Fundamental Characteristics and Kinetic Analysis of Lignocellulosic Woody and Herbaceous Biomass Fuels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Sample Preparation
2.2. Biomass Sample Analysis
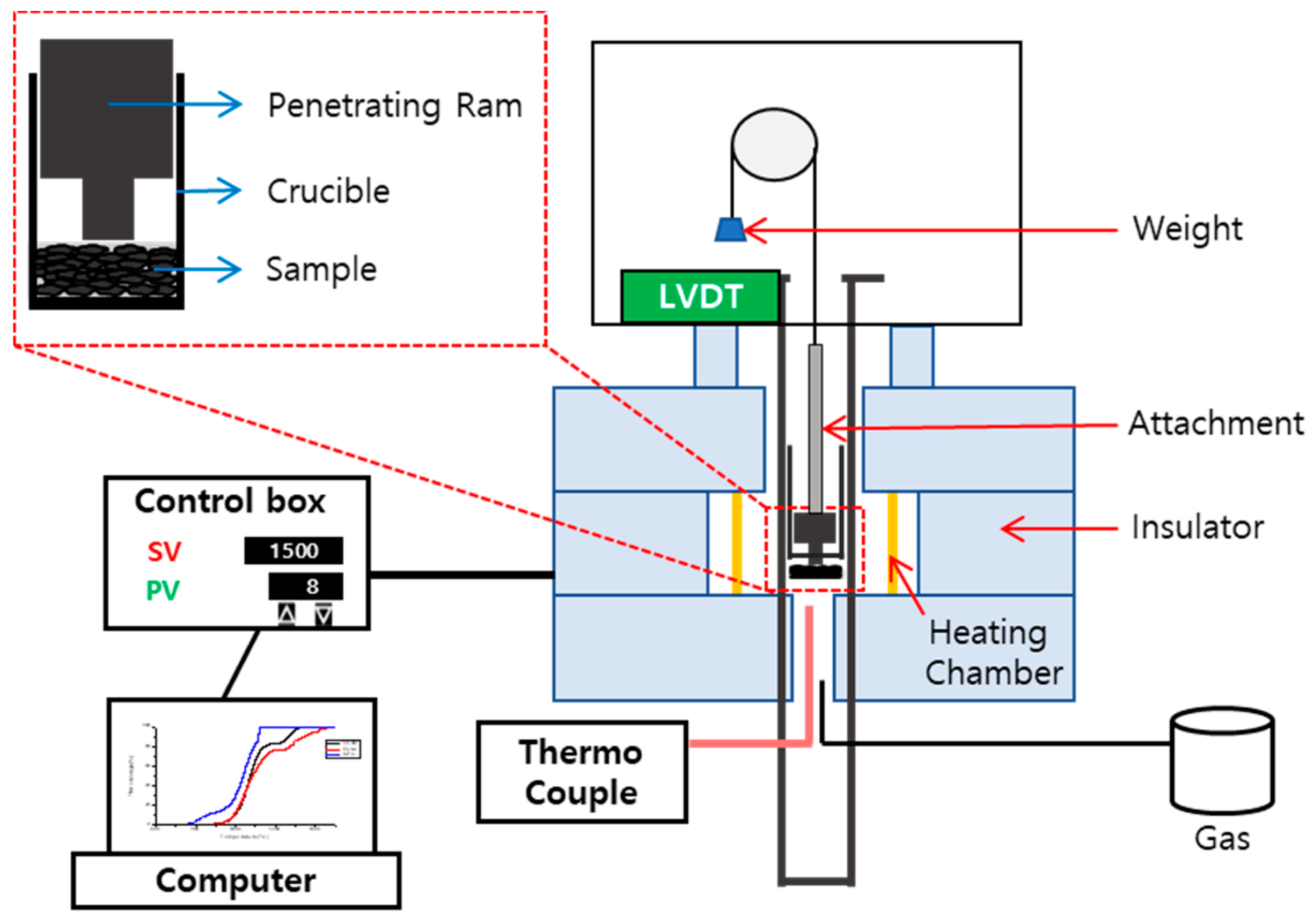
