Stability and Thermal Properties Study of Metal Chalcogenide-Based Nanofluids for Concentrating Solar Power †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of MoS2- and WS2-Based Nanofluids
2.3. Characterization of Nanofluids
3. Results
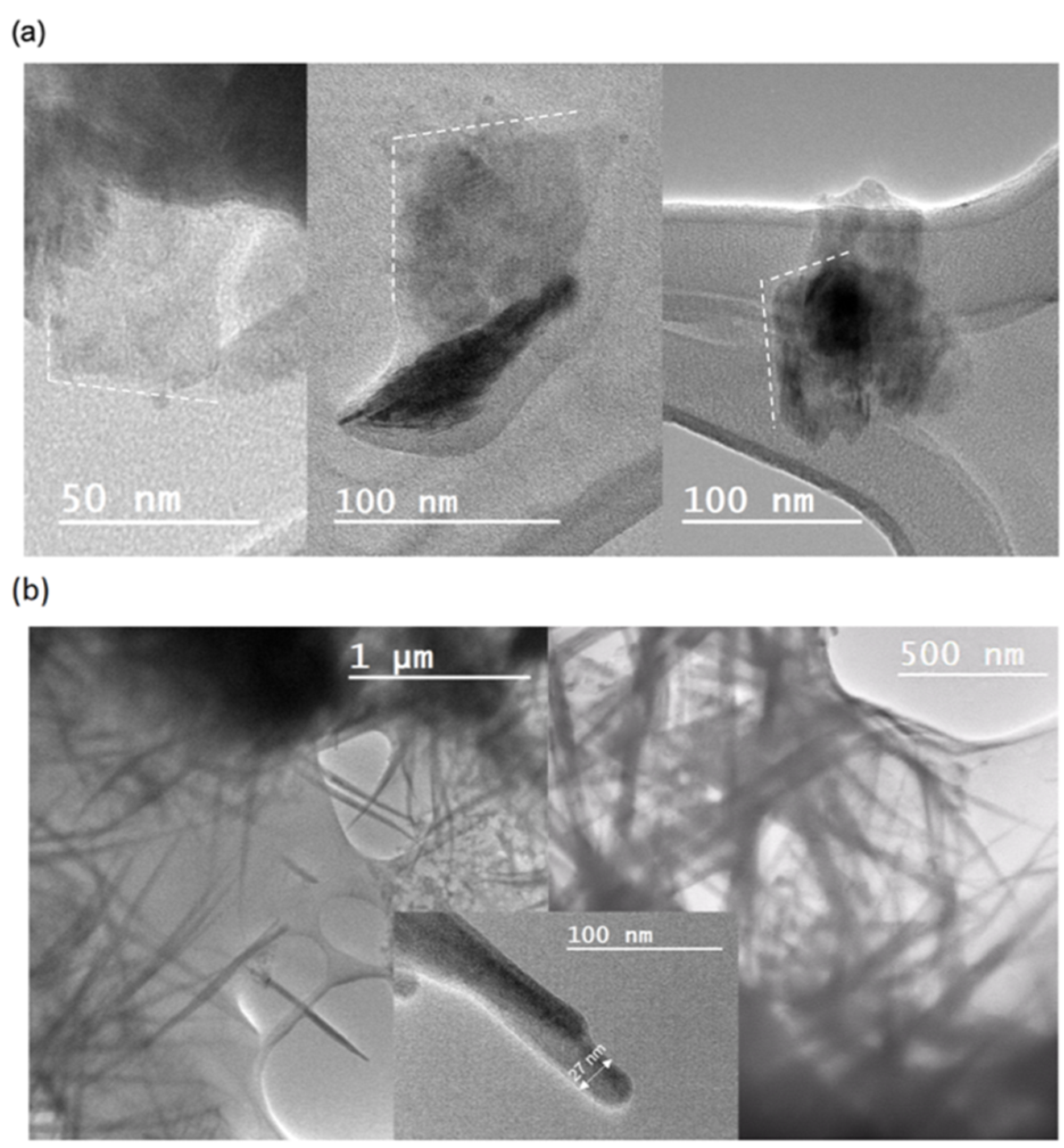
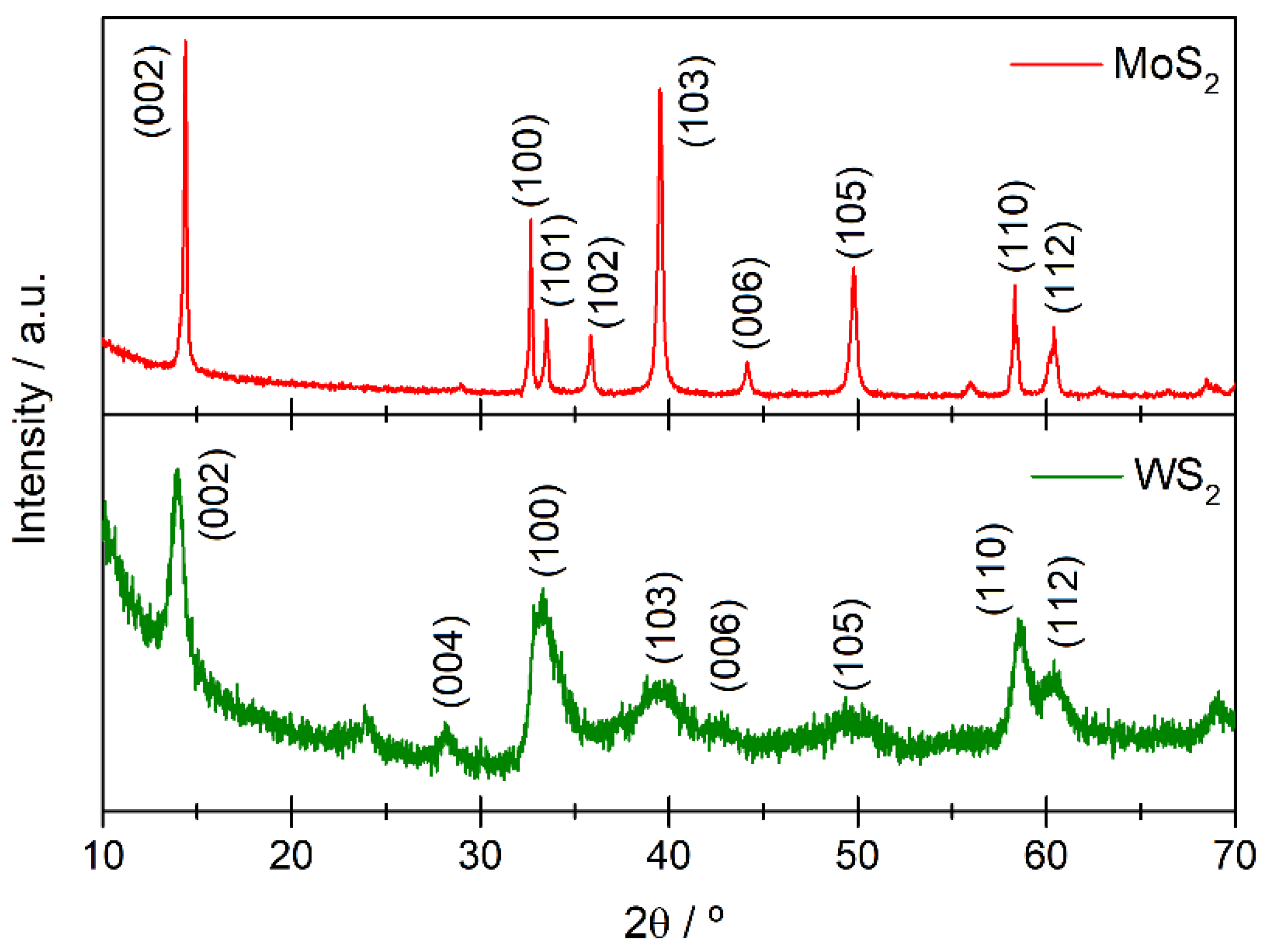
3.1. Morphological and Size Characterization of WS2 and MoS2 Nanostructures
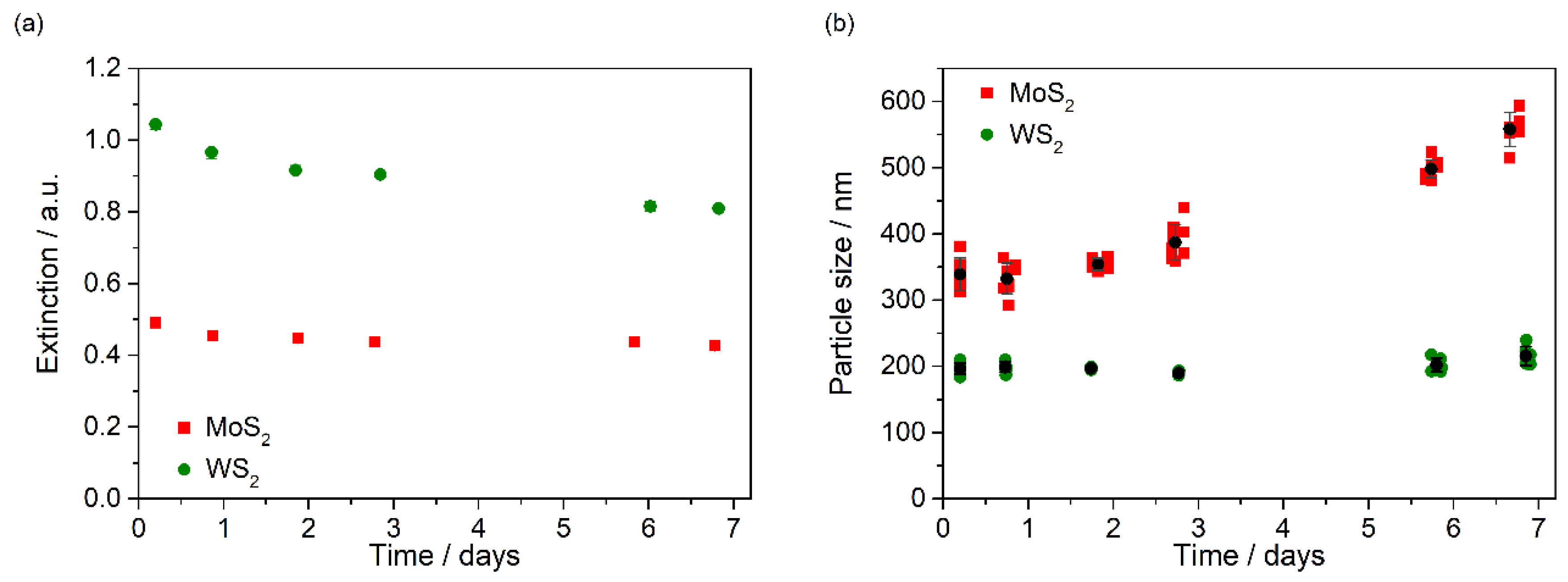
3.2. Stability of Nanofluids
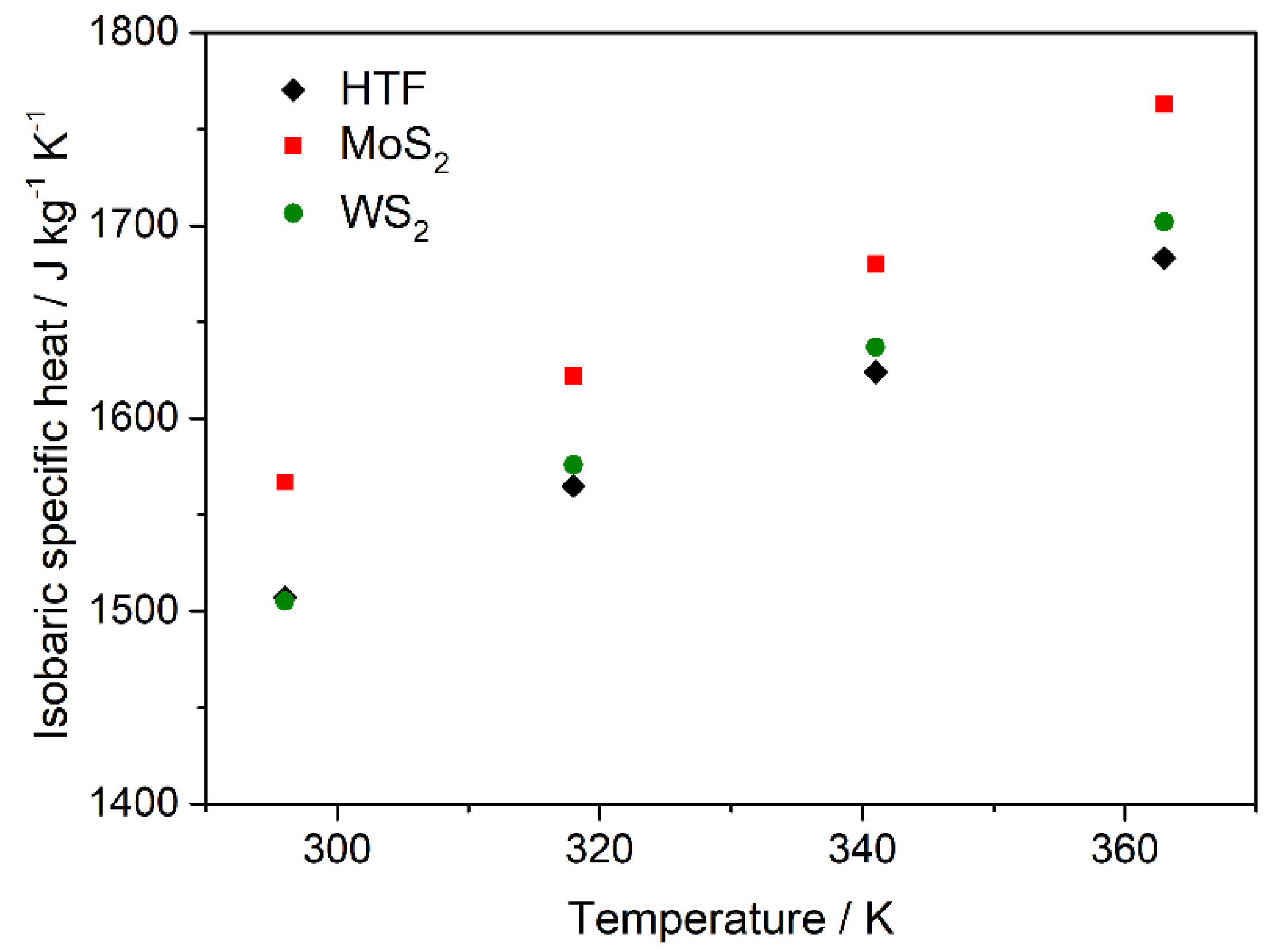
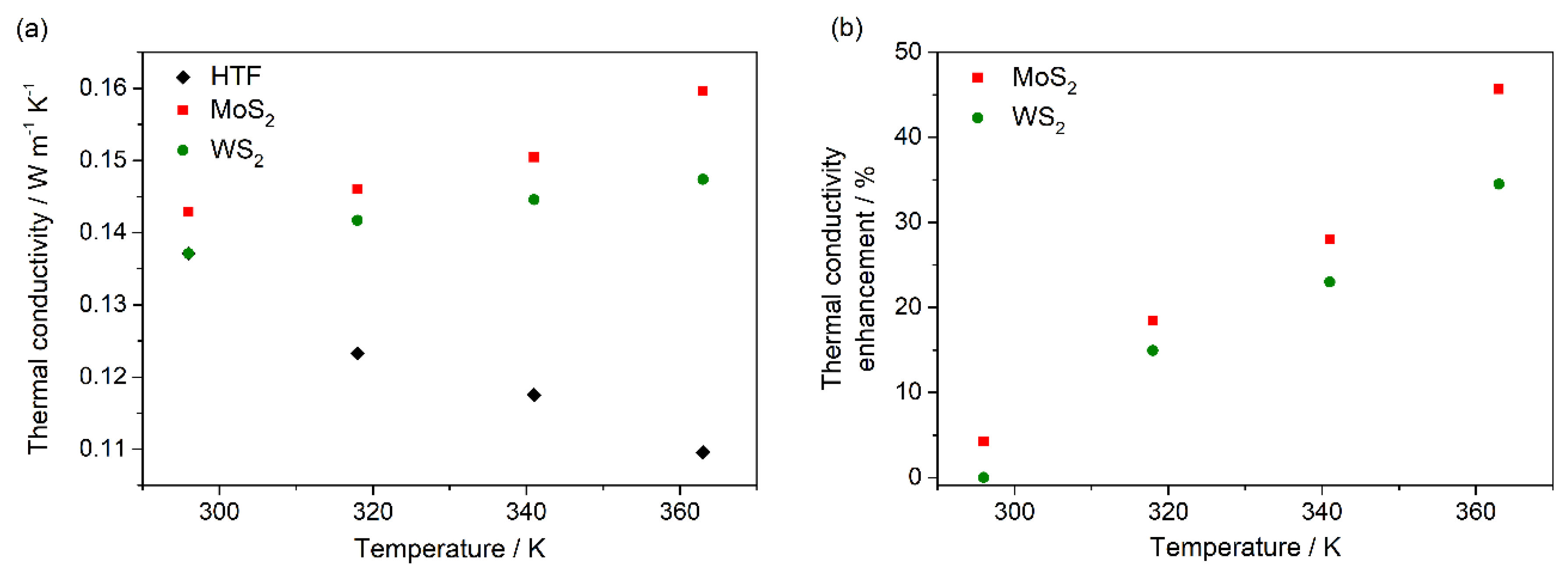
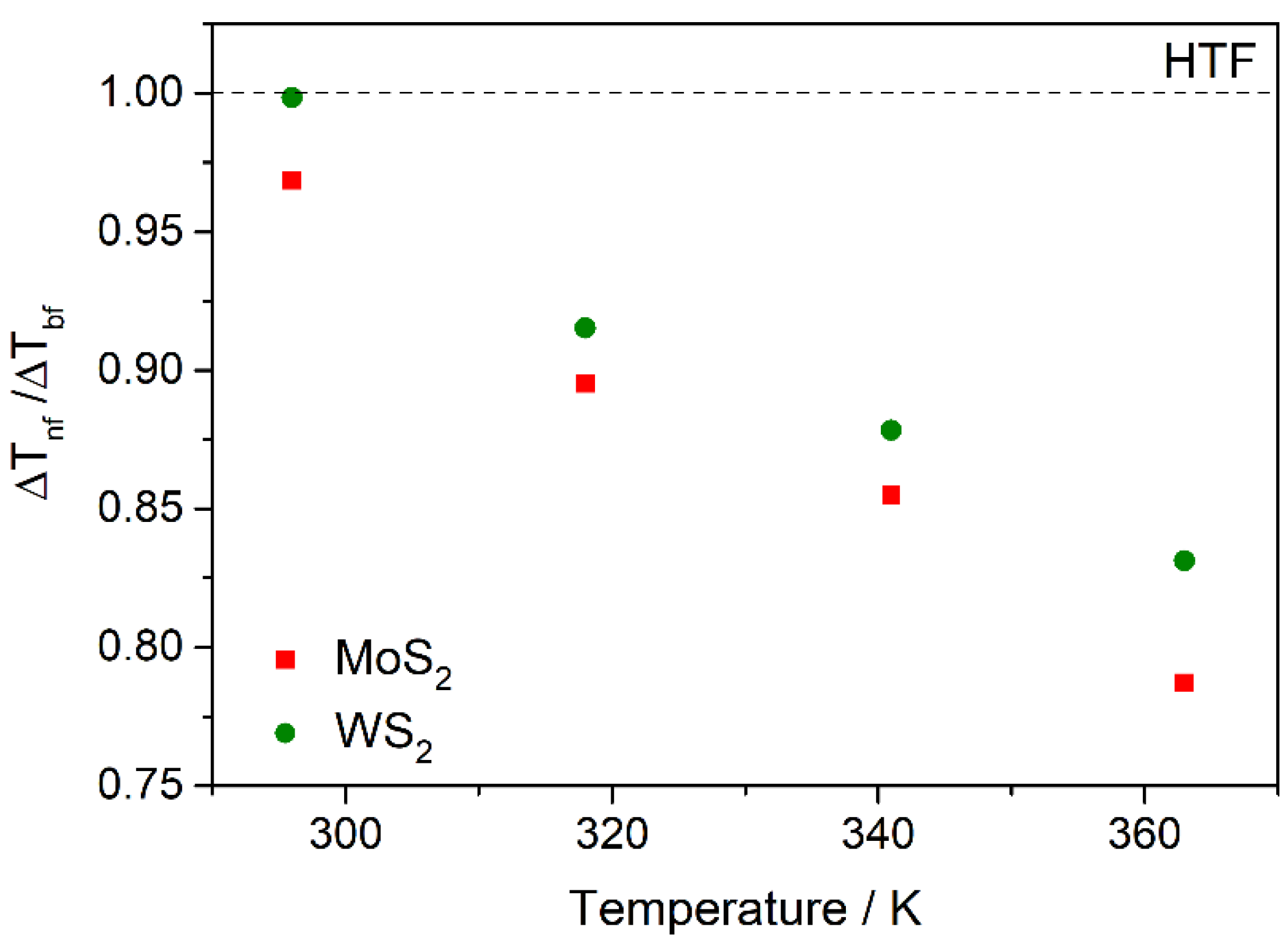
3.3. Nanofluid Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desideri, U.; Campana, P.E. Analysis and comparison between a concentrating solar and a photovoltaic power plant. Appl. Energy 2014, 113, 422–433. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.W.; Chan, C. Application of phase change materials for thermal Energyy storage in concentrated solar thermal power plants: A review to recent developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Rovense, F.; Reyes-Belmonte, M.A.; Gonzalez-Aguilar, J.; Amelio, M.; Bova, S.; Romero, M. Flexible electricity dispatch for CSP plant using un-fired closed air Brayton cycle with particles based thermal Energyy storage system. Energy 2019, 173, 971–984. [Google Scholar] [CrossRef]
