Luenberger-Sliding Mode Observer Based Backstepping Control for the SCR System in a Diesel Engine
Abstract
:1. Introduction
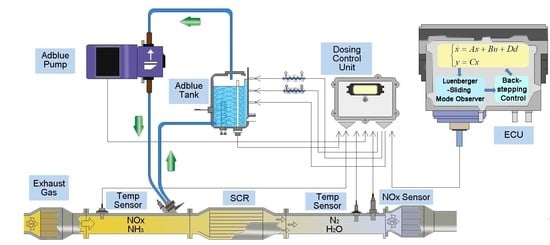
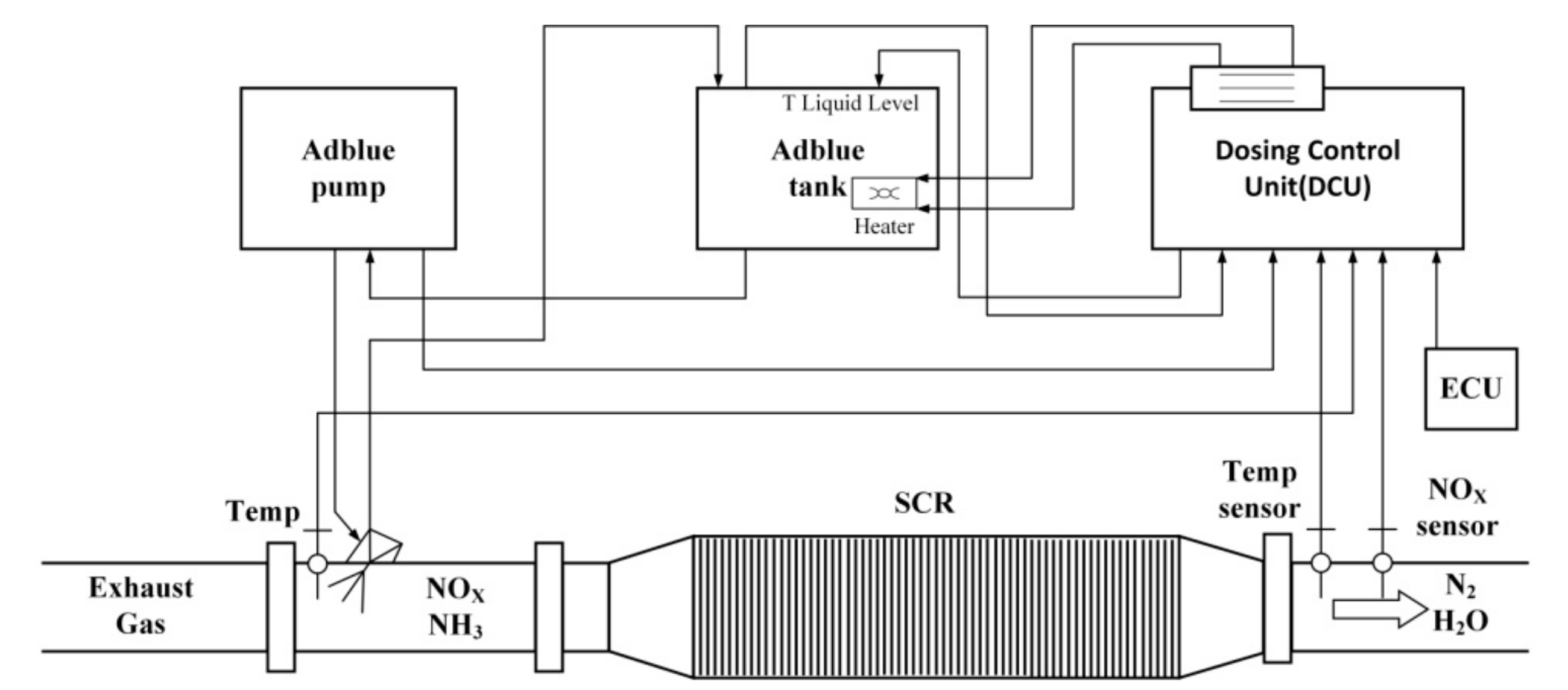
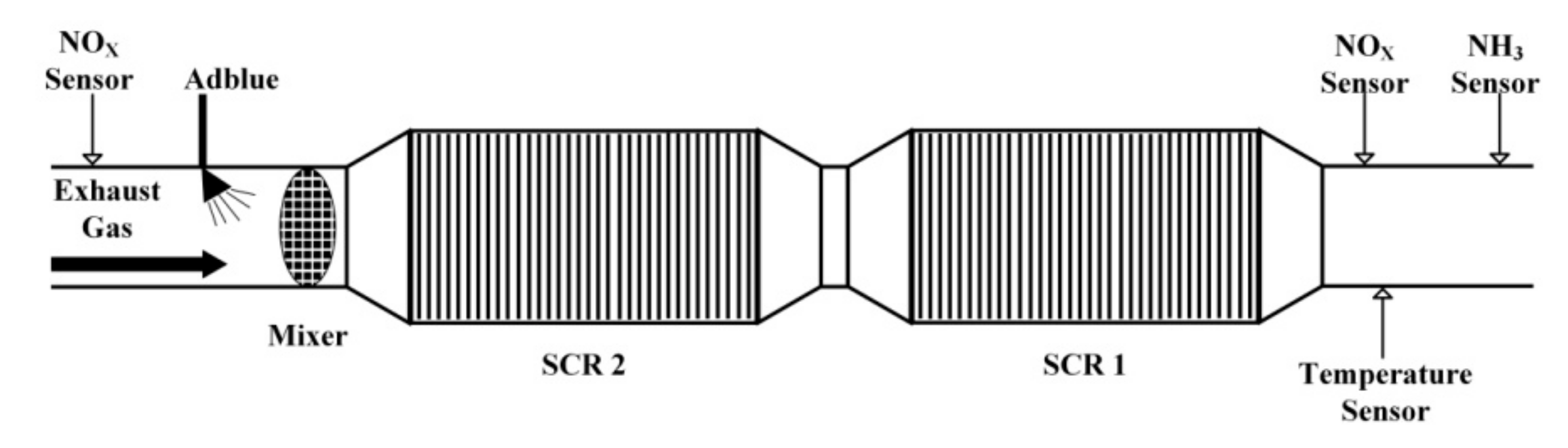
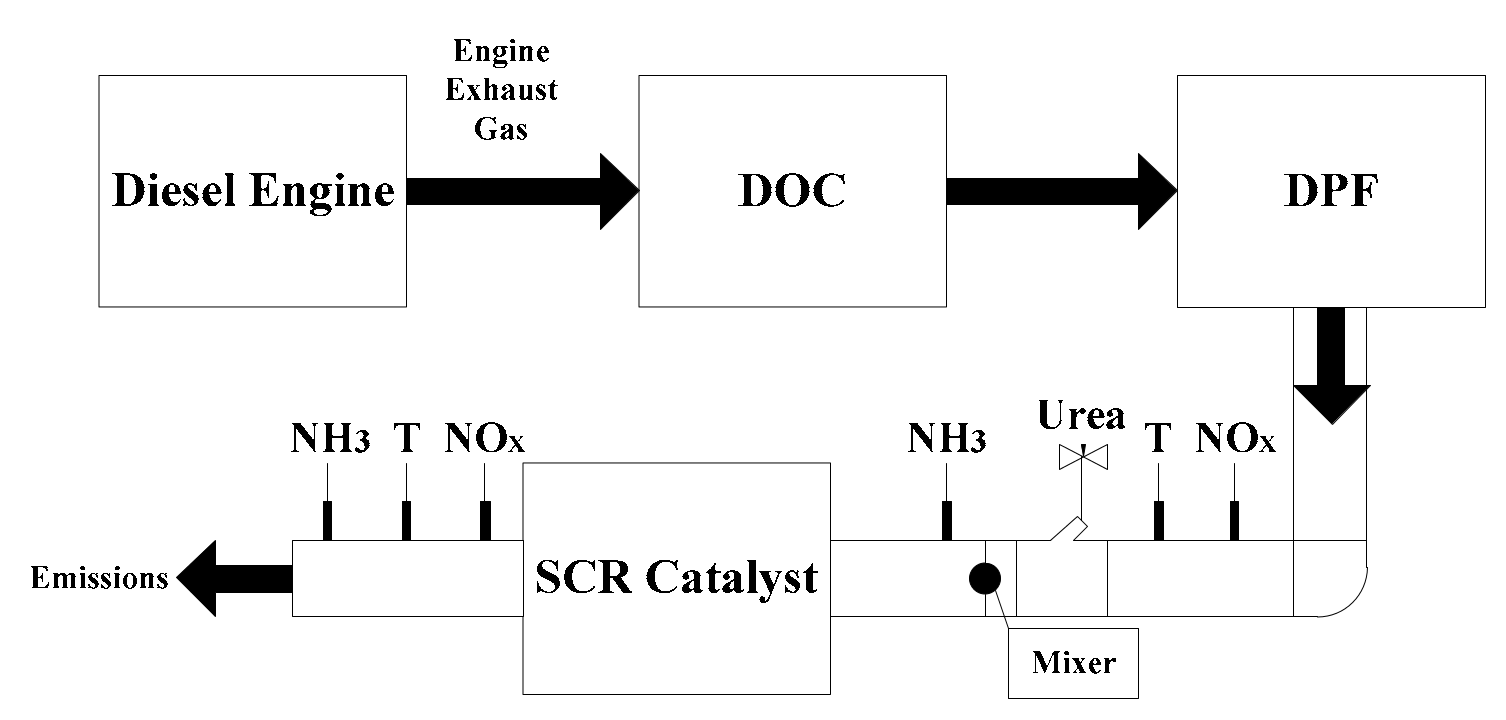
2. Selective Catalytic Reduction System
2.1. SCR System Operation Principles
2.2. SCR Dynamic Model and Analysis of Observability and Controllability
- (1)
- , ; the NH3 coverage ratio and the NOX concentration are uncontrollable. At that point, the NH3 coverage ratio reaches 100%. However, it will not happen in practice.
- (2)
- , ; the NOX is uncontrollable. In the meantime, , the reasonable working temperature, is below 600 °C. Therefore, the loss of controllability due to this condition is not expected operationally.
