 | 75.15 | 18.55 | 19.99 | 1.05 | 22.27 | 20.80 | 24.04 | 1.79 | 13.41 | |
| SW-QB | HRC-03 | 2.18 | 67.89 | 21.21 | 16.53 | 0.95 | 21.30 | 21.57 | 24.46 | 1.71 | 11.59 | |
| SW-QB | HRC-04 | 2.27 | 82.09 | 12.81 | 12.43 | 1.08 | 13.05 | 26.65 | 30.50 | 1.89 | 12.48 | |
| SW-QB | HRC-05 | 2.44 | 72.00 | 23.00 | 11.63 | 1.08 | 15.31 | 28.13 | 30.91 | 1.88 | 8.90 | |
| SW-QB | HRC-06 | 2.52 | 86.58 | 5.31 | 15.18 | 0.86 | 22.59 | 24.19 | 28.08 | 1.83 | 13.63 | |
| SW-QB | HRC-07 | 2.64 | 63.50 | 30.00 | 13.21 | 0.64 | 19.12 | 25.56 | 28.70 | 2.21 | 10.83 | |
| SW-QB | HRC-09 | 2.78 | 63.00 | 33.70 | 7.97 | 1.09 | 5.33 | 31.28 | 33.99 | 2.23 | 7.84 | |
| NE-QB | HRC-01 | 2.03 | 84.03 | 11.57 | 18.55 | 0.58 | 21.54 | 27.54 | 30.48 | 1.48 | 9.50 | |
| NE-QB | HRC-02 | 2.14 | 87.07 | 9.43 | 12.26 | 0.56 | 14.93 | 29.19 | 32.19 | 2.07 | 9.16 | |
| NE-QB | HRC-08 | 2.76 | 76.69 | 19.21 | 13.22 | 0.52 | 4.13 | 22.57 | 24.56 | 1.70 | 7.96 | |
| NE-QB | HRC-10 | 2.79 | 87.14 | 5.96 | 9.95 | 0.80 | 10.73 | 24.53 | 27.85 | 2.10 | 11.77 | |
| NE-QB | HRC-11 | 2.91 | 87.59 | 7.41 | 10.16 | 0.48 | 4.96 | 23.06 | 26.53 | 1.61 | 12.88 | |
| NE-QB | HRC-12 | 3.03 | 90.80 | 5.90 | 10.30 | 0.54 | 12.26 | 29.89 | 32.23 | 1.52 | 7.03 | |
| NE-QB | HRC-13 | 3.07 | 84.87 | 12.23 | 11.14 | 0.62 | 16.17 | 25.91 | 29.89 | 2.28 | 13.13 | |
| NE-QB | HRC-14 | 3.16 | 87.93 | 8.17 | 9.83 | 0.54 | 3.17 | 40.22 | 43.61 | 2.77 | 7.58 | |
| S-QB | HRC-15 | 3.22 | 72.50 | 24.30 | 6.94 | 1.02 | 8.59 | 30.47 | 33.88 | 3.00 | 9.97 | |
| S-QB | HRC-16 | 3.27 | 73.00 | 22.00 | 8.01 | 1.29 | 9.68 | 33.62 | 37.62 | 3.03 | 10.43 | |
| S-QB | HRC-17 | 3.38 | 71.97 | 24.63 | 9.82 | 0.93 | 10.34 | 34.81 | 39.51 | 2.40 | 11.81 | |
| S-QB | HRC-18 | 3.44 | 61.70 | 30.80 | 12.49 | 1.24 | 19.83 | 28.93 | 33.78 | 2.18 | 14.15 | |
| S-QB | HRC-19 | 3.59 | 71.33 | 24.67 | 10.23 | 0.86 | 16.26 | 29.73 | 32.76 | 1.85 | 9.11 | |
| S-QB | HRC-20 | 3.72 | 73.28 | 23.52 | 9.88 | 1.14 | 19.19 | 33.17 | 38.52 | 2.18 | 13.51 | |
| S-QB | HRC-21 | 3.80 | 70.72 | 24.98 | 9.12 | 1.40 | 14.58 | 37.38 | 43.17 | 2.51 | 13.19 | |
| S-QB | HRC-22 | 3.92 | 52.60 | 41.00 | 8.21 | 0.64 | 14.71 | 34.39 | 39.06 | 2.38 | 11.71 | |
| S-QB | HRC-23 | 4.04 | 70.60 | 27.00 | 6.94 | 1.68 | 8.93 | 31.03 | 36.25 | 2.41 | 13.78 | |
| S-QB | HRC-24 | 4.16 | 58.40 | 28.00 | 11.17 | 1.46 | 22.77 | 34.68 | 39.70 | 2.77 | 12.02 | |
| S-QB | HRC-25 | 4.26 | 59.90 | 36.30 | 8.58 | 1.22 | 16.93 | 31.61 | 36.64 | 2.56 | 12.88 | |
References
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Karacan, C.Ö.; Ruiz, F.A.; Cotè, M.; Sally, P. Coal mine methane: A review of capture and utilization practices with benefits to mining safety and to greenhouse gas reduction. Int. J. Coal Geol. 2011, 86, 121–156. [Google Scholar] [CrossRef]
- Zhou, F.B.; Xia, T.Q.; Wang, X.X.; Zhang, Y.F.; Sun, Y.N.; Liu, J.S. Recent developments in coal mine methane extraction and utilization in China: A review. J. Nat. Gas Sci. Eng. 2016, 31, 437–458. [Google Scholar] [CrossRef]
- Flores, R.M. Coalbed methane: From hazard to resource. Int. J. Coal Geol. 1998, 35, 3–26. [Google Scholar] [CrossRef]