- Zhang, H.L.; Baeyens, J.; Degreve, J.; Caceres, G. Concentrated solar power plants: Review and design methodology. Renew. Sust. Energy Rev. 2013, 22, 466–481. [Google Scholar] [CrossRef]
- Islam, M.T.; Huda, N.; Abdullah, A.B.; Saidur, R. A comprehensive review of state-of-the-art concentrating solar power (CSP) technologies: Current status and research trends. Renew. Sust. Energy Rev. 2018, 91, 987–1018. [Google Scholar] [CrossRef]
- Liu, M.; Tay, N.H.S.; Bell, S.; Belusko, M.; Jacob, R.; Will, G.; Saman, W.; Bruno, F. Review on concentrating solar power plants and new developments in high temperature thermal Energyy storage technologies. Renew. Sust. Energy Rev. 2016, 53, 1411–1432. [Google Scholar] [CrossRef]
- Wang, F.Q.; Cheng, Z.M.; Tan, J.Y.; Yuan, Y.; Shuai, Y.; Liu, L.H. Progress in concentrated solar power technology with parabolic trough collector system: A comprehensive review. Renew. Sust. Energy Rev. 2017, 79, 1314–1328. [Google Scholar]
- Fernandez-Garcia, A.; Zarza, E.; Valenzuela, L.; Perez, M. Parabolic-trough solar collectors and their applications. Renew. Sust. Energy Rev. 2010, 14, 1695–1721. [Google Scholar] [CrossRef]
- Price, H.; Lupfert, E.; Kearney, D.; Zarza, E.; Cohen, G.; Gee, R.; Mahoney, R. Advances in parabolic trough solar power technology. J. Sol. Energy T Asme 2002, 124, 109–125. [Google Scholar] [CrossRef]
