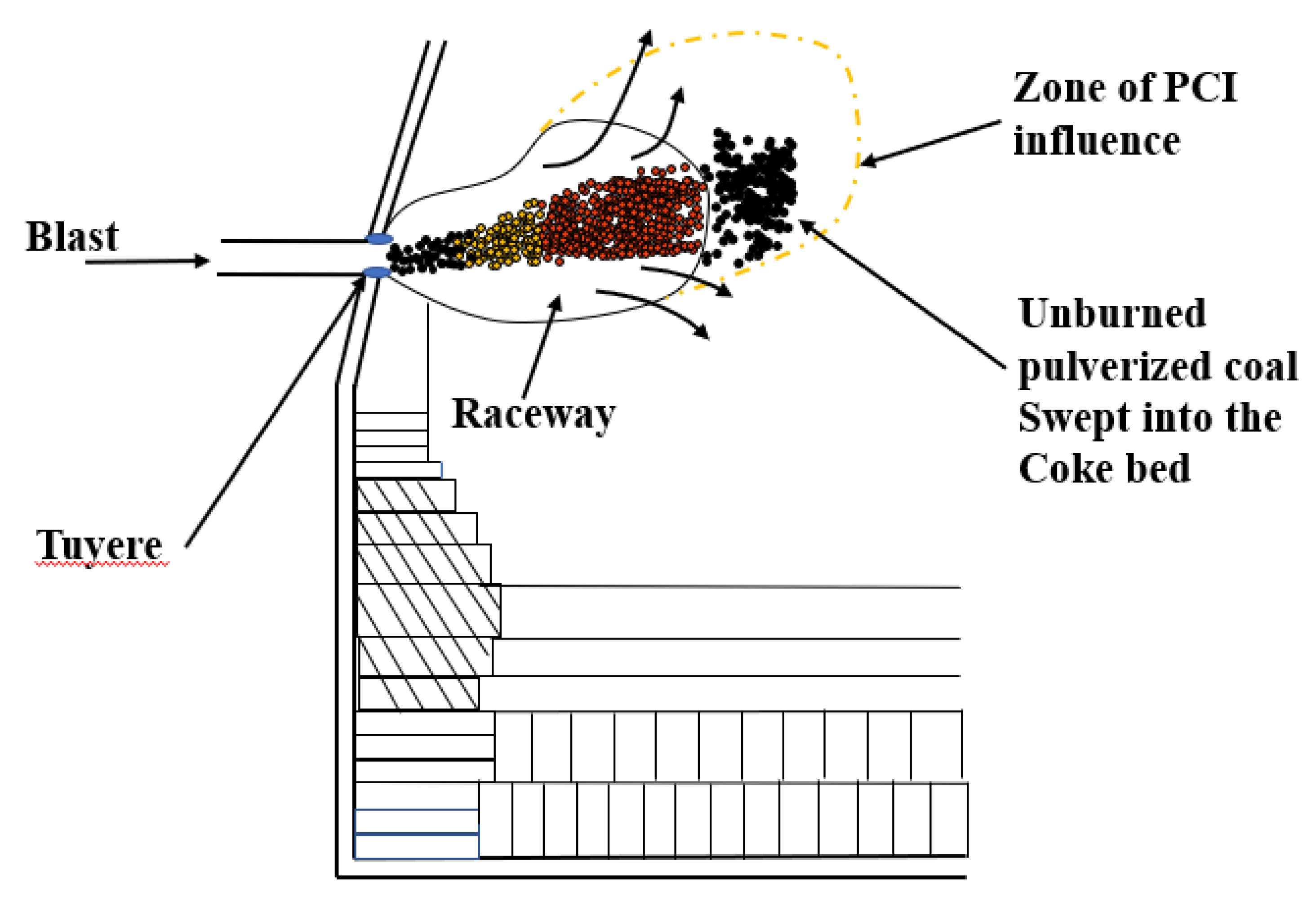
Figure 1.
Production of unburned pulverized coal (UPC) and movement behavior in a blast furnace (BF).
Figure 1.
Production of unburned pulverized coal (UPC) and movement behavior in a blast furnace (BF).
