Thermal Energy Storage in Solar Power Plants: A Review of the Materials, Associated Limitations, and Proposed Solutions
Abstract
1. Introduction
2. Materials for Thermal Fluids
3. Materials for Thermal Energy Storage
- (1)
- Phase transition temperature (solid–liquid in the case of PCM and chemical compositions in thermo-chemicals) should be in the operational temperature range of the CSP plant.
- (2)
- Volumetric density and energy storage density (latent heat, heat of reactions) should be maximum for a compact design.
- (3)
- Materials should have high and uniform specific heat capacity at different temperatures for accurate calculations in the design process.
- (4)
- Thermal conductivity of the material should be high for quick charging–discharging cycles.
- (5)
- It should have minimal or no super cooling of PCM and congruent melting.
- (6)
- It should be inexpensive and widely available.
- (7)
- The materials should have high thermal, chemical, and cyclic stability for an extended plant life.
- (8)
- In the case of PCM, there should be no volume changes during phase transition to curtail the issues of phase segregation.
- (9)
- The material should be non-flammable, non-toxic, and non-corrosive.
- (10)
- It should have a low vapor pressure.
3.1. Materials for Sensible Heat Storage
3.2. Materials for Latent Heat Storage
3.3. Materials for Thermochemical Energy Storage
4. Limitations of Thermal Energy Storage Systems and Their Proposed Solutions
4.1. High-Temperature Corrosion
- Tuning the composition of container by increasing the non-reactive content;
- Removal of impurities in molten salt systems or the addition of inhibitors;
- Surface treatment.
4.2. Life Cycle Assessment of TES
4.3. Economic Analysis of TES
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Acronyms | |
| CSP | Concentrated solar power |
| DOE | United States Department of Energy |
| HTF | Heat transfer fluid |
| ITC | Investment tax credit |
| LCA | Life cycle assessment |
| LCOE | Levelized cost of electricity |
| LHS | Latent heat storage |
| NGCC | Natural gas combined cycle |
| PCM | Phase change material |
| PV | Photovoltaics |
| SHS | Sensible heat storage |
| TES | Thermal energy storage |
| THS | Thermochemical heat storage |
| Measurement units | |
| Heat capacity | kJ/kg.K |
| Heat flux | kW/m2 |
| Heat transfer coefficient | W/m2.K |
| Latent heat of fusion | kJ/kg |
| Temperature | °C |
| Thermal conductivity | W/m.K |
| Volumetric energy density | MJ/m3 |
| Viscosity | cP |
References
- Chojnowski, T.; LaPlante, D.; Truong, J. Reverse Power Mitigation System for Photovoltaic Energy Resources; Worcester Polytechnic Institute: Worcester, MA, USA, 2015. [Google Scholar]
- Geng, K.; Ai, X.; Liu, B. A Two-Stage Scheduling Optimization Model and Corresponding Solving Algorithm for Power Grid Containing Wind Farm and Energy Storage System Considering Demand Response. DEStech Trans. Eng. Technol. Res. 2017. [Google Scholar] [CrossRef]
