Soybean Oil Transesterification for Biodiesel Production with Micro-Structured Calcium Oxide (CaO) from Natural Waste Materials as a Heterogeneous Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the CaO Catalyst
2.3. Catalyst Characterization
2.4. Soybean Oil Transesterification
3. Results
3.1. Characterization of the Catalyst
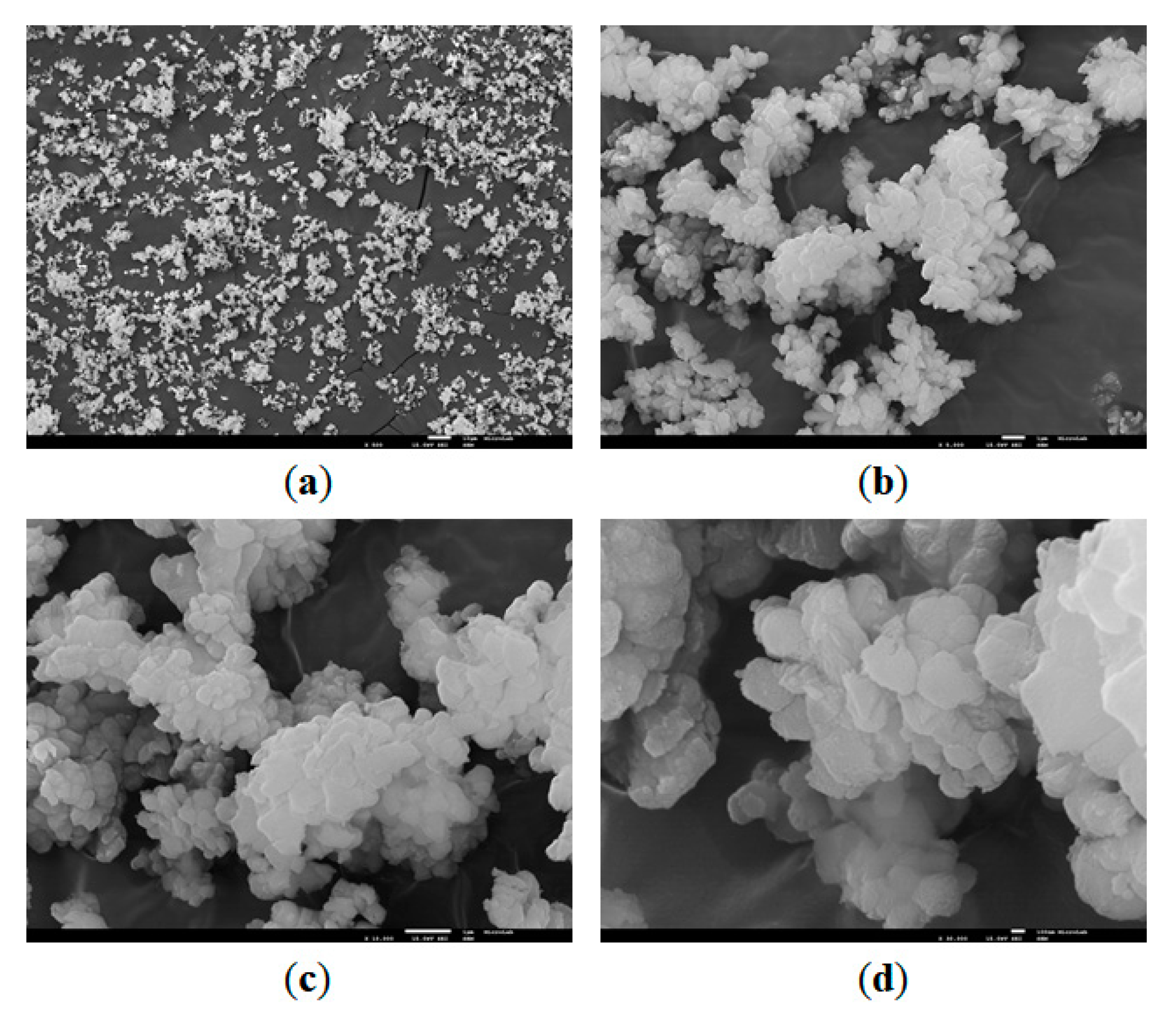
3.1.1. SEM Analysis
3.1.2. Dynamic Light Scattering (DLS) Analysis
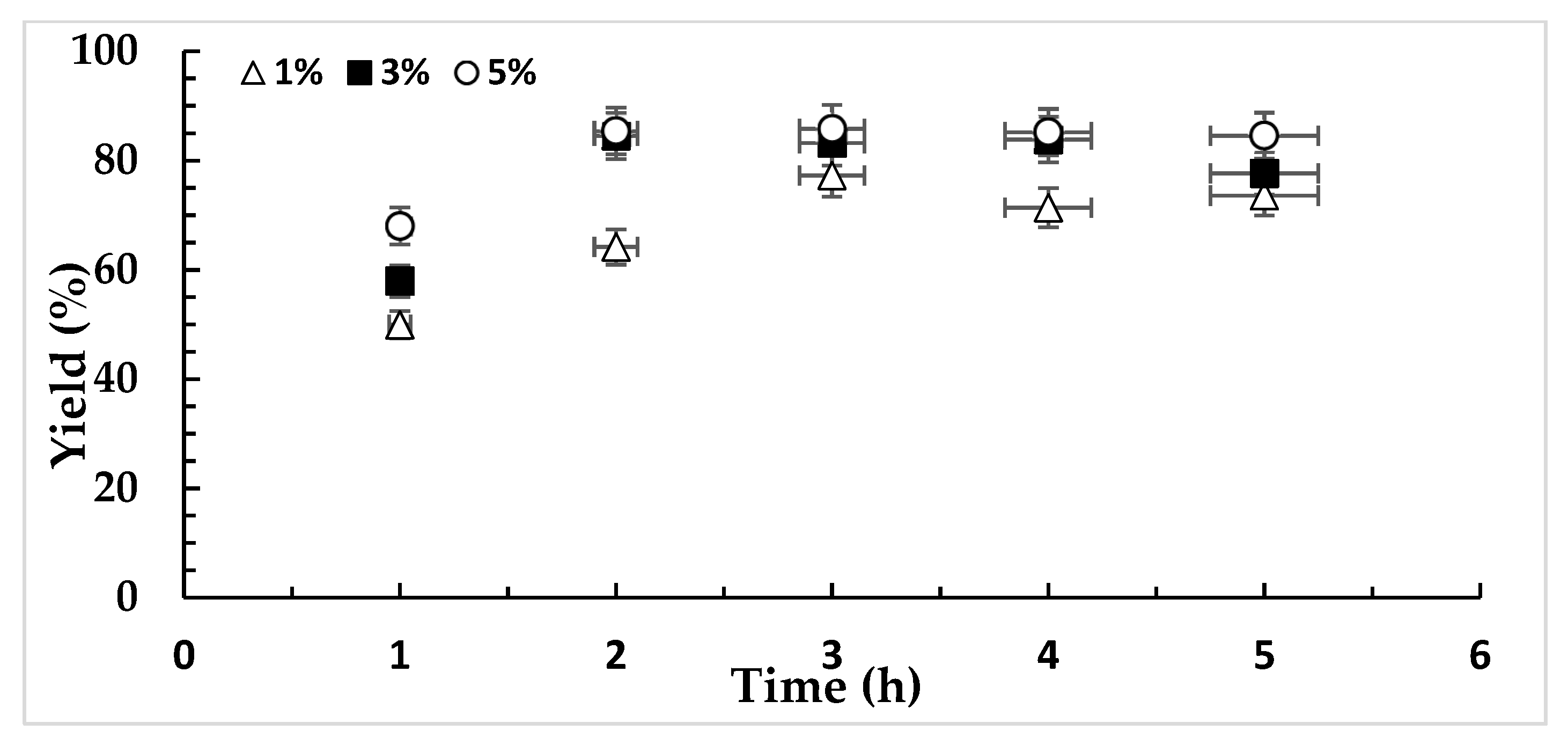
3.2. Catalytic Activity
4. Discussion
4.1. SEM Analysis
4.2. DLS Analysis
4.3. Catalytic Activity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Heterogeneous Solid Base Nanocatalyst: Preparation, Characterization and Application in Biodiesel Production. Bioresour. Technol. 2011, 102, 4150–4156. [Google Scholar] [CrossRef] [PubMed]
- Safaei-Ghomi, J.; Ghasemzadeh, M.A.; Mehrabi, M. Calcium Oxide Nanoparticles Catalyzed One-Step Multicomponent Synthesis of Highly Substituted Pyridines in Aqueous Ethanol Media. Sci. Iran. 2013, 20, 549–554. [Google Scholar] [CrossRef]
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical Aspects of Biodiesel Production by Transesterification—A Review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Islam, A.; Taufiq-Yap, Y.H.; Chan, E.S.; Moniruzzaman, M.; Islam, S.; Nabi, M.N. Advances in Solid-Catalytic and Non-Catalytic Technologies for Biodiesel Production. Energy Convers. Manag. 2014, 88, 1200–1218. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and Recent Trends in Biodiesel Fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Singh, A.K.; Fernando, S.D. Transesterification of Soybean Oil Using Heterogeneous Catalysts. Energy Fuels 2008, 22, 2067–2069. [Google Scholar] [CrossRef]
- Liu, C.; Lv, P.; Yuan, Z.; Yan, F.; Luo, W. The Nanometer Magnetic Solid Base Catalyst for Production of Biodiesel. Renew. Energy 2010, 35, 1531–1536. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J. Transesterification of Soybean Oil with Nano-MgO or Not in Supercritical and Subcritical Methanol. Fuel 2007, 86, 328–333. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and Adsorption Properties of Eggshells and Eggshell Membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef]
- Hamester, M.R.R.; Balzer, P.S.; Becker, D. Characterization of Calcium Carbonate Obtained from Oyster and Mussel Shells and Incorporation in Polypropylene. Mater. Res. 2012, 15, 204–208. [Google Scholar] [CrossRef]