3. Observer Design and Stability Analysis
3.1. Two-Cell SCR Catalyst Ammonia Concentration Observer Design
3.2. Observer Stability Analysis
3.2.1. Convergence Analysis of
3.2.2. Convergence Analysis of
3.2.3. Convergence Analysis of
4. Backstepping Control Law Design
4.1. Stability Analysis of Case 1
4.2. Stability Analysis of Case 2
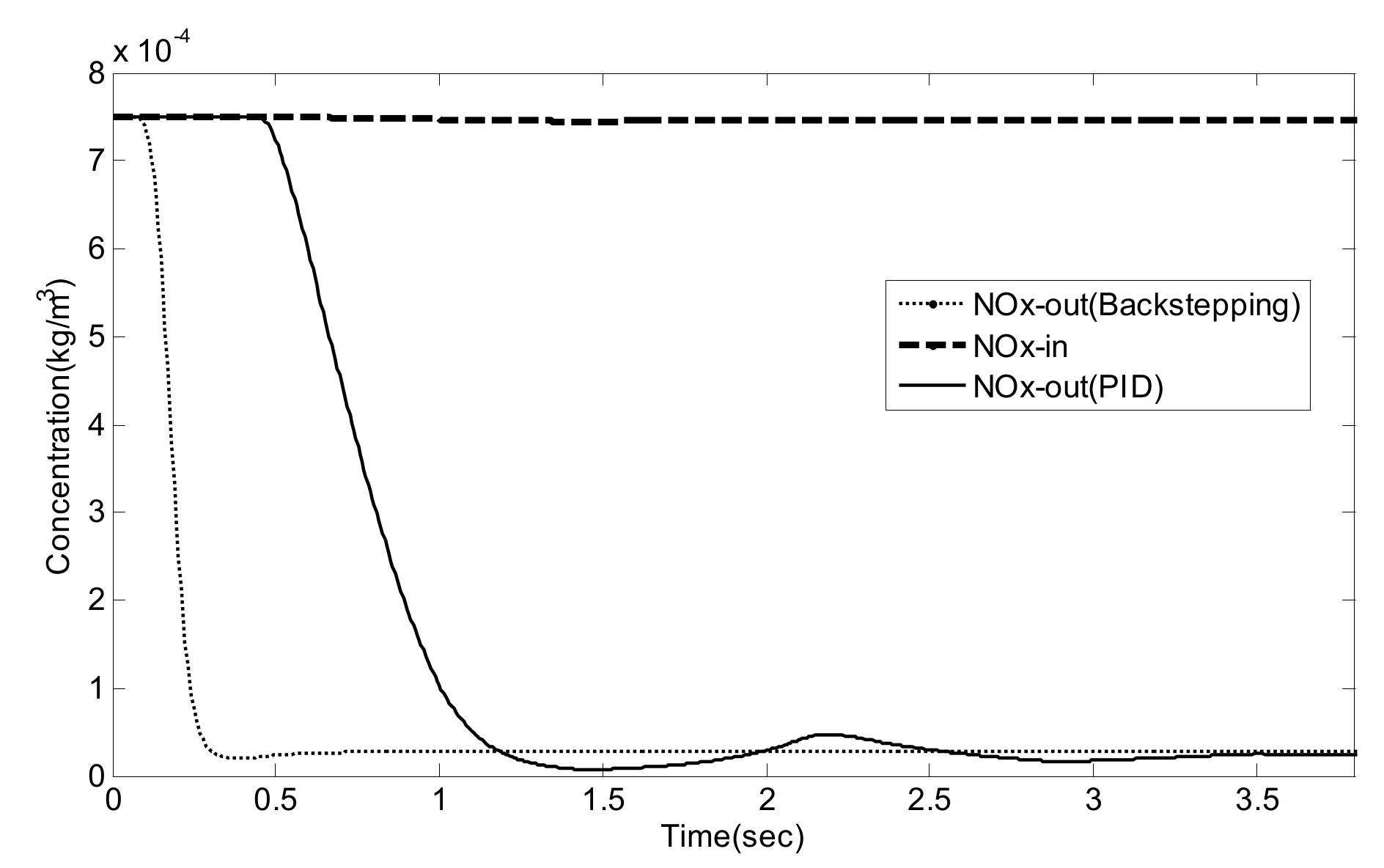
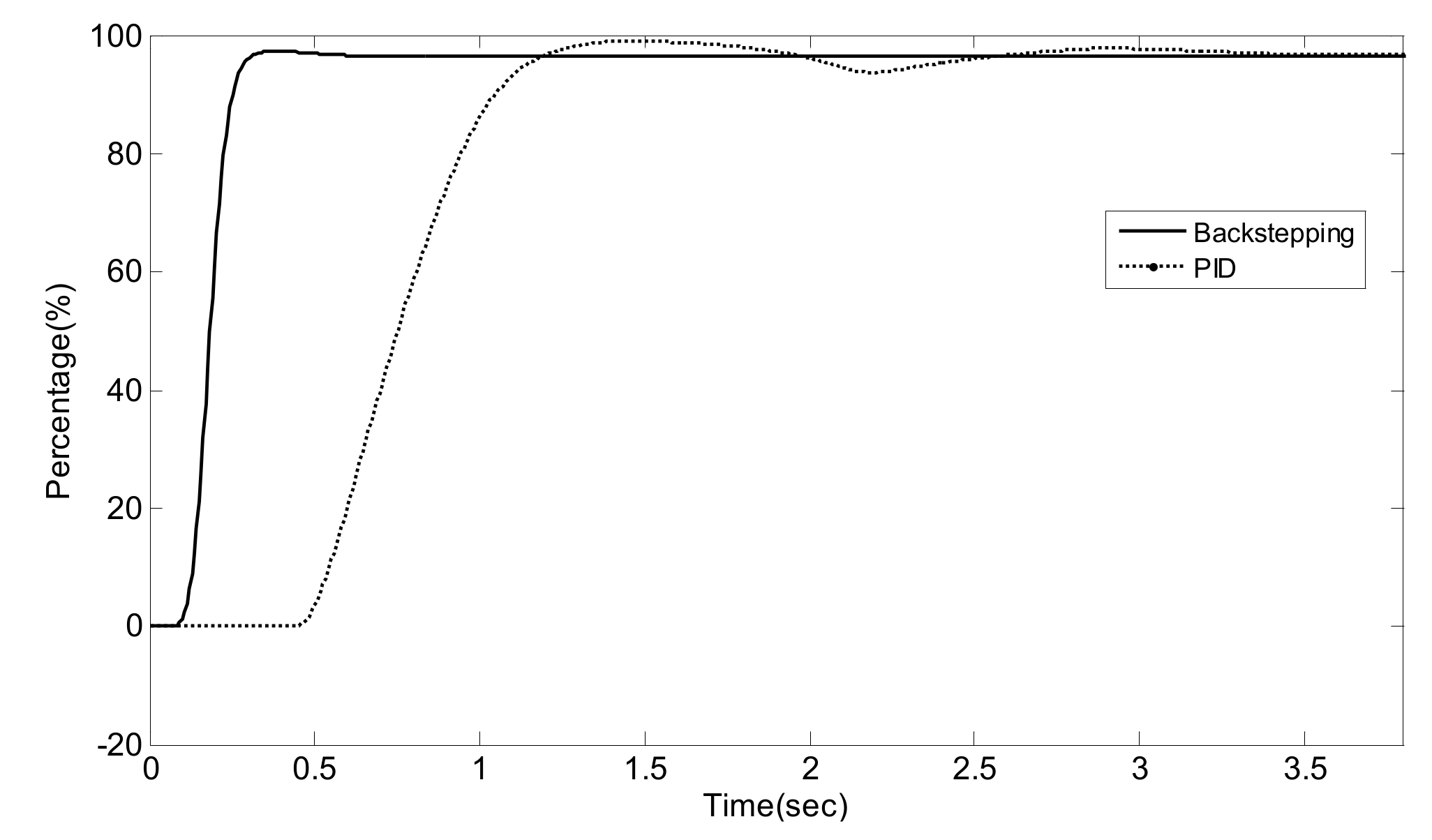
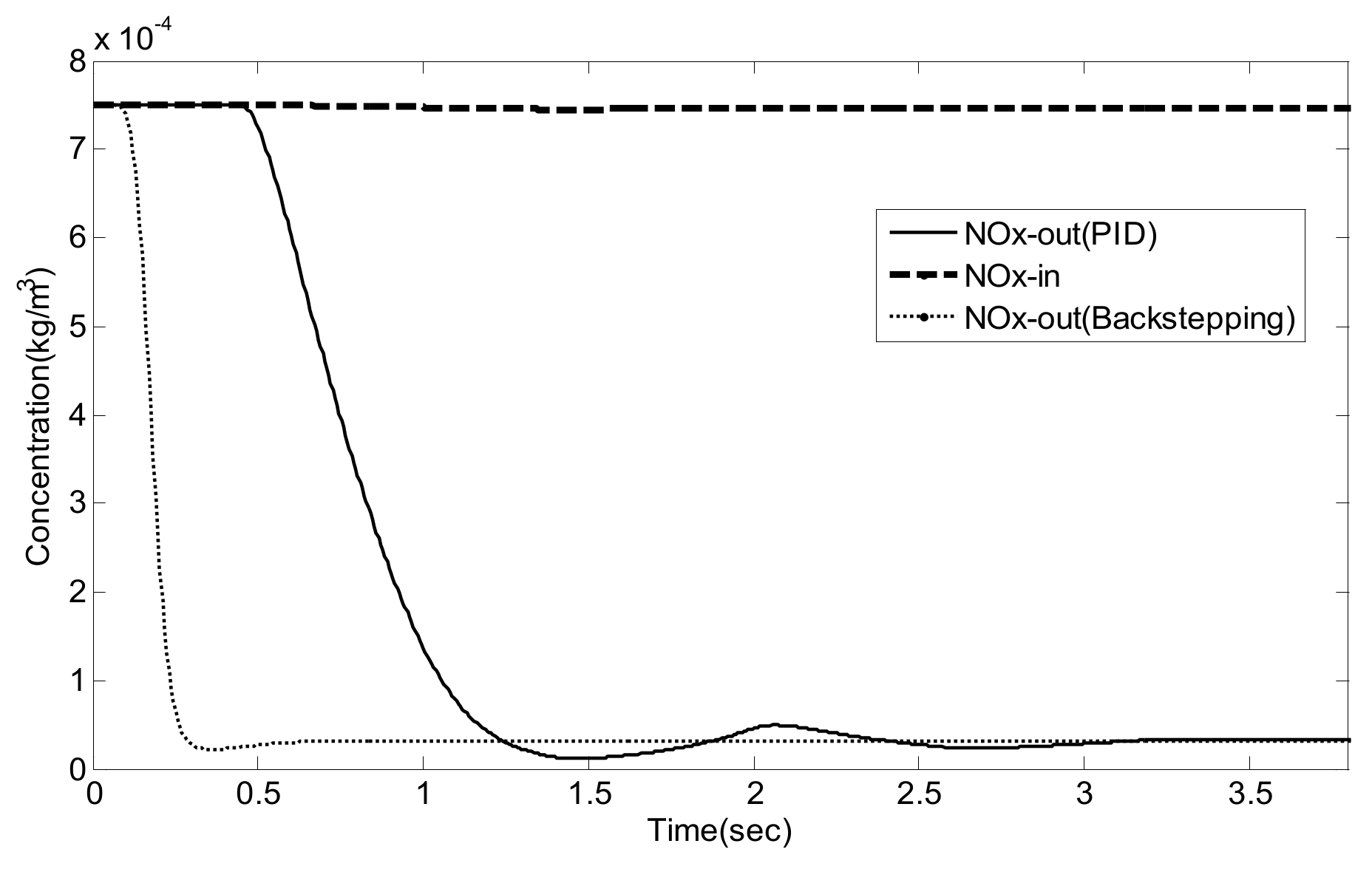
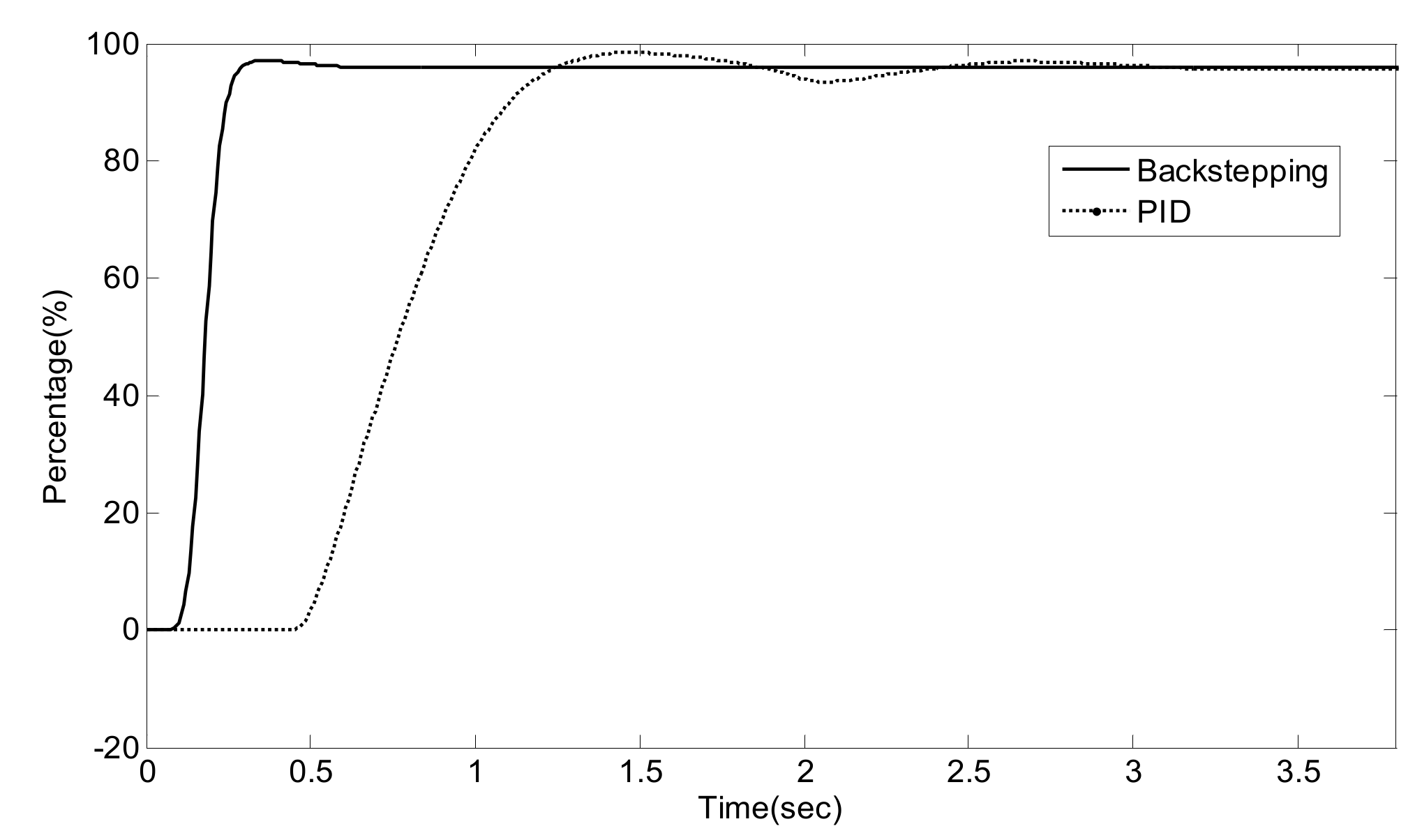
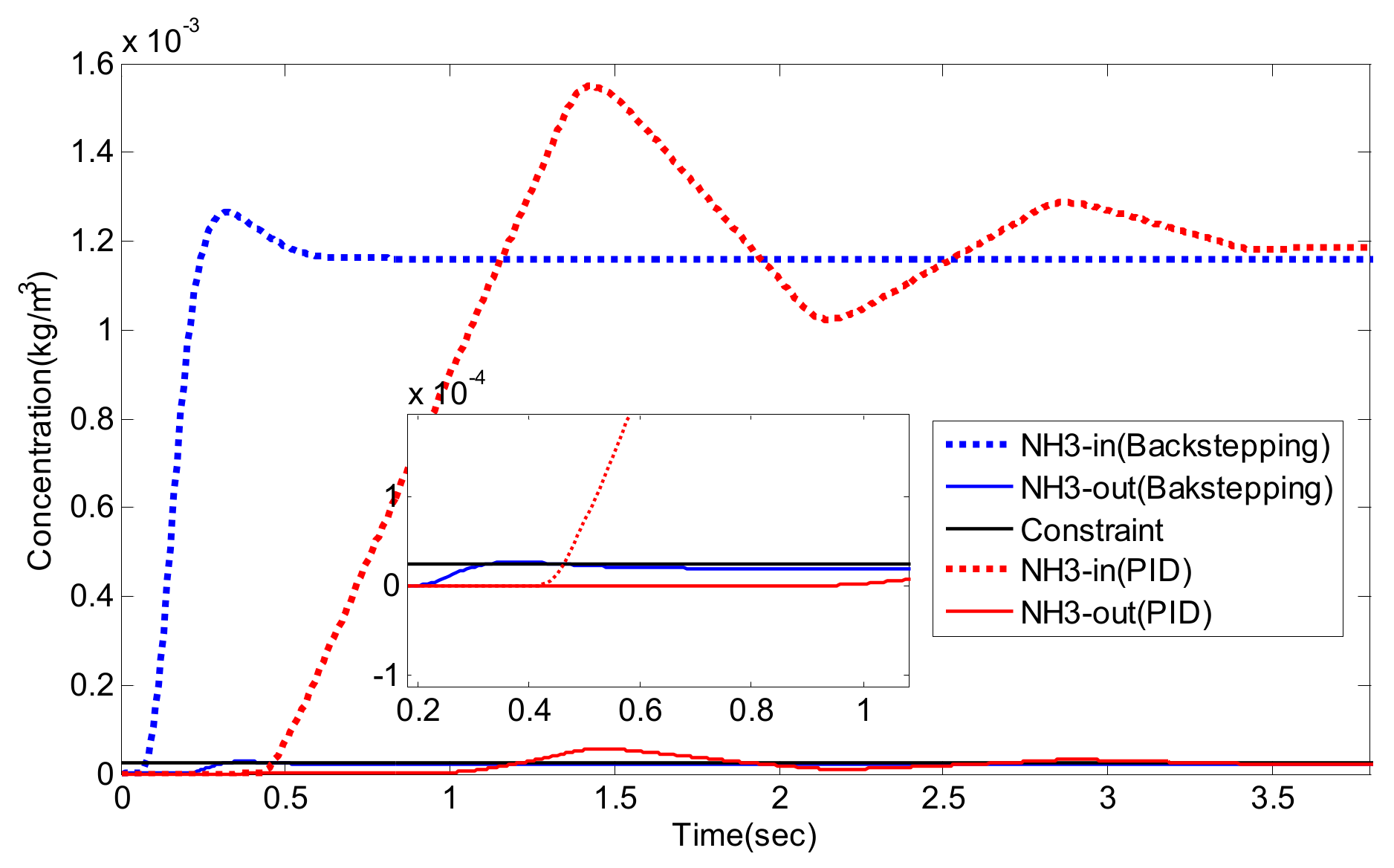
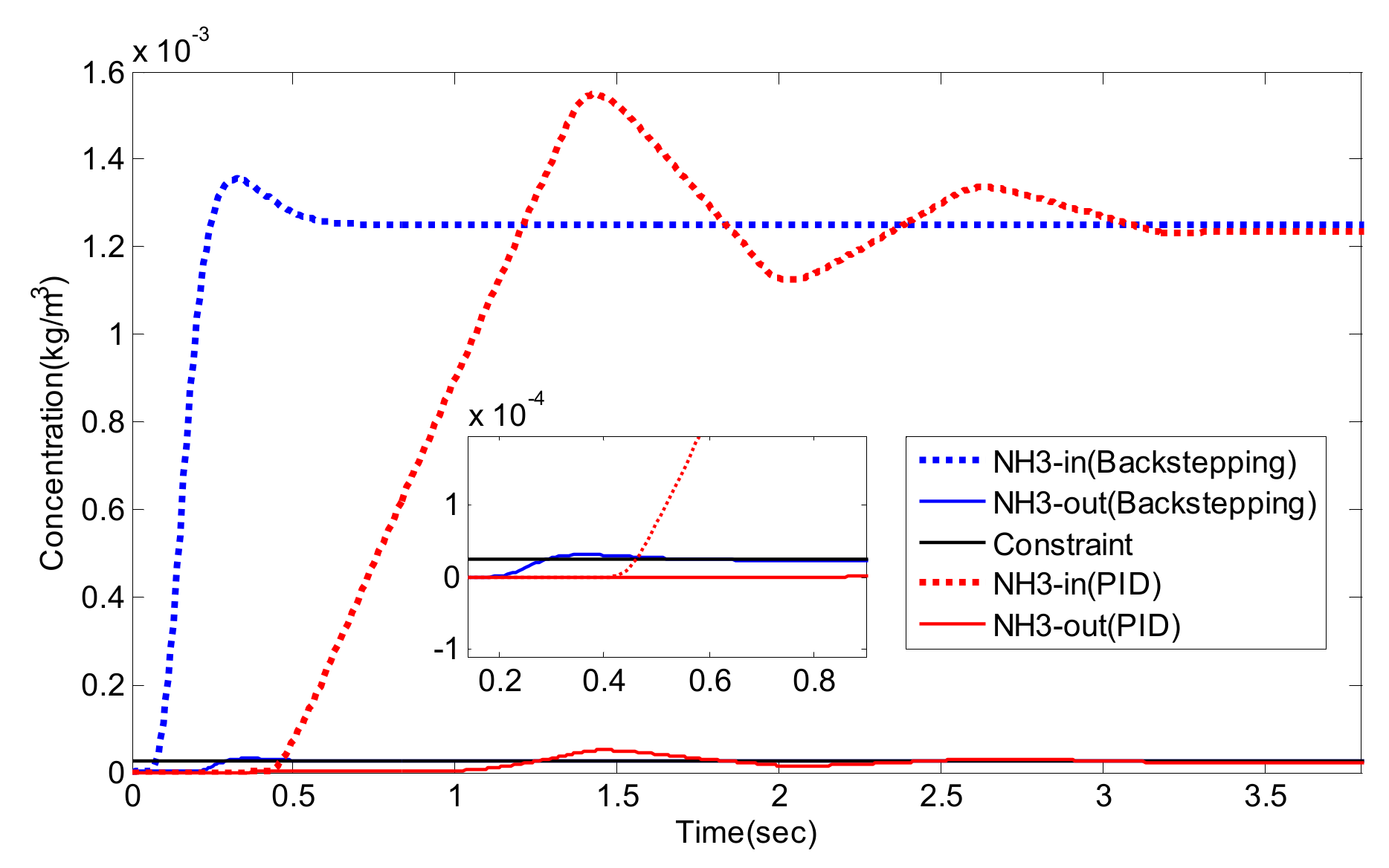
5. Experiment Results and Analysis
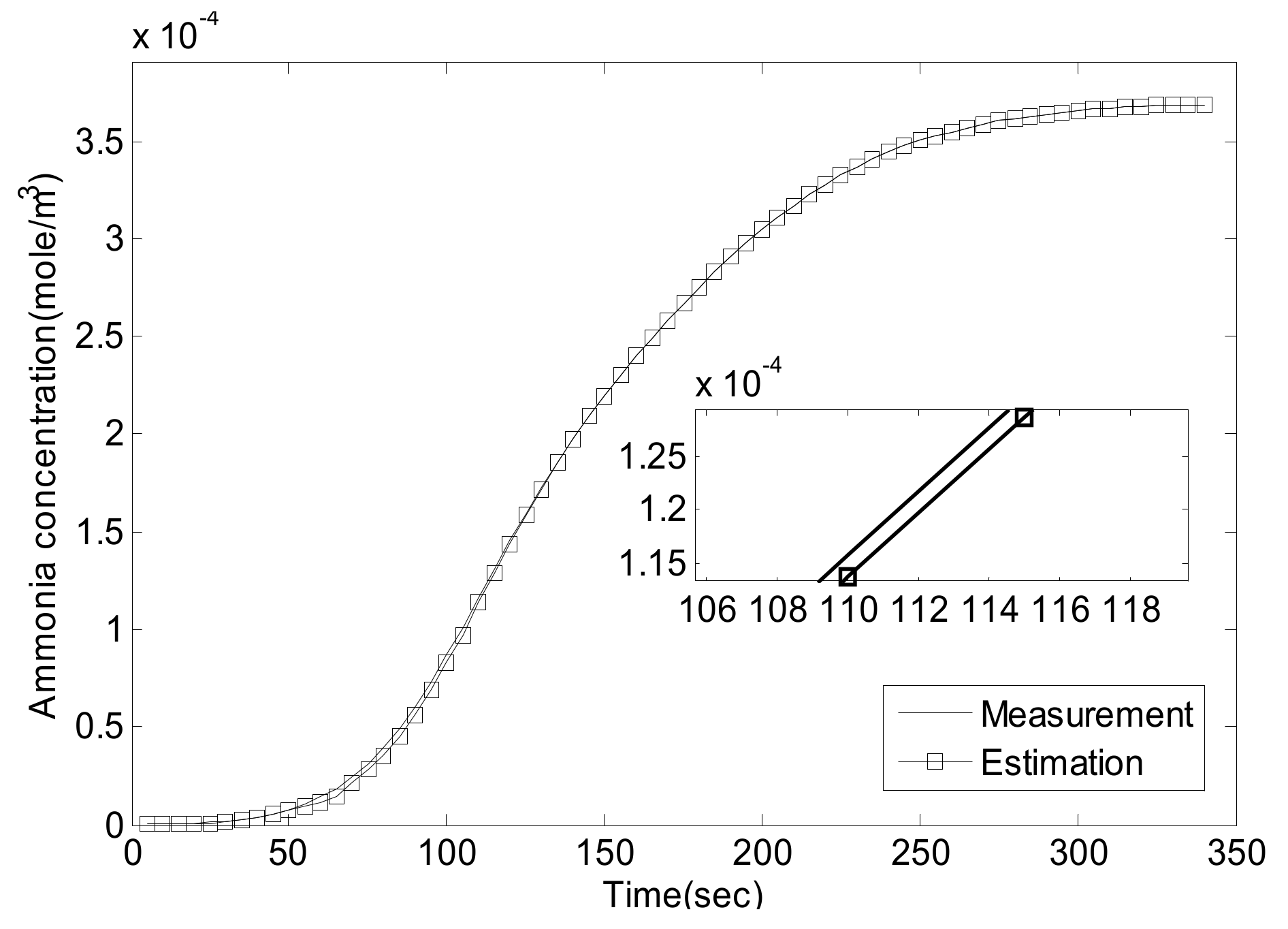
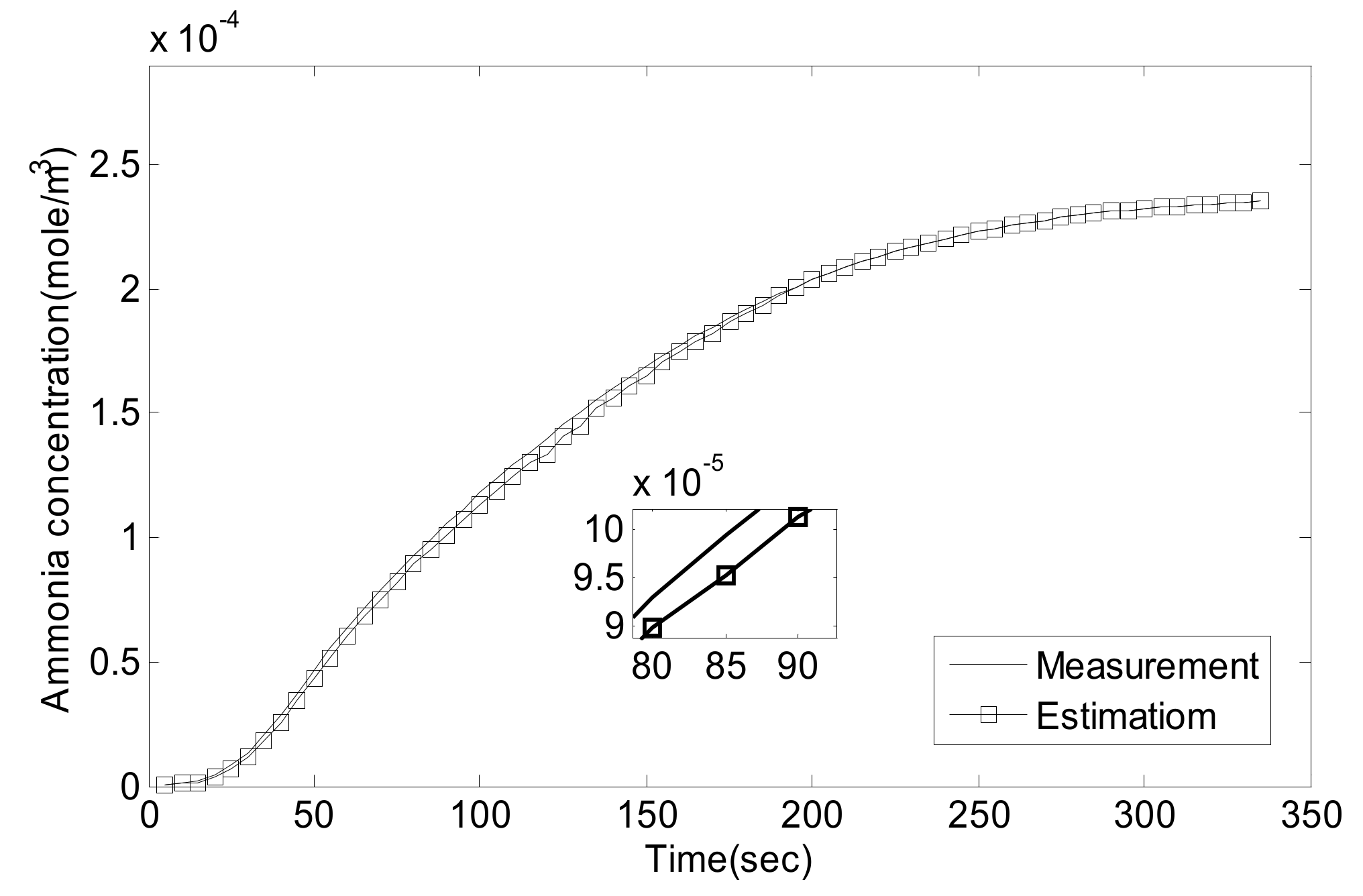
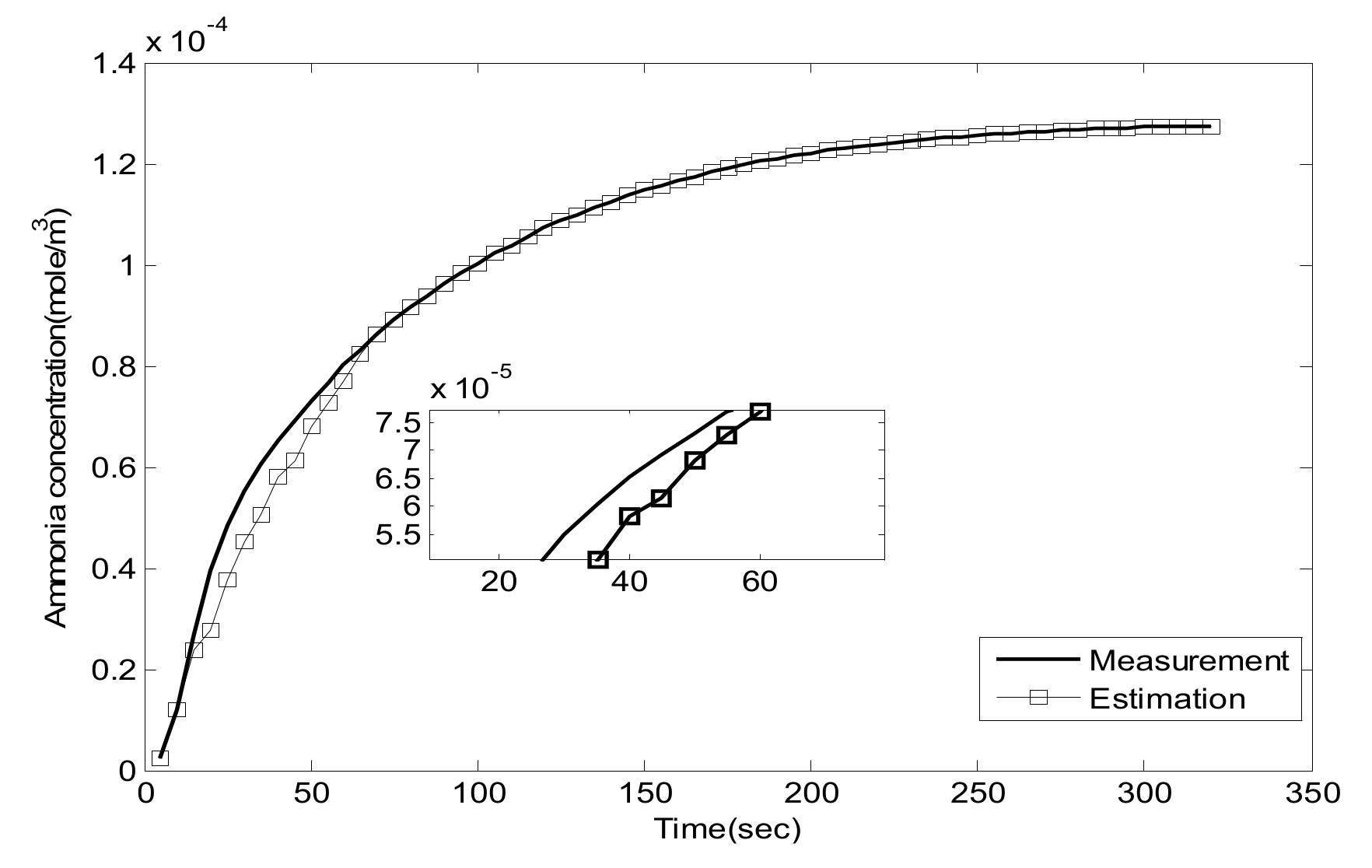
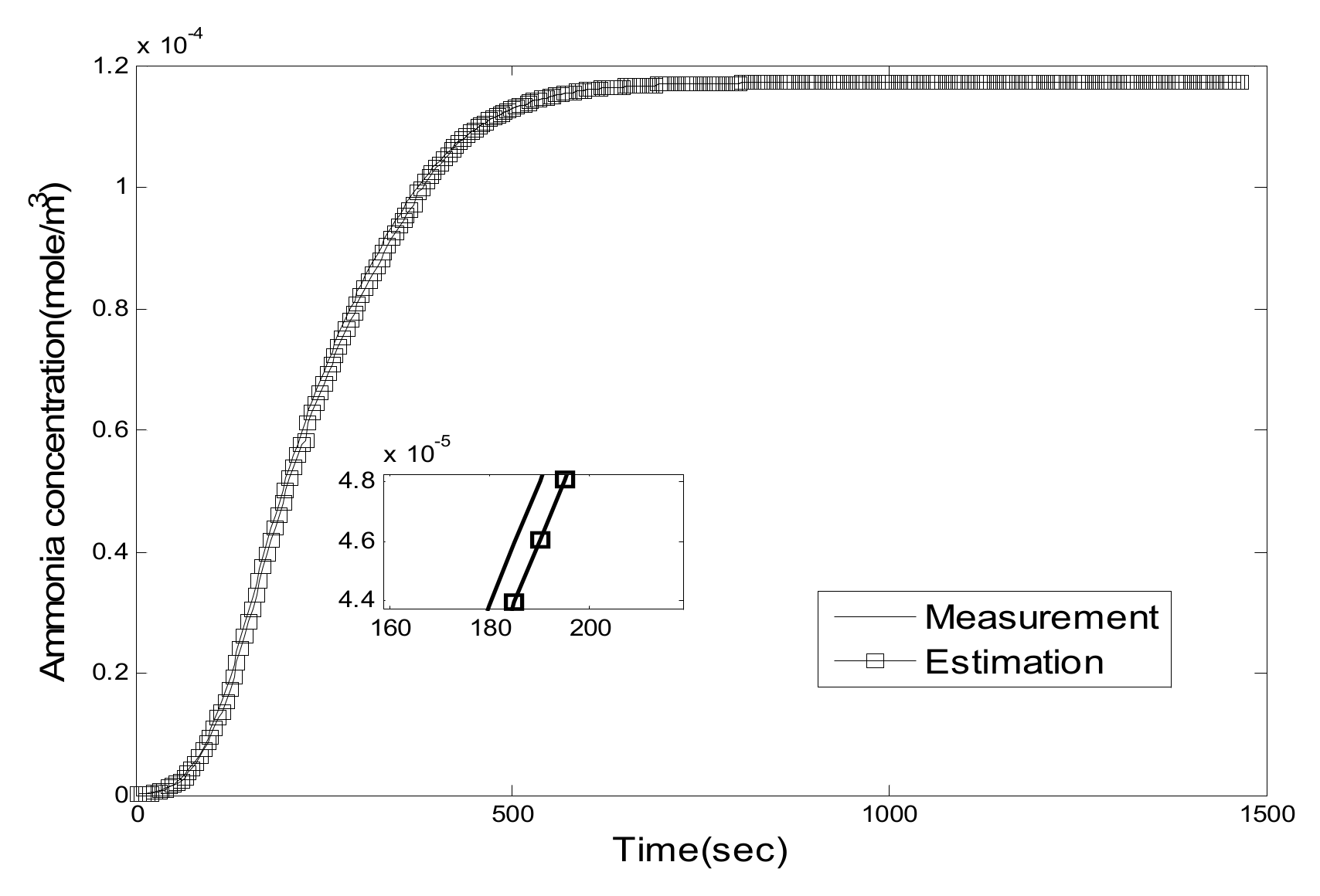
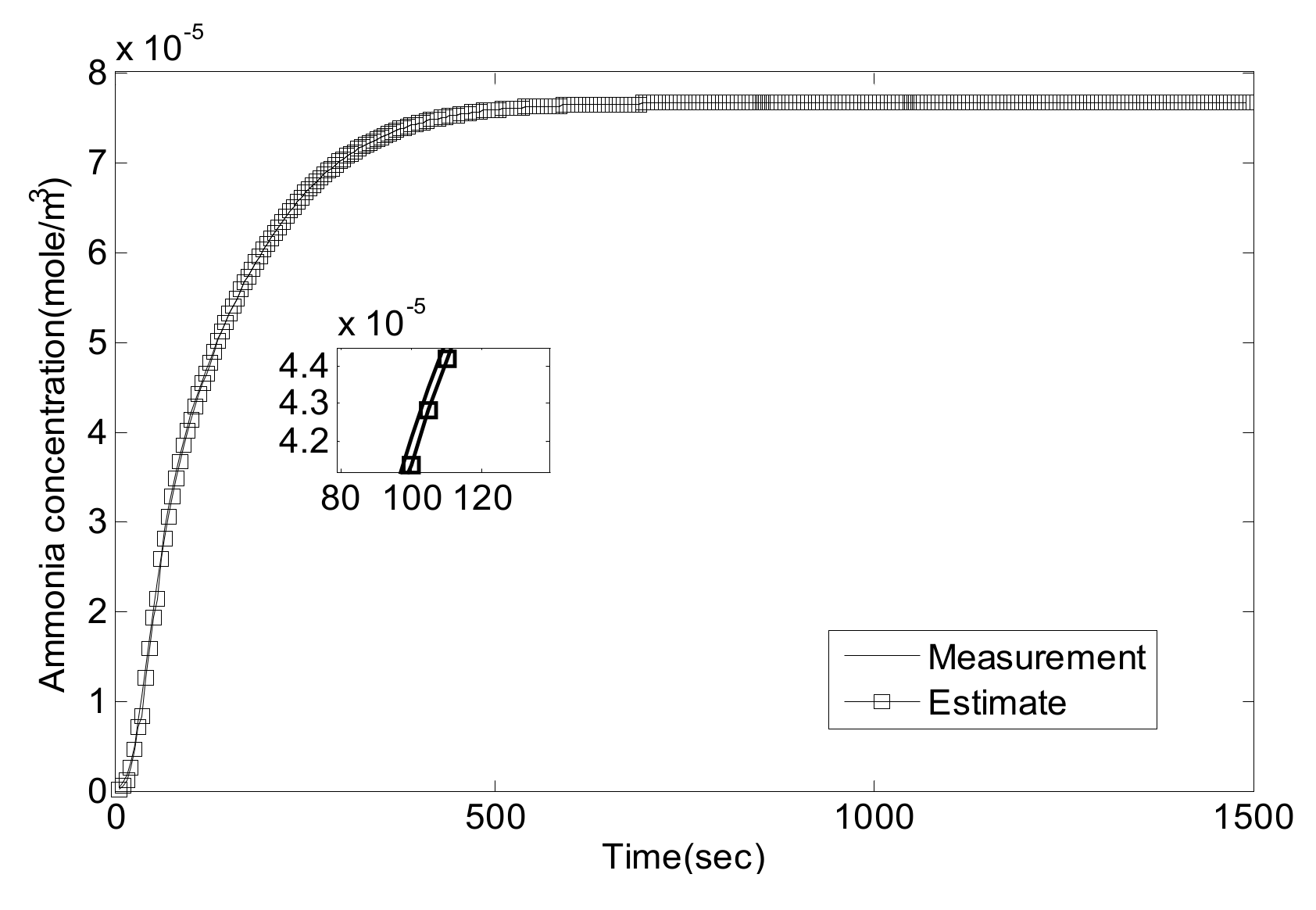
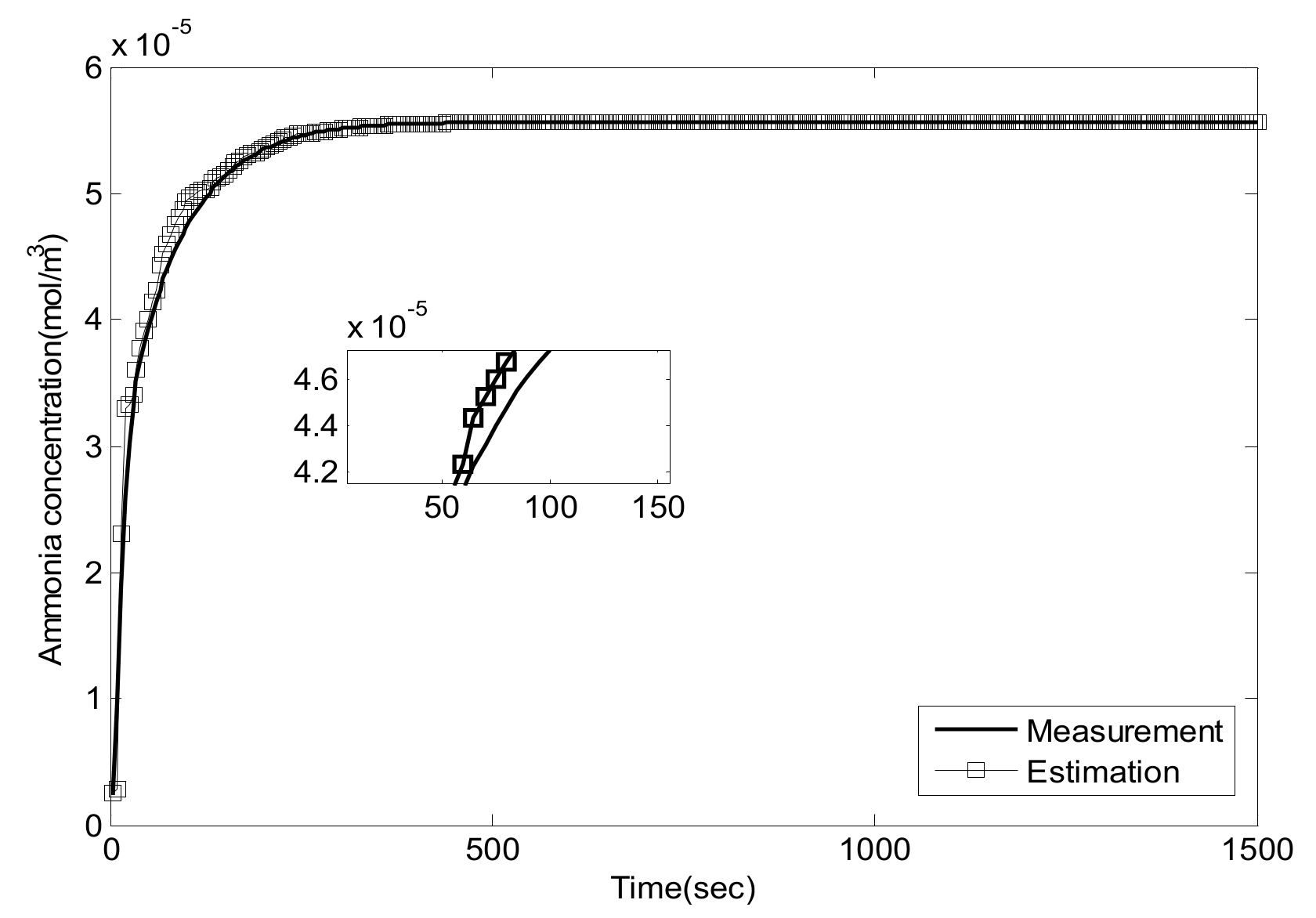
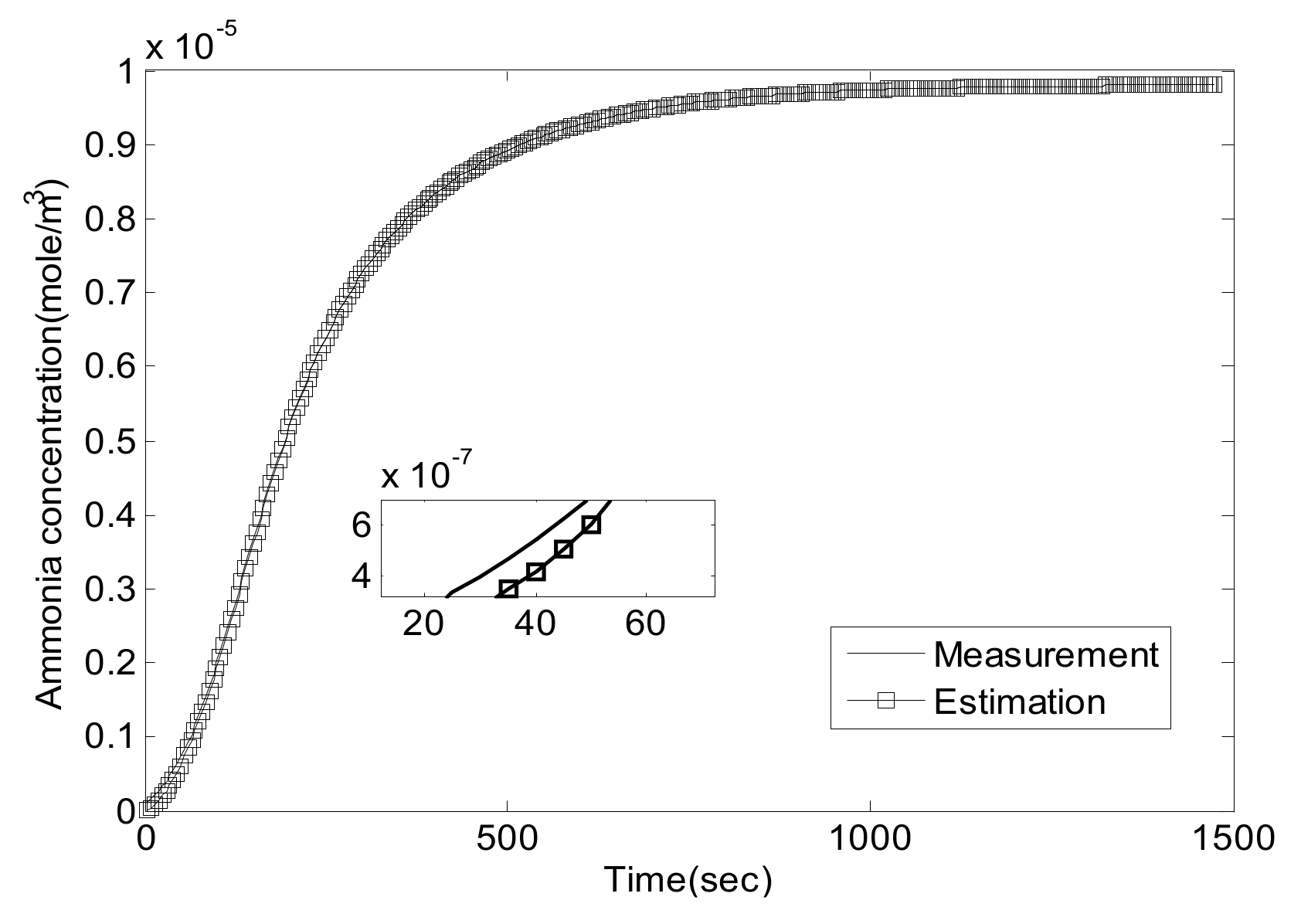
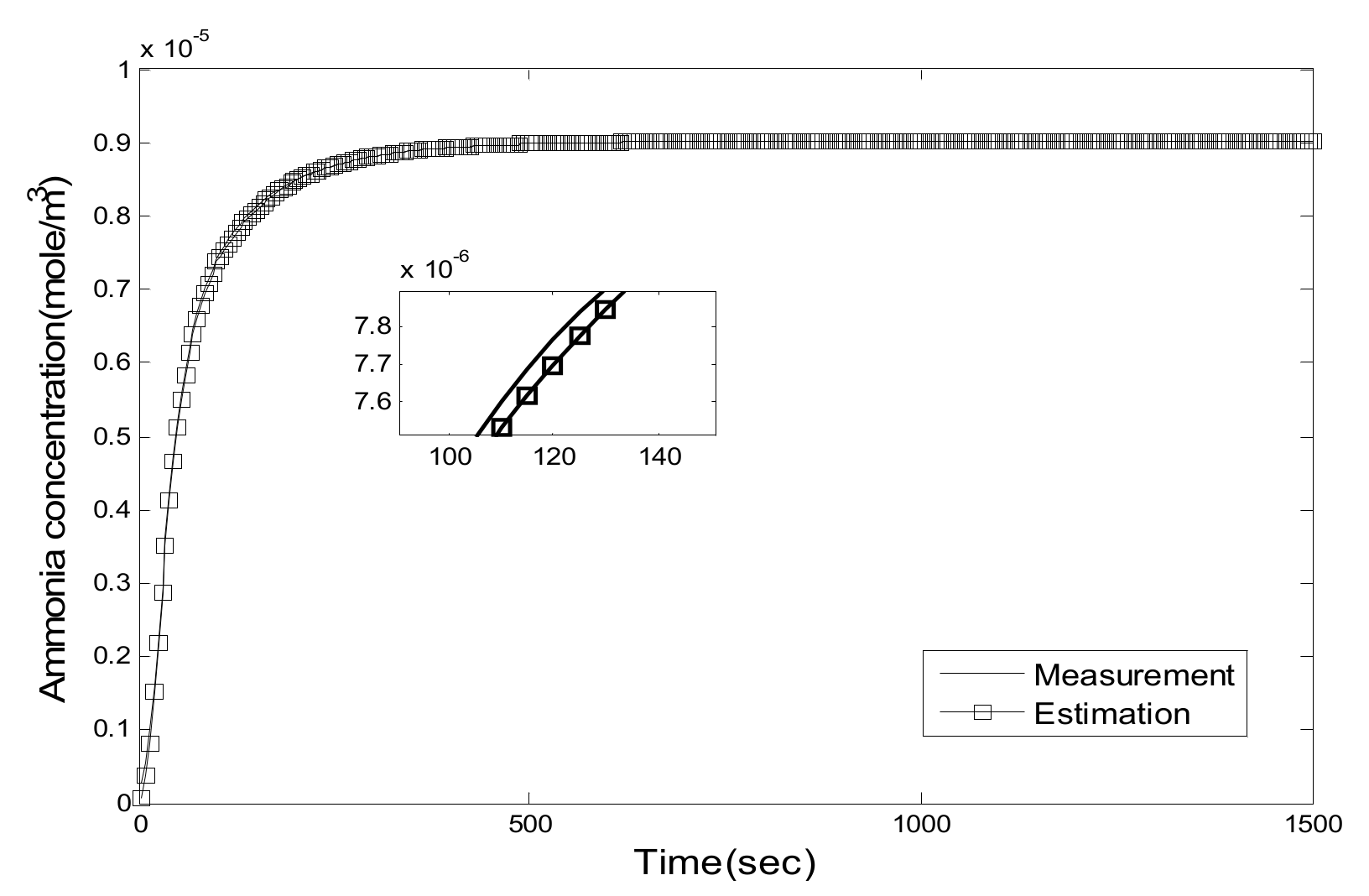
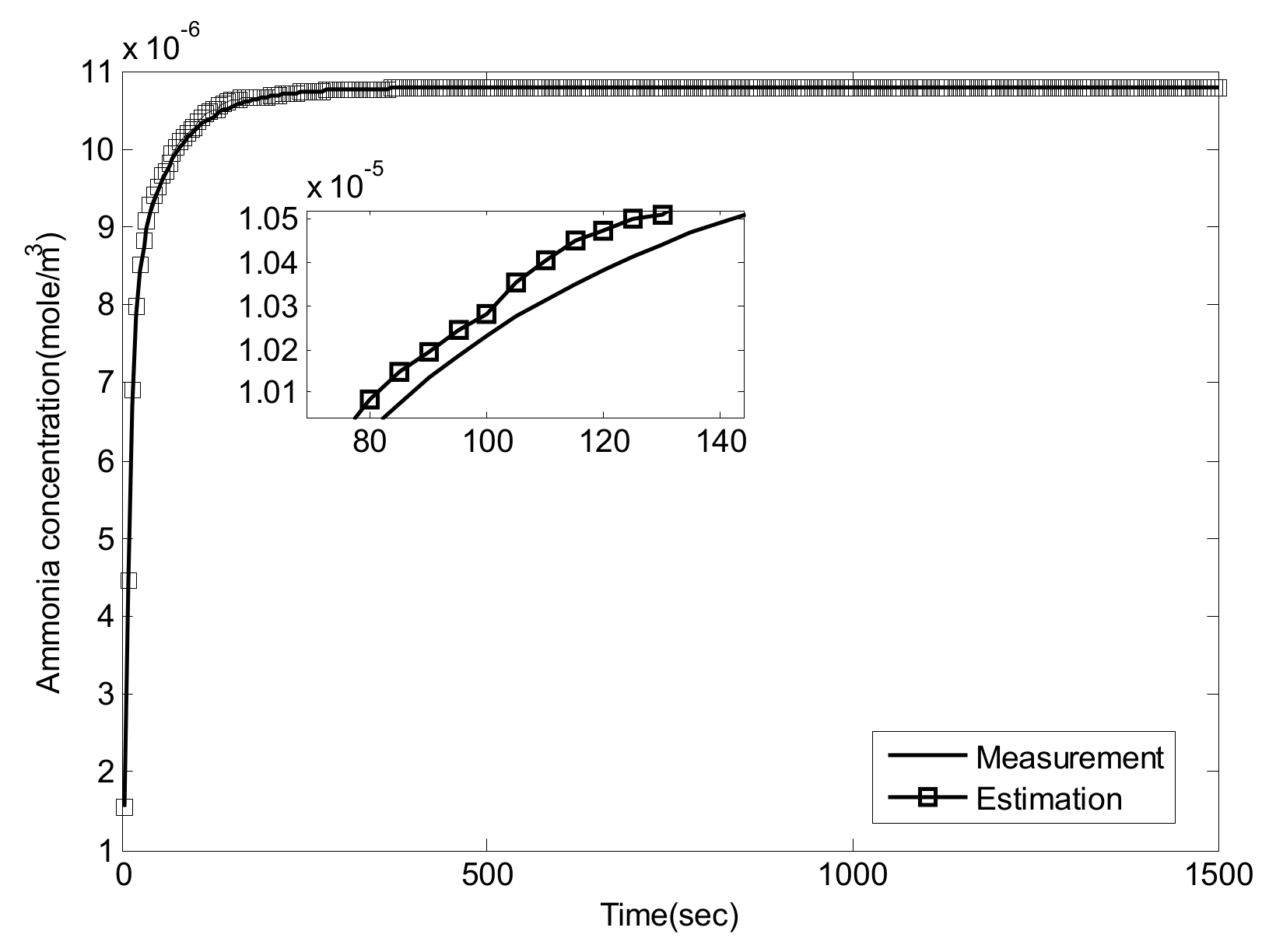
5.1. Experiment Validation of Luenberger-Sliding Mode Observer
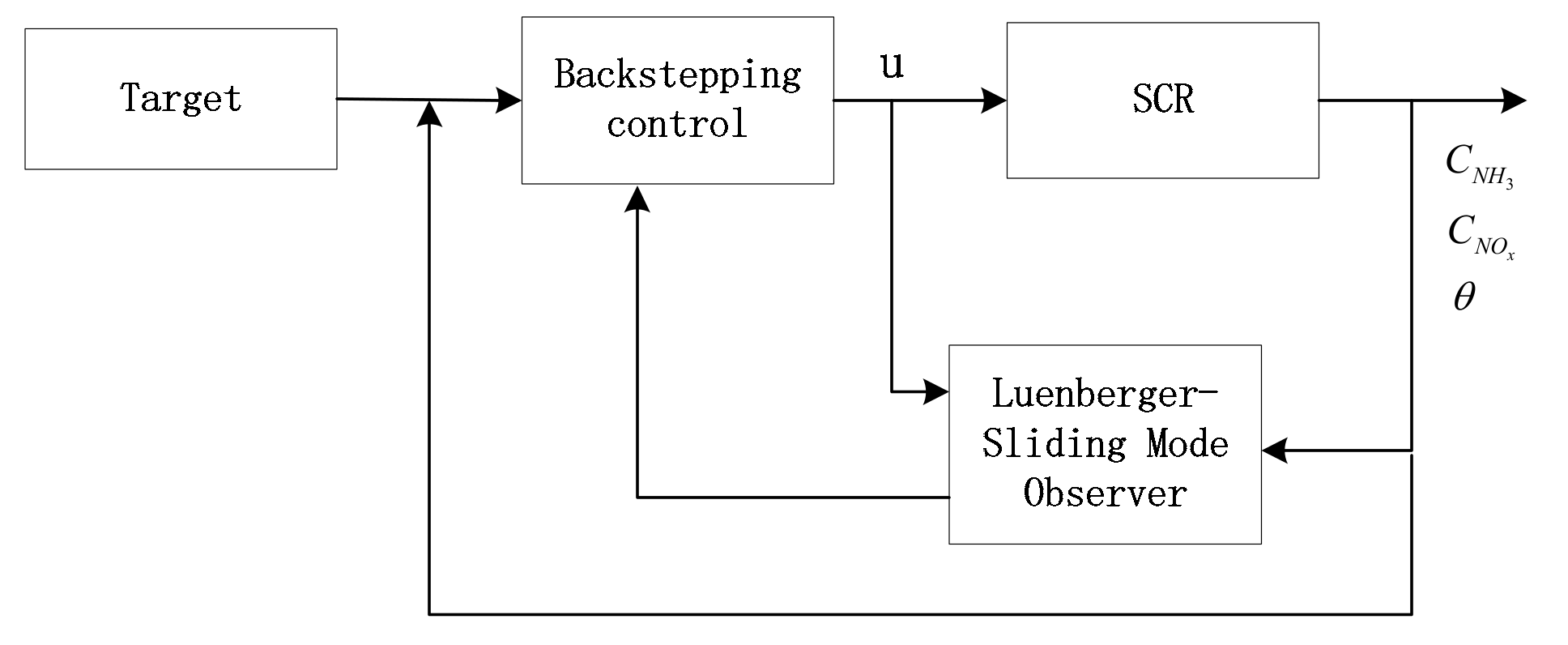
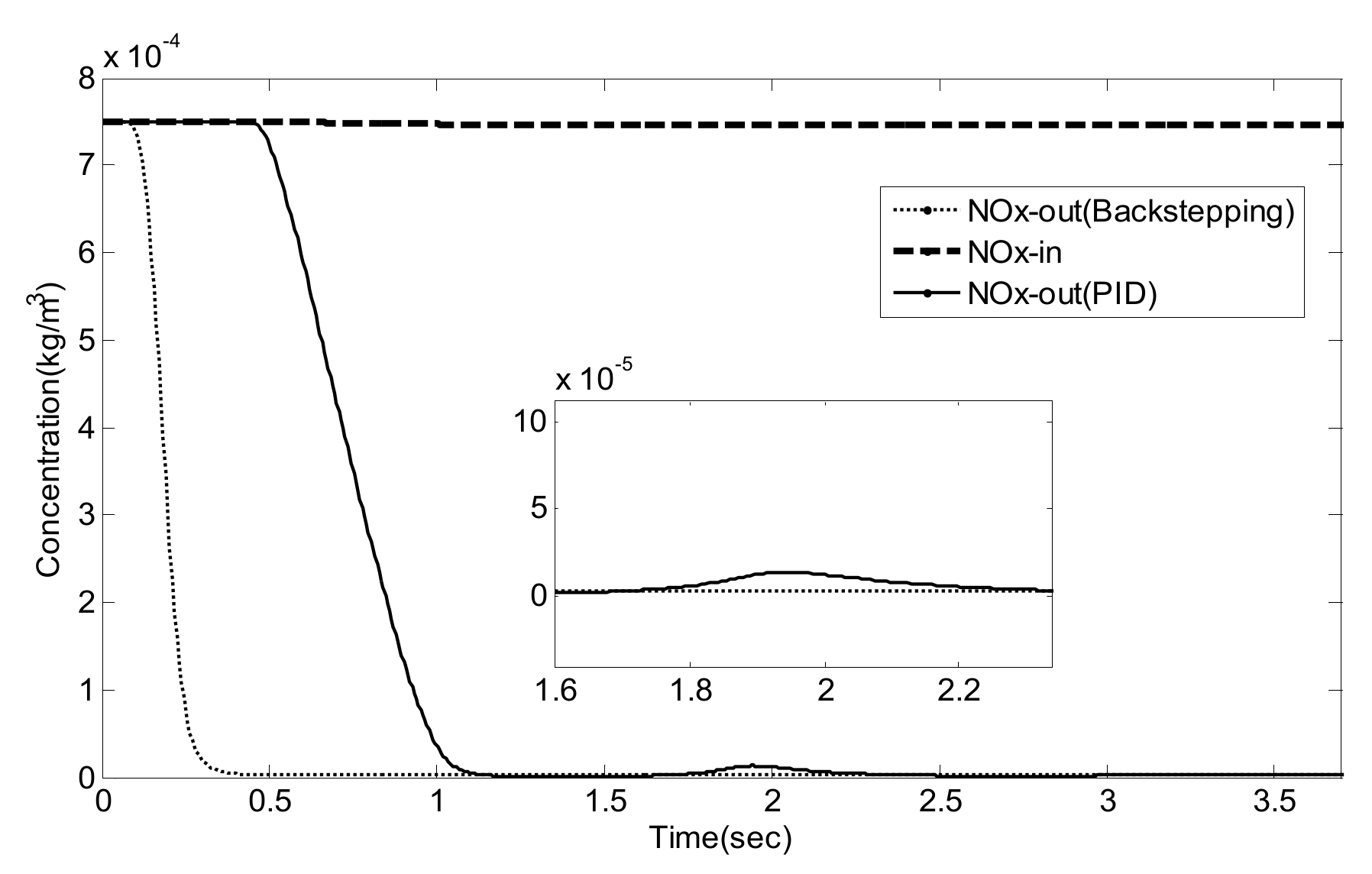
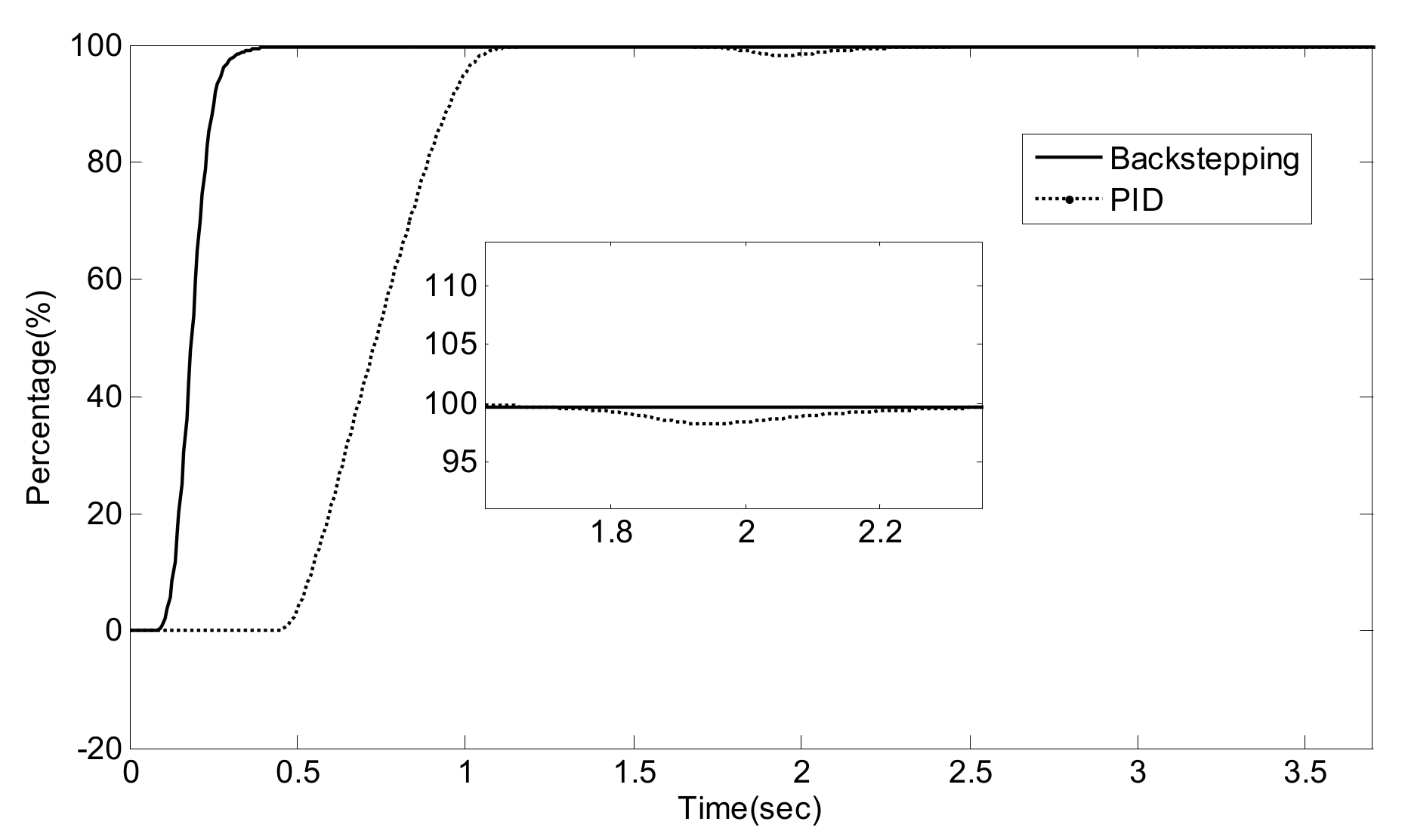
5.2. Simulation Validation of the Luenberger-Sliding Mode Observer Based Backstepping Control for SCR System
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Christoph, M.S.; Christopher, H.O.; Hans, P.G. Control of an SCR catalytic converter system for a mobile heavy-duty application. IEEE Trans. Control Syst. Technol. 2006, 14, 641–653. [Google Scholar]