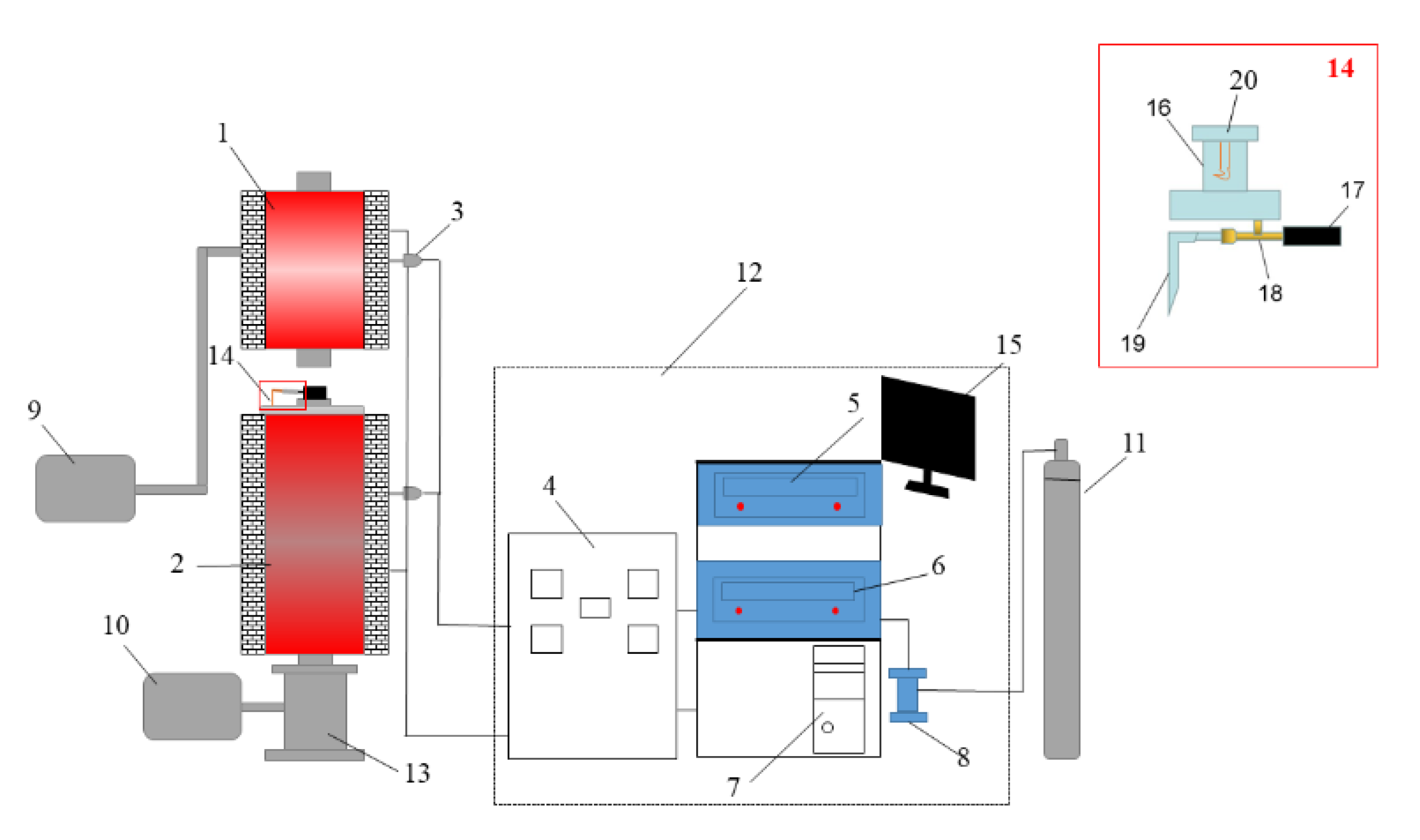
Figure 2.
Experimental simulation device for BF coal injection. 1. Hot blast stove, 2. Pulverized coal combustion furnace, 3. Thermocouple, 4. Current and voltage display meter, 5. Hot blast stove console, 6. Pulverized coal combustion furnace console, 7. Computer, 8. Air flow meter, 9. Air compressor, 10. Drawing fan, 11. N2 gas, 12. Data acquisition and control system, 13. UPC collecting tank, 14. Coal injection lance, 15. Computer monitor, 16. Coal bunker, 17. Sealed casing ring, 18. Copper injection pipe, 19. Gas nozzle and 20. Pulverized coal agitator.
Figure 2.