- Cisek, P.; Taler, D. Numerical and experimental study of a solid matrix Electric Thermal Storage unit dedicated to the environmentally friendly residential heating system. Energy Build. 2016, 130, 747–760. [Google Scholar] [CrossRef]
- Cisek, P.; Taler, D. Numerical analysis and performance assessment of the Thermal Energy Storage unit aimed to be utilized in Smart Electric Thermal Storage (SETS). Energy 2019, 173, 755–771. [Google Scholar] [CrossRef]
- Pelay, U.; Luo, L.; Fan, Y.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Barlev, D.; Vidu, R.; Stroeve, P. Innovation in concentrated solar power. Sol. Energy Mater. Sol. Cells 2011, 95, 2703–2725. [Google Scholar] [CrossRef]
- Arteconi, A.; Ciarrocchi, E.; Pan, Q.; Carducci, F.; Comodi, G.; Polonara, F.; Wang, R. Thermal energy storage coupled with PV panels for demand side management of industrial building cooling loads. Appl. Energy 2017, 185, 1984–1993. [Google Scholar] [CrossRef]
- Baeten, B.; Rogiers, F.; Helsen, L. Reduction of heat pump induced peak electricity use and required generation capacity through thermal energy storage and demand response. Appl. Energy 2017, 195, 184–195. [Google Scholar] [CrossRef]
- Patteeuw, D.; Bruninx, K.; Arteconi, A.; Delarue, E.; D’haeseleer, W.; Helsen, L. Integrated modeling of active demand response with electric heating systems coupled to thermal energy storage systems. Appl. Energy 2015, 151, 306–319. [Google Scholar] [CrossRef]
- Kim, Y.; Norford, L.K. Optimal use of thermal energy storage resources in commercial buildings through price-based demand response considering distribution network operation. Appl. Energy 2017, 193, 308–324. [Google Scholar] [CrossRef]
- Hassan, A.; Shakeel Laghari, M.; Rashid, Y. Micro-Encapsulated Phase Change Materials: A Review of Encapsulation, Safety and Thermal Characteristics. Sustainability 2016, 8, 1046. [Google Scholar] [CrossRef]
- Alva, G.; Liu, L.; Huang, X.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar] [CrossRef]
- Abedin, A.H. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef]
- Solé, A.; Martorell, I.; Cabeza, L.F. State of the art on gas–solid thermochemical energy storage systems and reactors for building applications. Renew. Sustain. Energy Rev. 2015, 47, 386–398. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-economic assessment of solid–gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- Powell, K.M.; Rashid, K.; Ellingwood, K.; Tuttle, J.; Iverson, B.D. Hybrid concentrated solar thermal power systems: A review. Renew. Sustain. Energy Rev. 2017, 80, 215–237. [Google Scholar] [CrossRef]
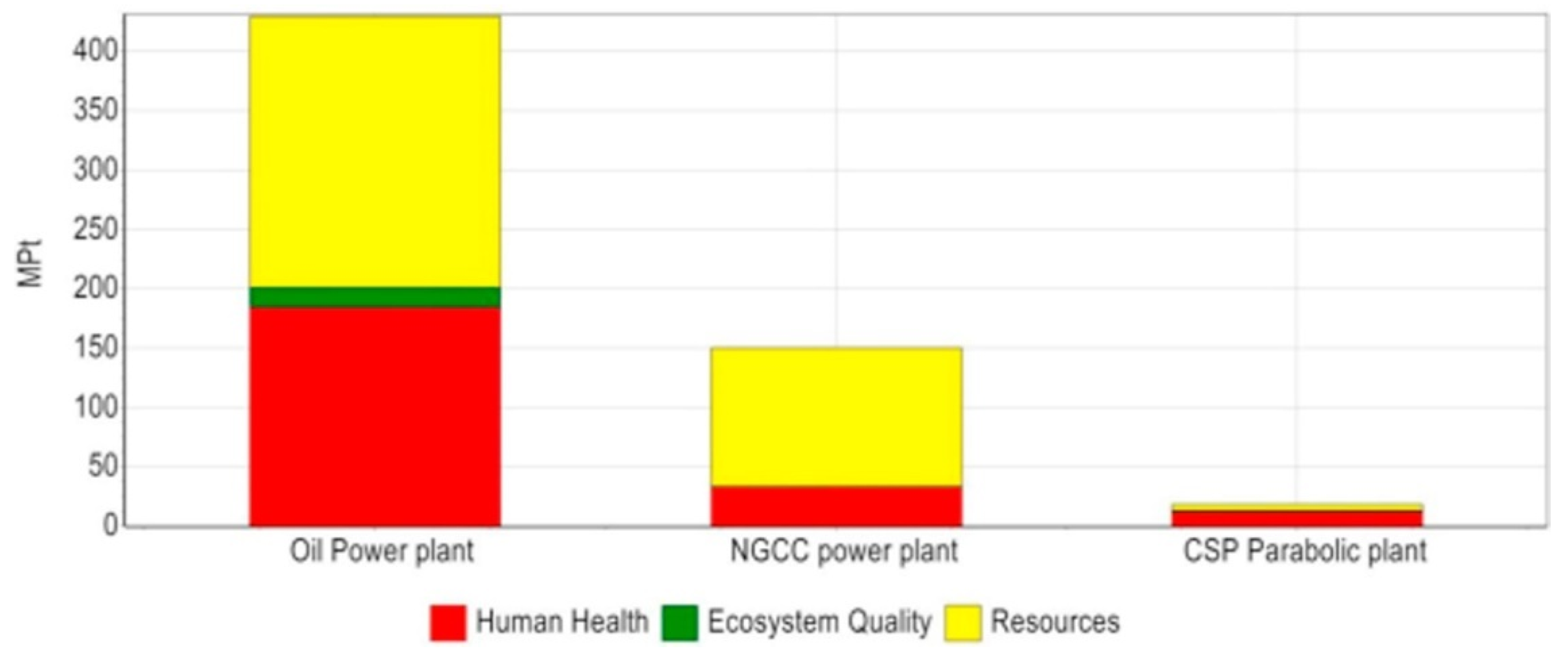
- Fuqiang, W.; Ziming, C.; Jianyu, T.; Yuan, Y.; Yong, S.; Linhua, L. Progress in concentrated solar power technology with parabolic trough collector system: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 79, 1314–1328. [Google Scholar] [CrossRef]
- Corona, B.; Bozhilova-Kisheva, K.P.; Olsen, S.I.; San Miguel, G. Social Life Cycle Assessment of a Concentrated Solar Power Plant in Spain: A Methodological Proposal: Social-LCA of a CSP Plant in Spain: Method Proposal. J. Ind. Ecol. 2017, 21, 1566–1577. [Google Scholar] [CrossRef]