- Yan, F.; Wang, J. Control of diesel engine dual-loop EGRair-path systems by a singular perturbation method. Control Eng. Pract. 2013, 21, 981–988. [Google Scholar] [CrossRef]
- Lee, S.; Park, S. Numerical analysis of internal flowcharacteristics of urea injectors for SCR dosing system. Fuel 2014, 129, 54–60. [Google Scholar] [CrossRef]
- Chen, P.; Wang, J. A novel cost-effective robust approach forselective catalytic reduction state estimations using dual nitrogenoxide sensors. J. Automob. Eng. 2015, 229, 83–96. [Google Scholar] [CrossRef]
- Devarakonda, M.; Parker, G.; Johnson, J.H.; Strots, V. Model-based control system design in a urea-SCR aftertreatmentsystem based on NH3 sensor feedback. Int. J. Automot. Technol. 2009, 10, 653–662. [Google Scholar] [CrossRef]
- Yan, F.; Wang, J. Design and robustness analysis of discreteobservers for diesel engine in-cylinder oxygen mass fractioncycle-by-cycle estimation. Control Syst. Technol. 2012, 20, 72–83. [Google Scholar]
- Ham, Y.; Park, S. Development of Map based Open Loop Control Algorithm for Urea-SCR System. Trans. Korean Soc. Automot. Eng. 2011, 19, 50–56. [Google Scholar]
- Zhang, S.M.; Tian, F.; Ren, G.F.; Yang, L.S. CR control strategy based on ANNs and Fuzzy PID in a heavy-duty diesel engine. Int. J. Automot. Technol. 2012, 13, 693–699. [Google Scholar] [CrossRef]