Experimental simulation device for BF coal injection. 1. Hot blast stove, 2. Pulverized coal combustion furnace, 3. Thermocouple, 4. Current and voltage display meter, 5. Hot blast stove console, 6. Pulverized coal combustion furnace console, 7. Computer, 8. Air flow meter, 9. Air compressor, 10. Drawing fan, 11. N2 gas, 12. Data acquisition and control system, 13. UPC collecting tank, 14. Coal injection lance, 15. Computer monitor, 16. Coal bunker, 17. Sealed casing ring, 18. Copper injection pipe, 19. Gas nozzle and 20. Pulverized coal agitator.
Figure 3.
SEM image of Shenhua and Qingding pulverized coal: (a) Shenhua and (b) Qingding.
Figure 3.
SEM image of Shenhua and Qingding pulverized coal: (a) Shenhua and (b) Qingding.
Figure 4.
Brunauer-Emmett-Teller (BET) results for two kinds of pulverized coal.
Figure 4.
Brunauer-Emmett-Teller (BET) results for two kinds of pulverized coal.
Figure 5.
Thermogravimetry (TG) and differential thermogravimetry (DTG) curves of Shenhua UPC.
Figure 5.
Thermogravimetry (TG) and differential thermogravimetry (DTG) curves of Shenhua UPC.
Figure 6.
TG and DTG curves for Qingding UPC.
Figure 6.
TG and DTG curves for Qingding UPC.
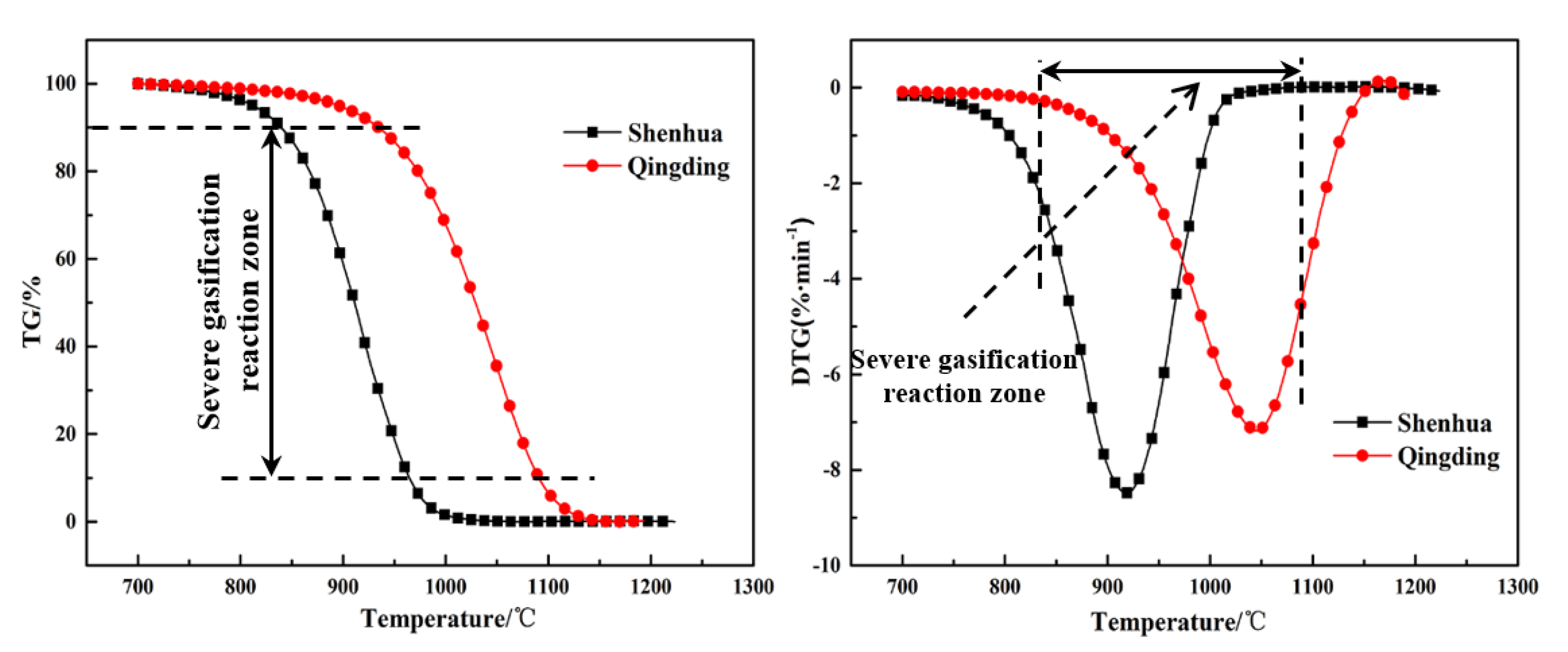
Figure 7.