2.3. Thermogravimetric Analysis
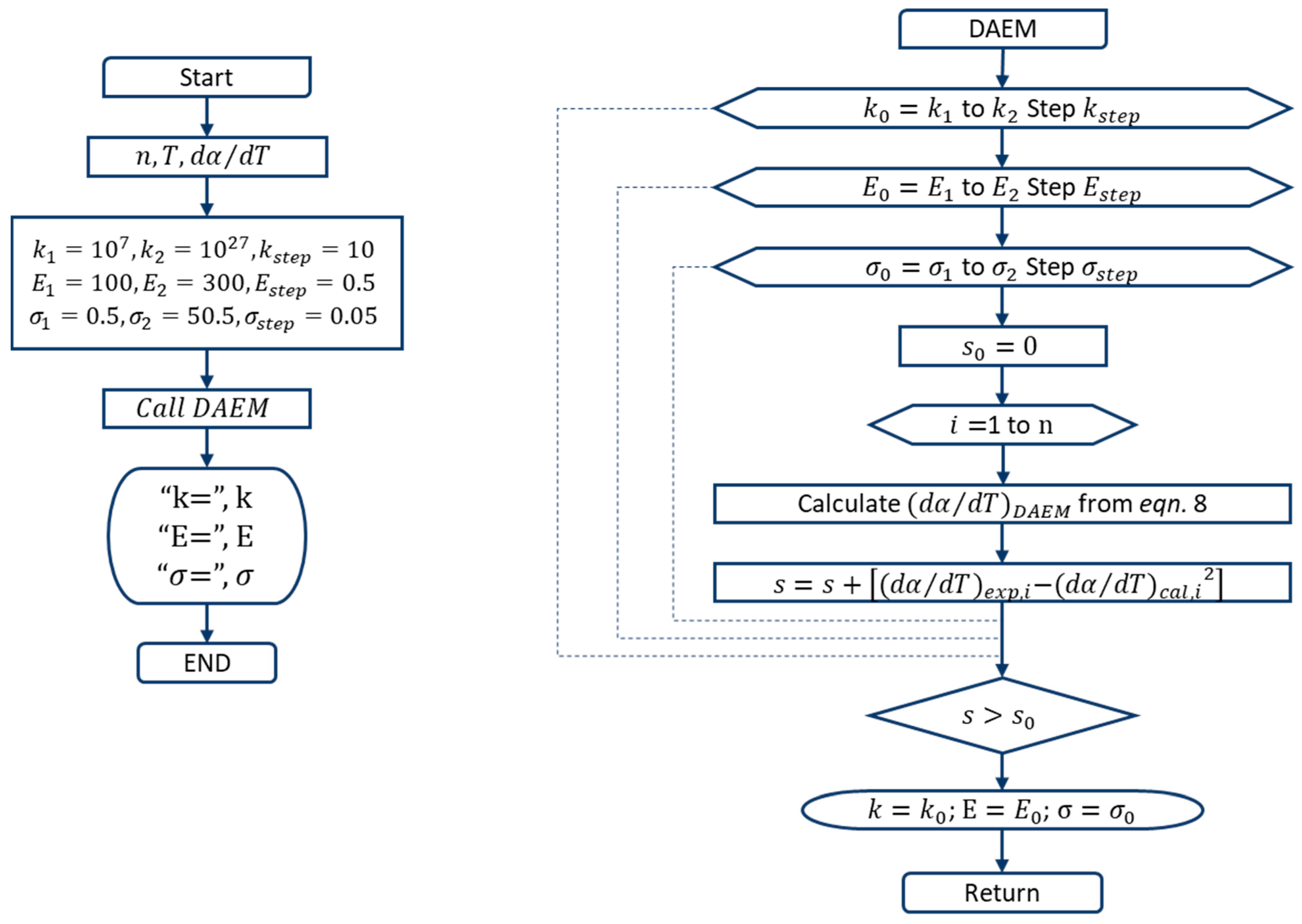
2.4. Multi-Gaussian Distributed Activation Energy Model
2.5. Char Oxidation Kinetics
3. Results and Discussion
3.1. Biomass Sample Analysis
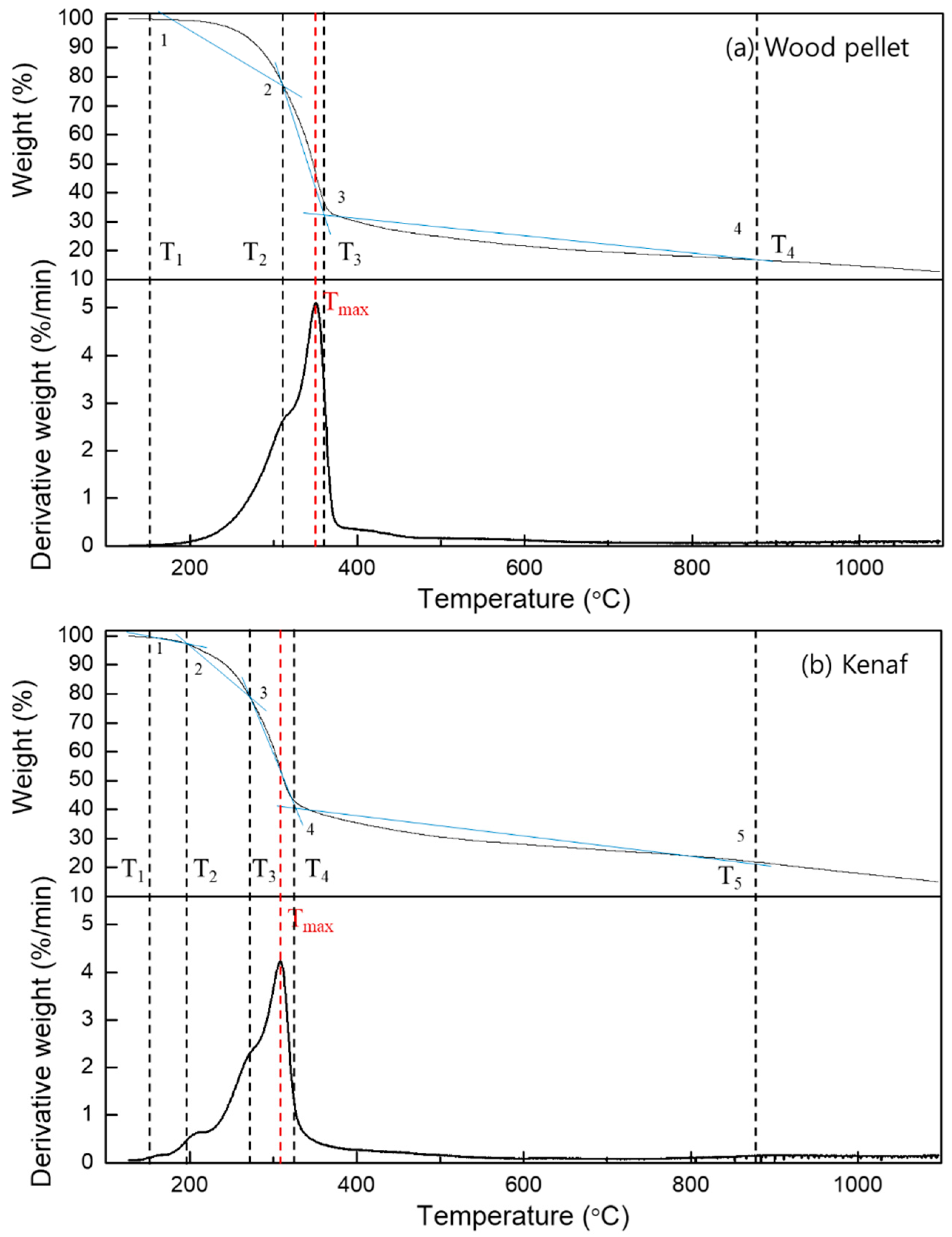