- Kim, Y.; Park, T.; Jung, C. Hybrid Nonlinear Model Predictive Control of LNT and Urealess SCR AftertreatmentSystem. IEEE Trans. Control Syst. Technol. 2019, 27, 2305–2313. [Google Scholar] [CrossRef]
- Zhao, J.H.; Hu, Y.F.; Gong, X.; Chen, H. Modelling and control of urea-SCR systems through the triple-step non-linear method in consideration of time-varying parameters and reference dynamics. Trans. Inst. Meas. Control 2018, 40, 287–302. [Google Scholar] [CrossRef]
- Yang, B.; Keqiang, L.; Ukawa, H.; Handa, M. Modelling and control of anon-linear dynamic system for heavy-duty trucks. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2006, 220, 1423–1435. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chan, W.S.; Chang, C.W. T-S fuzzy model-basedadaptive dynamic surface control for ball and beam system. IEEE Trans. Ind. Electron. 2013, 60, 2251–2263. [Google Scholar] [CrossRef]
- Chi, J.N.; DaCosta, H.F.M. Modeling and control of a urea-SCR aftertreatmentsystem. SAE Trans. 2005, 114, 449–465. [Google Scholar]
- Devarakonda, M.; Parker, G.; Johnson, J.H. Model-basedestimation and control system development in a urea-SCR aftertreatmentsystem. SAE Int. J. Fuels Lubr. 2009, 1, 646–661. [Google Scholar] [CrossRef]
- Liu, Q.F.; Chen, H.; Hu, Y.F.; Sun, P.Y.; Li, J. Modeling and control of the fuel injection system for rail pressure regulation in GDI engine. IEEE/ASME Trans. Mechatron. 2014, 19, 1501–1513. [Google Scholar]
