Biodiesel Production Processes and Sustainable Raw Materials
Abstract
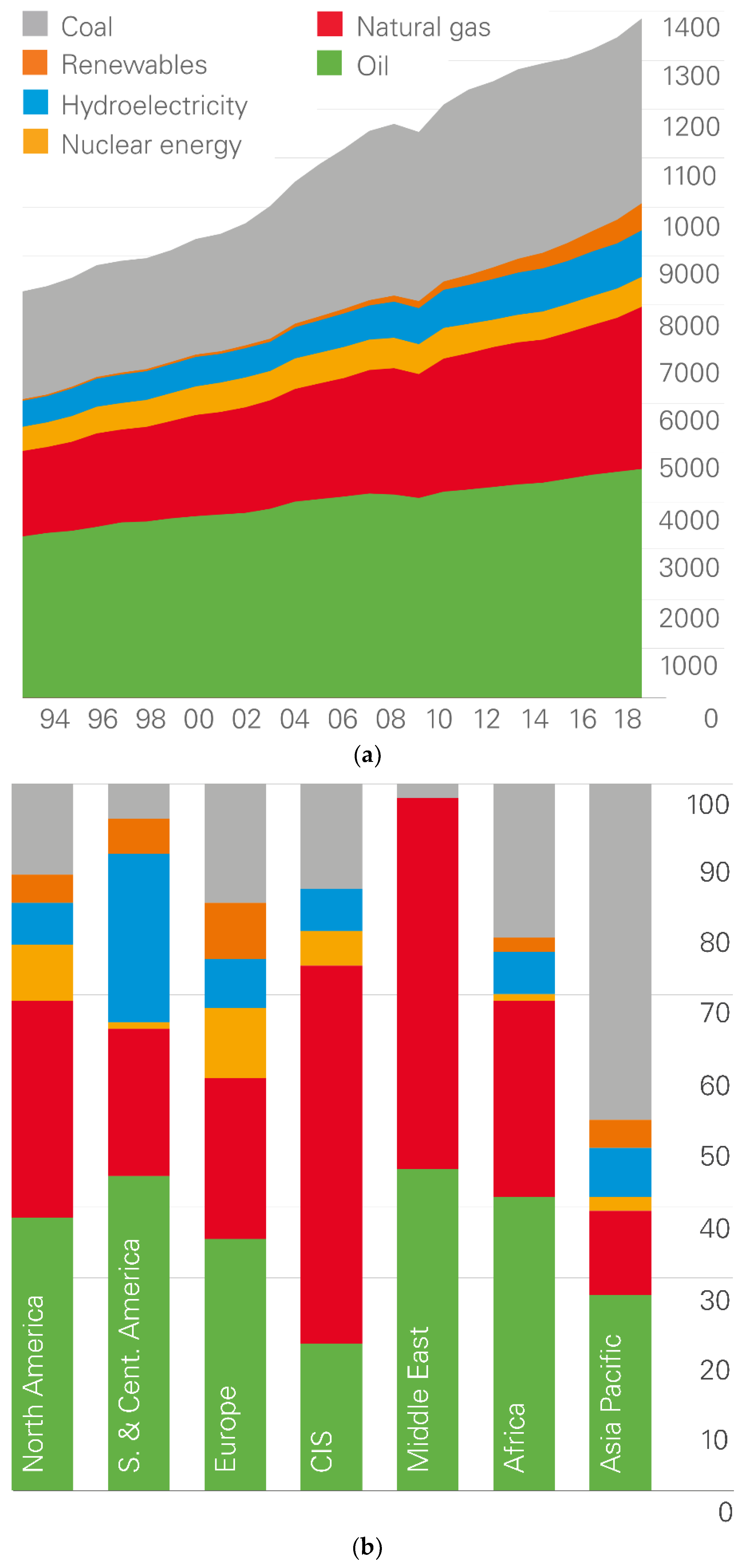
1. World Energy
- ✓
- 20% cut in greenhouse gas emissions (from 1990 levels)
- ✓
- 20% of EU energy from renewable sources in the energetic mix
- ✓
- 20% improvement in energy efficiency
- ✓
- At least 40% cuts in greenhouse gas emissions (from 1990 levels)
- ✓
- At least 32% share for renewable energy (upwards revision by 2023)
- ✓
- At least 32.5% improvement in energy efficiency
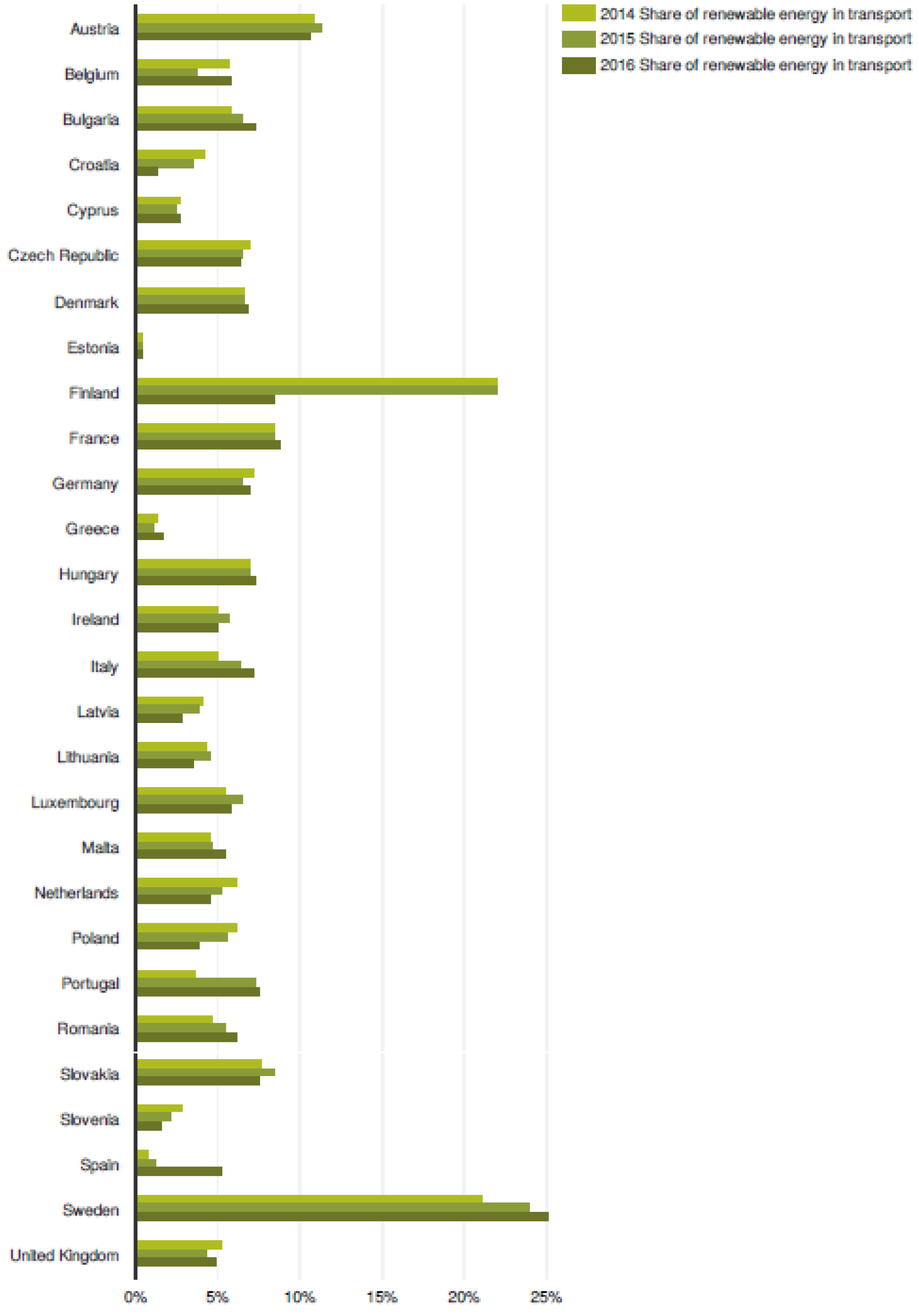
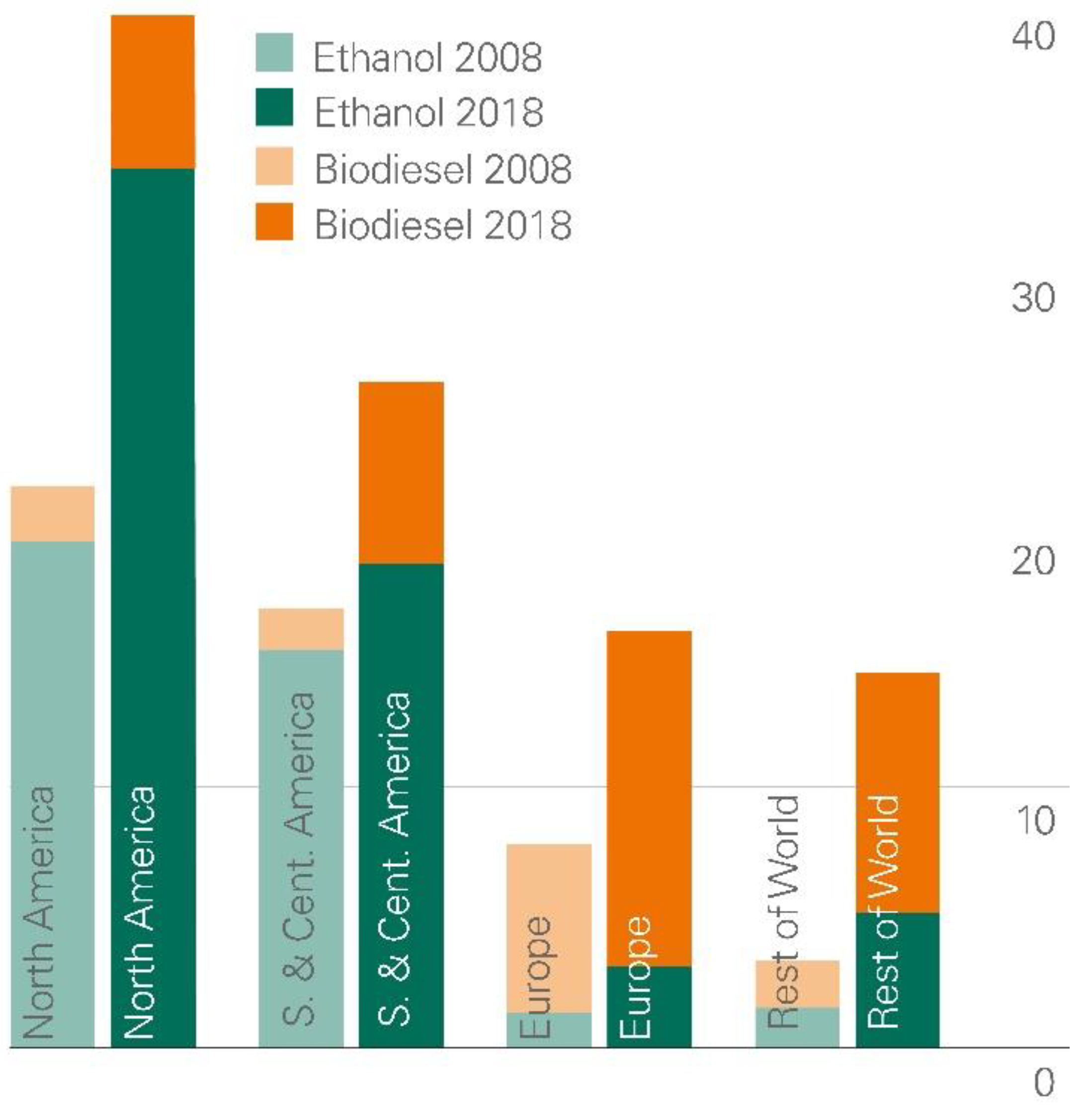
2. Biofuels
- ▪
- bioethanol: ethanol produced from biomass and/or the biodegradable fraction of waste;
- ▪
- biodiesel: a methyl-ester produced from vegetable or animal oil, of diesel quality;
- ▪
- biogas: a fuel gas produced from biomass and/or from the biodegradable fraction of waste, that can be purified to natural gas quality, to be used as biofuel, or wood gas;
- ▪
- biomethanol: methanol produced from biomass;
- ▪
- biodimethylether: dimethylether produced from biomass,
- ▪
- bio-ETBE (ethyl tert-butyl ether): ETBE produced based on bioethanol. The percentage by volume of bio-ETBE that is calculated as a biofuel is 47%;
- ▪
- bio-MTBE (methyl tert-butyl ether): a fuel produced based on biomethanol. The percentage by volume of bio-MTBE that is calculated as a biofuel is 36%;
- ▪
- synthetic biofuels: synthetic hydrocarbons or mixtures of synthetic hydrocarbons, which have been produced from biomass;
- ▪
- biohydrogen: hydrogen produced from biomass, and/or from the biodegradable fraction of waste;
- ▪
- pure vegetable oil: oil produced from oil plants through pressing, extraction or comparable procedures, crude or refined but chemically unmodified, when compatible with the type of engines involved and the corresponding emission requirements.