- Morin, G.; Dersch, J.; Platzer, W.; Eck, M.; Haberle, A. Comparison of Linear Fresnel and Parabolic Trough Collector power plants. Sol. Energy 2012, 86, 1–12. [Google Scholar] [CrossRef]
- Tyagi, H.; Phelan, P.; Prasher, R. Predicted Efficiency of a Low-Temperature Nanofluid-Based Direct Absorption Solar Collector. J. Sol. Energy Eng. 2009, 131, 041004. [Google Scholar] [CrossRef]
- Taylor, R.A.; Phelan, P.E.; Otanicar, T.P.; Walker, C.A.; Nguyen, M.; Trimble, S.; Prasher, R. Applicability of nanofluids in high flux solar collectors. J. Renew. Sustain. Energy 2011, 3, 023104. [Google Scholar] [CrossRef]
- Mwesigye, A.; Huan, Z.J.; Meyer, J.P. Thermodynamic optimisation of the performance of a parabolic trough receiver using synthetic oil-Al2O3 nanofluid. Appl. Energy 2015, 156, 398–412. [Google Scholar] [CrossRef]
- Peng, B.; Zhang, H.; Shao, H.Z.; Xu, Y.C.; Zhang, X.C.; Zhu, H.Y. Thermal conductivity of monolayer MoS2, MoSe2, and WS2: Interplay of mass effect, interatomic bonding and anharmonicity. RSC Adv. 2016, 6, 5767–5773. [Google Scholar] [CrossRef]
- Peimyoo, N.; Shang, J.Z.; Yang, W.H.; Wang, Y.L.; Cong, C.X.; Yu, T. Thermal conductivity determination of suspended mono- and bilayer WS2 by Raman spectroscopy. Nano Res. 2015, 8, 1210–1221. [Google Scholar] [CrossRef]