3.2. Thermogravimetric Analysis
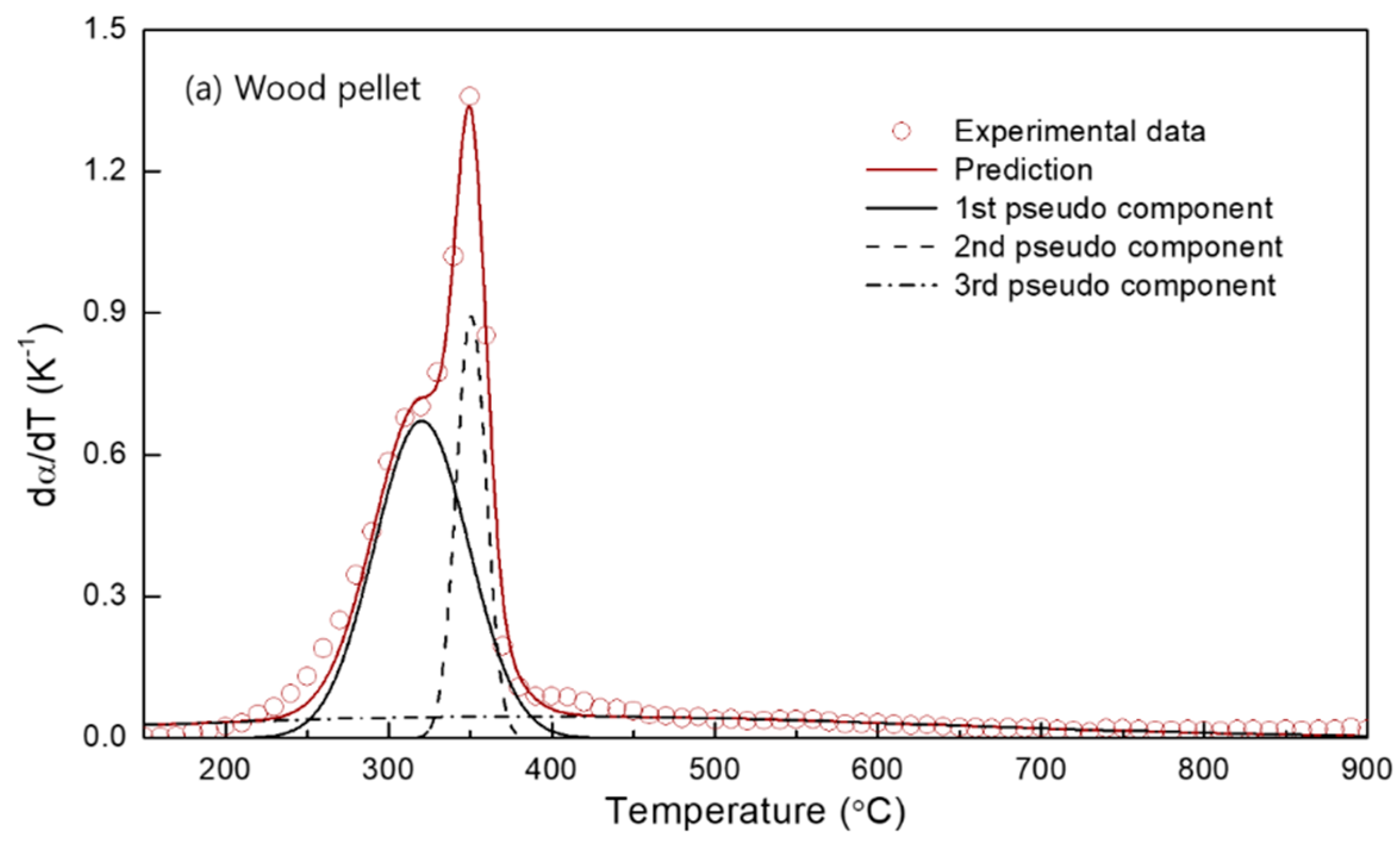
3.3. DAEM for Biomass Pyrolysis Kinetics
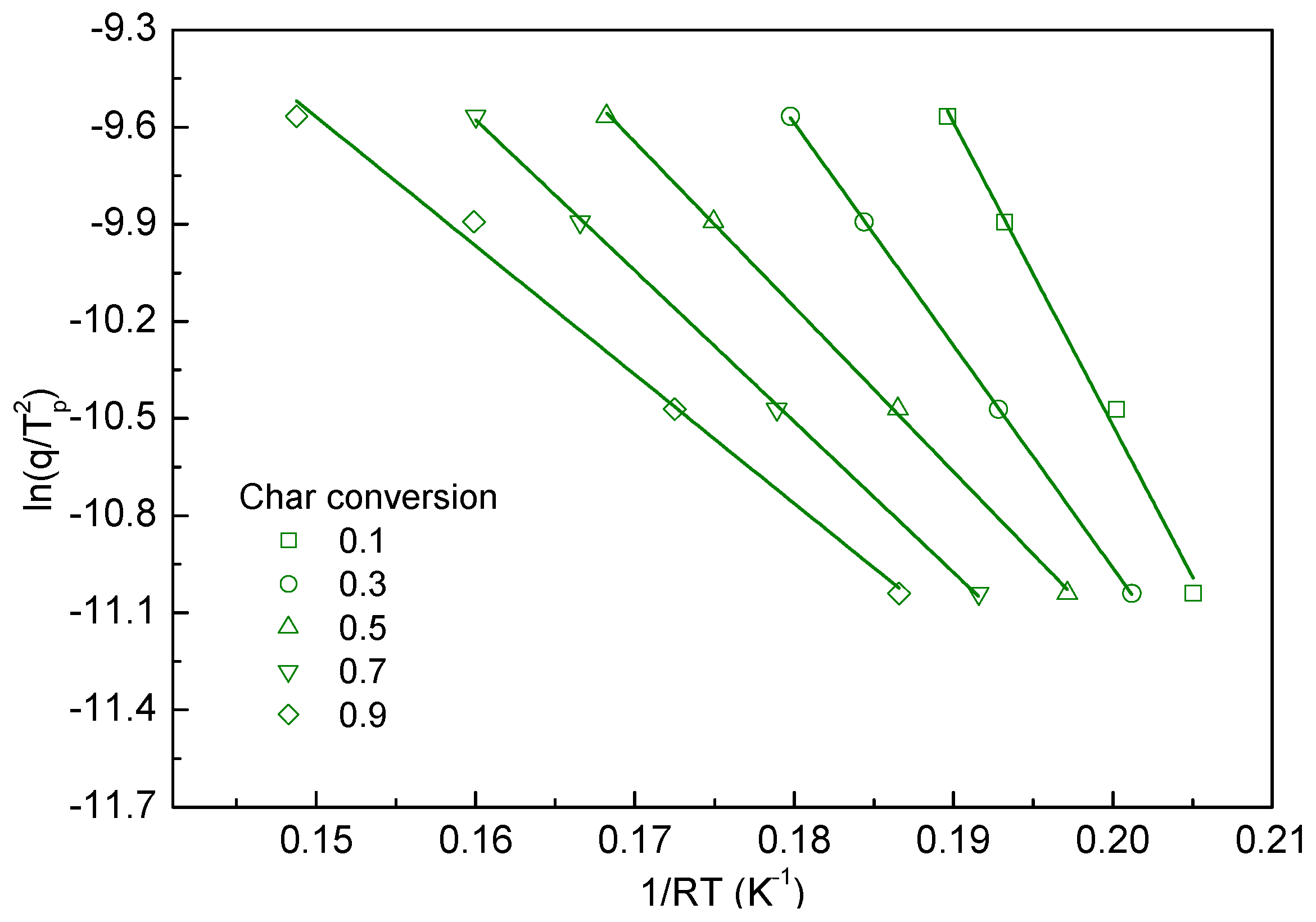
3.4. Kinetics Study of Biomass Char Oxidation
4. Conclusions
- (1).