- Dunlop, T.O.; Jarvis, D.J.; Voice, W.E.; Sullivan, J.H. Stabilization of molten salt materials using metal chlorides for solar thermal storage. Sci. Rep. 2018, 8, 8190. [Google Scholar] [CrossRef]
- Flamant, G.; Gauthier, D.; Benoit, H.; Sans, J.-L.; Garcia, R.; Boissière, B.; Ansart, R.; Hemati, M. Dense suspension of solid particles as a new heat transfer fluid for concentrated solar thermal plants: On-sun proof of concept. Chem. Eng. Sci. 2013, 102, 567–576. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Ren, N.; Ma, C. Experimental study of viscosity characteristics of high-temperature heat transfer molten salts. Sci. China Technol. Sci. 2011, 54, 3022–3026. [Google Scholar] [CrossRef]
- Peiró, G.; Gasia, J.; Miró, L.; Prieto, C.; Cabeza, L.F. Influence of the heat transfer fluid in a CSP plant molten salts charging process. Renew. Energy 2017, 113, 148–158. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, X.; Wei, X.; Yang, J.; Ding, J.; Lu, J. New molten salt heat transfer fluid for solar thermal power plant. In Proceedings of the 2013 International Conference on Materials for Renewable Energy and Environment, Chengdu, China, 19–21 August 2013; pp. 496–499. [Google Scholar]
- Peng, Q.; Ding, J.; Wei, X.; Yang, J.; Yang, X. The preparation and properties of multi-component molten salts. Appl. Energy 2010, 87, 2812–2817. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Khan, J.A. Thermal performance of Al2O3 Nanoparticle Enhanced Ionic Liquids (NEILs) for Concentrated Solar Power (CSP) applications. Int. J. Heat Mass Transf. 2015, 85, 585–594. [Google Scholar] [CrossRef]
- Paul, T.C.; Morshed, A.K.M.M.; Khan, J.A. Effect of Nanoparticle Dispersion on Thermophysical Properties of Ionic Liquids for its Potential Application in Solar Collector. Procedia Eng. 2014, 90, 643–648. [Google Scholar] [CrossRef][Green Version]
- Paul, T.C.; Morshed, A.K.M.M.; Fox, E.B.; Khan, J.A. Enhanced thermophysical properties of NEILs as heat transfer fluids for solar thermal applications. Appl. Therm. Eng. 2017, 110, 1–9. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Zhang, L.; Wang, X.; Ma, C. Experimental study on the specific heat and stability of molten salt nanofluids prepared by high-temperature melting. Sol. Energy Mater. Sol. Cells 2018, 176, 42–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, Y.; Hwang, S.; Wilk, G.; DeAngelis, F.; Henry, A.; Sandhage, K.H. Containment materials for liquid tin at 1350 °C as a heat transfer fluid for high temperature concentrated solar power. Sol. Energy 2018, 164, 47–57. [Google Scholar] [CrossRef]
- Montes, M.J.; Abánades, A.; Martínez-Val, J.M.; Valdés, M. Solar multiple optimization for a solar-only thermal power plant, using oil as heat transfer fluid in the parabolic trough collectors. Sol. Energy 2009, 83, 2165–2176. [Google Scholar] [CrossRef]
- Technical Data Sheet Therminol® VP-1 Heat Transfer Fluid. Available online: https://www.therminol.com/sites/therminol/files/documents/Therminol-VP1-TechDatasheet.pdf (accessed on 11 July 2019).
