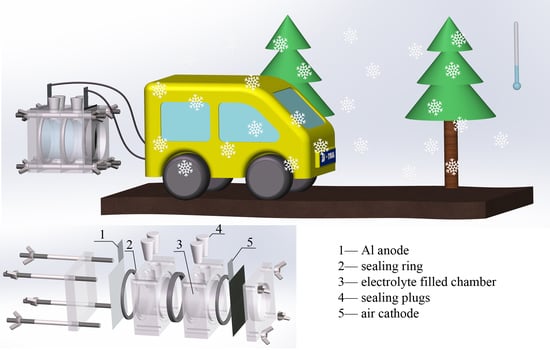
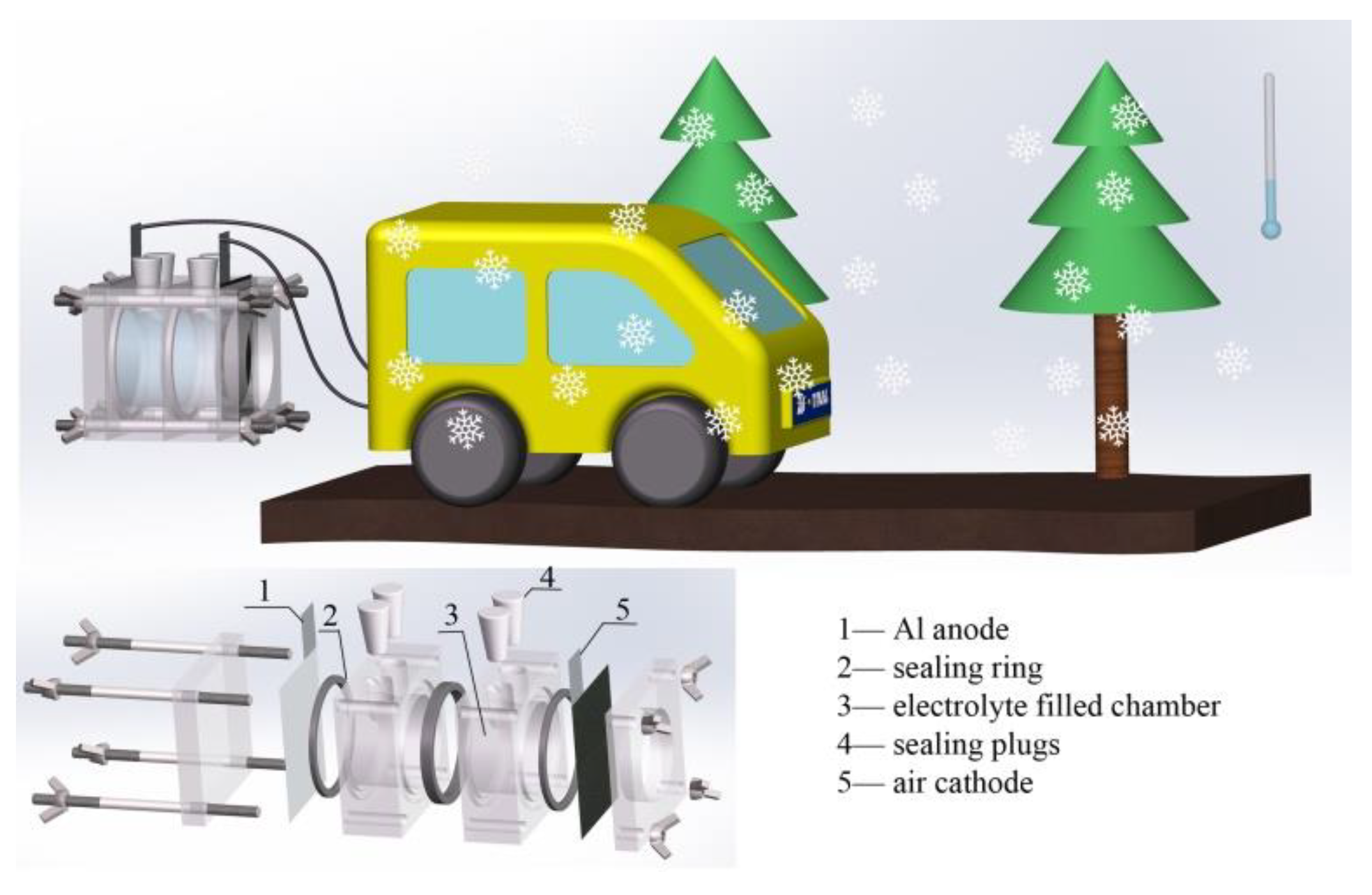
Low-Temperature Performance of Al-air Batteries
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Fabrication of Al-air Batteries
2.3. Electrochemical Tests
3. Results and Discussion
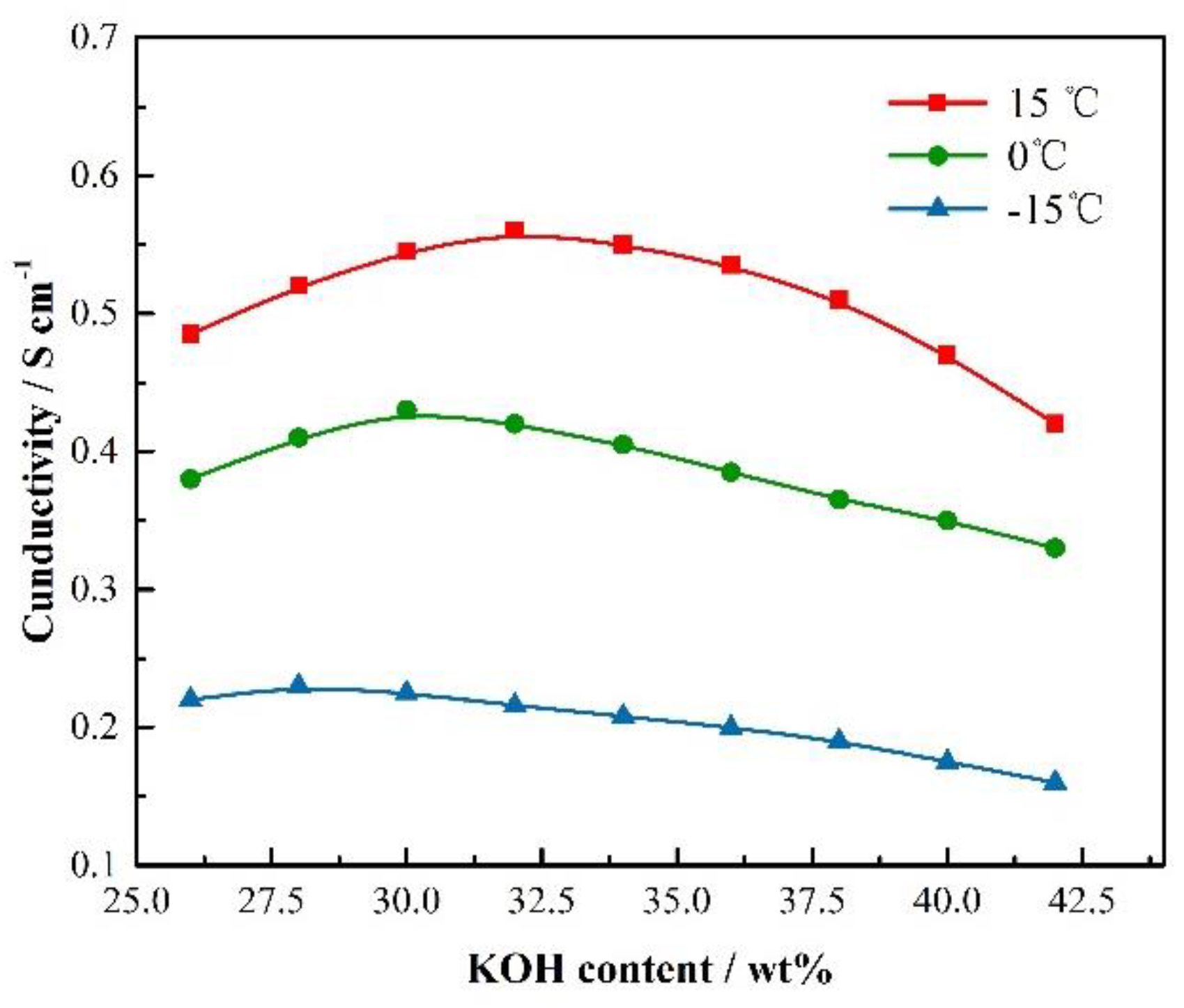
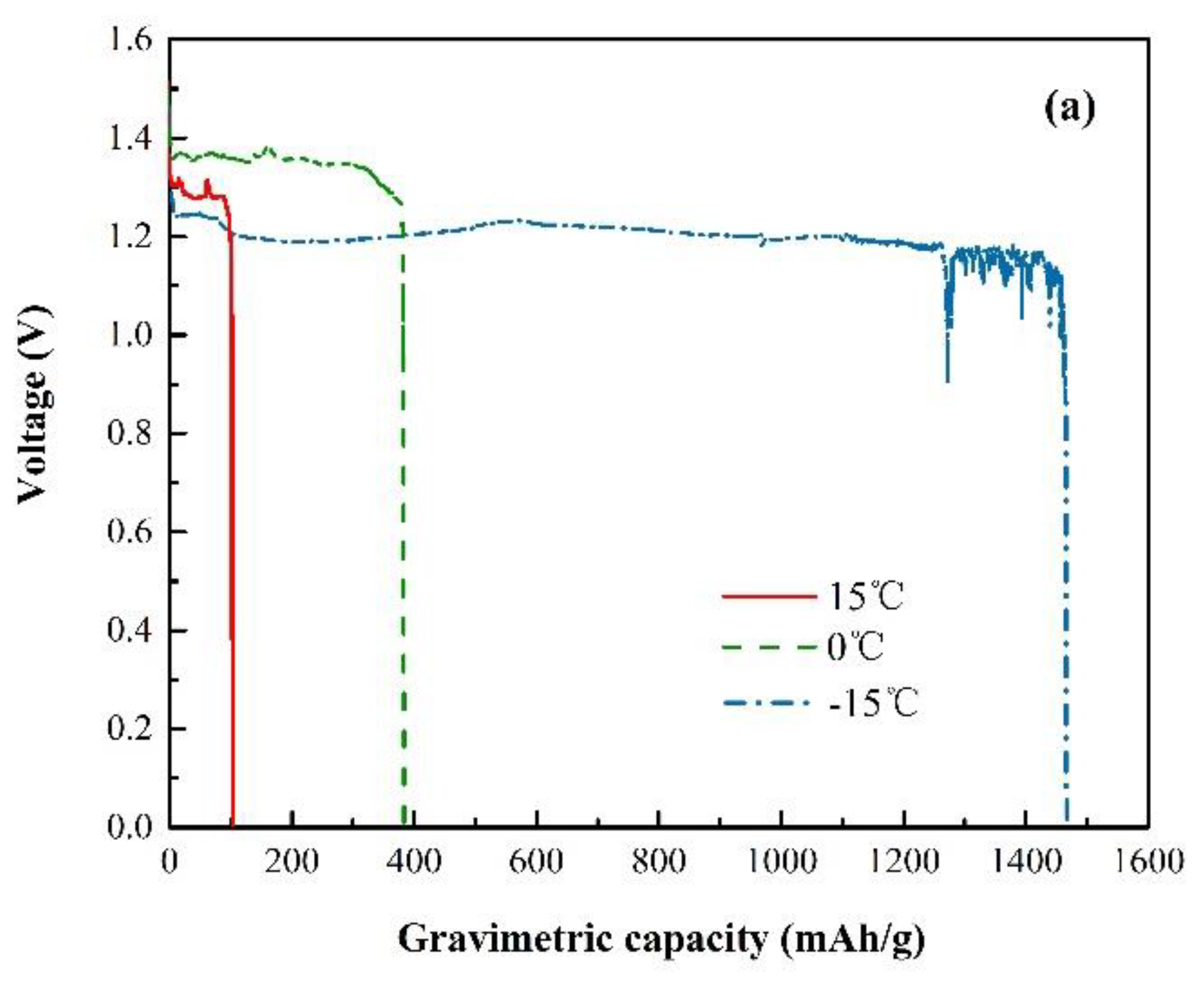
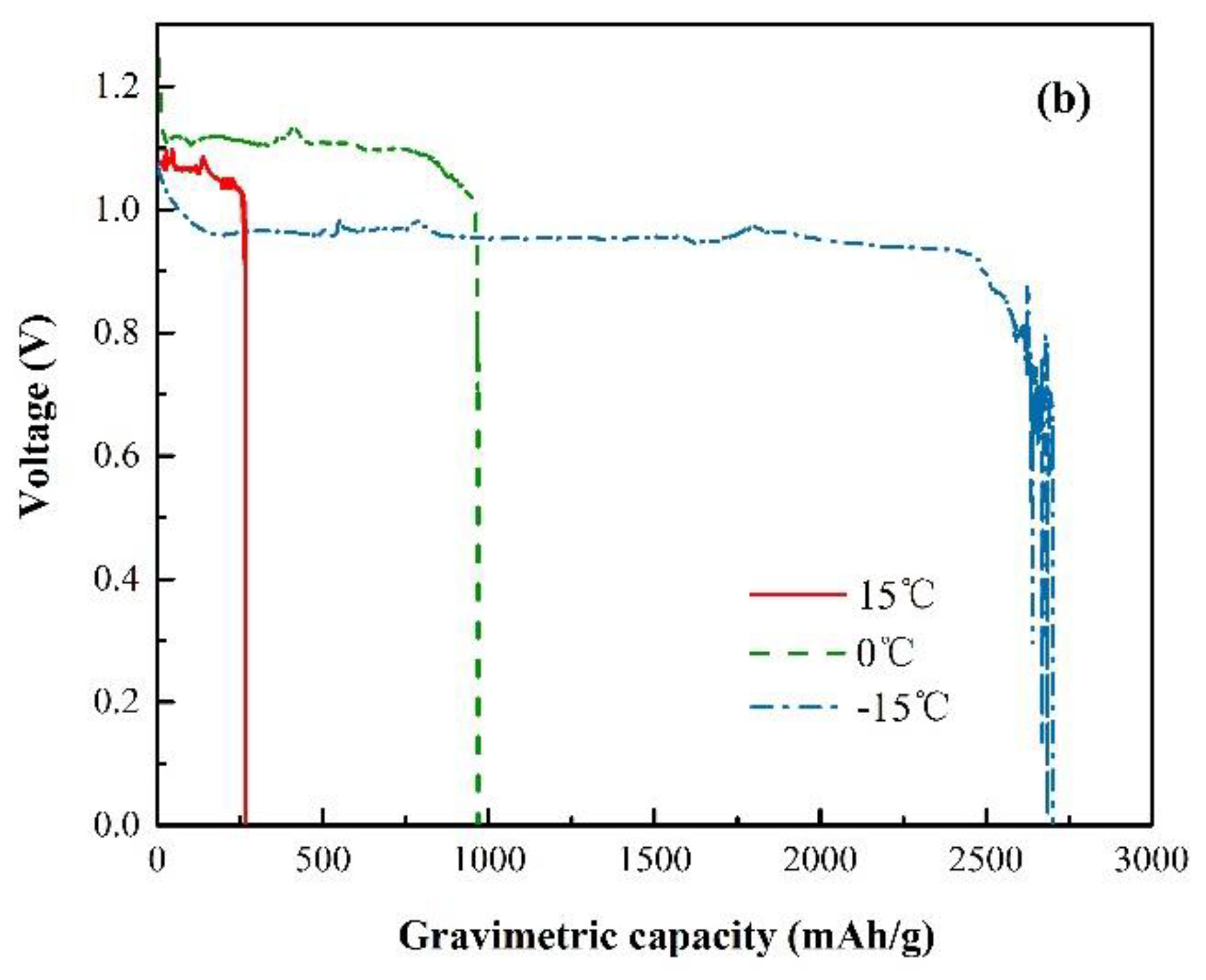
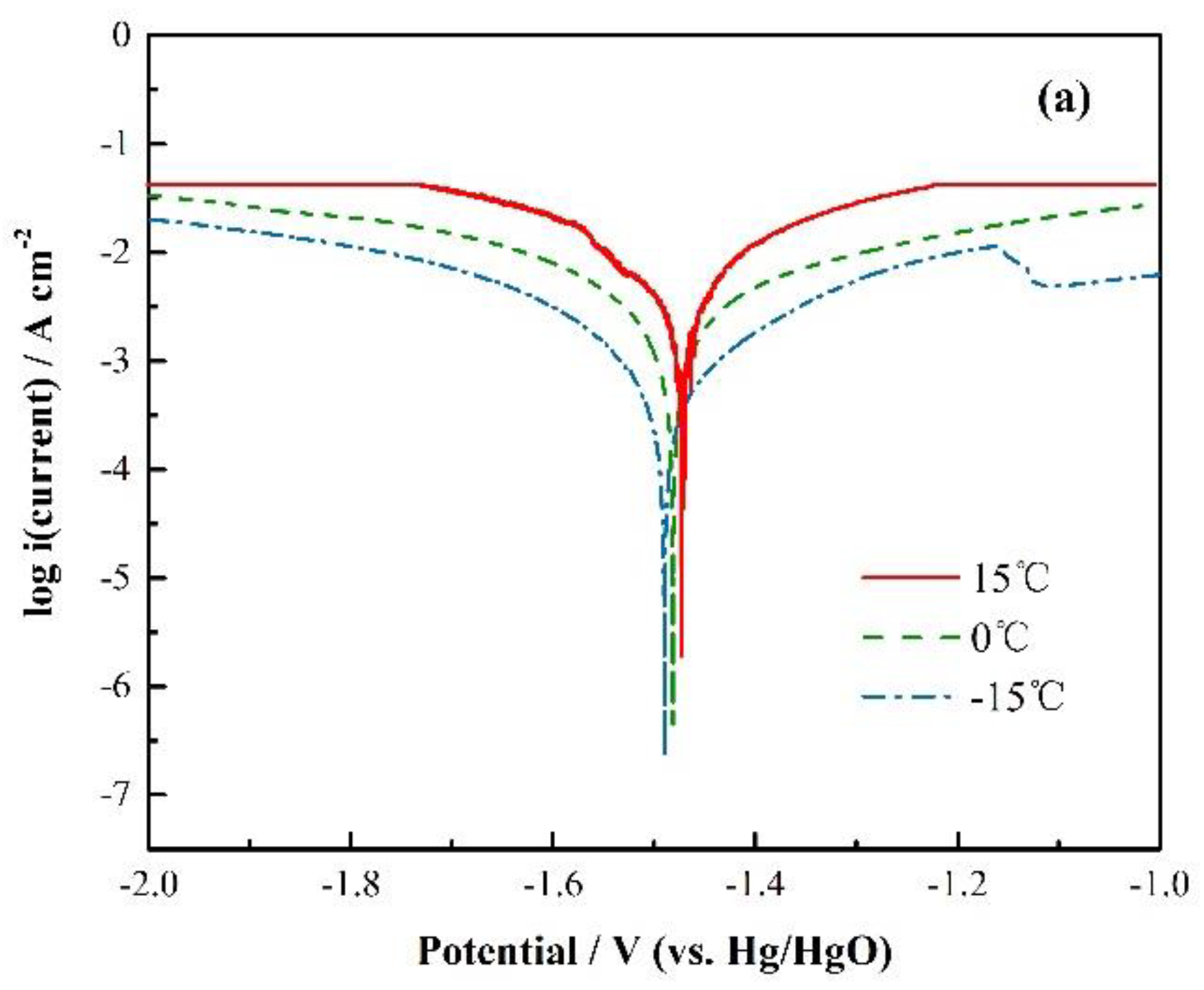
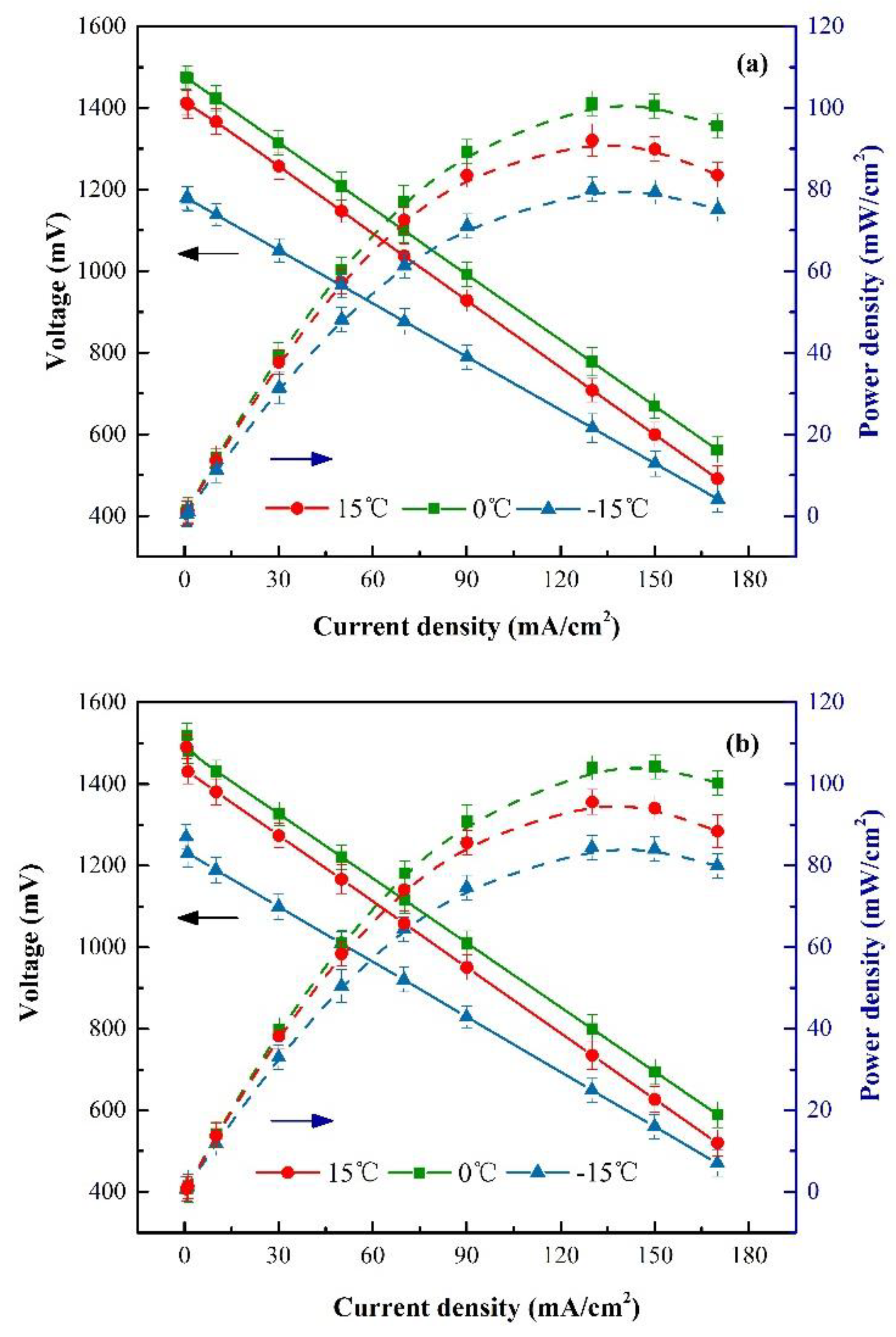
3.1. Ionic Conductivity
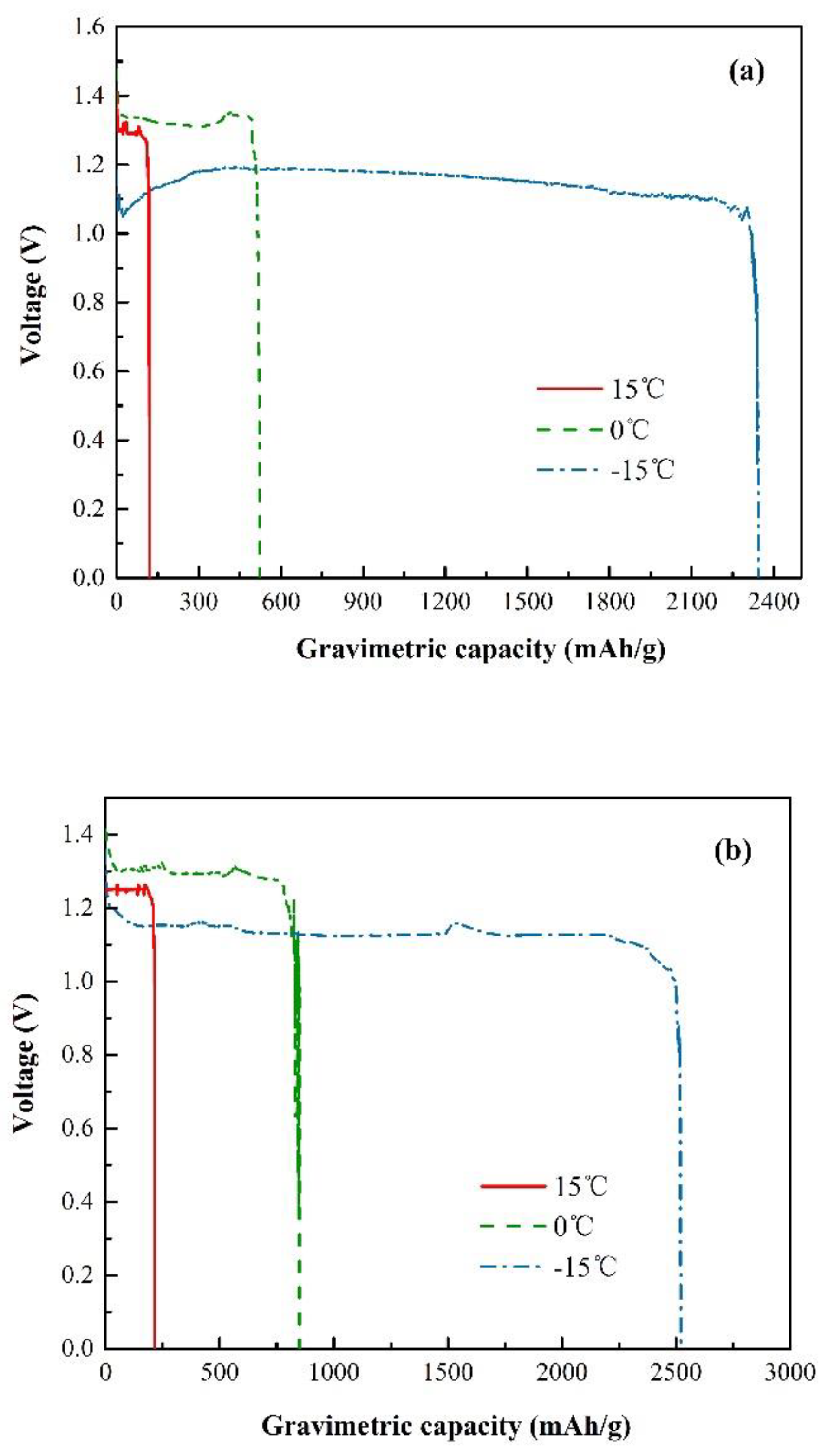
3.2. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.L.; Xu, D.; Zhong, H.X.; Wang, J.; Meng, F.L.; Zhang, X.B. Gelatin-derived sustainable carbon-based functional materials for energy conversion and storage with controllability of structure and component. Sci. Adv. 2015, 1, e1400035. [Google Scholar] [CrossRef]
- Gai, W.Z.; Deng, Z.Y. Effect of trace species in water on the reaction of Al with water. J. Power Sources 2014, 245, 721–729. [Google Scholar] [CrossRef]
- Zhang, Z.; Zuo, C.; Liu, Z.; Yu, Y.; Zuo, Y.; Song, Y. All-solid-state Al–air batteries with polymer alkaline gel electrolyte. J. Power Sources 2014, 251, 470–475. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Shao-Horn, Y.; Hart, D.P. Suppressing corrosion in primary aluminum–air batteries via oil displacemen. Science 2018, 362, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Gelman, D.; Lasman, I.; Elfimchev, S.; Starosvetsky, D.; Ein-Eli, Y. Aluminum corrosion mitigation in alkaline electrolytes containing hybrid inorganic/organic inhibitor system for power sources applications. J. Power Sources 2015, 285, 100–108. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H.; Leng, J. Performance of fine structured aluminum anodes in neutral and alkaline electrolytes for Al-air batteries. Electrochim. Acta 2015, 165, 22–28. [Google Scholar] [CrossRef]
- Grande, L.; Paillard, E.; Hassoun, J.; Park, J.B.; Lee, Y.J.; Sun, Y.K.; Passerini, S.; Scrosati, B. The lithium/air battery: Still an emerging system or a practical reality? Adv. Mater. 2015, 27, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Xu, K.; Jow, T.R. Electrochemical impedance study on the low temperature of Li-ion batteries. Electrochim. Acta 2004, 49, 1057–1061. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Zhang, Z.; Li, C.; Ma, J.; Wang, C.; Ge, X.; Dong, S.H.; Yin, L. Low-Temperature Solution-Based Phosphorization Reaction Route to Sn4P3/Reduced Graphene Oxide Nanohybrids as Anodes for Sodium Ion Batteries. Adv. Energy Mater. 2016, 6, 1600376. [Google Scholar] [CrossRef]