2.1. Biodiesel
2.2. Advantages and Disadvantages
- ⬬
- Biodegradability;
- ⬬
- Non-flammable and low toxicity;
- ⬬
- Safer to handle;
- ⬬
- Higher combustion efficiency, portability, availability, and renewability;
- ⬬
- Higher cetane number and flash point;
- ⬬
- Lower emissions such as CO2, CO, SO2, particulate matter (PM) and hydrocarbons (HC) compared to diesel;
- ⬬
- May be blended with diesel fuel at any proportion;
- ⬬
- No required engine modification up to B20;
- ⬬
- Excellent properties as a lubricant.
- ⬬
- Lower calorific value;
- ⬬
- Higher pour and cloud point fuel;
- ⬬
- Higher nitrous oxide (NOx) emissions (in some cases);
- ⬬
- Higher viscosity and less oxidative stability;
- ⬬
- Biodiesel is corrosive to copper and brass;
- ⬬
- May degrade plastic and natural rubber gaskets and hoses when used in pure form;
- ⬬
- Biodiesel causes excessive engine wear.
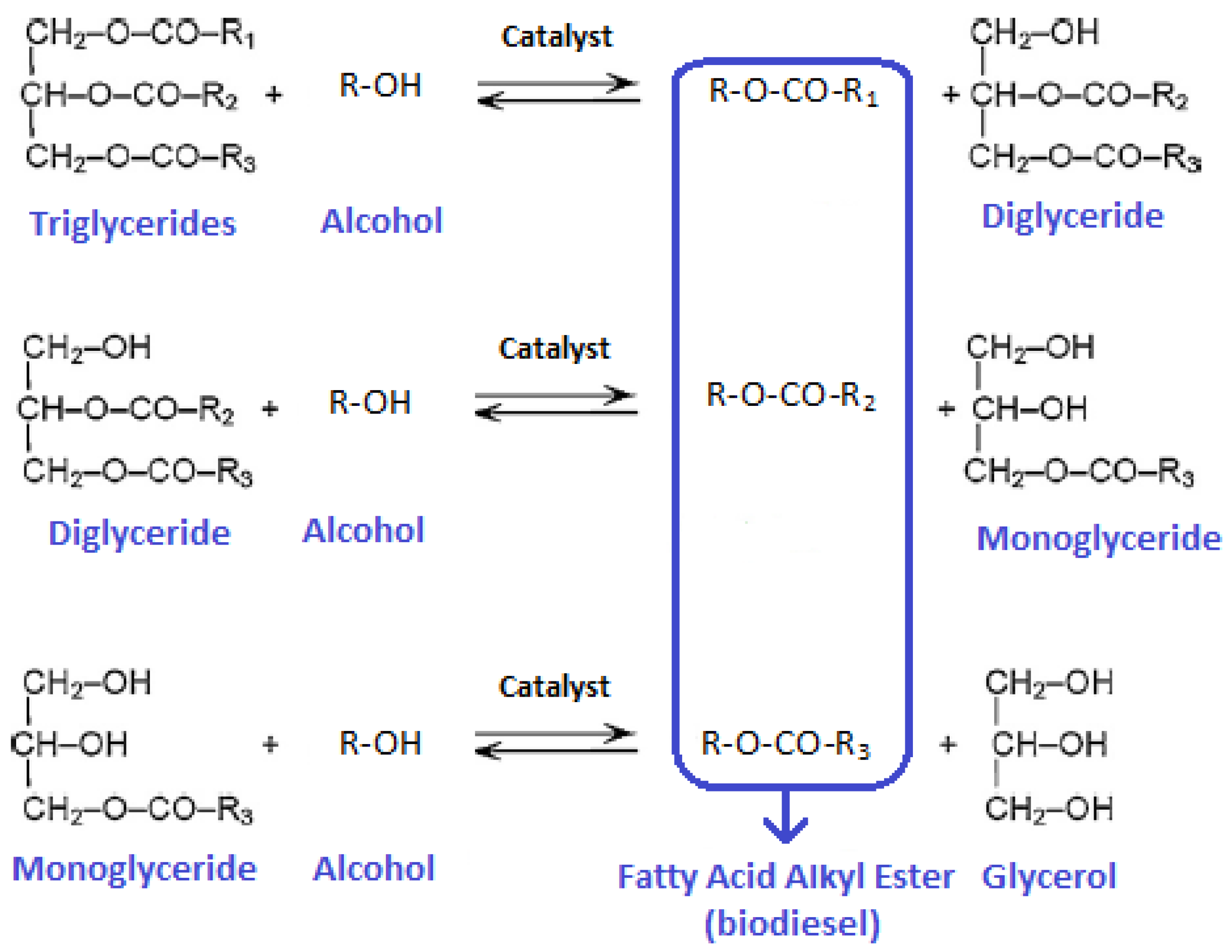
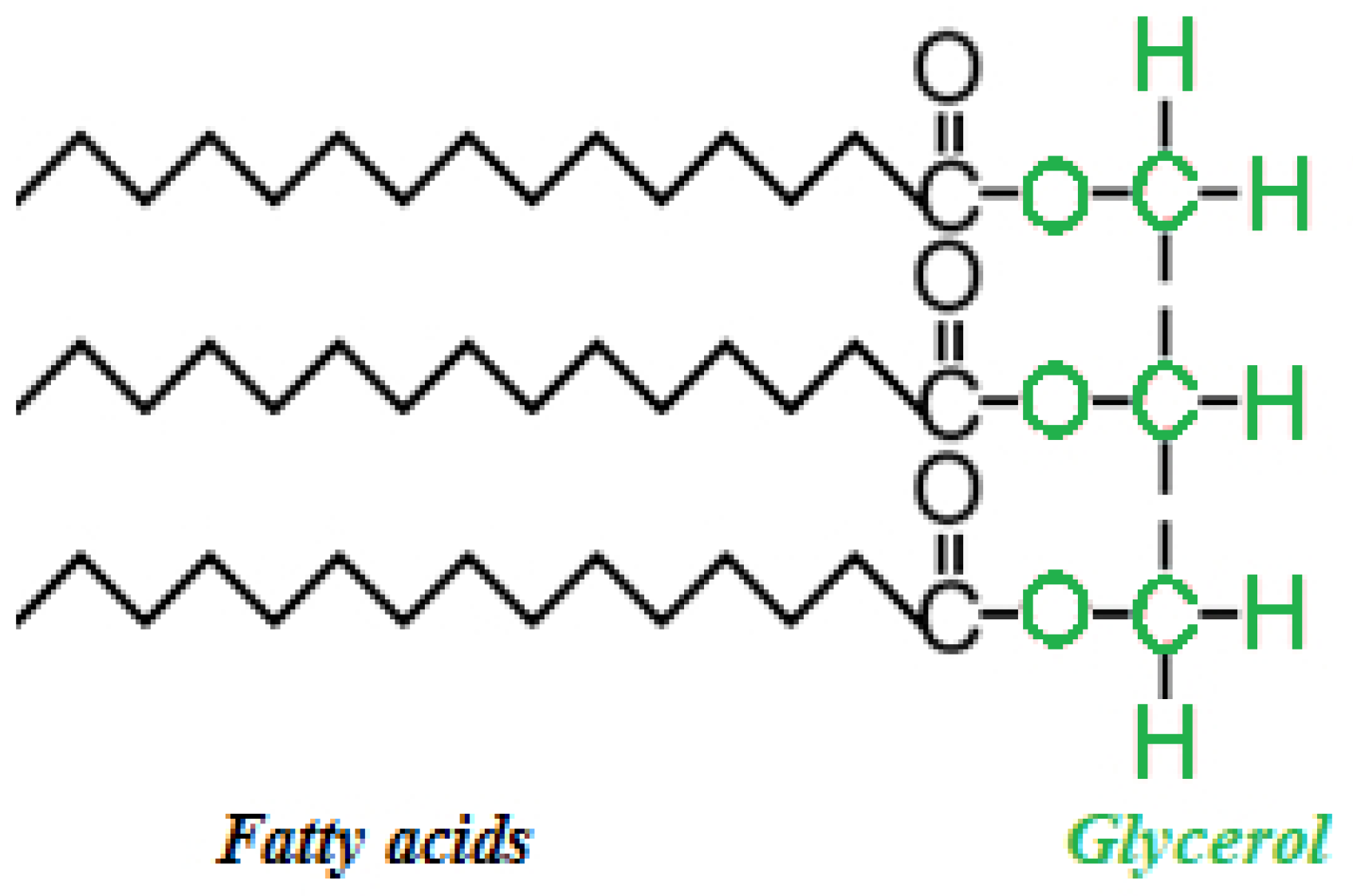
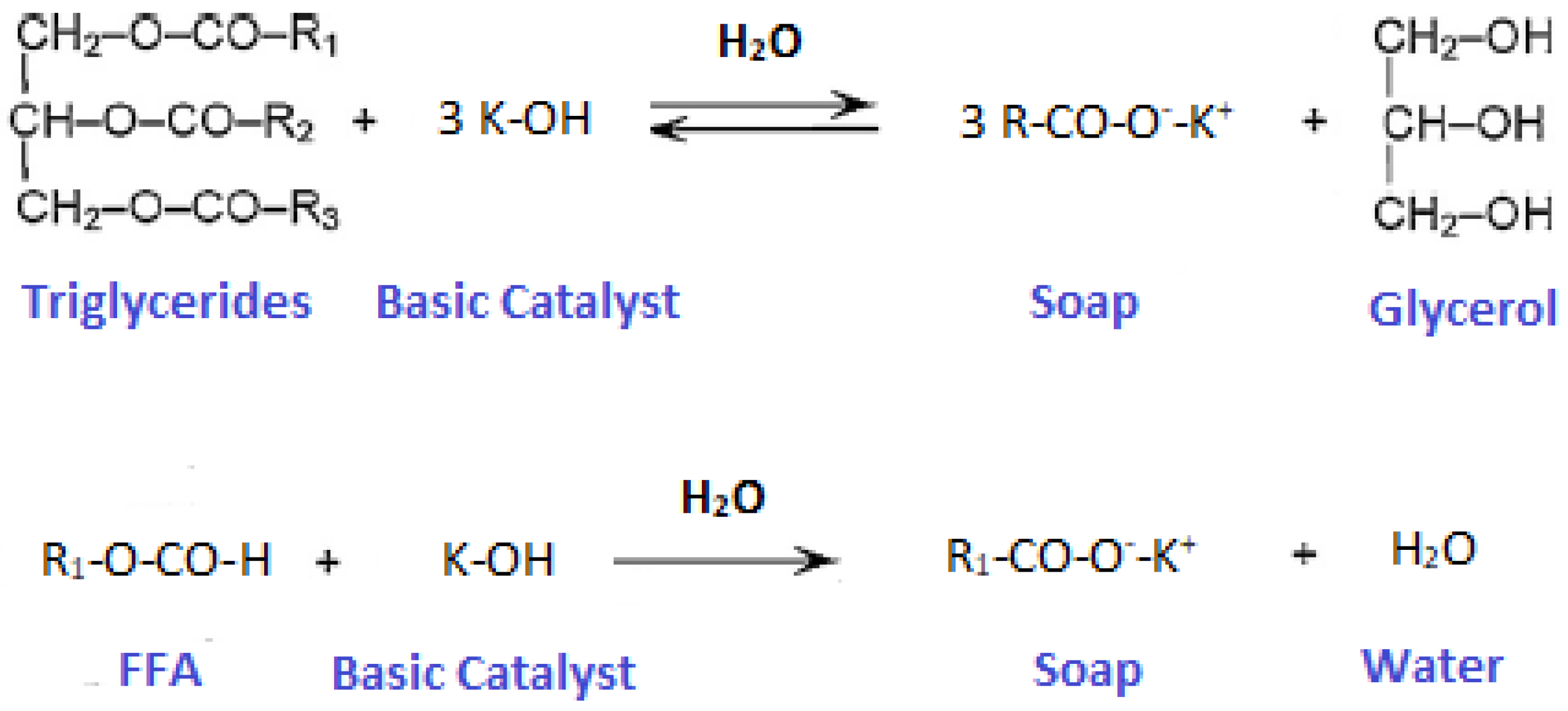
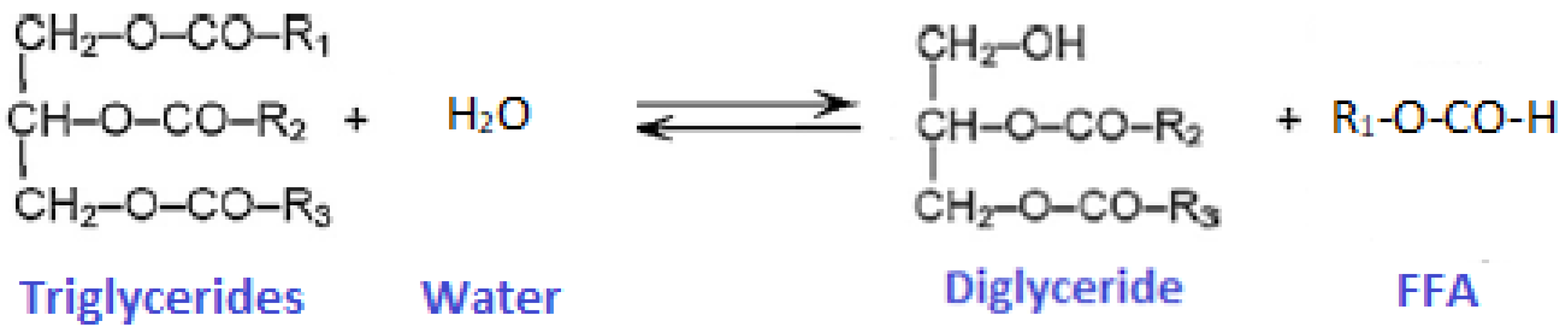
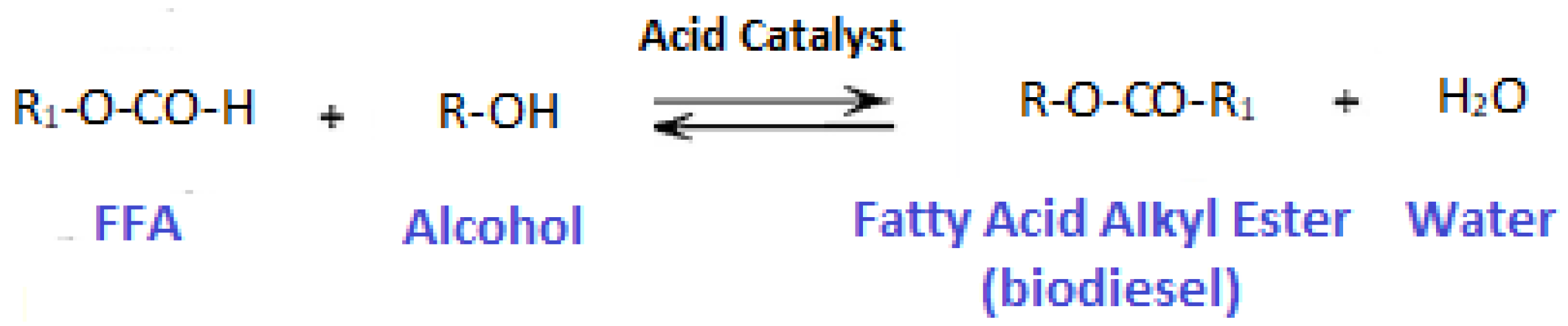
2.3. Transesterification
- (1)
- Free fatty acids, moisture and water content.
- (2)
- Type of alcohol and molar ratio employed.
- (3)
- Type and concentration of catalysts.
- (4)
- Reaction temperature and time.
- (5)
- Rate and mode of stirring.
- (6)
- Purification process of the final product.
- (7)
- Mixing intensity.
- (8)
- Effect of using organic co-solvents.
- (9)
- Specific gravity
2.4. Alcohol Used
2.5. Feedstocks
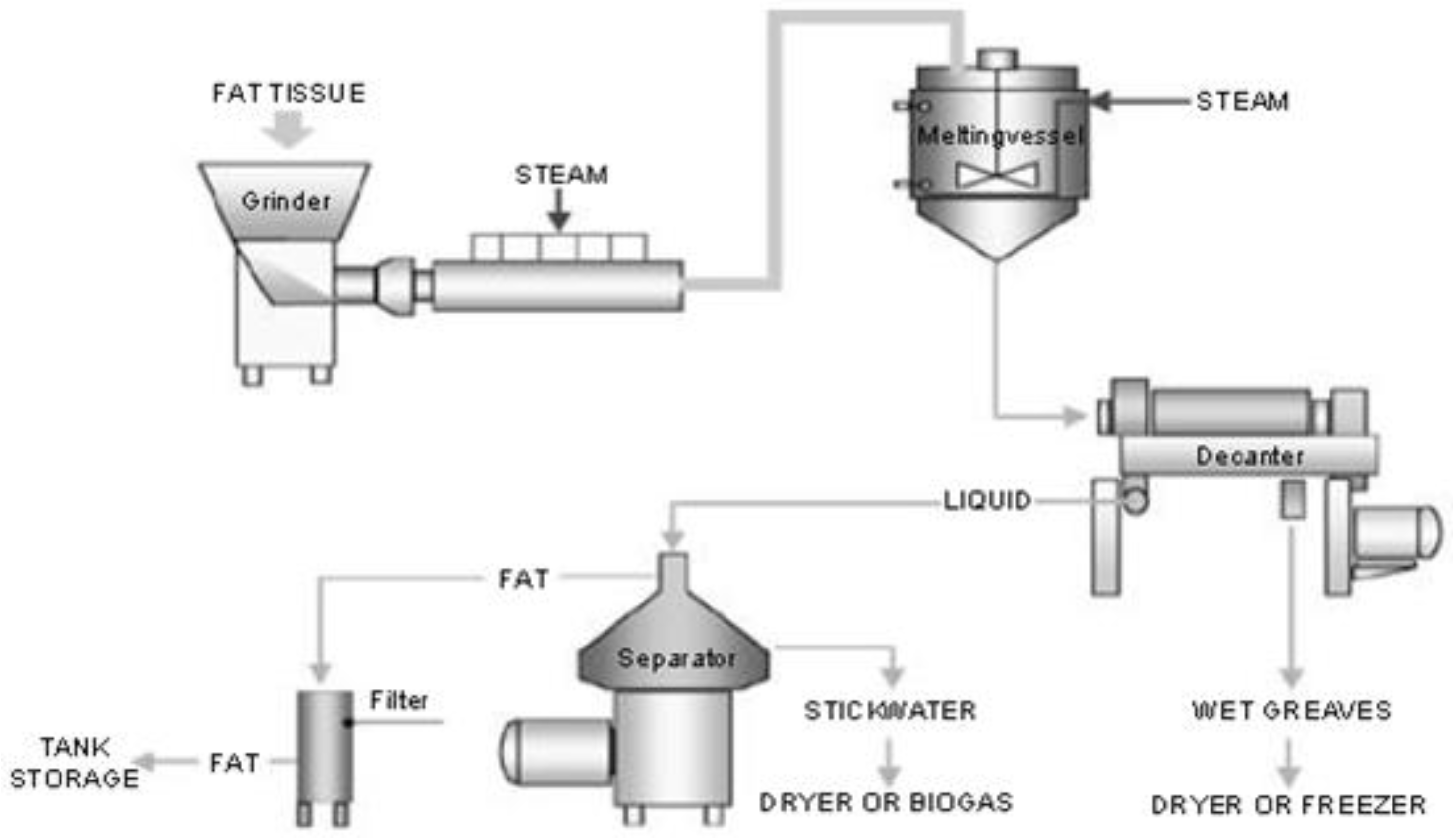
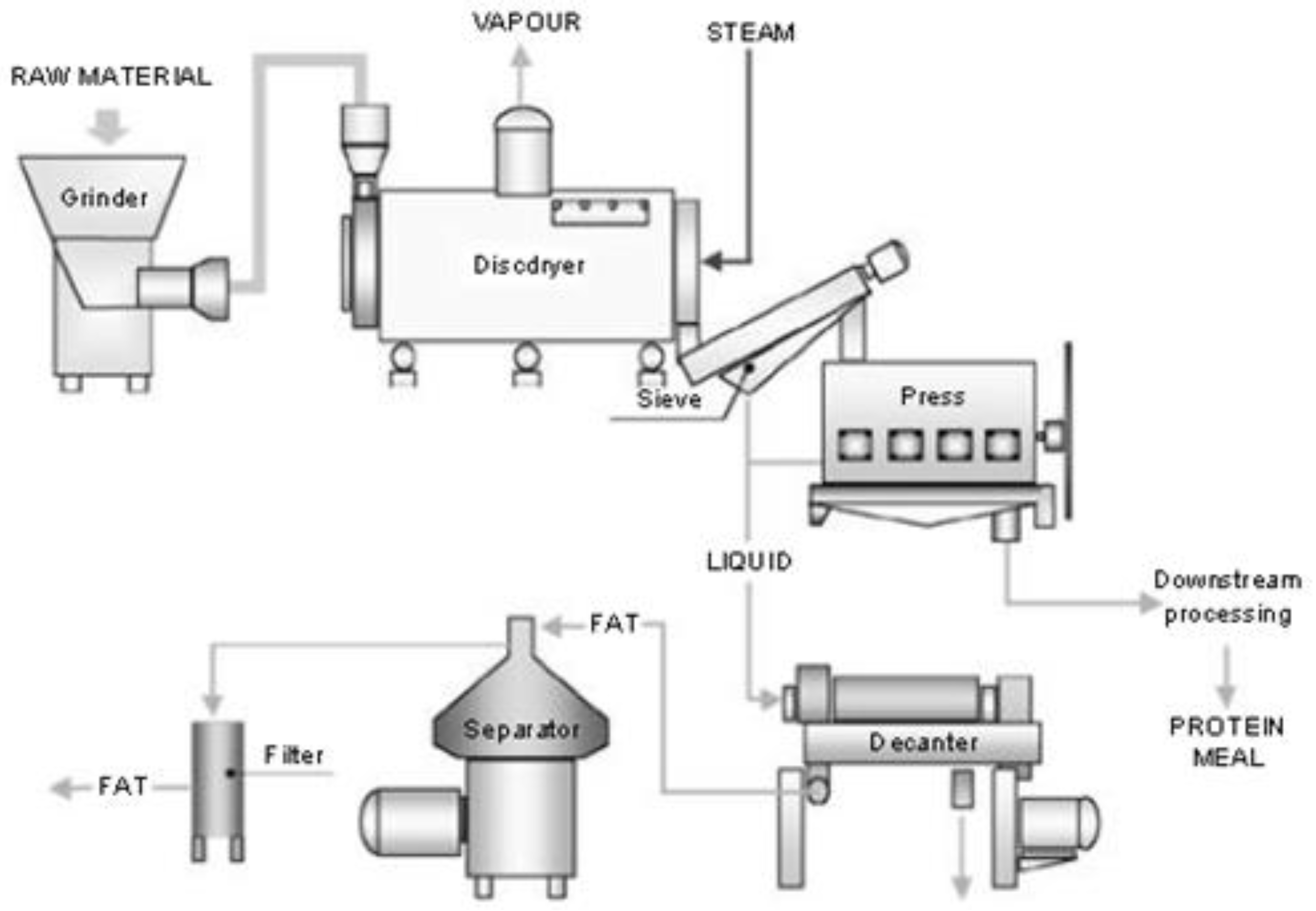
Animal Fats
- ✓
- Specified Risk Material (SRM) linked with the transmission of TSEs (Transmissible Spongiform Encephalopathies), this includes the spinal cord and brain.