- Vidal, J.C.; Klammer, N. Molten chloride technology pathway to meet the US DOE sunshot initiative with Gen3 CSP. In Proceedings of the AIP Conference 2019, Saint-Martin-d’Hères, France, 8–12 July 2019; Volume 2126, No. 1. p. 080006. [Google Scholar]
- Khanafer, K.; Vafai, K. A review on the applications of nanofluids in solar energy field. Renew. Energy 2018, 123, 398–406. [Google Scholar] [CrossRef]
- Bauer, T.; Pfleger, N.; Breidenbach, N.; Eck, M.; Laing, D.; Kaesche, S. Material aspects of Solar Salt for sensible heat storage. Appl. Energy 2013, 111, 1114–1119. [Google Scholar] [CrossRef]
- Awad, A.; Burns, A.; Waleed, M.; Al-Yasiri, M.; Wen, D. Latent and sensible energy storage enhancement of nano-nitrate molten salt. Sol. Energy 2018, 172, 191–197. [Google Scholar] [CrossRef]
- Xiao, G.; Guo, K.; Luo, Z.; Ni, M.; Zhang, Y.; Wang, C. Simulation and experimental study on a spiral solid particle solar receiver. Appl. Energy 2014, 113, 178–188. [Google Scholar] [CrossRef]
- Fasquelle, T.; Falcoz, Q.; Neveu, P.; Hoffmann, J.-F. A temperature threshold evaluation for thermocline energy storage in concentrated solar power plants. Appl. Energy 2018, 212, 1153–1164. [Google Scholar] [CrossRef]
- Li, B.; Ju, F. Thermal stability of granite for high temperature thermal energy storage in concentrating solar power plants. Appl. Therm. Eng. 2018, 138, 409–416. [Google Scholar] [CrossRef]
- Buscemi, A.; Panno, D.; Ciulla, G.; Beccali, M.; Lo Brano, V. Concrete thermal energy storage for linear Fresnel collectors: Exploiting the South Mediterranean’s solar potential for agri-food processes. Energy Convers. Manag. 2018, 166, 719–734. [Google Scholar] [CrossRef]
- Martins, M.; Villalobos, U.; Delclos, T.; Armstrong, P.; Bergan, P.G.; Calvet, N. New Concentrating Solar Power Facility for Testing High Temperature Concrete Thermal Energy Storage. Energy Procedia 2015, 75, 2144–2149. [Google Scholar] [CrossRef]
- Diago, M.; Iniesta, A.C.; Soum-Glaude, A.; Calvet, N. Characterization of desert sand to be used as a high-temperature thermal energy storage medium in particle solar receiver technology. Appl. Energy 2018, 216, 402–413. [Google Scholar] [CrossRef]
- Ortega-Fernández, I.; Calvet, N.; Gil, A.; Rodríguez-Aseguinolaza, J.; Faik, A.; D’Aguanno, B. Thermophysical characterization of a by-product from the steel industry to be used as a sustainable and low-cost thermal energy storage material. Energy 2015, 89, 601–609. [Google Scholar] [CrossRef]
- Mohan, G.; Venkataraman, M.B.; Coventry, J. Sensible energy storage options for concentrating solar power plants operating above 600 °C. Renew. Sustain. Energy Rev. 2019, 107, 319–337. [Google Scholar] [CrossRef]
- Rea, J.E.; Oshman, C.J.; Olsen, M.L.; Hardin, C.L.; Glatzmaier, G.C.; Siegel, N.P.; Parilla, P.A.; Ginley, D.S.; Toberer, E.S. Performance modeling and techno-economic analysis of a modular concentrated solar power tower with latent heat storage. Appl. Energy 2018, 217, 143–152. [Google Scholar] [CrossRef]
- Risueño, E.; Doppiu, S.; Rodríguez-Aseguinolaza, J.; Blanco, P.; Gil, A.; Tello, M.; Faik, A.; D’Aguanno, B. Experimental investigation of Mg-Zn-Al metal alloys for latent heat storage application. J. Alloys Compd. 2016, 685, 724–732. [Google Scholar] [CrossRef]