- Qi, Y.; Mu, L.; Zhao, J.; Hu, Y.S.; Liu, H.; Dai, S. Superior Na-Storage Performance of Low-Temperature-Synthesized Na3(VO1−xPO4)2F1+2x (0 ≤ x ≤ 1) Nanoparticles for Na-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 9911–9916. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wen, K.; Lv, W.; Zhou, X.; Liang, Y.; Yang, F.; Chen, Z.; Zou, M.; Li, J.; Zhang, Y.; et al. Materials insights into low-temperature performances of lithium-ion batteries. J. Power Sources 2015, 300, 29–40. [Google Scholar] [CrossRef]
- Shang, H.; Zuo, Z.; Yu, L.; Wang, F.; He, F.; Li, Y. Low-Temperature Growth of All-Carbon Graphdiyne on a Silicon Anode for High-Performance Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1801459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Hou, B.H.; Guo, J.Z.; Ning, Q.L.; Pang, W.L.; Wang, J.; Lü, C.L.; Wu, X.L. An Ultralong Lifespan and Low-Temperature Workable Sodium-Ion Full Battery for Stationary Energy Storage. Adv. Energy Mater. 2018, 8, 1703252. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.; Wang, C.Y. Li-ion cell operation at low temperatures. J. Electrochem. Soc. 2013, 160, A636–A649. [Google Scholar] [CrossRef]
- Mousa, A.; Skyllas-Kazacos, M. Effect of additives on the low-temperature stability of vanadium redox flow battery negative half-cell electrolyte. ChemElectroChem 2015, 2, 1742–1751. [Google Scholar] [CrossRef]