TG and DTG curves for Shenhua UPC and Qingding UPC at a heating rate of 10 °C/min.
Figure 7.
TG and DTG curves for Shenhua UPC and Qingding UPC at a heating rate of 10 °C/min.
Figure 8.
SEM image of (a,b) Shenhua UPC and (c,d) Qingding UPC.
Figure 8.
SEM image of (a,b) Shenhua UPC and (c,d) Qingding UPC.
Figure 9.
Linear fitting of the mechanism function for the second stage of the Shenhua UPC gasification reaction at 5 °C/min.
Figure 9.
Linear fitting of the mechanism function for the second stage of the Shenhua UPC gasification reaction at 5 °C/min.
Figure 10.
Linear fitting of the mechanism function for the second stage of the two UPCs at 5 °C/min, 10 °C/min, 15 °C/min and 20 °C/min: (a) Shenhua UPC at 5 °C/min, (b) Shenhua UPC at 10 °C/min, (c) Shenhua UPC at 15 °C/min, (d) Shenhua UPC at 20 °C/min, (e) Qingding UPC at 5 °C/min, (f) Qingding UPC at 10 °C/min, (g) Qingding UPC at 15 °C/min, and (h) Qingding UPC at 20 °C/min.
Figure 10.
Linear fitting of the mechanism function for the second stage of the two UPCs at 5 °C/min, 10 °C/min, 15 °C/min and 20 °C/min: (a) Shenhua UPC at 5 °C/min, (b) Shenhua UPC at 10 °C/min, (c) Shenhua UPC at 15 °C/min, (d) Shenhua UPC at 20 °C/min, (e) Qingding UPC at 5 °C/min, (f) Qingding UPC at 10 °C/min, (g) Qingding UPC at 15 °C/min, and (h) Qingding UPC at 20 °C/min.
Table 1.
Proximate and ultimate analysis of Shenhua and Qingding pulverized coal (mass %), air dry basis.
Table 1.
Proximate and ultimate analysis of Shenhua and Qingding pulverized coal (mass %), air dry basis.
| Sample | Proximate Analysis | Ultimate Analysis |
|---|
| Moisture | Volatiles | Ash | Fixed Carbon | C | H | O | N | St |
|---|
| Shenhua | 5.27 | 23.94 | 4.98 | 65.81 | 68.31 | 4.89 | 10.23 | 0.86 | 0.26 |
| Qingding | 1.20 | 7.99 | 12.25 | 78.56 | 83.57 | 0.17 | 1.99 | 0.97 | 0.71 |
Table 2.
Combustion conversion degree of Shenhua and Qingding pulverized coal (%).
Table 2.
Combustion conversion degree of Shenhua and Qingding pulverized coal (%).
| Sample | Ash Content of UPC | Combustion Conversion Degree |
|---|
| Shenhua | 13.17 | 65.43 |
| Qingding | 21.08 | 47.74 |
Table 3.
Characteristic temperatures of the gasification reaction of the different UPCs at 10 °C/min heating rate.
Table 3.
Characteristic temperatures of the gasification reaction of the different UPCs at 10 °C/min heating rate.
| Sample | T10, °C | Tm, °C | T90, °C |
|---|
| Shenhua | 839 | 919 | 964 |
| Qingding | 934 | 1047 | 1090 |
Table 4.
Characteristic temperature of gasification reaction of UPC under different heating rates.
Table 4.
Characteristic temperature of gasification reaction of UPC under different heating rates.
| Sample | Heating Rate (°C/min) | T10, °C | Tm, °C | T90, °C |
|---|
| Shenhua UPC | 5 | 824 | 893 | 941 |
| 10 | 839 | 919 | 964 |
| 15 | 863 | 945 | 1004 |
| 20 | 871 | 961 | 1019 |
| Qingding UPC | 5 | 908 | 998 | 1047 |
| 10 | 934 | 1047 | 1090 |
| 15 | 949 | 1072 | 1123 |
| 20 | 979 | 1099 | 1146 |
Table 5.