- Risueño, E.; Faik, A.; Gil, A.; Rodríguez-Aseguinolaza, J.; Tello, M.; D’Aguanno, B. Zinc-rich eutectic alloys for high energy density latent heat storage applications. J. Alloys Compd. 2017, 705, 714–721. [Google Scholar] [CrossRef]
- Risueño, E.; Faik, A.; Gil, A.; Rodríguez-Aseguinolaza, J.; Tello, M.; D’Aguanno, B. Thermal cycling testing of Zn–Mg–Al eutectic metal alloys as potential high-temperature phase change materials for latent heat storage. J. Therm. Anal. Calorim. 2017, 129, 885–894. [Google Scholar] [CrossRef]
- Niedermeier, K.; Marocco, L.; Flesch, J.; Mohan, G.; Coventry, J.; Wetzel, T. Performance of molten sodium vs. molten salts in a packed bed thermal energy storage. Appl. Therm. Eng. 2018, 141, 368–377. [Google Scholar] [CrossRef]
- Coventry, J.; Pye, J.; Kumar, A.; Iyer, S.; Kee, Z.; Lipiński, W. A sodium boiler and phase-change energy storage system. In Proceedings of the AIP Conference 2019, Saint-Martin-d’Hères, France, 8–12 July 2019; Volume 2126, No. 1. p. 060002. [Google Scholar]
- Rea, J.E.; Oshman, C.J.; Singh, A.; Alleman, J.; Parilla, P.A.; Hardin, C.L.; Olsen, M.L.; Siegel, N.P.; Ginley, D.S.; Toberer, E.S. Experimental demonstration of a dispatchable latent heat storage system with aluminum-silicon as a phase change material. Appl. Energy 2018, 230, 1218–1229. [Google Scholar] [CrossRef]
- Singh, D.; Yu, W.; Zhao, W.; Kim, T.; France, D.M.; Smith, R.K. Development and prototype testing of MgCl2/graphite foam latent heat thermal energy storage system. Sol. Energy 2018, 159, 270–282. [Google Scholar] [CrossRef]
- Dheep, G.R.; Sreekumar, A. Investigation on thermal reliability and corrosion characteristics of glutaric acid as an organic phase change material for solar thermal energy storage applications. Appl. Therm. Eng. 2018, 129, 1189–1196. [Google Scholar] [CrossRef]
- Mojiri, A.; Grbac, N.; Bourke, B.; Rosengarten, G. D-mannitol for medium temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 150–156. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Alelyani, S.; Cao, X.; Phelan, P.E. Thermophysical properties enhancement of ternary carbonates with carbon materials for high-temperature thermal energy storage. Sol. Energy 2017, 155, 661–669. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Y.; Ding, B.; Yu, G.; Ye, F.; Xu, C. Structure and hydration state characterizations of MgSO4-zeolite 13x composite materials for long-term thermochemical heat storage. Sol. Energy Mater. Sol. Cells 2019, 200, 110047. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Calcium-Looping performance of mechanically modified Al2O3-CaO composites for energy storage and CO2 capture. Chem. Eng. J. 2018, 334, 2343–2355. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Low-cost Ca-based composites synthesized by biotemplate method for thermochemical energy storage of concentrated solar power. Appl. Energy 2018, 210, 108–116. [Google Scholar] [CrossRef]
- Fernández, Á.G.; Cabeza, L.F. Molten salt corrosion mechanisms of nitrate based thermal energy storage materials for concentrated solar power plants: A review. Sol. Energy Mater. Sol. Cells 2019, 194, 160–165. [Google Scholar] [CrossRef]