- ✓
- Fallen stock with SRM
- ✓
- Catering waste
- ✓
- Anything handled with Category 1
- ✓
- Material not fit for human consumption and posing a risk to animals and humans
- ✓
- Fallen stock without SRM
- ✓
- Fit for human consumption at the point of slaughter
2.6. Biodiesel Production from Animal Fats Versus Vegetable Oils
2.7. Catalysts for Biodiesel Production
2.8. Homogeneous and Heterogeneous Catalytic Conversion of Animal Fats
2.9. Biodiesel Purification
2.10. Quality Specifications
2.11. Properties of Biodiesel from Different Feedstocks
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| CFPP | Cold Filter Plugging Point |
| CIS | Commonwealth of Independent States |
| EIA | U.S. Energy Information Administration |
| ETBE | Ethyl tert-butyl ether |
| FAEE | Fatty acid ethyl ester |
| FAME | Fatty acid methyl ester |
| FFA | Free fatty acid |
| GHG | Greenhouse gas |
| HC | Hydrocarbons |
| ISO | European Committee of Standardization |
| MTBE | Methyl tert-butyl ether |
| NOx | Nitrous oxide |
| PM | Particulate matter |
| SRM | Specified Risk Material |
| TSE | Transmissible Spongiform Encephalopathies |
| UCO | Used cooking oil |
| WAF | Waste animal fats |
| WCO | Waste cooking oil |
References
- U.S. Energy Information Administration. International Energy Outlook 2017; U.S. Energy Information Administration: Washington, DC, USA, 2017.
- BP Statistical Review of World Energy 2019, 68th ed.; BP: London, UK, 2019.
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- European Commission 2020 Climate & Energy Package. Available online: https://ec.europa.eu/clima/policies/strategies/2020_en?fbclid=IwAR3DhvhN-_IcDcqtIu0_sgWiB7cctQrqR90JKNs5dMRxOCxtGV_FQQVw4QM (accessed on 5 February 2019).
- European Union Directive (EU) 2009/28. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:140:0016:0062:EN:PDF (accessed on 12 February 2019).
- European Environment Agency (EEA). Use of Renewable Fuels in Transport. Available online: https://www.eea.europa.eu/data-and-maps/indicators/use-of-cleaner-and-alternative-fuels/use-of-cleaner-and-alternative-4 (accessed on 12 February 2019).
- European Commission Directive (EU) 2015/1513. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015L1513 (accessed on 13 February 2019).
- European Commission 2030 Climate & Energy Framework. Available online: https://ec.europa.eu/clima/policies/strategies/2030_en (accessed on 11 March 2019).
- European Commission. The Commission Presents Strategy for a Climate Neutral Europe by 2050—Questions and Answers. Available online: https://ec.europa.eu/clima/policies/strategies/2050_en (accessed on 13 March 2019).
- European Commission Directive (EU) 2003/30. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0028 (accessed on 13 March 2019).
- Biodiesel Explained—U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/energyexplained/biofuels/biodiesel.php (accessed on 14 April 2019).
- Phillips, S.; Flach, B.; Lieberz, S.; Lappin, J.; Bolla, S. EU Biofuels Annual 2018. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Biofuels%20Annual_The%20Hague_EU-28_7-3-2018.pdf (accessed on 14 April 2019).
- Direcção Geral de Energía e Geología. Renováveis-Estatísticas Rápidas—No 169; Direcção Geral de Energía e Geología: Lisbon, Portugal, 2018.
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Romano, S.D.; Sorichetti, P.A. Dielectric Spectroscopy in Biodiesel Production and Characterization; Springer: London, UK, 2011. [Google Scholar]
- Islam, A.; Ravindra, P. Biodiesel Production with Green Technologies; Springer: Basel, Switzerland, 2016; ISBN 9783319452722. [Google Scholar]
- Moser, B.R. Biodiesel production, properties, and feedstocks. Vitr. Cell. Dev. Biol. Plant 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Banković-Ilić, I.B.; Stojković, I.J.; Stamenković, O.S.; Veljkovic, V.B.; Hung, Y.T. Waste animal fats as feedstocks for biodiesel production. Renew. Sustain. Energy Rev. 2014, 32, 238–254. [Google Scholar] [CrossRef]
- Banerjee, A.; Chakraborty, R. Parametric sensitivity in transesterification of waste cooking oil for biodiesel production—A review. Resour. Conserv. Recycl. 2009, 53, 490–497. [Google Scholar] [CrossRef]
- Shahid, E.M.; Jamal, Y. Production of biodiesel: A technical review. Renew. Sustain. Energy Rev. 2011, 15, 4732–4745. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. Innovation in solid heterogeneous catalysis for the generation of economically viable and ecofriendly biodiesel: A review. Catal. Rev. 2016, 58, 157–208. [Google Scholar] [CrossRef]
- Ajala, O.E.; Aberuagba, F.; Odetoye, T.E.; Ajala, A.M. Biodiesel: Sustainable energy replacement to petroleum-based diesel fuel—A review. Chembioeng Rev. 2015, 2, 145–156. [Google Scholar] [CrossRef]
- Mathiyazhagan, M.; Ganapathi, A. Factors affecting biodiesel production. Res. Plant Biol. 2011, 1, 1–5. [Google Scholar]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Avramović, J.M.; Veličković, A.V.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. Optimization of sunflower oil ethanolysis catalyzed by calcium oxide: RSM versus ANN-GA. Energy Convers. Manag. 2015, 105, 1149–1156. [Google Scholar] [CrossRef]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Methanol production and applications: An overview. In Methanol: Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–28. [Google Scholar]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Watcharathamrongkul, K. Calcium oxide based catalysts for ethanolysis of soybean oil. Songklanakarin J. Sci. Technol. 2010, 32, 627–634. [Google Scholar]
- Velickovic, A.; Avramovic, J.; Stamenkovic, O.; Veljkovic, V. Kinetics of the sunflower oil ethanolysis using CaO as catalyst. Chem. Ind. Chem. Eng. Q. 2016, 22, 409–418. [Google Scholar] [CrossRef]
- Rashid, U.; Ibrahim, M.; Ali, S.; Adil, M.; Hina, S.; Bukhari, I.H.; Yunus, R. Comparative study of the methanolysis and ethanolysis of Maize oil using alkaline catalysts. Grasas Aceites 2012, 63, 35–43. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P. Comparative analysis of effect of methanol and ethanol on Karanja biodiesel production and its optimisation. Fuel 2016, 180, 164–174. [Google Scholar] [CrossRef]
- García, M.; Gonzalo, A.; Sánchez, J.L.; Arauzo, J.; Simoes, C. Metanolysis and ethanolysis of animal fats: A comparative study of the influence of alcohols. Chem. Ind. Chem. Eng. Q. 2011, 17, 91–97. [Google Scholar] [CrossRef]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Wolf, C.R.; Silva, E.C.; Lima, G.E.S.; de Lira Silva, L.; Serra, T.M.; Cauduro, F.; de Oliveira, L.G. Biodiesel from castor oil: A comparison of ethanolysis versus methanolysis. Energy Fuels 2006, 20, 2262–2265. [Google Scholar] [CrossRef]
- Adewale, P.; Dumont, M.J.; Ngadi, M. Recent trends of biodiesel production from animal fat wastes and associated production techniques. Renew. Sustain. Energy Rev. 2015, 45, 574–588. [Google Scholar] [CrossRef]
- Fat Minami-Nutrition. Available online: https://socratic.org/questions/how-do-lipids-affect-the-digestive-systemhttp://www.minami-nutrition.co.uk/website/absorption.php (accessed on August 2006).