- You, Y.; Yao, H.R.; Xin, S.; Yin, Y.X.; Zuo, T.T.; Yang, C.P.; Guo, Y.-G.; Cui, Y.; Wan, L.-J.; Goodenough, J.-B. Subzero-Temperature Cathode for a Sodium-Ion Battery. Adv. Mater. 2016, 28, 7243–7248. [Google Scholar] [CrossRef] [PubMed]
- Senyshyn, A.; Mühlbauer, M.J.; Dolotko, O.; Ehrenberg, H. Low-temperature performance of Li-ion batteries: The behavior of lithiated graphite. J. Power Sources 2015, 282, 235–240. [Google Scholar] [CrossRef]
- Lee, M.J.; Lho, E.; Bai, P.; Chae, S.; Li, J.; Cho, J. Low-temperature carbon coating of nanosized Li1. 015Al0. 06Mn1. 925O4 and high-density electrode for high-power Li-Ion batteries. Nano Lett. 2017, 17, 3744–3751. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dubé, Y. A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures. Appl. Energy 2016, 164, 99–114. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V.; Chin, K.B.; Whitcanack, L.D. Lithium-ion electrolytes containing ester cosolvents for improved low temperature performance. J. Electrochem. Soc. 2010, 157, A1361–A1374. [Google Scholar] [CrossRef]
- Sides, C.R.; Martin, C.R. Nanostructured Electrodes and the Low-Temperature Performance of Li-Ion Batteries. Adv. Mater. 2005, 17, 125–128. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. A new approach toward improved low temperature performance of Li-ion battery. Electrochem. Commun. 2002, 4, 928–932. [Google Scholar] [CrossRef]