- Westerlund, C.; Westerberg, B.; Ingemar, O.; Egnell, R. Model predictive control of a combined EGR/SCR HD diesel engine[C]. SAE2010 World Congr. Exhib. 2010, 13–15. [Google Scholar] [CrossRef]
- Ebrahimian, V.; Habchi, C.; Nicolle, A. Detailed modeling of the evaporation and thermal decomposition of urea-water solution in SCR systems. AIChE J. 2012, 58, 1998–2009. [Google Scholar] [CrossRef]
- Chen, P.; Wang, J. Observer-based estimation of air-fractionsfor a diesel engine coupled with aftertreatment systems. Control Syst. Technol. 2013, 21, 2239–2250. [Google Scholar] [CrossRef]
- Bonfils, A.; Creff, Y.; Lepreux, O.; Petit, N. Closed-loop controlof a SCR system using a NOx sensor cross-sensitive to NH3. J. Process Control 2014, 24, 368–378. [Google Scholar] [CrossRef]
- Davila, J.; Fridman, L.; Levant, A. Second-Order Sliding-Mode-observer for Mechanical Systems. IEEE Trans. Autom. Control 2005, 50, 1785–1789. [Google Scholar] [CrossRef]
- Foo, G.; Rahman, M.F. Sensorless sliding-mode MTPA control of an IPM synchronous motor drive using a sliding-mode observer and HF signal injection. IEEE Trans. Ind. Electron. 2010, 57, 1270–1278. [Google Scholar] [CrossRef]
- Kubinski, D.J.; Visser, J.H. Sensor and method for determining the ammonia loading of a zeolite SCR catalyst. Sens. Actuators B Chem. 2008, 130, 425–429. [Google Scholar] [CrossRef]
- Hsieh, M.; Wang, J. Sliding-mode observer for urea-selectivecatalytic reduction (SCR) mid-catalyst ammonia concentrationestimation. Int. J. Automot. Technol. 2011, 12, 321–329. [Google Scholar] [CrossRef]