- Woodgate, S.L.; Van der Veen, J.T. Fats and Oils—Animal Based. In Food Processing: Principles and Applications, 2nd ed.; Clark, S., Jung, S., Lamsal, B., Eds.; John Wiley & Sons.: Hoboken, NJ, USA, 2014; pp. 481–499. [Google Scholar]
- Azócar, L.; Ciudad, G.; Heipieper, H.J.; Muñoz, R.; Navia, R. Improving fatty acid methyl ester production yield in a lipase-catalyzed process using waste frying oils as feedstock. J. Biosci. Bioeng. 2010, 109, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, V.T.; Hess, M.A.; Dunn, R.O.; Foglia, T.A.; Haas, M.J.; Marmer, W.N. Fuel properties and nitrogen oxide emission levels of biodiesel produced from animal fats. J. Amer Oil Chem Soc. 2005, 82, 585–591. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-ferraz, M.C.M.; Almeida, M.F. Mixtures of vegetable oils and animal fat for biodiesel production: Influence on product composition and quality. Energy Fuels 2008, 20, 3889–3893. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Charoenchaitrakool, M.; Thienmethangkoon, J. Statistical optimization for biodiesel production from waste frying oil through two-step catalyzed process. Fuel Process. Technol. 2011, 92, 112–118. [Google Scholar] [CrossRef]
- Chakraborty, R.; Gupta, A.K.; Chowdhury, R. Conversion of slaughterhouse and poultry farm animal fats and wastes to biodiesel: Parametric sensitivity and fuel quality assessment. Renew. Sustain. Energy Rev. 2014, 29, 120–134. [Google Scholar] [CrossRef]
- Espinosa, M.; Canielas, L.; Silvana, M.; Moraes, A.; Schmitt, C.; Assis, R.; Rodrigues, S.; Regina, M.; Rodrigues, A.; Bastos, E. Beef tallow biodiesel produced in a pilot scale. Fuel Process. Technol. 2009, 90, 570–575. [Google Scholar]
- Ito, T.; Sakurai, Y.; Kakuta, Y.; Sugano, M.; Hirano, K. Biodiesel production from waste animal fats using pyrolysis method. Fuel Process. Technol. 2012, 94, 47–52. [Google Scholar] [CrossRef]
- Dias, J.M.; Ferraz, C.A.; Almeida, M.F. Using mixtures of waste frying oil and pork lard to produce biodiesel. Energy Fuels 2008, 22, 3889–3893. [Google Scholar]
- Boey, P.L.; Maniam, G.P.; Hamid, S.A.; Ali, D.M.H. Crab and cockle shells as catalysts for the preparation of methyl esters from low free fatty acid chicken fat. J. Am. Oil Chem. Soc. 2011, 88, 283–288. [Google Scholar] [CrossRef]
- Maniam, G.P.; Hindryawati, N.; Nurfitri, I.; Manaf, I.S.A.; Ramachandran, N.; Rahim, M.H.A. Utilization of waste fat from catfish (Pangasius) in methyl esters preparation using CaO derived from waste marine barnacle and bivalve clam as solid catalysts. J. Taiwan Inst. Chem. Eng. 2015, 49, 58–66. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Simpson, B.K.; Cue, R.I.; Phillip, L.E. Enzymatic transesterification of fats and oils from animal discards to fatty acid ethyl esters for potential fuel use. Biomass Bioenergy 2011, 35, 4149–4157. [Google Scholar] [CrossRef]
- Behçet, R. Performance and emission study of waste anchovy fish biodiesel in a diesel engine. Fuel Process. Technol. 2011, 92, 1187–1194. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Živković, S.B.; Veljković, M.V.; Banković-Ilić, I.B.; Krstić, I.M.; Konstantinović, S.S.; Ilić, S.B.; Avramović, J.M.; Stamenković, O.S.; Veljković, V.B. Technological, technical, economic, environmental, social, human health risk, toxicological and policy considerations of biodiesel production and use. Renew. Sustain. Energy Rev. 2017, 79, 222–247. [Google Scholar] [CrossRef]
- Canakci, M. The potential of restaurant waste lipids as biodiesel feedstocks. Bioresour. Technol. 2007, 98, 183–190. [Google Scholar] [CrossRef]
- Index Mundi Commodity Prices. Available online: https://www.indexmundi.com/commodities/ (accessed on 5 February 2019).
- Grenea WASTE-BASED MARKET PERFORMANCE. Available online: https://www.greenea.com/en/market-analysis/ (accessed on 5 February 2019).
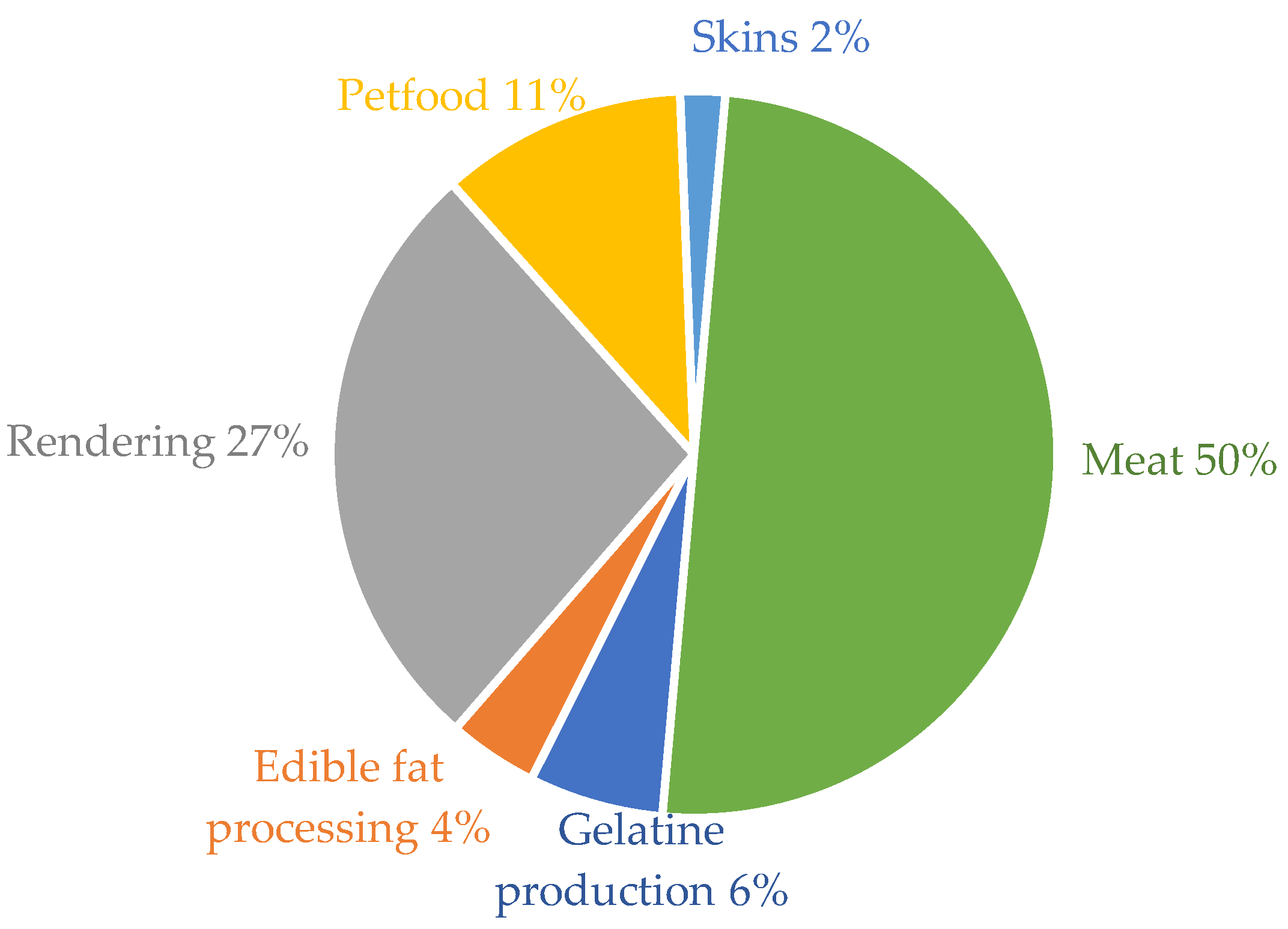
- EFPRA. What are the Three Categories? Available online: http://efpra.eu/which-byproducts-rendered/ (accessed on 22 November 2018).
- Meeker, D.L. Essential Rendering, All About The Animal By-Products Industry; The National Renderers Association: Alexandria, VA, USA, 2006; ISBN 0965466035. [Google Scholar]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef]
- Woodgate, S.; van der Veen, J. The role of fat processing and rendering in the European Union animal production industry. Biotechnol. Agron. Soc. Environ. 2004, 8, 283–294. [Google Scholar]
- Giriprasad, R.; Sharma, H.; Goswami, M. Animal fat-processing and its quality control. J. Food Process. Technol. 2013, 4, 252. [Google Scholar] [CrossRef]
- Knothe, G.; Krahl, J.; Van Gerpen, J. The Biodiesel Handbook; AOCS Press: Urbana, IL, USA, 2005; Volume 1. [Google Scholar]
- Encinar, J.M.; Sánchez, N.; Martínez, G.; García, L. Study of biodiesel production from animal fats with high free fatty acid content. Bioresour. Technol. 2011, 102, 10907–10914. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.M.; Alvim-Ferraz, M.C.M.; Almeida, M.F.; Méndez Díaz, J.D.; Polo, M.S.; Utrilla, J.R. Selection of heterogeneous catalysts for biodiesel production from animal fat. Fuel 2012, 94, 418–425. [Google Scholar] [CrossRef]
- Avhad, M.R.; Marchetti, J.M. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Pinzi, S.; Leiva-Candia, D.; López-García, I.; Redel-Macías, M.; Dorado, M. Latest trends in feedstocks for bioidesel production. Biofuels Bioprod. Biorefin. 2014, 8, 126–143. [Google Scholar] [CrossRef]
- Pereira, G.G.; Garcia, R.K.A.; Ferreira, L.L.; Barrera-Arellano, D. Soybean and soybean/beef-tallow biodiesel: A comparative study on oxidative degradation during long-term storage. J. Am. Oil Chem. Soc. 2017, 94, 587–593. [Google Scholar] [CrossRef]
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef]
- Sendzikiene, E.; Makareviciene, V.; Janulis, P. Oxidation stability of biodiesel fuel produced from fatty wastes. Pol. J. Environ. Stud. 2005, 14, 335–339. [Google Scholar]
- Kumar, M.; Sharma, M.P. Selection of potential oils for biodiesel production. Renew. Sustain. Energy Rev. 2016, 56, 1129–1138. [Google Scholar] [CrossRef]
- Gorji, A. Animal renewable waste resource as catalyst in biodiesel production. J. Biodivers. Environ. Sci. 2015, 7, 36–49. [Google Scholar]
- Sharma, Y.C.; Singh, B.; Korstad, J. Latest developments on application of heterogenous basic catalysts for an efficient and eco friendly synthesis of biodiesel: A review. Fuel 2011, 90, 1309–1324. [Google Scholar] [CrossRef]
- Shan, R.; Zhao, C.; Lv, P.; Yuan, H.; Yao, J. Catalytic applications of calcium rich waste materials for biodiesel: Current state and perspectives. Energy Convers. Manag. 2016, 127, 273–283. [Google Scholar] [CrossRef]
- Nasreen, S.; Nafees, M.; Qureshi, L.A.; Asad, M.S.; Sadiq, A.; Ali, S.D. Review of catalytic transesterifcation methods for biodiesel production. Intechopen 2015, 2, 64. [Google Scholar]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.S. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Fjerbaek, L.; Christensen, K.V.; Norddahl, B. A review of the current state of biodiesel production using enzymatic transesterification. Biotechnol. Bioeng. 2009, 102, 1298–1315. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Mendes, A.M.; Caetano, N.S.; Martins, A.A. Properties and sustainability of biodiesel from animal fats and fish oil. Chem. Eng. Trans. 2014, 38, 175–180. [Google Scholar]
- Alptekin, E.; Canakci, M.; Sanli, H. Biodiesel production from vegetable oil and waste animal fats in a pilot plant. Waste Manag. 2014, 34, 2146–2154. [Google Scholar] [CrossRef]
- Araújo, B.Q.; Nunes, R.C.D.R.; De Moura, C.V.R.; De Moura, E.M.; Citó, A.M.D.G.L.; Dos Santos Júnior, J.R. Synthesis and characterization of beef tallow biodiesel. Energy Fuels 2010, 24, 4476–4480. [Google Scholar] [CrossRef]
- Mata, T.M.; Cardoso, N.; Ornelas, M.; Neves, S.; Caetano, N.S. Evaluation of two purification methods of biodiesel from pork lard, beef tallow, and chicken fat. Energy Fuels 2011, 25, 4756–4762. [Google Scholar] [CrossRef]