- Liu, M.; Steven Tay, N.H.; Bell, S.; Belusko, M.; Jacob, R.; Will, G.; Saman, W.; Bruno, F. Review on concentrating solar power plants and new developments in high temperature thermal energy storage technologies. Renew. Sustain. Energy Rev. 2016, 53, 1411–1432. [Google Scholar] [CrossRef]
- Pierce, S.; Lukiman, C.; Shah, T.; Ravi, V.A. Selection of salts and containment materials for solar thermal energy storage. In Proceedings of the NACE International Corrosion 2018, Phoenix, AZ, USA, 15–19 April 2018. [Google Scholar]
- Rashmi, W.; Khalid, M.; Ong, S.S.; Saidur, R. Preparation, thermo-physical properties and heat transfer enhancement of nanofluids. Mater. Res. Express 2014, 1, 032001. [Google Scholar] [CrossRef]
- Rashmi, W.; Ismail, A.F.; Sopyan, I.; Jameel, A.T.; Yusof, F.; Khalid, M.; Mubarak, N.M. Stability and thermal conductivity enhancement of carbon nanotube nanofluid using gum arabic. J. Exp. Nanosci. 2011, 6, 567–579. [Google Scholar] [CrossRef]
- Ren, N.; Wu, Y.; Ma, C.; Sang, L. Preparation and thermal properties of quaternary mixed nitrate with low melting point. Sol. Energy Mater. Sol. Cells 2014, 127, 6–13. [Google Scholar] [CrossRef]
- Walczak, M.; Pineda, F.; Fernández, Á.G.; Mata-Torres, C.; Escobar, R.A. Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renew. Sustain. Energy Rev. 2018, 86, 22–44. [Google Scholar] [CrossRef]
- Grosu, Y.; Udayashankar, N.; Bondarchuk, O.; González-Fernández, L.; Faik, A. Unexpected effect of nanoparticles doping on the corrosivity of molten nitrate salt for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 178, 91–97. [Google Scholar] [CrossRef]
- Grosu, Y.; Nithiyanantham, U.; Zaki, A.; Faik, A. A simple method for the inhibition of the corrosion of carbon steel by molten nitrate salt for thermal storage in concentrating solar power applications. NPJ Mater. Degrad. 2018, 2, 34. [Google Scholar] [CrossRef]
- Binder, S.; Haussener, S. Design guidelines for Al-12% Si latent heat storage encapsulations to optimize performance and mitigate degradation. Appl. Surf. Sci. 2019. [Google Scholar] [CrossRef]
- Fernández, Á.G.; Cabeza, L.F. Corrosion monitoring and mitigation techniques on advanced thermal energy storage materials for CSP plants. Sol. Energy Mater. Sol. Cells 2019, 192, 179–187. [Google Scholar] [CrossRef]
- Fernández, A.G.; Pineda, F.; Walczak, M.; Cabeza, L.F. Corrosion evaluation of alumina-forming alloys in carbonate molten salt for CSP plants. Renew. Energy 2019, 140, 227–233. [Google Scholar] [CrossRef]
- Ding, W.; Shi, H.; Xiu, Y.; Bonk, A.; Weisenburger, A.; Jianu, A.; Bauer, T. Hot corrosion behavior of commercial alloys in thermal energy storage material of molten MgCl2/KCl/NaCl under inert atmosphere. Sol. Energy Mater. Sol. Cells 2018, 184, 22–30. [Google Scholar] [CrossRef]
- Fernández, Á.G.; Fullana, M.; Calabrese, L.; Proverbio, E.; Cabeza, L.F. Corrosion characterization in components for thermal energy storage applications. In Recent Advancements in Materials and Systems for Thermal Energy Storage; Springer: Cham, Switzerland, 2019; pp. 139–169. [Google Scholar]
- Zhang, H.; Balram, A.; Tiznobaik, H.; Shin, D.; Santhanagopalan, S. Microencapsulation of molten salt in stable silica shell via a water-limited sol-gel process for high temperature thermal energy storage. Appl. Therm. Eng. 2018, 136, 268–274. [Google Scholar] [CrossRef]