- Hasan, S.N.; Husain, I. A Luenberger-sliding mode observer for online parameter estimation and adaptation in high-performance induction motor drives. IEEE Trans. Ind. Appl. 2009, 45, 772–781. [Google Scholar] [CrossRef]
- Sun, W.C.; Gao, H.J.; Kaynak, O. Adaptive backstepping control for active suspension systems with hard constraints. IEEE/ASME Trans. Mechatron. 2013, 18, 1072–1079. [Google Scholar] [CrossRef]
- Hamida, A.; Leon, J.; Glumineau, A. Experimental sensorless control for IPMSM by using integral backstepping strategy and adaptive high gain observer. Control Eng. Pract. 2017, 59, 64–76. [Google Scholar] [CrossRef]
- Hsieh, M.; Wang, J. A two-cell backstepping-based control strategy for diesel engine selective catalytic reduction systems. Control Syst. Technol. 2014, 24, 1504–1515. [Google Scholar] [CrossRef]
- Olsson, L.; Sjövall, H.; Blint, R.J. A kinetic model for ammonia selective catalytic reduction over Cu-ZSM-5. Appl. Catal. B Environ. 2008, 81, 203–217. [Google Scholar] [CrossRef]
- Zheng, T.; Han, W.; Li, Y.; Yang, B.; Shi, L. Luenberger-sliding modeobserver based ammonia concentration estimation for selectivecatalyst reduction system[C]. In Proceedings of the 2016 12th World Congress on Intelligent Control and Automation (WCICA), Guilin, China, 12–15 June 2016; pp. 3021–3026. [Google Scholar]