- Bet-Moushoul, E.; Farhadi, K.; Mansourpanah, Y.; Nikbakht, A.M.; Molaei, R.; Forough, M. Application of CaO-Based/Au Nanoparticles as Heterogeneous Nanocatalysts in Biodiesel Production. Fuel 2016, 164, 119–127. [Google Scholar] [CrossRef]
- Pandit, P.R.; Fulekar, M.H. Egg Shell Waste as Heterogeneous Nanocatalyst for Biodiesel Production: Optimized by Response Surface Methodology. J. Environ. Manag. 2017, 198, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Baskar, G.; Aiswarya, R. Trends in Catalytic Production of Biodiesel from Various Feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N.; Shah, N.K. Applications of Nano-Catalyst in New Era. J. Saudi Chem. Soc. 2012, 16, 307–325. [Google Scholar] [CrossRef]
- Anr, R.; Saleh, A.A.; Islam, M.S.; Hamdan, S.; Maleque, M.A. Biodiesel Production from Crude Jatropha Oil Using a Highly Active Heterogeneous Nanocatalyst by Optimizing Transesterification Reaction Parameters. Energy Fuels 2016, 30, 334–343. [Google Scholar] [CrossRef]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-Magnetic Catalyst KF/CaO–Fe3O4 for Biodiesel Production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Lu, D.; Hu, S.; Han, H. Preparation of KF/CaO Nanocatalyst and Its Application in Biodiesel Production from Chinese Tallow Seed Oil. Fuel 2010, 89, 2267–2271. [Google Scholar] [CrossRef]
- Kaur, M.; Ali, A. Lithium Ion Impregnated Calcium Oxide as Nano Catalyst for the Biodiesel Production from Karanja and Jatropha Oils. Renew. Energy 2011, 36, 2866–2871. [Google Scholar] [CrossRef]
- Lee, L.Y.; Wang, C.H.; Smith, K.A. Supercritical Antisolvent Production of Biodegradable Micro- and Nanoparticles for Controlled Delivery of Paclitaxel. J. Control. Release 2008, 125, 96–106. [Google Scholar] [CrossRef]
- Chattopadhyay, P.; Gupta, R.B. Production of Griseofulvin Nanoparticles Using Supercritical CO2 Antisolvent with Enhanced Mass Transfer. Int. J. Pharm. 2001, 228, 19–31. [Google Scholar] [CrossRef]
- Kim, M.-S.; Jin, S.-J.; Kim, J.-S.; Park, H.J.; Song, H.-S.; Neubert, R.H.H.; Hwang, S.-J. Preparation, Characterization and in Vivo Evaluation of Amorphous Atorvastatin Calcium Nanoparticles Using Supercritical Antisolvent (SAS) Process. Eur. J. Pharm. Biopharm. 2008, 69, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Della Porta, G.; Sannino, D.; Ciambelli, P. Supercritical Antisolvent Precipitation of Nanoparticles of a Zinc Oxide Precursor. Powder Technol. 1999, 102, 127–134. [Google Scholar] [CrossRef]