- Crosdale, P.J.; Beamish, B.B.; Valix, M. Coalbed methane sorption related to coal composition. Int. J. Coal Geol. 1998, 35, 147–158. [Google Scholar] [CrossRef]
- Mares, T.E.; Moore, T.A.; Moore, C.R. Uncertainty of gas saturation estimates in a subbituminous coal seam. Int. J. Coal Geol. 2009, 77, 320–327. [Google Scholar] [CrossRef]
- Joubert, J.I.; Grein, C.T.; Bienstock, D. Effect of moisture on methane capacity of American coals. Fuel 1974, 53, 186–191. [Google Scholar] [CrossRef]
- Hao, S.; Wen, J.; Yu, X.; Chu, W. Effect of the surface oxygen groups on methane adsorption on coals. Appl. Surf. Sci. 2013, 264, 433–442. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Huang, W. Influences of igneous intrusions on coal rank, coal quality and adsorption capacity in Hongyang, Handan and Huaibei coalfields, North China. Int. J. Coal Geol. 2011, 88, 135–146. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, S.; Pang, Y.; Li, J.; Xin, H. Effects of Coal Functional Groups on Adsorption Microheat of Coal Bed Methane. Energy Fuels 2015, 29, 1550–1557. [Google Scholar] [CrossRef]
- Fu, H.J.; Tang, D.Z.; Xu, T.; Xu, H.; Tao, S.; Li, S.; Yin, Z.Y.; Chen, B.L.; Zhang, C.; Wang, L.L. Characteristics of pore structure and fractal dimension of low-rank coal: A case study of Lower Jurassic Xishanyao coal in the southern Junggar Basin, NW China. Fuel 2017, 193, 254–264. [Google Scholar] [CrossRef]
- Levy, J.; Day, S.J.; Killingley, J.S. Methane capacity of Bowen Basin coals related to coal properties. Fuel 1997, 74, 1–7. [Google Scholar] [CrossRef]
- Ritter, U.; Arnt, G. Adsorption of petroleum compounds in vitrinite: Implications for petroleum expulsion from coal. Int. J. Coal Geol. 2005, 62, 183–191. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, C.; Song, Y.; Wang, H.; Wang, Y. Differences and origin of physical properties of low-rank and high-rank coal-bed methanes. Acta Petrol. Sin. 2008, 2, 179–184, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Yee, D.; Seidle, J.P.; Hanson, W.B. Gas sorption on coal and measurement of gas content. Hydrocarb. Coal AAPG Stud. Geol. 1993, 38, 203–218. [Google Scholar]
- Levine, J.R. Coalification: The evolution of coal as a source rock and reservoir rock for oil and gas. In SG 38: Hydrocarbons from Coal; Law, B.E., Rice, D.D., Eds.; AAPG: Tulsa, OK, USA, 1993; Volume 38, pp. 39–77. [Google Scholar]
- Laxminarayana, C.; Crosdale, P.J. Controls on methane sorption capacity of Indian coals. AAPG Bull. 2002, 86, 201–212. [Google Scholar]
- Keshavarz, A.; Sakurovs, R.; Grigore, M.; Sayyafzadeh, M. Effect of maceral composition and coal rank on gas diffusion in Australian coals. Int. J. Coal Geol. 2017, 173, 65–75. [Google Scholar] [CrossRef]
- Su, X. Influence of coal rank on coal adsorption capacity. Nat. Gas Ind. 2005, 25, 19–21, (In Chinese with English abstract). [Google Scholar]
- Zhang, K.; Tang, D.; Tao, S.; Liu, Y.; Chen, S. Study on influence factors of adsorption capacity of different metamorphic degree coals. Coal Sci. Technol. 2017, 45, 192–197, (In Chinese with English abstract). [Google Scholar]