- The proximate, ultimate, and oxide analysis results as well as the calorific values of the wood pellets and kenaf were obtained using the ASTM methods. Additionally, ash fusion temperatures of the biomass samples were successfully derived using TMA. The results for the detection of 19 elements in the biomass samples were observed by ICP-OES analysis.
- (2).
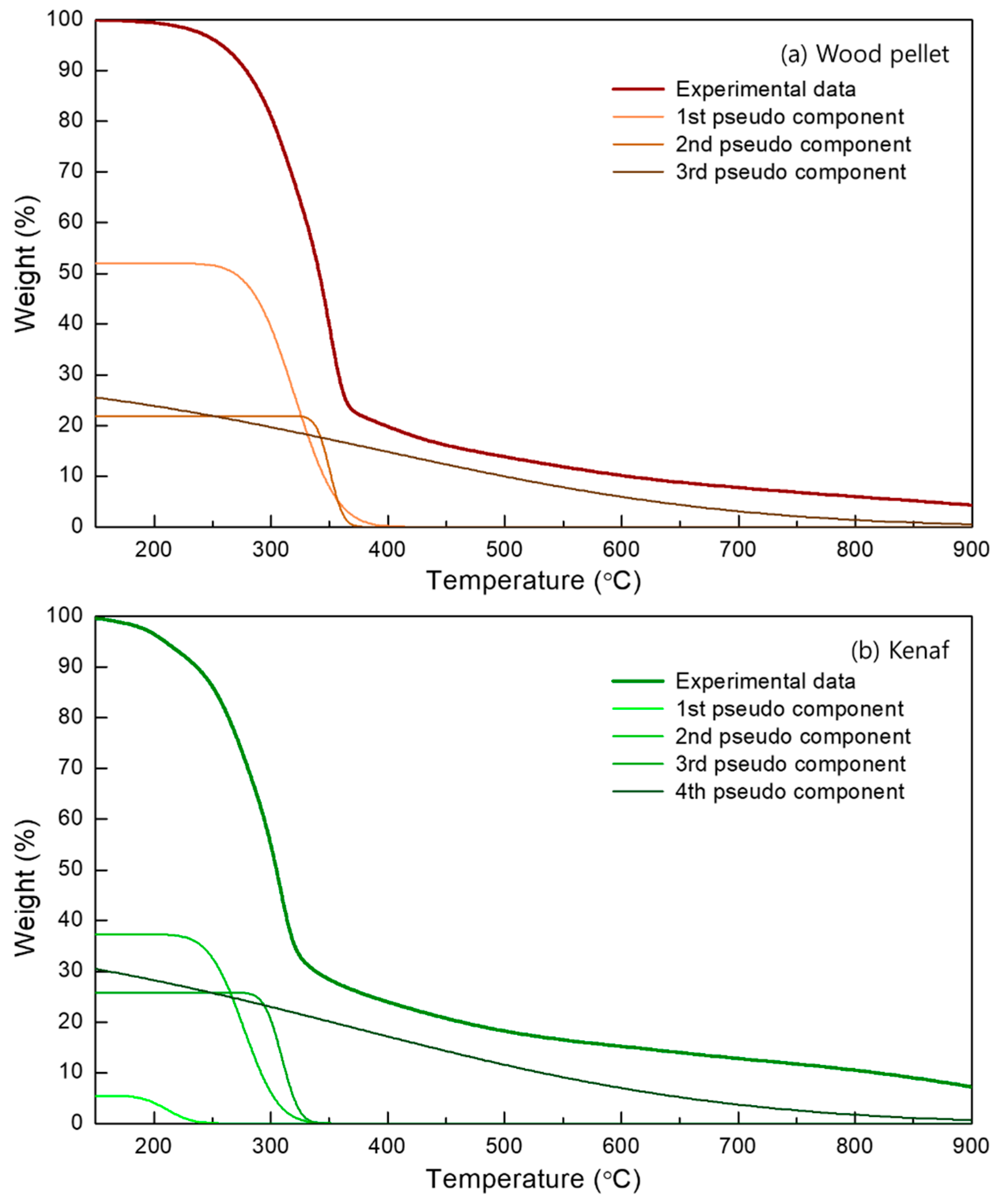
- Thermogravimetry (TG) and derivative thermogravimetry (DTG) curves at pyrolysis conditions were performed, and the trend in mass reduction was similar for both wood pellets and kenaf; the overall pyrolysis tended to occur more rapidly at a lower temperature for kenaf.