- Yan, R.S.; Simpson, J.R.; Bertolazzi, S.; Brivio, J.; Watson, M.; Wu, X.F.; Kis, A.; Luo, T.F.; Walker, A.R.H.; Xing, H.G. Thermal Conductivity of Monolayer Molybdenum Disulfide Obtained from Temperature-Dependent Raman Spectroscopy. ACS Nano 2014, 8, 986–993. [Google Scholar] [CrossRef]
- Navas, J.; Sanchez-Coronilla, A.; Martin, E.I.; Teruel, M.; Gallardo, J.J.; Aguilar, T.; Gomez-Villarejo, R.; Alcantara, R.; Fernandez-Lorenzo, C.; Pinero, J.C.; et al. On the enhancement of heat transfer fluid for concentrating solar power using Cu and Ni nanofluids: An experimental and molecular dynamics study. Nano Energy 2016, 27, 213–224. [Google Scholar] [CrossRef]
- Mishra, A.K.; Lakshmi, K.V.; Huang, L.P. Eco-friendly synthesis of metal dichalcogenides nanosheets and their environmental remediation potential driven by visible light. Sci. Rep. UK 2015, 5, 15718. [Google Scholar] [CrossRef]
- Song, H.L.; Yu, X.F.; Chen, M.; Qiao, M.; Wang, T.J.; Zhang, J.; Liu, Y.; Liu, P.; Wang, X.L. Modification of WS2 nanosheets with controllable layers via oxygen ion irradiation. Appl. Surf. Sci. 2018, 439, 240–245. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. Investigations of thermal conductivity and viscosity of nanofluids. Int. J. Therm. Sci. 2008, 47, 560–568. [Google Scholar] [CrossRef]