- Liu, J.; Lin, X.; Han, T.; Li, X.; Gu, C.; Li, J. A novel litchi-like LiFePO4 sphere/reduced graphene oxide composite Li-ion battery cathode with high capacity, good rate-performance and low-temperature property. Appl. Surf. Sci. 2018, 459, 233–241. [Google Scholar] [CrossRef]
- Tripathy, Y.; McGordon, A.; Low, C. A New Consideration for Validating Battery Performance at Low Ambient Temperatures. Energies 2018, 11, 2439. [Google Scholar] [CrossRef]
- Li, Y.; Qian, K.; He, Y.B.; Kaneti, Y.V.; Liu, D.; Luo, D.; Li, H.; Li, B.; Kang, F. Study on the reversible capacity loss of layered oxide cathode during low-temperature operation. J. Power Sources 2017, 342, 24–30. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Cheng, Y.; Feng, K.; Li, X.; Zhang, H. All-NASICON LVP-LTP aqueous lithium ion battery with excellent stability and low-temperature performance. Electrochim. Acta 2018, 278, 279–289. [Google Scholar] [CrossRef]
- Reyes, J.R.M.D.; Parsons, R.V.; Hoemsen, R. Winter happens: The effect of ambient temperature on the travel range of electric vehicles. IEEE Trans. Veh. Technol. 2016, 65, 4016–4022. [Google Scholar] [CrossRef]