- Weniger, P.; Francu, J.; Hemza, P.; Krooss, B.M. Investigations on the methane and carbon dioxide sorption capacity of coals from the SW Upper Silesian Coal Basin, Czech Republic. Int. J. Coal Geol. 2010, 84, 23–39. [Google Scholar] [CrossRef]
- Li, Z.T.; Yao, Y.B.; Zhou, H.P.; Bai, X.H. Study on Coal and Rock Maceral Composition Affected to Methane Adsorption Capacity. Coal Sci. Technol. 2012, 40, 125–128, (In Chinese with English abstract). [Google Scholar]
- Li, J.; Yao, Y.; Cai, Y.; Qiu, Y. Discussion on coal physical properties and formation with different metamorphic degree in North China. Coal Sci. Technol. 2012, 4, 111–115, (In Chinese with English abstract). [Google Scholar]
- An, F.; Cheng, Y.; Wu, D.; Wang, L. The effect of small micropores on methane adsorption of coals from Northern China. Adsorption 2013, 19, 83–90. [Google Scholar] [CrossRef]
- Fu, H.; Tang, D.; Xu, H.; Tao, S.; Xu, T.; Chen, B.; Yin, Z. Abrupt Changes in Reservoir Properties of Low-Rank Coal and Its Control Factors for Methane Adsorbability. Energy Fuels 2016, 30, 2084–2094. [Google Scholar] [CrossRef]
- Li, Y.; Tang, D.; Xu, H.; Elsworth, D.; Meng, Y. Geological and hydrological controls on water co-produced with coalbed methane in Liulin, eastern Ordos Basin, China. AAPG Bull. 2015, 99, 207–229. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Cai, Y.; Yao, Y.; Zhou, Y. Evaluation of structured coal evolution and distribution by geophysical logging methods in the Gujiao Block, northwest Qinshui basin, China. J. Nat. Gas. Sci. Eng. 2018, 51, 210–222. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Cai, Y.; Pan, Z.; Yao, Y.; Wang, Y. Geological and hydrological controls on the accumulation of coalbed methane within the No. 3 coal seam of the southern Qinshui Basin. Int. J. Coal Geol. 2017, 182, 94–111. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Tang, D.; Gan, Q.; Niu, X.; Wang, K. Coal pore size distributions controlled by the coalification process: An experimental study of coals from the Junggar, Ordos and Qinshui basins in China. Fuel 2017, 206, 352–363. [Google Scholar] [CrossRef]
- O’Keefe, J.M.K.; Bechtel, A.; Christanis, K.; Dai, S. On the fundamental difference between coal rank and coal type. Int. J. Coal Geol. 2013, 118, 58–87. [Google Scholar] [CrossRef]
- Pickel, W.; Kus, J.; Flores, D.; Kalaitzidis, S.; Christanis, K.; Cardott, B.J.; Misz-Kennan, M.; Rodrigues, S.; Hentschel, A.; Hamor-Vido, M.; et al. Classification of liptinite—ICCP System 1994. Int. J. Coal Geol. 2017, 169, 40–61. [Google Scholar] [CrossRef]
- Hou, H.; Shao, L.; Li, Y.; Li, Z.; Wang, S.; Zhang, W.; Wang, X. Influence of coal petrology on methane adsorption capacity of the Middle Jurassic coal in the Yuqia coalfield, northern Qaidam Basin, China. J. Petrol. Sci. Eng. 2017, 149, 218–227. [Google Scholar] [CrossRef]
- Gagarin, S.G. Relation between the petrographic content of mineral components in coal and its ash content. Coke Chem. 2008, 51, 241–246. [Google Scholar] [CrossRef]