- Prasher, R.; Song, D.; Wang, J.L.; Phelan, P. Measurements of nanofluid viscosity and its implications for thermal applications. Appl. Phys. Lett. 2006, 89, 133108. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K. A critical synthesis of thermophysical characteristics of nanofluids. Int. J. Heat Mass Trans. 2011, 54, 4410–4428. [Google Scholar] [CrossRef]
- Shen, J.F.; He, Y.M.; Wu, J.J.; Gao, C.T.; Keyshar, K.; Zhang, X.; Yang, Y.C.; Ye, M.X.; Vajtai, R.; Lou, J.; et al. Liquid Phase Exfoliation of Two-Dimensional Materials by Directly Probing and Matching Surface Tension Components. Nano Lett. 2015, 15, 5449–5454. [Google Scholar] [CrossRef] [PubMed]
- Teruel, M.; Aguilar, T.; Martinez-Merino, P.; Carrillo-Berdugo, I.; Gallardo-Bernal, J.J.; Gomez-Villarejo, R.; Alcantara, R.; Fernandez-Lorenzo, C.; Navas, J. 2D MoSe2-based nanofluids prepared by liquid phase exfoliation for heat transfer applications in concentrating solar power. Sol. Energy Mat. Sol. Cells 2019, 200, 109972. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.M.; McGovern, I.T.; et al. Liquid Phase Production of Graphene by Exfoliation of Graphite in Surfactant/Water Solutions. J. Am. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef]