- (3).
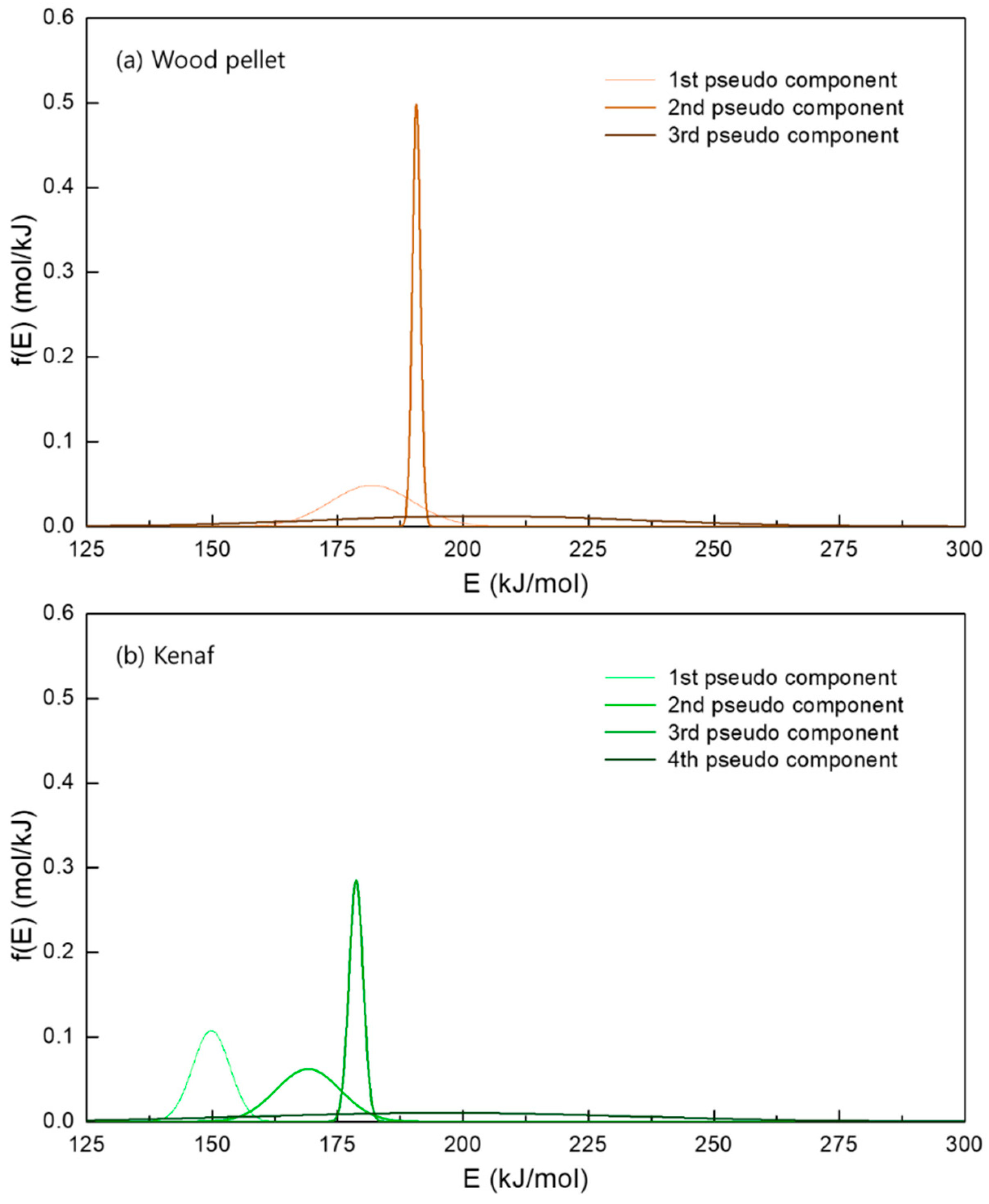
- The multi-gaussian DAEM was successfully applied to the 3 or 4 pseudocomponents for the biomass samples in order to obtain the parallel descriptions of each independent pyrolysis reaction.
- (4).