- Tavares Cardoso, M.A.; Antunes, S.; van Keulen, F.; Ferreira, B.S.; Geraldes, A.; Cabral, J.M.S.; Palavra, A.M.F. Supercritical Antisolvent Micronisation of Synthetic All-Trans-β-Carotene with Tetrahydrofuran as Solvent and Carbon Dioxide as Antisolvent. J. Chem. Technol. Biotechnol. 2009, 84, 215–222. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I.; Torino, E. Nanoparticles Production by Supercritical Antisolvent Precipitation: A General Interpretation. J. Supercrit. Fluids 2007, 43, 126–138. [Google Scholar] [CrossRef]
- Fahim, T.K.; Zaidul, I.S.M.; Abu Bakar, M.R.; Salim, U.M.; Awang, M.B.; Sahena, F.; Jalal, K.C.A.; Sharif, K.M.; Sohrab, M.H. Particle Formation and Micronization Using Non-Conventional Techniques—Review. Chem. Eng. Process. Process Intensif. 2014, 86, 47–52. [Google Scholar] [CrossRef]
- Catarino, M.; Ramos, M.; Dias, A.P.S.; Santos, M.T.; Puna, J.F.; Gomes, J.F. Calcium Rich Food Wastes Based Catalysts for Biodiesel Production. Waste Biomass Valoriz. 2017, 8, 1699–1707. [Google Scholar] [CrossRef]
- Soares Dias, A.P.; Puna, J.; Neiva Correia, M.J.; Nogueira, I.; Gomes, J.; Bordado, J. Effect of the Oil Acidity on the Methanolysis Performances of Lime Catalyst Biodiesel from Waste Frying Oils (WFO). Fuel Process. Technol. 2013, 116, 94–100. [Google Scholar] [CrossRef]
- Puna, J.F.; Gomes, J.F.; Bordado, J.C.; Correia, M.J.N.; Dias, A.P.S. Biodiesel Production over Lithium Modified Lime Catalysts: Activity and Deactivation. Appl. Catal. A Gen. 2014, 470, 451–457. [Google Scholar] [CrossRef]
- Puna, J.F.; Correia, M.J.N.; Dias, A.P.S.; Gomes, J.; Bordado, J. Biodiesel Production from Waste Frying Oils over Lime Catalysts. React. Kinet. Mech. Catal. 2013, 109, 405–415. [Google Scholar] [CrossRef][Green Version]
- Ilgen, O. Dolomite as a Heterogeneous Catalyst for Transesterification of Canola Oil. Fuel Process. Technol. 2011, 92, 452–455. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Eggshell Waste as Catalyst: A Review. J. Environ. Manag. 2017, 197, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Badrul, H.M.; Rahmat, N.; Steven, S.; Syarifah, F.; Shelly, W.; Agung, P.F. Synthesis and Characterization of Nano Calcium Oxide from Eggshell to Be Catalyst of Biodiesel Waste Oil. Int. Energy Conf. 2014, 340–345. [Google Scholar]