- Keblinski, P.; Eastman, J.A.; Cahill, D.G. Nanofluids for thermal transport. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Yu, W.H.; France, D.M.; Singh, D.; Routbort, J.L. Nanofluids for heat transfer: An engineering approach. Nanoscale Res. Lett. 2011, 6, 182. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Hasan, H.A.; Wahid, M.A. Heat transfer enhancement of nanofluids in a double pipe heat exchanger with louvered strip inserts. Int. Commun. Heat Mass 2013, 40, 36–46. [Google Scholar] [CrossRef]
- Taylor, R.; Coulombe, S.; Otanicar, T.; Phelan, P.; Gunawan, A.; Lv, W.; Rosengarten, G.; Prasher, R.; Tyagi, H. Critical Review of the Novel Applications and Uses of Nanofluids. In Proceedings of the Asme Micro/Nanoscale Heat and Mass Transfer International Conference, Atlanta, GA, USA, 3–6 March 2012; pp. 219–234. [Google Scholar]
- Zhou, S.Q.; Ni, R. Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl. Phys. Lett. 2008, 92, 093123. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Specific Heat Measurement of Three Nanofluids and Development of New Correlations. J. Heat Transf. 2009, 131, 071601. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.W.; Ma, H.B.; Yang, M. Molecular dynamics simulation of effect of liquid layering around the nanoparticle on the enhanced thermal conductivity of nanofluids. J. Nanopart. Res. 2010, 12, 811–821. [Google Scholar] [CrossRef]
- Oh, S.H.; Kauffmann, Y.; Scheu, C.; Kaplan, W.D.; Ruhle, M. Ordered liquid aluminum at the interface with sapphire. Science 2005, 310, 661–663. [Google Scholar] [CrossRef]