- Morandi, S.; Prinetto, F.; Ghiotti, G.; Castoldi, L.; Lietti, L.; Forzatti, P.; Daturi, M.; Blasin-Aubé, V. The influence of CO2 and H2O on the storage properties of Pt-Ba/Al2O3 LNT catalyst studied by FT-IR spectroscopy and transient microreactor experiments. Catal. Today 2014, 231, 116–124. [Google Scholar] [CrossRef]
- Shimizu, K.; Satsuma, A. Hydrogen assisted urea-SCR and NH3-SCR with silver–alumina as highly active and SO2-tolerant de-NOx catalysis. Appl. Catal. B Environ. 2007, 77, 202–205. [Google Scholar] [CrossRef]
- Doronkin, D.E.; Fogel, S.; Tamm, S.; Olsson, L.; Khan, T.S.; Bligaard, T.; Gabrielsson, P.; Dahl, S. Study of the “Fast SCR”-like mechanism of H2-assisted SCR of NOx with ammonia over Ag/Al2O3. Appl. Catal. B Environ. 2012, 113, 228–236. [Google Scholar] [CrossRef]

| Item | Quantity |
|---|---|
| Engine type | 4-cylinder |
| Bore (mm) | 100 |
| Stroke (mm) | 110 |
| Connecting rod length (mm) | 152 |
| Compression ratio | 18 |
| Engine displacement (liter) | 3 |
| Item | Quantity |
|---|---|
| Cell density (1/inch2) | 400 |
| Length (mm) | 250 |
| Diamater (mm) | 25 |
| Active surface site density (mole/m3) | 125 |
| Item | Quantity |
|---|---|
| Channel geometry | square |
| Front area (mm2) | 20000 |
| Cell density (1/inch2) | 400 |
| Length (mm) | 150 |
| Item | Quantity |
|---|---|
| Trap diameter (mm) | 130 |
| Filter wall thickness (inch) | 0.014 |
| Channel length (mm) | 260 |
| Inlet cell density (1/inch2) | 95 |