- Zhang, H.; Shin, D.; Santhanagopalan, S. Microencapsulated binary carbonate salt mixture in silica shell with enhanced effective heat capacity for high temperature latent heat storage. Renew. Energy 2019, 134, 1156–1162. [Google Scholar] [CrossRef]
- Oró, E.; Gil, A.; de Gracia, A.; Boer, D.; Cabeza, L.F. Comparative life cycle assessment of thermal energy storage systems for solar power plants. Renew. Energy 2012, 44, 166–173. [Google Scholar] [CrossRef]
- Corona, B.; Ruiz, D.; San Miguel, G. Life Cycle Assessment of a HYSOL Concentrated Solar Power Plant: Analyzing the Effect of Geographic Location. Energies 2016, 9, 413. [Google Scholar] [CrossRef]
- Piemonte, V.; Falco, M.D.; Tarquini, P.; Giaconia, A. Life Cycle Assessment of a high temperature molten salt concentrated solar power plant. Sol. Energy 2011, 85, 1101–1108. [Google Scholar] [CrossRef]
- Ehtiwesh, I.A.S.; Coelho, M.C.; Sousa, A.C.M. Exergetic and environmental life cycle assessment analysis of concentrated solar power plants. Renew. Sustain. Energy Rev. 2016, 56, 145–155. [Google Scholar] [CrossRef]
- Miró, L.; Oró, E.; Boer, D.; Cabeza, L.F. Embodied energy in thermal energy storage (TES) systems for high temperature applications. Appl. Energy 2015, 137, 793–799. [Google Scholar] [CrossRef]
- Lechón, Y.; de la Rúa, C.; Sáez, R. Life Cycle Environmental Impacts of Electricity Production by Solarthermal Power Plants in Spain. J. Sol. Energy Eng. 2008, 130, 021012. [Google Scholar] [CrossRef]
- Heath, G.; Turchi, C.; Decker, T.; Burkhardt, J.; Kutscher, C. Life Cycle Assessment of Thermal Energy Storage: Two-Tank Indirect and Thermocline. In Proceedings of the American Society of Mechanical Engineers (ASME) Third International Conference on Energy Sustainability, San Francisco, CA, USA, 19–23 July 2009; pp. 689–690. [Google Scholar]
- Kuenlin, A.; Augsburger, G.; Gerber, L.; Maréchal, F. Life Cycle Assessment and Environomic Optimization of Concentrating Solar Thermal Power Plants. In Proceedings of the 26th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems (ECOS2013), Guilin, China, 16–19 July 2013. [Google Scholar]
- Klein, S.J.W.; Rubin, E.S. Life cycle assessment of greenhouse gas emissions, water and land use for concentrated solar power plants with different energy backup systems. Energy Policy 2013, 63, 935–950. [Google Scholar] [CrossRef]
- Corona, B.; Miguel, G.S.; Cerrajero, E. Life cycle assessment of concentrated solar power (CSP) and the influence of hybridising with natural gas. Int. J. Life Cycle Assess. 2014, 19, 1264–1275. [Google Scholar] [CrossRef]
- Zhai, R.; Li, C.; Chen, Y.; Yang, Y.; Patchigolla, K.; Oakey, J.E. Life cycle assessment of solar aided coal-fired power system with and without heat storage. Energy Convers. Manag. 2016, 111, 453–465. [Google Scholar] [CrossRef]
- Burkhardt, J.J.; Heath, G.A.; Turchi, C.S. Life Cycle Assessment of a Parabolic Trough Concentrating Solar Power Plant and the Impacts of Key Design Alternatives. Environ. Sci. Technol. 2011, 45, 2457–2464. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Mao, T.; Sui, J.; Jin, H. Life cycle assessment (LCA) optimization of solar-assisted hybrid CCHP system. Appl. Energy 2015, 146, 38–52. [Google Scholar] [CrossRef]