- De Almeida, V.F.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Biodiesel production from mixtures of waste fish oil, palm oil and waste frying oil: Optimization of fuel properties. Fuel Process. Technol. 2015, 133, 152–160. [Google Scholar] [CrossRef]
- Janchiv, A.; Oh, Y.; Choi, S. High quality biodiesel production from pork lard by high solvent additive. ScienceAsia 2012, 38, 95–101. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Al-tikrity, E.T.B.; Albadree, M.A. Transesterification of a novel feedstock, Cyprinus carpio fish oil: Influence of co-solvent and characterization of biodiesel. Fuel 2015, 162, 215–223. [Google Scholar] [CrossRef]
- Ejikeme, P.M.; Anyaogu, I.D.; Egbuonu, C.A.C.; Eze, V.C. Pig-fat (Lard) derivatives as alternative diesel fuel in compression ignition engines. J. Pet. Technol. Altern. Fuels 2013, 4, 7–11. [Google Scholar]
- Jeong, G.T.; Yang, H.S.; Park, D.H. Optimization of transesterification of animal fat ester using response surface methodology. Bioresour. Technol. 2009, 100, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Fadhil, A.B.; Ali, L.H. Alkaline-catalyzed transesterification of Silurus triostegus Heckel fish oil: Optimization of transesterification parameters. Renew. Energy 2013, 60, 481–488. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M.; Sanli, H. Methyl ester production from chicken fat with high FFA. World Renew. Energy Congr. 2011, 1, 319–326. [Google Scholar]
- Fayyazi, E.; Ghobadian, B.; Najafi, G.; Hosseinzadeh, B.; Mamat, R.; Hosseinzadeh, J. An ultrasound-assisted system for the optimization of biodiesel production from chicken fat oil using a genetic algorithm and response surface methodology. Ultrason. Sonochem. 2015, 26, 312–320. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Oh, J.H.; Herring, J.L. Fast biodiesel production from beef tallow with radio frequency heating. Renew. Energy 2011, 36, 1003–1007. [Google Scholar] [CrossRef]
- Mata, T.M.; Cardoso, N.; Ornelas, M.; Neves, S.; Caetano, N.S. Sustainable production of biodiesel from tallow, lard and poultry fat and its quality evaluation. Chem. Eng. Trans. 2010, 19, 13–18. [Google Scholar]
- Panneerselvam, S.I.; Miranda, L.R. Biodiesel production from mutton. In Proceedings of the 2011 IEEE Conference on Clean Energy and Technology (CET), Kuala Lumpur, Malaysia, 27–29 June 2011; pp. 83–86. [Google Scholar]
- Cunha, A.; Feddern, V.; De Prá, M.C.; Higarashi, M.M.; De Abreu, P.G.; Coldebella, A. Synthesis and characterization of ethylic biodiesel from animal fat wastes. Fuel 2013, 105, 228–234. [Google Scholar] [CrossRef]
- Stojković, I.J.; Miladinović, M.R.; Stamenković, O.S.; Banković-Ilić, I.B.; Povrenović, D.S.; Veljković, V.B. Biodiesel production by methanolysis of waste lard from piglet roasting over quicklime. Fuel 2016, 182, 454–466. [Google Scholar] [CrossRef]
- Vafakish, B.; Barari, M. Biodiesel production by transesterification of tallow fat using heterogeneous catalysis. Kem. Ind. 2017, 66, 47–52. [Google Scholar] [CrossRef]
- Soldi, R.A.; Oliveira, A.R.S.; Ramos, L.P.; César-Oliveira, M.A.F. Soybean oil and beef tallow alcoholysis by acid heterogeneous catalysis. Appl. Catal. A Gen. 2009, 361, 42–48. [Google Scholar] [CrossRef]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO-Fe3O4for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Xu, G.; Cui, X.; Fan, S.; Zhang, B.; Song, H.; Yan, X.; Ma, X.; Kong, D. Optimization of transesterification of beef tallow for biodiesel production. In Proceedings of the 2011 Asia-Pacific Power and Energy Engineering Conference, Wuhan, China, 25–28 March 2011. [Google Scholar]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. Biodiesel separation and purification: A review. Renew. Energy 2011, 36, 437–443. [Google Scholar] [CrossRef]
- Alves, M.J.; Nascimento, S.M.; Pereira, I.G.; Martins, M.I.; Cardoso, V.L.; Reis, M. Biodiesel purification using microand ultrafiltration membranes. Renew. Energy 2013, 58, 15–20. [Google Scholar] [CrossRef]
- Berrios, M.; Skelton, R.L. Comparison of purification methods for biodiesel. Chem. Eng. J. 2008, 144, 459–465. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.R.A.; Sulaiman, N.M.N. Refining technologies for the purification of crude biodiesel. Appl. Energy 2011, 88, 4239–4251. [Google Scholar] [CrossRef]
- Veljković, V.B.; Banković-Ilić, I.B.; Stamenković, O.S. Purification of crude biodiesel obtained by heterogeneously-catalyzed transesterification. Renew. Sustain. Energy Rev. 2015, 49, 500–516. [Google Scholar] [CrossRef]
- Atadashi, I.M. Purification of crude biodiesel using dry washing and membrane technologies. Alex. Eng. J. 2015, 54, 1265–1272. [Google Scholar] [CrossRef]
- Reis, M.H.M.; Cardoso, V.L. Biodiesel production and purification using membrane technology. In Membrane Technologies for Biorefining; Elsevier: Amsterdam, The Netherlands, 2016; pp. 289–307. [Google Scholar]
- European Committee for Standardization. European Standard EN 14214: 2012+A1; European Committee for Standardization: Brussels, Belgium, 2014; pp. 1–21. [Google Scholar]
- U.S. Department of Energy. ASTM Biodiesel Specifications. Available online: https://afdc.energy.gov/fuels/biodiesel_specifications.html (accessed on 5 November 2018).
- Sakthivel, R.; Ramesh, K.; Purnachandran, R.; Mohamed Shameer, P. A review on the properties, performance and emission aspects of the third generation biodiesels. Renew. Sustain. Energy Rev. 2018, 82, 2970–2992. [Google Scholar] [CrossRef]


| Biodiesel Production (ton); Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Feedstock | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 |
| Fresh oils | 316,507 | 365,622 | 304,190 | 299,404 | 324,200 | 287,329 | 205,594 | 175,954 | 151,078 |
| WCO+ animal fat | 4810 | 4639 | 4869 | 11,044 | 16,906 | 75,737 | 131,226 | 179,875 | 176,023 |
| Total | 321,317 | 370,261 | 309,059 | 310,448 | 341,106 | 363,066 | 336,820 | 355,828 | 327,101 |
| Fatty Acid | Rapeseed Oil [18,38,39] | Soybean Oil [18,38,40,41] | WFO [39,41,42,43] | Beef Tallow [38,40,44,45,46] | Lard [18,38,40,44,47] | Poultry Fat | Fish Oil | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Chicken Fat [18,38,40,44,48] | Duck Tallow [44] | Catfish Fat [49] | Salmon Oil [50] | Anchovy Oil [51] | ||||||
| Lauric (C12:0) | - | - | nm–0.4 | - | - | nm–1.0 | - | 11.5 | 0.1 | - |
| Myristic (C14:0) | - | nm–1.0 | nm–1.1 | 2.6–3.5 | 1.3–1.7 | 0.5–1.0 | - | 11.7 | 5.8 | 6.7 |
| Myristoleic (C14:1) | - | - | - | 0.5–1.3 | - | 0.1–0.2 | - | 2.0 | - | - |
| Pentadecanoic (C15:0) | - | - | - | 0.5–1.0 | - | - | - | 1.9 | - | - |
| Palmitic (C16:0) | 3.5–4.5 | 10.5–11.0 | 8.4–25.8 | 23.8–27.0 | 23.2–25.5 | 20.9–24.7 | 17.0 | 28.1 | 16.9 | 20.2 |
| Palmitoleic (C16:1) | nm–0.5 | - | 0.2–4.6 | 0.5–4.7 | 2.2–2.7 | 5.0–7.7 | - | - | 5.4 | 6.6 |
| Margaric (C17:0) | - | - | - | 1.1–2.5 | nm–0.4 | - | - | - | - | 0.2 |
| Heptadecenoic (C17:1) | - | - | - | 0.5–1.7 | nm–0.4 | - | - | - | - | - |
| Stearic (C18:0) | 0.9–1.5 | 3.3–4.8 | 3.7–4.8 | 12.7–34.7 | 10.4–17.0 | 4.5–5.8 | 4.0 | - | 4.3 | 4.2 |
| Oleic (C18:1 cis) | - | 22.0–25.4 | 28.5–52.9 | 29.9–47.2 | 40.0–42.8 | 38.2–48.5 | 59.4 | 26.8 | 19.2 | 19.7 |
| Linoleic (C18:2) | 18.7–22.3 | 52.3–54.5 | 13.5–50.5 | 0.8–2.7 | 10.7–21.0 | 17.3–23.8 | 19.6 | 6.7 | 16.1 | 2.6 |
| Linolenic (C18:3) | 7.7–9.0 | 5.3–7.5 | 0.6–3.5 | nm–0.8 | nm–64.7 | nm–2.5 | - | - | 2.8 | 1.6 |
| Arachidic (C20:0) | 0.4–0.5 | 0.4–0.5 | 0.1–0.4 | nm–0.3 | nm–0.2 | - | - | - | - | - |
| Gadoleic acid (C20:1) | 1.0–2.0 | nm–0.3 | 0.1–0.8 | nm–0.5 | 0.9–1.0 | 0.5–1.0 | - | 2.7 | - | - |
| Eicosadienoic (C20:2) | - | - | - | - | 0.5–0.7 | - | - | 0.8 | - | 0.2 |
| Eicosatrienoic (C20:3) | - | - | - | - | nm–0.2 | - | - | 0.5 | - | - |
| Eicosapentaenoic (C20:5) | nm–0.1 | - | 0.2 | - | - | - | - | - | 15.6 | 10.4 |
| Behenic (C22:0) | nm–0.5 | 0.4–0.5 | nm–0.8 | - | - | - | - | - | - | - |
| Erucic (C22:1) | nm–0.1 | - | - | - | - | - | - | - | - | - |
| Docosapentaenoic (C22:5) | - | - | - | - | - | - | - | - | 2.5 | 0.8 |
| Docosahexanoic (C22:6) | - | - | - | - | - | - | - | - | 11.4 | 21.6 |