| NO2/NO = 0/1 | NO2/NO = 1/2 | NO2/NO = 1/1 | |
|---|---|---|---|
| 300 °C | 1.9 × 10−6 | 1.6 × 10−6 | 1.1 × 10−7 |
| 350 °C | 3.1 × 10−6 | 1.7 × 10−6 | 0.6 × 10−7 |
| 400 °C | 4.2 × 10−6 | 2.1 × 10−6 | 1.6 × 10−7 |
| NO/NO2 | Settling Time (s) | Overshoot (%) | Integrated Absolute Error (IAE) | |
|---|---|---|---|---|
| PID | 1/0 | 3.2 | 27.8 | 0.1482 |
| 2/1 | 3.3 | 30.0 | 0.1784 | |
| 1/1 | 3.1 | 25.2 | 0.1649 | |
| Luenberger-Sliding Mode Observer Based Backstepping | 1/0 | 0.49 | 9.3 | 0.0373 |
| 2/1 | 0.49 | 8.9 | 0.0295 | |
| 1/1 | 0.48 | 8.3 | 0.0310 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, T.; Yang, B.; Li, Y.; Ma, Y. Luenberger-Sliding Mode Observer Based Backstepping Control for the SCR System in a Diesel Engine. Energies 2019, 12, 4270. https://doi.org/10.3390/en12224270
Zheng T, Yang B, Li Y, Ma Y. Luenberger-Sliding Mode Observer Based Backstepping Control for the SCR System in a Diesel Engine. Energies. 2019; 12(22):4270. https://doi.org/10.3390/en12224270
Chicago/Turabian StyleZheng, Taixiong, Bin Yang, Yongfu Li, and Ying Ma. 2019. "Luenberger-Sliding Mode Observer Based Backstepping Control for the SCR System in a Diesel Engine" Energies 12, no. 22: 4270. https://doi.org/10.3390/en12224270
APA StyleZheng, T., Yang, B., Li, Y., & Ma, Y. (2019). Luenberger-Sliding Mode Observer Based Backstepping Control for the SCR System in a Diesel Engine. Energies, 12(22), 4270. https://doi.org/10.3390/en12224270