- Inoishi, A.; Nishio, A.; Yoshioka, Y.; Kitajou, A.; Okada, S. A single-phase all-solid-state lithium battery based on Li1.5Cr0.5Ti1.5(PO4)3 for high rate capability and low temperature operation. Chem. Commun. 2018, 54, 3178–3181. [Google Scholar] [CrossRef] [PubMed]
- Zaromb, S. The use and behavior of aluminum anodes in alkaline primary batteries. J. Electrochem. Soc. 1962, 109, 1125–1130. [Google Scholar] [CrossRef]
- Patnaik, R.S.M.; Ganesh, S.; Ashok, G.; Ganesan, M.; Kapali, V. Heat management in aluminium/air batteries: Sources of heat. J. Power Sources 1994, 50, 331–342. [Google Scholar] [CrossRef]
- Li, Q.; Bjerrum, N.J. Aluminum as anode for energy storage and conversion: A review. J. Power Sources 2002, 110, 1–10. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Whiting, G.L.; Steingart, D.A.; Arias, A.C. Highly flexible, printed alkaline batteries based on mesh-embedded electrodes. Adv. Mater. 2011, 23, 3251–3255. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Majlan, E.H.; Ahmad, A.; Tasirin, S.M.; Daud, W.R.W. Effect of ZnO filler on PVA-alkaline solid polymer electrolyte for aluminum-air battery applications. J. Electrochem. Soc. 2018, 165, A2483–A2492. [Google Scholar] [CrossRef]