- Prasher, R.; Bhattacharya, P.; Phelan, P.E. Brownian-motion-based convective-conductive model for the effective thermal conductivity of nanofluids. J. Heat Transf. 2006, 128, 588–595. [Google Scholar] [CrossRef]
- Xue, L.; Keblinski, P.; Phillpot, S.R.; Choi, S.U.S.; Eastman, J.A. Effect of liquid layering at the liquid-solid interface on thermal transport. Int. J. Heat Mass Trans. 2004, 47, 4277–4284. [Google Scholar] [CrossRef]
- Starace, A.K.; Gomez, J.C.; Wang, J.; Pradhan, S.; Glatzmaier, G.C. Nanofluid heat capacities. J. Appl. Phys. 2011, 110, 124323. [Google Scholar] [CrossRef]
- De Castro, C.A.N.; Murshed, S.M.S.; Lourenco, M.J.V.; Santos, F.J.V.; Lopes, M.L.M.; Franca, J.M.P. Enhanced thermal conductivity and specific heat capacity of carbon nanotubes ionanofluids. Int. J. Therm. Sci. 2012, 62, 34–39. [Google Scholar] [CrossRef]
- Zare, V.; Moalemian, A. Parabolic trough solar collectors integrated with a Kalina cycle for high temperature applications: Energyy, exergy and economic analyses. Energy Convers. Manag. 2017, 151, 681–692. [Google Scholar] [CrossRef]


| Sample | Density/kg m−3 | Dynamic viscosity /mPa s |
|---|---|---|
| HTF | 1056.6 ± 0.5 | 3.70 ± 0.02 |
| Nanofluid of MoS2 | 1061.6 ± 0.2 | 3.82 ± 0.02 |