- Islam, A.; Taufiq-Yap, Y.H.; Chu, C.M.; Chan, E.S.; Ravindra, P. Studies on Design of Heterogeneous Catalysts for Biodiesel Production. Process Saf. Environ. Prot. 2013, 91, 131–144. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M. Preparation and Characterizaton of CaO Nanoparticle for Biodiesel Production. AIP Conf. Proc. 2016, 1724. [Google Scholar] [CrossRef]
- Viriya-Empikul, N.; Krasae, P.; Nualpaeng, W.; Yoosuk, B.; Faungnawakij, K. Biodiesel Production over Ca-Based Solid Catalysts Derived from Industrial Wastes. Fuel 2012, 92, 239–244. [Google Scholar] [CrossRef]
- Khemthong, P.; Luadthong, C.; Nualpaeng, W.; Changsuwan, P.; Tongprem, P.; Viriya-Empikul, N.; Faungnawakij, K. Industrial Eggshell Wastes as the Heterogeneous Catalysts for Microwave-Assisted Biodiesel Production. Catal. Today 2012, 190, 112–116. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.H.; Park, K. Smart Nanoparticles for Drug Delivery: Boundaries and Opportunities. Chem. Eng. Sci. 2015, 125, 158–164. [Google Scholar] [CrossRef]
- Nakatani, N.; Takamori, H.; Takeda, K.; Sakugawa, H. Transesterification of Soybean Oil Using Combusted Oyster Shell Waste as a Catalyst. Bioresour. Technol. 2009, 100, 1510–1513. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of Waste Eggshell as Low-Cost Solid Catalyst for Biodiesel Production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Taufiq-Yap, Y.H. Waste Ostrich- and Chicken-Eggshells as Heterogeneous Base Catalyst for Biodiesel Production from Used Cooking Oil: Catalyst Characterization and Biodiesel Yield Performance. Appl. Energy 2015, 160, 58–70. [Google Scholar] [CrossRef]
- Yin, X.; Duan, X.; You, Q.; Dai, C.; Tan, Z.; Zhu, X. Biodiesel Production from Soybean Oil Deodorizer Distillate Usingcalcined Duck Eggshell as Catalyst. Energy Convers. Manag. 2016, 112, 199–207. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Volli, V.; Shu, C.-M. Transesterification of Waste Cooking Oil Using Pyrolysis Residue Supported Eggshell Catalyst. Sci. Total Environ. 2019, 661, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Correia, L.M.; Saboya, R.M.A.; de Sousa Campelo, N.; Cecilia, J.A.; Rodríguez-Castellón, E.; Cavalcante, C.L.; Vieira, R.S. Characterization of Calcium Oxide Catalysts from Natural Sources and Their Application in the Transesterification of Sunflower Oil. Bioresour. Technol. 2014, 151, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.B.; Seo, G. High Activity of Acid-Treated Quail Eggshell Catalysts in the Transesterification of Palm Oil with Methanol. Bioresour. Technol. 2010, 101, 8515–8519. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Synthesis of Biodiesel from Low FFA Waste Frying Oil Using Calcium Oxide Derived from Mereterix Mereterix as a Heterogeneous Catalyst. J. Clean. Prod. 2012, 29, 82–90. [Google Scholar] [CrossRef]