Mechanism functions of common gas-solid reaction models.
Table 5.
Mechanism functions of common gas-solid reaction models.
| Mechanism | Code | Differential Form | Integral Form |
|---|
| Chemical reaction | N = 1 | F1 | (1 − ) | −In(1 − ) |
| N = 2 | F2 | (1 − )2 | (1 − )−1 − 1 |
| N = 3 | F3 | (1 − )3 | [(1 − )−2 − 1]/2 |
| Diffusion | The two-dimensional diffusion control | D2 | [−In(1 − )]−1 | + (1 − )In(1 − ) |
| The two-dimensional diffusion control (Jander function) | D3 | 1.5(1 − )2/3[1 − (1 − )1/3] | [1 − (1 )1/3]2 |
| The three-dimensional diffusion control (Ginstling-Brounshten function) | D4 | 1.5[1 − (1 − )1/3 − 1]−1 | (1 − 2/3) − (1 − )2/3 |
| Shrinking core | Shrinking core | R1 | 2(1 − x)1/2 | 1 − (1 − x)1/2 |
| Shrinking core | R2 | 3(1 − x)2/3 | 1 − (1 − x)1/3 |
| Shrinking core (n = 2) | R3 | (1 − x)−1 | 1 − (1 − x)2 |
| Random nucleation and nuclei growth | Two-dimensional | A2 | 2(1 − )[−In(1 − )]1/2 | [−In(1 − )]1/2 |
| Three-dimensional | A3 | 3(1 − )[−In(1 − )]2/3 | [−In(1 − )]1/3 |
Table 6.
Kinetic parameters of different mechanism functions of the Shenhua UPC in the second stage at 5 °C/min.
Table 6.
Kinetic parameters of different mechanism functions of the Shenhua UPC in the second stage at 5 °C/min.
| Code | Stage 2 |
|---|
| E/(kJ/mol) | A/s−1 | R2 |
|---|
| F1 | 269.89 | 1.12 1011 | 0.9972 |
| F2 | 380.22 | 2.40 1016 | 0.9933 |
| F3 | 3.95 10−4 | 2.83 10−1 | 0.9873 |
| D2 | 442.50 | 2.03 1018 | 0.9805 |
| D3 | 498.68 | 2.26 1020 | 0.9915 |
| D4 | 461.01 | 3.50 1018 | 0.9849 |
| A2 | 125.35 | 1.99 104 | 0.9966 |
| A3 | 77.17 | 8.92 10 | 0.9959 |
| R1 | 2.07 10−4 | 1.24 10−1 | 0.9791 |
| R2 | 2.22 10−4 | 1.34 10−1 | 0.9844 |
| R3 | 1.25 10−4 | 7.52 10−2 | 0.9074 |
Table 7.
Optimum mechanism function kinetic parameters for the second stage of the UPC gasification reaction at different heating rates.
Table 7.
Optimum mechanism function kinetic parameters for the second stage of the UPC gasification reaction at different heating rates.
| Sample | Heating Rate | Stage/°C | Reaction Mechanism | E/(kJ/mol) | A/s−1 | R2 |
|---|
| Shenhua UPC | 5 °C/min | 2 | F1 | 269.89 | 1.12 × 1011 | 0.9972 |
| 10 °C/min | 2 | F1 | 265.16 | 7.73 × 1010 | 0.9995 |
| 15 °C/min | 2 | F1 | 233.74 | 2.26 × 109 | 0.9941 |
| 20 °C/min | 2 | F1 | 223.41 | 8.16 × 108 | 0.9922 |
| Qingding UPC | 5 °C/min | 2 | F1 | 266.70 | 7.63 × 109 | 0.9998 |
| 10 °C/min | 2 | F1 | 252.01 | 1.63 × 109 | 0.9993 |
| 15 °C/min | 2 | F1 | 235.43 | 3.10 × 108 | 0.9989 |
| 20 °C/min | 2 | F1 | 251.54 | 1.43 × 109 | 0.9998 |
Table 8.
Verification results of Equation (10).
Table 8.
Verification results of Equation (10).
| Shenhua UPC | 5 °C/min | 10 °C/min |
| T(K) | R J/(mol*K) | E(kJ/mol) | T(K) | R J/(mol*K) | E(kJ/mol) |
| 1155.5 | 8.314 | 269.89 | 1174.5 | 8.314 | 265.16 |
| |