- Yashonath, S.; Ghorai, P.K. Diffusion in nanoporous phases: Size dependence and levitation effect. J. Phys. Chem. B 2008, 112, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.L.; Jamaludin, A.; Alias, Y.; Basirun, W.J.; Ahmad, Z.A.; Mohamad, A.A. Effect of KOH concentration in the gel polymer electrolyte for direct borohydride fuel cell. J. Appl. Polym. Sci. 2001, 123, 2662–2666. [Google Scholar] [CrossRef]
- Iwakura, C.; Nohara, S.; Furukawa, N.; Inoue, H. The possible use of polymer gel electrolytes in nickel/metal hydride battery. Solid State Ionics 2002, 148, 487–492. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the temperature dependence of soil respiration. Funct. Ecol. 1994, 8, 315–323. [Google Scholar] [CrossRef]
- Elia, G.A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R.; Knipping, E.; Peters, W.; Drillet, J.-F.; Passerini, S.; Hahn, R. An overview and future perspectives of aluminum batteries. Adv. Mater. 2016, 28, 7564–7579. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.N.; Kang, S.H.; Prasanna, K.; Eom, S.W.; Lee, C.W. Shield effect of polyaniline between zinc active material and aqueous electrolyte in zinc-air batteries. Appl. Surf. Sci. 2017, 422, 406–412. [Google Scholar] [CrossRef]
- Migliardini, F.; Di Palma, T.M.; Gaele, M.F.; Corbo, P. Solid and acid electrolytes for Al-air batteries based on xanthan-HCl hydrogels. J. Solid State Electrochem. 2018, 22, 2901–2916. [Google Scholar] [CrossRef]