| Lignoceric (C24:0) | - | nm–0.1 | 0.04–0.3 | - | - | - | - | - | - | |
| 1st Generation Edible Oils | 2nd Generation | 3rd Generation Microalgal Oils | |
|---|---|---|---|
| Non-Edible Oils | Animal Fats | ||
| Soybeans (Glycine max) | Jatropha (Jatropha curcas L.) | Pork lard | Bacteria |
| Rapeseed (Brassica napus L.) | Mahua (Madhuca longifolia) | Beef tallow | Microalgae (Chlorella prothecoides) |
| Safflower (Carthamus tinctorius L.) | Coffee grounds | Poultry fat | Microalgae (Chlorella vulgaris) |
| Rice bran oil (Oryza sativa L.) | Camelina (Camelina sativa) | Fish oil | Microalgae (Botryococcus braunii) |
| Barley (Hordeum vulgare L.) | Cottonseed (Gossypium hirsutum) | Chicken fat | Microalgae (Chlorella sorokiana) |
| Sorghum (Sorghum bicolor) Wheat (Triticum aestivum) | Tall fescue (Festuca arundinacea) | ||
| Corn (Zea mays) | Neem (Azadirachta indica) | ||
| Coconut (Cocos nucifera) | Jojoba (Simmondsia chinensis) | ||
| Canola (Brassica napus) | Passion seed | ||
| Peanut (Arachis hypogaea) | Moringa (Moringa oleifera) | ||
| Palm (Arecaceae) | Tobacco seed (Nicotiana tabacum) | ||
| Sunflower (Helianthus annuus) | Rubber tree seed (Hevea brasiliensis) | ||
| Palm kernel (Elaeis guineensis) | Nag champa (Plumeria) | ||
| Type | Price |
|---|---|
| soybean oil | 728 USD per ton 1 |
| rapeseed oil | 827 USD per ton 1 |
| palm oil | 535 USD per ton 1 |
| WCO | 610 USD per ton 2 |
| tallow (category 1) | 400 € per ton 2 |
| Advantages | Disadvantages | |
|---|---|---|
| Homogeneous | Less time required with a higher yield Mild operation condition Base catalysts are more active than acid Acid catalysts are not affected by the content of FFA or water simultaneously capacity to catalyzed transesterification and esterification reaction | Not separable from the reaction mixture, cannot be reuse A large amount of water is needed to neutralize and purify the biodiesel Base catalysts are affected by high FFA and water content |
| Heterogeneous | Easily recovered, regenerated and reused Available to batch or continuous fixed bed reactors Requires fewer process units with a simpler separation and purification processes The amount of water is reduced Base catalysts are more active than acid Acid catalysts are not affected by the FFA or water amount, capacity to catalyzed both transesterification and esterification reaction | Lower conversions requiring more severe reaction conditions to achieve the same conversions of homogeneous ones Mass transfer resistance due to the presence of three phases (oil/alcohol/catalyst) in the reaction mixture Base catalysts are affected by high FFA and water content |
| Reaction Conditions | Optimized Condition | Ref. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feedstock | Alcohol Type | Catalyst/Co-Solvent | Reaction | Alcohol:Oil (Molar Ratio) | Cat. Conc. (wt % Oil) | Temp. (°C) and Impeller Speed | Time (h) | Catalyst | Alcohol:Oil (Molar Ratio) | Cat. Conc. (wt % Oil) | Temp. (°C) and Impeller Speed | Time (h) | Y = Yield; C = Conversion; P = Purity (%) | |
| Mix of WFO and pork lard (fat fraction of mix 0–1(w/w)) | MeOH | NaOH | - | 6:1 | 0.8 | 60 | 1 | - | 6:1 | 0.8 | 60 | 1 | Y = 81.7–87.7 P = 93.9–96.3 | [47] |
| beef tallow | MeOH | KOH | - | 6:1 | 1 | 60 and 60 | 2 | - | 6:1 | 1 | 60 and 60 | 2 | Y = 90.6 | [78] |
| pork lard | Y = 91.4 | |||||||||||||
| chicken fat | Y = 76.8 | |||||||||||||
| sardine oil | Y = 89.5 | |||||||||||||
| corn oil | MeOH | KOH | - | - | - | 60 and 300 rpm | 2 | KOH | 6:1 | 1 | 60 and 300 rpm | 2 | Y = 91 | [79] |
| chicken fat | 1st PT: H2SO4 | 1 | 1st PT: H2SO4 | 45 kg oil/40.5 kg alcohol | 2.4 kg | 1 | Y (experiment 1) = 80.4; Y (experiment 2) = 81.5 | |||||||
| 2nd PT: H2SO4 | 1 | 2nd PT: H2SO4 | 40 kg fat/3.1 kg alcohol | 0.1 kg | 1 | |||||||||
| KOH | 2 | KOH | 6:1 | 1 | 2 | |||||||||
| fleshing oil | 1st PT: H2SO4 | 1 | 1st PT: H2SO4 | 50 kg oil/21.1 kg alcohol | 0.62 kg | 1 | Y (experiment 1) = 81.6; Y (experiment 2) = 82.3 | |||||||
| KOH | 2 | KOH | 7.5:1 | 1 | 2 | |||||||||
| beef tallow | MeOH | methanolic KOH | - | - | - | - | - | - | - | - | 70-90 | 65 min. | Y = 96.26 | [80] |
| beef tallow, | MeOH | KOH | - | 6:1 | 0.8 | 60 and 60 | 2 | - | 6:1 | 0.8 | 60 and 60 | 2 | Y = 90.8 | [81] |
| pork lard | Y = 91.5 | |||||||||||||
| chicken fat | Y = 76.8 | |||||||||||||
| Mix of waste fish oil(WFO); palm oil (PO) and WFO | MeOH | 1st: H2SO4 | - | 6:1 | 1 | 60 | 1 | - | 6:1 | 1 | 60 | 1 | FAME content (33.3 wt % PO; 66.7 wt % WFO) = 80 | [82] |
| 2nd: NaOH | - | 9:1 | 0.5 | 60 | 1 | - | 9:1 | 0.5 | 60 | 1 | Y (33.3 wt % WFO; 66.7 wt % PO) = 98.5 | |||
| pork lard blended with n-hexane solvent | MeOH | KOH | - | 6:1–18:1 | 0.48–3.05 | 50–60 | 2 | - | 10:1 | 2.0 | 60 | 2 | Y (65 wt % solvent) = 98.2 | [83] |
| Cyprinus carpio fish oil | MeOH | KOH; CH3ONa; NaOH; EtONa/n-hexane; pet. ether; acetone; cyclohexane; diethyl ether | - | - | - | - | - | KOH | 5:1 | 0.6 | 50 | 0.5 | Y (1.5:1 hexane to methanol volume ratio) = 98.55 ± 1.02 | [84] |
| beef tallow | MeOH | KOH | - | 6:1 | 1.5 | 65 and 400 rpm | 180 min | - | 6:1 | 1.5 | 65 and 400 rpm | 180 min | ester content = 95–97 | [45] |
| lard | MeOH | NaOH | - | 180 cm3 fat/138 cm3 MeOH | 1.4 g | 40–70 | 1.5 | - | 180 cm3 fat/138 cm3 MeOH | 1.4 g | 70 | 1.5 | Y = 73 | [85] |
| lard | MeOH | KOH | - | 3.48–8.52 | 0.16–1.84 | 24.8–75.2 | 20 min. | - | 7.5:1 | 1.26 | 65 | 20 min. | Y = 97.8 ± 0.6 | [86] |
| Silurus triostegus heckel fish oil | MeOH | KOH | single and two-step transesterification | 3:1–12:1 | 0.25–1.0 | 32–60 | 0.5–2 | - | 6:1 | 0.50 | 32 | 0.5 | Y KOH = 96 | [87] |
| NaOH | 6:1 | 0.5 | 32–60 | 1 | ||||||||||
| chicken fat | MeOH | KOH | Sulfuric acid, hydrochloric acid and sulfamic acid with methanol | 6:1 | - | 25–60 | 1–4 | K methoxide | 6:1 | 1 | 60 | 1 | Y KOMe = 88.5 | [88] |
| NaOH | ||||||||||||||
| KOMe (32% in MeOH) | ||||||||||||||
| NaOMe (30% in MeOH) | ||||||||||||||
| chicken fat | MeOH | KOH | pre-treatment sulfuric acid and methanol | 4:1–8:1 | 0.75–1.25 | 45 | 3–9 min | - | 7:1 | 1 | 45 | 9 min | Y = 94.8 | [89] |
| beef tallow | MeOH | NaOH | radio frequency heating | 5:1–9:1 | 0.2–0.6 | - | RF heating 1–5 min | - | 9:1 | 0.6 | - | RF heating 5 min. | Y = 96.3 ± 0.5 | [90] |
| tallow | MeOH | KOH | - | 6:1 | 0.8 | 60 | 2 | - | 6:1 | 0.8 | 60 | 2 | Y = 90.8 | [91] |
| lard | Y = 91.4 | |||||||||||||
| poultry | Y = 76.8 | |||||||||||||
| mutton tallow | MeOH | KOH | pre-treatment sulfuric acid and MeOH | 6:1 | 0.35–0.4 g | 60 and 900 rpm | 1.5 | - | 6:1 | 0.39 g | 60 and 900 rpm | 1.5 | Y = 93.2 | [92] |
| mix of chicken and swine fat residues | EtOH | KOH | animal fats pre-treated | 6:1–8:1 | 0.44–1.32 | 30–70 | 1 | - | 7:1 | 0.96 | 30 | 1 | C = 83.5 | [93] |
| Reaction Conditions | Optimized Condition | Ref. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feedstock | Alcohol Type | Catalyst/Co-Solvent | Catalyst Preparation | Alcohol:Oil (Molar Ratio) | Cat. Conc. (wt % Oil) | Temp. (°C) and Impeller Speed | Time (h) | Catalyst Preparation | Alcohol:Oil (Molar Ratio) | Cat. Conc. (wt % Oil) | Temp. (°C) and Impeller Speed | Time (h) | Y = Yield; C = Conversion; P = Purity (%) | |
| catfish fat (Pangasius) | MeOH | barnacle | 900 °C | 6:1–15:1 | 2–7 | 65 | 2–8 | 900 | 12:1 | 5 | 65 | 4 | FAME content = 97.2 ± 0.04 wt % | [49] |
| bivalve clam | FAME content = 96.9 ± 0.03 wt % | |||||||||||||
| waste lard from piglet roasting (compared to heated and unheated lard | MeOH | quicklime and CaO | Pure CaO 550 °C, 2 h Quicklime 550 °C, 4 h | 6:1 | 5 | 40; 50; 60 and 900 rpm | up to 3 | Quicklime 550 °C 4 h | 6.1 | 5 | 60; 900 rpm | 1 | FAME concentration waste lard = 97.5% | [94] |
| chicken fat | MeOH | crab, cockle shells and mix | 900 °C, 2 h | - | - | - | - | 900 °C, 2 h | 13.8:1 | 4.9% (1:1 crab:cockle) | 65 | 3 | P = 98.8 | [48] |
| commercial-grade fat | MeOH | Amberlyst™ A26 OH | - | 6:1 | 1–2.7 mol/L of fat | 65 | 60–500 min | - | 6:1 | 2.2 mol/L | 65 | 6 | Y = 90–95 | [95] |
| pork lard | MeOH | CaMnOx | - | 9:1–27:1 | 1:5 | 40–60 | 4–8 | - | 21:1 | 1 | 60 | 8 | Y = 99.6 | [64] |