| Nanofluid of WS2 | 1069.3 ± 0.7 | 3.77 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Merino, P.; Alcántara, R.; Aguilar, T.; Gallardo, J.J.; Carrillo-Berdugo, I.; Gómez-Villarejo, R.; Rodríguez-Fernández, M.; Navas, J. Stability and Thermal Properties Study of Metal Chalcogenide-Based Nanofluids for Concentrating Solar Power. Energies 2019, 12, 4632. https://doi.org/10.3390/en12244632
Martínez-Merino P, Alcántara R, Aguilar T, Gallardo JJ, Carrillo-Berdugo I, Gómez-Villarejo R, Rodríguez-Fernández M, Navas J. Stability and Thermal Properties Study of Metal Chalcogenide-Based Nanofluids for Concentrating Solar Power. Energies. 2019; 12(24):4632. https://doi.org/10.3390/en12244632
Chicago/Turabian StyleMartínez-Merino, Paloma, Rodrigo Alcántara, Teresa Aguilar, Juan Jesús Gallardo, Iván Carrillo-Berdugo, Roberto Gómez-Villarejo, Mabel Rodríguez-Fernández, and Javier Navas. 2019. "Stability and Thermal Properties Study of Metal Chalcogenide-Based Nanofluids for Concentrating Solar Power" Energies 12, no. 24: 4632. https://doi.org/10.3390/en12244632
APA StyleMartínez-Merino, P., Alcántara, R., Aguilar, T., Gallardo, J. J., Carrillo-Berdugo, I., Gómez-Villarejo, R., Rodríguez-Fernández, M., & Navas, J. (2019). Stability and Thermal Properties Study of Metal Chalcogenide-Based Nanofluids for Concentrating Solar Power. Energies, 12(24), 4632. https://doi.org/10.3390/en12244632