- Kang, Q.X.; Wang, Y.; Zhang, X.Y. Experimental and theoretical investigation on calcium oxide and L-aspartic as an effective hybrid inhibitor for aluminum-air batteries. J. Alloys Compd. 2019, 774, 1069–1080. [Google Scholar] [CrossRef]


| KOH Content | Temperature (°C) | −Ecorr (V vs. Hg/HgO) | Icorr (mA/cm2) | η% |
|---|---|---|---|---|
| 25 wt% | −15 | −1.490 | 0.232 | 84.33 |
| 0 | −1.480 | 0.695 | 53.02 | |
| 15 | −1.471 | 1.479 | -- | |
| 31 wt% | −15 | −1.487 | 0.448 | 44.54 |
| 0 | −1.479 | 0.534 | 33.86 | |
| 15 | −1.466 | 0.807 | -- |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, Y.; Yu, Y.; Zuo, C.; Ning, C.; Liu, H.; Gu, Z.; Cao, Q.; Shen, C. Low-Temperature Performance of Al-air Batteries. Energies 2019, 12, 612. https://doi.org/10.3390/en12040612
Zuo Y, Yu Y, Zuo C, Ning C, Liu H, Gu Z, Cao Q, Shen C. Low-Temperature Performance of Al-air Batteries. Energies. 2019; 12(4):612. https://doi.org/10.3390/en12040612
Chicago/Turabian StyleZuo, Yuxin, Ying Yu, Chuncheng Zuo, Chuanlong Ning, Hao Liu, Zhiqing Gu, Qianqian Cao, and Ciming Shen. 2019. "Low-Temperature Performance of Al-air Batteries" Energies 12, no. 4: 612. https://doi.org/10.3390/en12040612
APA StyleZuo, Y., Yu, Y., Zuo, C., Ning, C., Liu, H., Gu, Z., Cao, Q., & Shen, C. (2019). Low-Temperature Performance of Al-air Batteries. Energies, 12(4), 612. https://doi.org/10.3390/en12040612