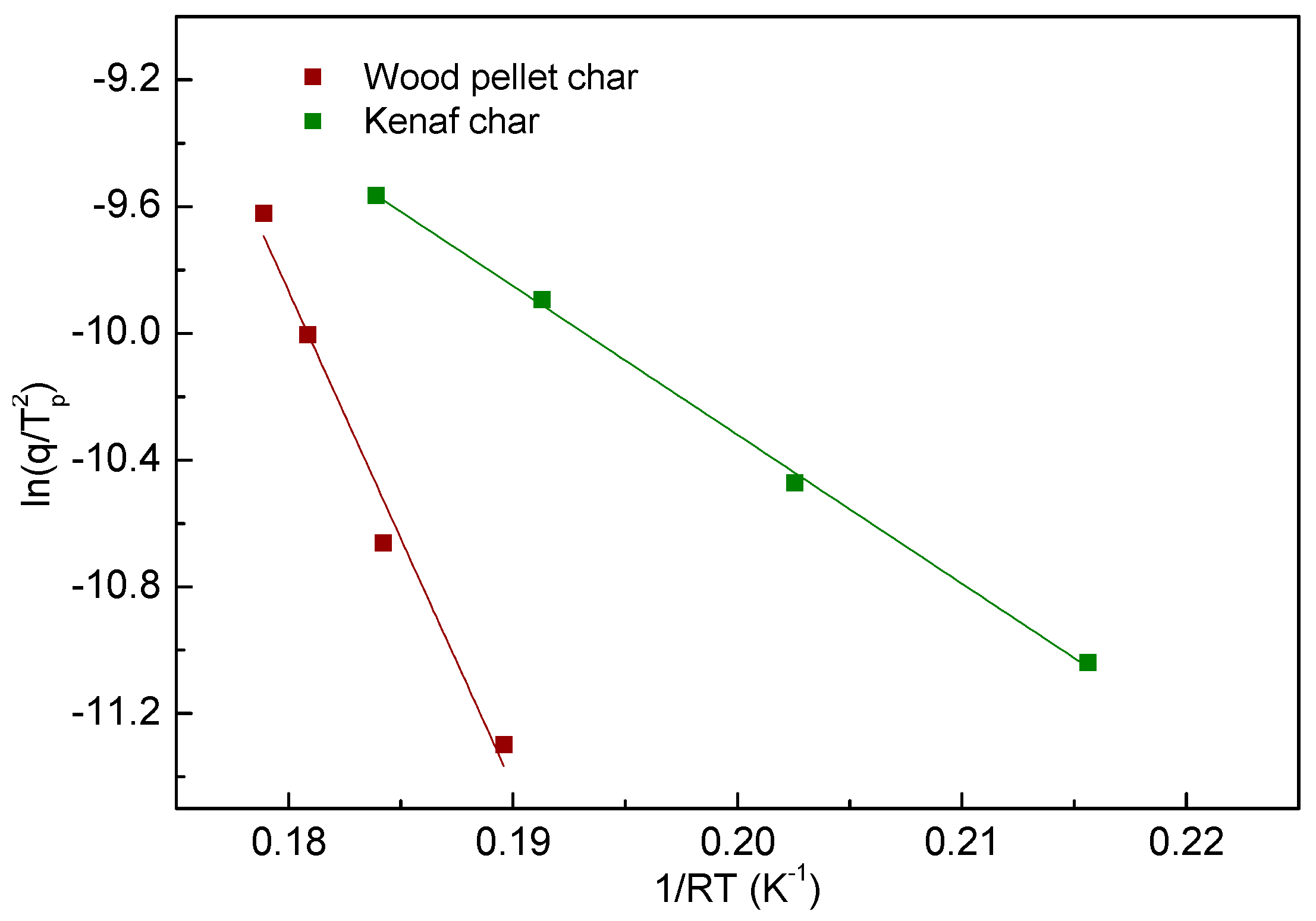
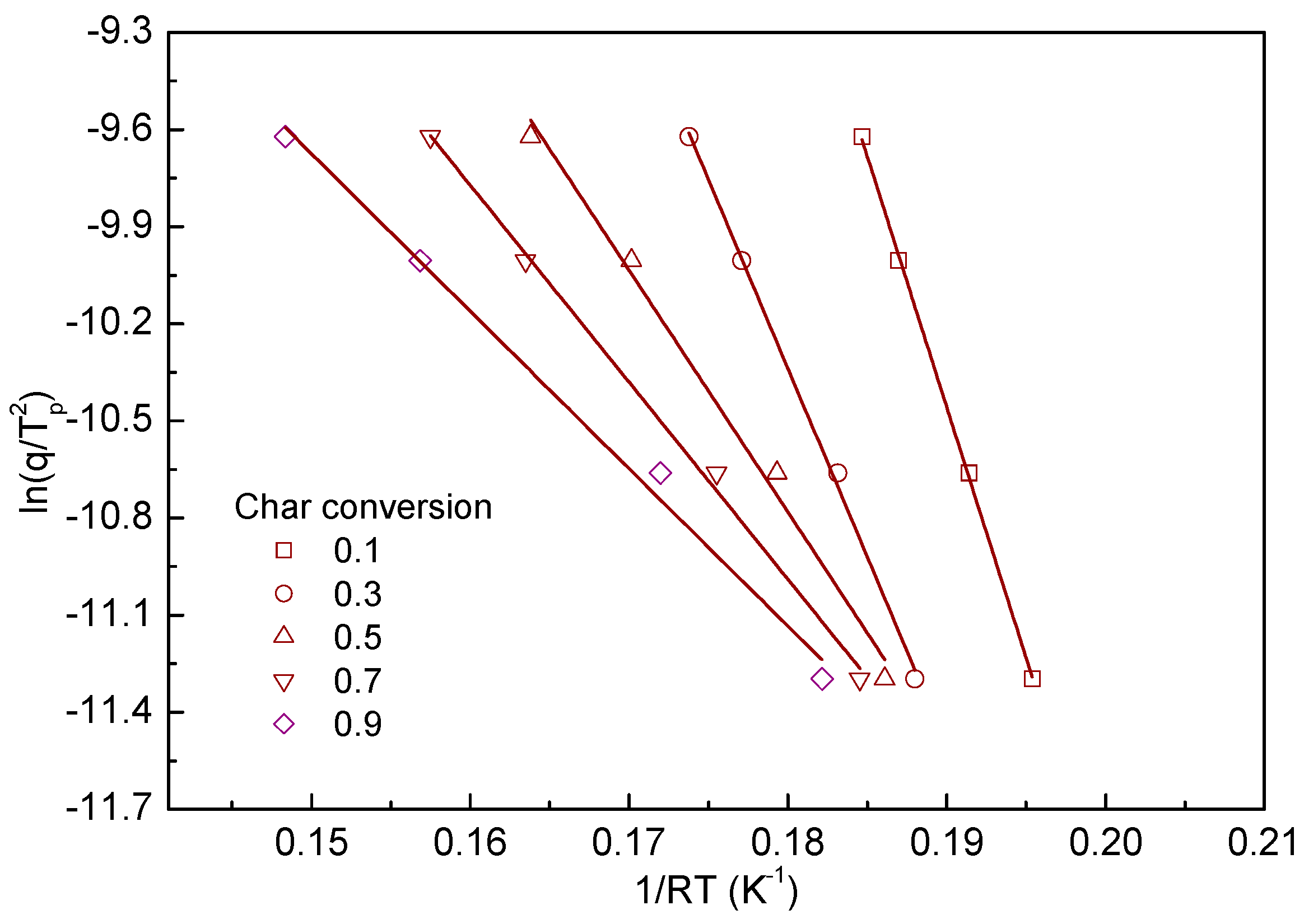
- The reaction rate of char oxidation was taken into account by using the Kissinger equation, and it shows appropriate linearity for the derived kinetic parameters.