- Gao, Z.; Yang, W. Adsorption mechanism of water molecule on different rank coals molecular surface. J. China Coal Soc. 2017, 42, 753–759, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Li, X.; Zeng, F.; Si, J.; Wang, W.; Dong, X.; Cheng, L. High resolution TEM image analysis of coals with different metamorphic degrees. J. Fuel Chem. Technol. 2016, 44. (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Su, X.; Si, Q.; Song, J. Characteristics of coal Raman spectrum. J. China Coal Soc. 2016, 42, 1197–1202, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Xu, H.; Tang, D.; Mathews, J.P.; Zhao, J.; Li, B.; Tao, S.; Li, S. Evaluation of coal macrolithotypes distribution by geophysical logging data in the Hancheng Block, eastern margin, Ordos Basin, China. Int. J. Coal Geol. 2016, 165, 265–277. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, D.; Pan, Z.; Yao, Y.; Li, J.; Qiu, Y. Pore structure and its impact on CH4 adsorption capacity and flow capability of bituminous and subbituminous coals from Northeast China. Fuel 2013, 103, 258–268. [Google Scholar] [CrossRef]
- Meng, Y.; Tang, D.; Xu, H.; Qu, Y.; Li, Y.; Zhang, W. Division of coalbed methane desorption stages and its significance. Petrol. Explor. Dev. 2014, 41, 671–677. [Google Scholar] [CrossRef]
- Zhang, L.; Su, X.; Zeng, S. Discussion on the controlling effects of coal properties on coal adsorption capacity. Acta Geol. Sin. 2006, 6, 910–915, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Pan, Z.; Connell, L.D.; Camilleri, M.; Connelly, L. Effects of matrix moisture on gas diffusion and flow in coal. Fuel 2010, 89, 3207–3217. [Google Scholar] [CrossRef]
- Liu, A.; Fu, X.; Wang, K.; An, H.; Wang, G. Investigation of coalbed methane potential in low-rank coal reservoirs—Free and soluble gas contents. Fuel 2013, 112, 14–22. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 1. Isotherms and pore volume distributions. Fuel 1999, 78, 1333–1344. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, D.; Cai, Y.; Li, X. Variation of Petrophysical Properties and Adsorption Capacity in Different Rank Coals: An Experimental Study of Coals from the Junggar, Ordos and Qinshui Basins in China. Energies 2019, 12, 986. https://doi.org/10.3390/en12060986
Wang Y, Liu D, Cai Y, Li X. Variation of Petrophysical Properties and Adsorption Capacity in Different Rank Coals: An Experimental Study of Coals from the Junggar, Ordos and Qinshui Basins in China. Energies. 2019; 12(6):986. https://doi.org/10.3390/en12060986
Chicago/Turabian StyleWang, Yingjin, Dameng Liu, Yidong Cai, and Xiawei Li. 2019. "Variation of Petrophysical Properties and Adsorption Capacity in Different Rank Coals: An Experimental Study of Coals from the Junggar, Ordos and Qinshui Basins in China" Energies 12, no. 6: 986. https://doi.org/10.3390/en12060986
APA StyleWang, Y., Liu, D., Cai, Y., & Li, X. (2019). Variation of Petrophysical Properties and Adsorption Capacity in Different Rank Coals: An Experimental Study of Coals from the Junggar, Ordos and Qinshui Basins in China. Energies, 12(6), 986. https://doi.org/10.3390/en12060986