| Feedstock | Catalyst | MeOH/Oil Molar Ratio | Catalyst Loading (%wt) | Yield (%) 1/Conversion (%FAME) 2 | Reference |
|---|---|---|---|---|---|
| Soybean oil | Oyster shell (CaO) | 6:1 | 20.0 | 99.56 1 | [38] |
| Soybean oil | Chicken eggshell (CaO) | 9:1 | 3.0 | >95.00 1 | [39] |
| Used cooked oil | Ostrich/chicken eggshell (CaO) | 9:1 | 1.5 | 96.00 2 | [40] |
| Soybean oil Deodorizer Distillate | Duck eggshell (CaO) | 10:1 | 10.0 | 94.60 1 | [41] |
| Waste cooking oil | Chicken eggshell (CaO) supported char | 12:1 | 10.0 | >95.00 1 | [42] |
| Sunflower oil | Crab shell (CaO)/Chicken eggshell (CaO) | 6:1/9:1 | 3.0 | 83.10 2/97.75 2 | [43] |
| Palm oil | Acid-treated quail eggshell (CaO) | 12:1 | 1.5 | >98.00 2 | [44] |
| Waste frying oil (WFO) | Commercial CaO | 12:1 | 5.0 | 87.00 2 | [29] |
| Rapeseed oil | 97.00 2 | ||||
| Soybean oil | 98.00 2 | ||||
| Low FFA (free fatty acid) WFO | Clamshell (Meretrix meretrix) (CaO) | 12:1 | 4.0 | 83.75 1/90.13 2 | [45] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.; Nobre, L.; Gomes, J.; Puna, J.; Quinta-Ferreira, R.; Bordado, J. Soybean Oil Transesterification for Biodiesel Production with Micro-Structured Calcium Oxide (CaO) from Natural Waste Materials as a Heterogeneous Catalyst. Energies 2019, 12, 4670. https://doi.org/10.3390/en12244670
Santos S, Nobre L, Gomes J, Puna J, Quinta-Ferreira R, Bordado J. Soybean Oil Transesterification for Biodiesel Production with Micro-Structured Calcium Oxide (CaO) from Natural Waste Materials as a Heterogeneous Catalyst. Energies. 2019; 12(24):4670. https://doi.org/10.3390/en12244670
Chicago/Turabian StyleSantos, Samuel, Luís Nobre, João Gomes, Jaime Puna, Rosa Quinta-Ferreira, and João Bordado. 2019. "Soybean Oil Transesterification for Biodiesel Production with Micro-Structured Calcium Oxide (CaO) from Natural Waste Materials as a Heterogeneous Catalyst" Energies 12, no. 24: 4670. https://doi.org/10.3390/en12244670
APA StyleSantos, S., Nobre, L., Gomes, J., Puna, J., Quinta-Ferreira, R., & Bordado, J. (2019). Soybean Oil Transesterification for Biodiesel Production with Micro-Structured Calcium Oxide (CaO) from Natural Waste Materials as a Heterogeneous Catalyst. Energies, 12(24), 4670. https://doi.org/10.3390/en12244670