Author Contributions
Funding
Conflicts of Interest
References
- Park, S.-W.; Lee, S.-Y.; Jeong, Y.-O.; Han, G.-H.; Seo, Y.-C. Effects of oxygen enrichment in air oxidants on biomass gasification efficiency and the reduction of tar emissions. Energies 2018, 11, 2664. [Google Scholar] [CrossRef]
- Norhasyima, R.S.; Mahlia, T.M.I. Advances in CO2 utilization technology: A patent landscape review. J. CO2 Util. 2018, 26, 323–335. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Damanik, N.; Ong, H.C.; Tong, C.W.; Mahlia, T.M.I.; Silitonga, A.S. A review on the engine performance and exhaust emission characteristics of diesel engines fueled with biodiesel blends. Environ. Sci. Pollut. Res. 2018, 25, 15307–15325. [Google Scholar] [CrossRef]
- Białowiec, A.; Micuda, M.; Koziel, J.A. Waste to carbon: Densification of torrefied refuse-derived fuel. Energies 2018, 11, 3233. [Google Scholar] [CrossRef]
- Hasan, M.; Haseli, Y.; Karadogan, E. Correlations to predict elemental compositions and heating value of torrefied biomass. Energies 2018, 11, 2443. [Google Scholar] [CrossRef]
- Poudel, J.; Karki, S.; Oh, S.C. Valorization of waste wood as a solid fuel by torrefaction. Energies 2018, 11, 1641. [Google Scholar] [CrossRef]
- De Filippis, P.; de Caprariis, B.; Scarsella, M.; Verdone, N. Double distribution activation energy model as suitable tool in explaining biomass and coal pyrolysis behavior. Energies 2015, 8, 1730–1744. [Google Scholar] [CrossRef]
- Cai, J.; Wu, W.; Liu, R. An overview of distributed activation energy model and its application in the pyrolysis of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 236–246. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Liang, L.; Yang, J.; Hao, X.; Guan, G.; Huang, W. Kinetics modeling of low-rank coal pyrolysis based on a three-Gaussian distributed activation energy model (DAEM) reaction model. Energy Fuels 2016, 30, 9693–9702. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.; Mahlia, T.M.I.; Azad, A.K. Elemental, morphological and thermal analysis of mixed microalgae species from drain water. Renew. Energy 2019, 131, 617–624. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass chars: The effects of pyrolysis conditions on their morphology, structure, chemical properties and reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R. Effects of biomass feedstocks and gasification conditions on the physiochemical properties of char. Energies 2013, 6, 3972–3986. [Google Scholar] [CrossRef]
- Ogbonnaya, C.I.; Roy-Macauley, H.; Nwalozie, M.C.; Annerose, D.J.M. Physical and histochemical properties of kenaf (Hibiscus cannabinus L.) grown under water deficit on a sandy soil. Ind. Crops Prod. 1997, 7, 9–18. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, G.-B.; Jeon, C.-H. Prediction of correlation between ash fusion temperature of ASTM and Thermo-Mechanical Analysis. Appl. Therm. Eng. 2017, 125, 1291–1299. [Google Scholar] [CrossRef]
- Song, H.; Liu, G.; Zhang, J.; Wu, J. Pyrolysis characteristics and kinetics of low rank coals by TG-FTIR method. Fuel Process. Technol. 2017, 156, 454–460. [Google Scholar] [CrossRef]
- Vyazovkin, S. Computational aspects of kinetic analysis: Part C. The ICTAC Kinetics Project—The light at the end of the tunnel? Thermochim. Acta 2000, 355, 155–163. [Google Scholar] [CrossRef]
- Miura, K.; Mae, K.; Shimada, M.; Minami, H. Analysis of formation rates of sulfur-containing gases during the pyrolysis of various coals. Energy Fuels 2001, 15, 629–636. [Google Scholar] [CrossRef]
- Miura, K.; Maki, T. A simple method for estimating f(E) and k0(E) in the distributed activation energy model. Energy Fuels 1998, 12, 864–869. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Liu, N.; Jørgensen, U.; Lærke, P.-E. Quality Determination of Biomass for Combustion: A New High-Throughput Microwave Digestion Method Prior to Elemental Analysis by Inductively Coupled Plasma−Optical Emission Spectroscopy. Energy Fuels 2013, 27, 7485–7488. [Google Scholar] [CrossRef]
- Cai, J.; Wu, W.; Liu, R.; Huber, G.-W. A distributed activation energy model for the pyrolysis of lignocellulosic biomass. Green Chem. 2013, 15, 1331–1340. [Google Scholar] [CrossRef]
- Meng, A.; Chen, S.; Long, Y.; Zhou, H.; Zhang, Y.; Li, Q. Pyrolysis and gasification of typical components in wastes with macro-TGA. Waste Manag. 2015, 46, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.-Q.; Bach, Q.-V.; Trinh, T.-T.; Seisenbaeva, G. Non-isothermal pyrolysis of torrefied stump—A comparative kinetic evaluation. Appl. Energy 2014, 136, 759–766. [Google Scholar] [CrossRef]