| soybean oil (SBO) and beef tallow (bf) | MeOH | Sulfonated polystyrene compounds | - | 3:1–9:1; 100:1 | 20 mol % of –SO3H groups in relation to the oil mass | 28–64 | 3–18 | - | 100:1 | 20 mol % of –SO3H groups in relation to the oil mass | 64 | 18 | CSBO = 85 | [96] |
| EtOH | - | 100:1 | 64 | 18 | - | - | - | - | CBeef tallow = 85 and 75 | |||||
| beef tallow | MeOH | KF/CaO-Fe3O4 | - | 3:1–12:1 | 1–6 g | 40–65 | 20–70 min. | - | 10:1 | 5 g | 55 | 1 | Y = 94 | [97] |
| beef tallow | MeOH | Cs2O/γ-Al2O3 | wet impregnation with aqueous solution of Cs2CO3. | 8:1–12:1 | 4–6 g | 55–75 | 80–160 min. | - | 10.5:1 | 5.3% | 66 | 2 | Y = 95.5 | [98] |
| Impurities | Effects | |
|---|---|---|
| Biodiesel | Engines | |
| FFA | Low oxidation stability | Corrosion |
| water | Reduces the heat of combustion Hydrolysis (FFA production) | Corrosion Bacteriological growth (filter blockage) |
| methanol | Low values of density and viscosity Low flash point (transport, storage, and use problems) | Corrosion of Al and Zn pieces |
| glycerides | High viscosity | Deposits in the injectors (carbon residue) Crystallization |
| metals (soap, catalyst) | - | Deposits in the injectors (carbon residue) Filter blockage (sulfated ashes) Engine weakening |
| glycerin | Decantation storage problem | Increase aldehydes and acrolein emissions |
| Property Specification | ASTM D6751 Limit | Test Methods | EN 14214 Limit | Test Methods |
|---|---|---|---|---|
| Ester content (% (m/m)) | - | - | 96.5 | EN 14103 |
| Density at 15 °C (kg/m3) | 880 | D1298 | 860–900 | EN ISO 3675/12185 |
| Viscosity at 40 °C (mm2/s) | 1.9–6.0 | D445 | 3.5–5.0 | EN ISO 3104 |
| Cetane number | Min. 47 | D613 | Min. 51.0 | EN ISO 5165 |
| Iodine number (g I2/100 g) | - | - | Max. 120 | EN 14111/16300 |
| Acid value (mg KOH/g) | Max. 0.50 | D664 | Max. 0.50 | EN 14104 |
| Pour point (°C) | -15 to -16 | D97 | - | - |
| Flash point (°C) | Min. 130 | D93 | Min. 101 | EN ISO 2719/3679 |
| Cloud point (°C) | -3 to -12 | D2500 | - | - |
| Cold filter plugging point (°C) | Max. +5 | D6371 | - | EN 116/16329 |
| Copper strip corrosion (3 h at 50 °C) | No 3 | D130 | class 1 | EN ISO 2160 |
| Carbon residue (% (m/m)) | Max. 0.05 | D4530 | - | - |
| Methanol content (% (m/m)) | Max. 0.20 | EN 14110 | Max. 0.20 | EN 14110 |
| Water content (mg/kg) | Max. 500 | D2709 | Max. 500 | EN ISO 12937 |
| Sulfur (mg/kg)) | S15 Max. 15 S500 Max. 500 | D5453 | Max. 10.0 | EN ISO 20846/20884 |
| Sulfated ash (% (m/m)) | Max. 0.02 | D874 | Max. 0.02 | EN ISO 3987 |
| Phosphorus content (mg/kg) | Max. 10 | D4951 | Max. 4.0 | EN 14107/16294 |
| Free glycerol (% (m/m)) | Max. 0.02 | D6584 | Max. 0.02 | EN 14105/EN 14106 |
| Total glycerol (% (m/m)) | Max. 0.24 | D6548 | Max. 0.25 | EN 14105 |
| Monoglyceride (% (m/m)) | Max. 0.40 | D6584 | Max. 0.70 | EN 14105 |
| Diglyceride (% (m/m)) | - | - | Max. 0.20 | EN 14105 |
| Triglyceride (% (m/m)) | - | - | Max. 0.20 | EN 14105 |
| Distillation temperature, 90% recovered (°C) | Max. 360 | D1160 | - | - |
| Oxidation stability h (at 110 °C) | Min. 3 | EN 15751 | Min. 8 | EN 14112/15751 |
| Linolenic acid methyl ester (% (m/m)) | - | - | Max. 12.0 | EN 14103 |
| Polyunsaturated (≥4 double bonds) Methyl esters (% (m/m)) | - | - | Max. 1.00 | EN 15779 |
| Alkaline metals (Na+ K) (mg/kg) | Max. 5.0 | EN 14538 | Max. 5.0 | EN 14108/14109/14538 |
| Alkaline earth metals (Ca + Mg) (mg/kg) | Max. 5.0 | EN 14538 | Max. 5.0 | EN 14538 |
| Total contamination (mg/kg) | - | - | Max. 24 | EN 12662 |
| Property Specification | ASTM D6751 Limit | EN 14214 Limit | Rapeseed Oil [3] | Soybean Oil [3] | Palm Oil [3] | Chicken Fat [79] | Fleshing Oil [79] | Beef Tallow [3,18] | Mutton Tallow [18] | Lard [18] | Fish Oil [108] | WCO [108] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density at 15 °C (kg/m3) | 880 | 860–900 | 882 | 914 | 864 | 867–889.7 | 875.5–876.7 | 832–872 | 856–882 | 873–877.4 | 881–890 | 875–888 |
| Viscosity at 40 °C (mm2/s) | 1.9–6.0 | 3.5–5.0 | 4.44 | 4.04 | 4.5 | 4.94–6.25 | 4.7–4.77 | 4.89–5.35 | 4.75–5.98 | 4.59–5.08 | 3.82–7.2 | 3.66–6.8 |
| Cetane number | Min. 47 | Min. 51.0 | 54.4 | 37.9 | 54.6 | 52.3 | 58.8 | 60.36 | 59–59 | - | 50.9–52.6 | 41–66 |
| Iodine number (g I2/100 g) | - | Max. 120 | - | 128–143 | 54 | 95.5–130 | 53.6–61 | nm–44.4 | 40–126 | 67–77 | nm–185 | 60–125.21 |
| Acid value (mg KOH/g) | Max. 0.50 | Max. 0.50 | - | 0.266 | 0.24 | 0.22–0.8 | 0.28–0.32 | 0.147–0.2 | 0.3–0.65 | 0.04–1.13 | 0.35–1.32 | 0.27–1.31 |
| Pour point (°C) | −15 to −16 | - | −12 | 15 | −6–12.3 | 10–15 | −5 | 5–7 | −14–4 | −2.5–9 | ||
| Flash point (°C) | Min. 130 | Min. 101 | 170 | 254 | 135 | 169–174 | 168–175 | 152–171 | - | 143.5–147 | 114–176 | 70.6–190 |
| Cloud point (°C) | −3 to −12 | - | −3.3 | 0.9 | 16 | −5–14 | nm–16 | −4 | - | −5 | −12–13 | |
| Cold filter plugging point (°C) | Max. +5 | - | −13 | −4 | 12 | 2–3 | 10–11 | nm–14 | - | - | - | −5–12 |
| Copper strip corrosion (3 h at 50 °C) | No 3 | class 1 | 1 | 1 | No 1 | No 1 | No 1 | - | No 1 | - | - | |
| Carbon residue (% (m/m)) | Max. 0.05 | - | 81 | - | - | nm–0.024 | - | - | - | nm–0.21 | 76.53–80.01 | 0.0004–77.38 |
| Methanol content (% (m/m)) | Max. 0.20 | Max. 0.20 | - | - | - | 0.01–0.06 | 0.01–0.01 | nm–0.1 | - | - | - | - |
| Water content (mg/kg) | Max. 500 | Max. 500 | - | <0.005 %vol | 200–440 | 326–410 | nm–374.2 | - | 184–1100 | - | ||
| Sulfur (mg/kg)) | S15 Max. 15 S500 Max. 500 | Max. 10.0 | - | 0.8 | 0.003 | nm–81.5 | 138.1–141 | nm–7.0 | - | - | - | 0–12.5 |
| Sulfated ash (% (m/m)) | Max. 0.02 | Max. 0.02 | - | <0.005 | 0.002 | - | 0.03 | nm–<0.005 | nm–0.025 | nm–0.002 | - | - |
| Phosphorus content (mg/kg) | Max. 10 | Max. 4.0 | - | 0.1 | <0.001 | - | 100 | nm–<0.1 | nm–16 | - | - | - |
| Free glycerol (% (m/m)) | Max. 0.02 | Max. 0.02 | - | 0.012 | 0.01 | 0.008–0.02 | 0.01–0.01 | 0.008–0.01 | - | - | - | - |
| Total glycerol (% (m/m)) | Max. 0.24 | Max. 0.25 | - | 0.149 | 0.01 | 0.03–0.19 | 0.10–0.05 | 0.076–0.33 | - | - | - | - |
| Monoglyceride (% (m/m)) | Max. 0.40 | Max. 0.70 | 0.473 | - | 0.02–0.56 | 0.06–0.27 | 0.13–0.223 | - | - | - | - | |
| Diglyceride (% (m/m)) | - | Max. 0.20 | 0.088 | - | 0.05–0.09 | 0.02–0.09 | 0.63–0.12 | - | - | - | - | |
| Triglyceride (% (m/m)) | - | Max. 0.20 | 0.019 | - | 0.06–0.12 | 0.04–0.20 | 0–0.07 | - | - | - | - | |
| Distillation temperature, 90 % recovered (°C) | Max. 360 | - | - | - | - | - | - | 307–344 | - | nm–352.5 | - | - |
| Oxidation stability h (at 110 °C) | Min. 3 | Min. 8 | 7.6 | 2.1 | 10.3 | nm–6 | - | nm–1.6 | - | - | - | 0.43–15.9 |
| Linolenic acid methyl ester (% (m/m)) | - | Max. 12.0 | - | - | - | - | - | - | - | 0.9–1.4 | - | - |
| Polyunsaturated (≥4 double bonds) Methyl esters (% (m/m)) | - | Max. 1.00 | - | - | - | - | - | - | - | - | - | - |
| Alkaline metals (Na+ K) (mg/kg) | Max. 5.0 | Max. 5.0 | - | - | - | - | 5 | 2–2.63 | - | nm–17.2 | - | - |
| Alkaline metals (Ca + Mg) (mg/kg) | Max. 5.0 | Max. 5.0 | - | - | - | - | - | - | - | - | - | - |
| Total contamination (mg/kg) | - | Max. 24 | - | - | - | - | - | - | - | - | - | - |
| Heat of combustion (MJ/kg) | - | - | 37 | 39.76 | - | 39.34–40.17 | 39.89–39.95 | 40.23 | - | 36.5–40.10 | 37.79–42.24 | 35.40–43.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, M.; Dias, A.P.S.; Puna, J.F.; Gomes, J.; Bordado, J.C. Biodiesel Production Processes and Sustainable Raw Materials. Energies 2019, 12, 4408. https://doi.org/10.3390/en12234408
Ramos M, Dias APS, Puna JF, Gomes J, Bordado JC. Biodiesel Production Processes and Sustainable Raw Materials. Energies. 2019; 12(23):4408. https://doi.org/10.3390/en12234408
Chicago/Turabian StyleRamos, Marta, Ana Paula Soares Dias, Jaime Filipe Puna, João Gomes, and João Carlos Bordado. 2019. "Biodiesel Production Processes and Sustainable Raw Materials" Energies 12, no. 23: 4408. https://doi.org/10.3390/en12234408
APA StyleRamos, M., Dias, A. P. S., Puna, J. F., Gomes, J., & Bordado, J. C. (2019). Biodiesel Production Processes and Sustainable Raw Materials. Energies, 12(23), 4408. https://doi.org/10.3390/en12234408