- Prince, M.; Ptasinski, K.; Janssen, F. Torrefaction of wood: Part 1. Weight loss kinetics. J. Anal. Appl. Pyrolysis 2006, 77, 28–34. [Google Scholar] [CrossRef]
- Fletcher, T.H.; Hillier, J.L. Pyrolysis Kinetics of a Green River Oil Shale Using a Pressurized TGA. Energy Fuels 2011, 25, 232–239. [Google Scholar]


| Properties | Samples | |
|---|---|---|
| Endpoint | Wood Pellets | Kenaf |
| Total moisture (as-rec a, wt.%) | ||
| Total moisture c | 9.46 | 11.52 |
| Proximate analysis (adb a, wt.%) | ||
| Moisture % c | 2.76 | 3.73 |
| Volatiles % | 75.65 | 73.64 |
| Fixed carbon % | 19.41 | 17.18 |
| Ash yield % | 2.18 | 5.45 |
| Ultimate analysis (daf a, wt.%) | ||
| Carbon % | 48.07 | 46.71 |
| Hydrogen % | 6.62 | 6.71 |
| Nitrogen % | 0.57 | 1.21 |
| Oxygen a % | 44.68 | 45.32 |
| Sulfur % | 0.06 | 0.05 |
| Oxide analysis (wt.%) | ||
| SiO2 | 2.87 | 6.73 |
| Al2O3 | 0.58 | 1.07 |
| TiO2 | 0.16 | 0.25 |
| Fe2O3 | 4.34 | 27.82 |
| CaO | 60.62 | 26.07 |
| MgO | 4.07 | 2.39 |
| Cr2O3 | 3.69 | 7.61 |
| K2O | 14.09 | 12.61 |
| P2O5 | 3.73 | 12.35 |
| SO3 | 4.66 | 2.58 |
| MnO | 1.19 | 0.52 |
| Calorific value (kcal/kg) | ||
| LHV b | 4210 | 3869 |
| HHV b | 4481 | 4160 |
| Index | TMA Shrinkage Temperature (°C) | |
|---|---|---|
| Parameter | Wood Pellets | Kenaf |
| T25 | 1124 | 977 |
| T50 | 1278 | 1043 |
| T75 | 1494 | 1102 |
| T90 | 1537 | 1135 |
| Sample | Element | Wavelength (nm) | Measured (ppm) a |
|---|---|---|---|
| Wood pellet | Cu | 327.393 | 30.1 |
| Cd | 228.802 | n.d. | |
| Cr | 267.716 | n.d. | |
| Fe | 238.204 | 213 | |
| Pb | 220.353 | n.d. | |
| Mn | 257.61 | 86.2 | |
| Zn | 206.2 | n.d. | |
| Al | 396.153 | 197 | |
| As | 188.979 | n.d. | |
| Ba | 233.527 | 14.6 | |
| Ca | 317.933 | 4750 | |
| Mg | 285.213 | 990 | |
| K | 766.49 | 1856 | |
| Na | 589.592 | 437 | |
| Sr | 407.771 | 18.6 | |
| P | 213.617 | 682 | |
| Si | 251.611 | 324 | |
| S | 180.669 | 554 | |
| Ti | 334.94 | n.d. | |
| Kenaf | Cu | 327.393 | 41.3 |
| Cd | 228.802 | n.d. | |
| Cr | 267.716 | 29.3 | |
| Fe | 238.204 | 1738 | |
| Pb | 220.353 | 25.8 | |
| Mn | 257.61 | 90.0 | |
| Zn | 206.2 | 210 | |
| Al | 396.153 | 892 | |
| As | 188.979 | n.d. | |
| Ba | 233.527 | 40.3 | |
| Ca | 317.933 | 7979 | |
| Mg | 285.213 | 3124 | |
| K | 766.49 | 3090 | |
| Na | 589.592 | 354 | |
| Sr | 407.771 | 28.8 | |
| P | 213.617 | 7177 | |
| Si | 251.611 | 3049 | |
| S | 180.669 | 1406 | |
| Ti | 334.94 | 60.1 |
| Samples | Stage Temp. | Activation Energy, E | Frequency Factor, A | R2 |
|---|---|---|---|---|
| (°C) | (kJ/mol) | (min−1) | (-) | |
| Wood pellet | 150–310 | 69.0 | 433,870 | 0.9982 |
| 310–360 | 81.4 | 7,061,872 | 0.9948 | |
| 360–875 | 7.6 | 16.42 | 0.9959 | |
| Kenaf | 150–193 | 69.3 | 1,037,931 | 0.9944 |
| 193–264 | 58.0 | 61,031 | 0.9967 | |
| 264–320 | 69.8 | 855,788 | 0.9981 | |
| 320–875 | 6.8 | 13.07 | 0.9885 |
| Wood Pellet Component Description | Kenaf Component Description | ||||||
|---|---|---|---|---|---|---|---|
| Species | T (°C) | E0 (kJ/mol) | (kJ/mol) | Species | T (°C) | E0 (kJ/mol) | (kJ/mol) |
| 1st | 237–400 | 181.8 | 8.2 | 1st | 132–287 | 149.8 | 3.2 |
| 2nd | 340–360 | 190.7 | 0.8 | 2nd | 180–375 | 169.2 | 6.4 |
| 3rd | 162–897 | 203.2 | 32 | 3rd | 290–327 | 178.7 | 1.4 |
| - | - | - | - | 4th | 167–877 | 196.4 | 3.8 |
| Char Conversion Value | ||
|---|---|---|
| Endpoint | Wood Pellet | Kenaf |
| 0.1 | 155.0 | 93.5 |
| 0.3 | 116.6 | 68.7 |
| 0.5 | 74.7 | 50.9 |
| 0.7 | 60.9 | 46.6 |
| 0.9 | 48.7 | 39.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-M.; Lee, D.-G.; Jeon, C.-H. Fundamental Characteristics and Kinetic Analysis of Lignocellulosic Woody and Herbaceous Biomass Fuels. Energies 2019, 12, 1008. https://doi.org/10.3390/en12061008
Kim G-M, Lee D-G, Jeon C-H. Fundamental Characteristics and Kinetic Analysis of Lignocellulosic Woody and Herbaceous Biomass Fuels. Energies. 2019; 12(6):1008. https://doi.org/10.3390/en12061008
Chicago/Turabian StyleKim, Gyeong-Min, Dae-Gyun Lee, and Chung-Hwan Jeon. 2019. "Fundamental Characteristics and Kinetic Analysis of Lignocellulosic Woody and Herbaceous Biomass Fuels" Energies 12, no. 6: 1008. https://doi.org/10.3390/en12061008
APA StyleKim, G.-M., Lee, D.-G., & Jeon, C.-H. (2019). Fundamental Characteristics and Kinetic Analysis of Lignocellulosic Woody and Herbaceous Biomass Fuels. Energies, 12(6), 1008. https://doi.org/10.3390/en12061008