- Jacob, R.; Belusko, M.; Inés Fernández, A.; Cabeza, L.F.; Saman, W.; Bruno, F. Embodied energy and cost of high temperature thermal energy storage systems for use with concentrated solar power plants. Appl. Energy 2016, 180, 586–597. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Chan, C. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: A review to recent developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Romaní, J.; Gasia, J.; Solé, A.; Takasu, H.; Kato, Y.; Cabeza, L.F. Evaluation of energy density as performance indicator for thermal energy storage at material and system levels. Appl. Energy 2019, 235, 954–962. [Google Scholar] [CrossRef]
- Pacheco, J.E.; Showalter, S.K.; Kolb, W.J. Development of a Molten-Salt Thermocline Thermal Storage System for Parabolic Trough Plants. J. Sol. Energy Eng. 2002, 124, 153. [Google Scholar] [CrossRef]
- Zurita, A.; Mata-Torres, C.; Valenzuela, C.; Cardemil, J.M.; Escobar, R.A. Techno-economic analysis of a hybrid CSP+ PV plant integrated with TES and BESS in Northern Chile. In Proceedings of the AIP Conference, Santiago, Chile, 26–29 September 2017; Volume 2033, No. 1. p. 180013. [Google Scholar]
- Strasser, M.N.; Selvam, R.P. A cost and performance comparison of packed bed and structured thermocline thermal energy storage systems. Sol. Energy 2014, 108, 390–402. [Google Scholar] [CrossRef]
- Nithyanandam, K.; Pitchumani, R.; Mathur, A. Analysis of a latent thermocline storage system with encapsulated phase change materials for concentrating solar power. Appl. Energy 2014, 113, 1446–1460. [Google Scholar] [CrossRef]
- Nithyanandam, K.; Pitchumani, R. Cost and performance analysis of concentrating solar power systems with integrated latent thermal energy storage. Energy 2014, 64, 793–810. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Chan, C.; Tumilowicz, E. General volume sizing strategy for thermal storage system using phase change material for concentrated solar thermal power plant. Appl. Energy 2015, 140, 256–268. [Google Scholar] [CrossRef]
- Pihl, E.; Kushnir, D.; Sandén, B.; Johnsson, F. Material constraints for concentrating solar thermal power. Energy 2012, 44, 944–954. [Google Scholar] [CrossRef]
- Zhao, B.; Cheng, M.; Liu, C.; Dai, Z. System-level performance optimization of molten-salt packed-bed thermal energy storage for concentrating solar power. Appl. Energy 2018, 226, 225–239. [Google Scholar] [CrossRef]
- Ciani Bassetti, M.; Consoli, D.; Manente, G.; Lazzaretto, A. Design and off-design models of a hybrid geothermal-solar power plant enhanced by a thermal storage. Renew. Energy 2018, 128, 460–472. [Google Scholar] [CrossRef]
- The SunShot Initiative. Available online: https://www.energy.gov/eere/solar/sunshot-initiative (accessed on 3 October 2019).
- Rea, J.E.; Glatzmaier, G.C.; Oshman, C.; Parilla, P.A.; Siegel, N.P.; Ginley, D.S.; Toberer, E.S. Techno-economic analysis of a small scale solar power tower at varied locations. In Proceedings of the AIP Conference, Santiago, Chile, 26–29 September 2017; Volume 2033, No. 1. p. 040034. [Google Scholar]
- Tehrani, S.S.M.; Shoraka, Y.; Nithyanandam, K.; Taylor, R.A. Shell-and-tube or packed bed thermal energy storage systems integrated with a concentrated solar power: A techno-economic comparison of sensible and latent heat systems. Appl. Energy 2019, 238, 887–910. [Google Scholar] [CrossRef]
- Le, P.T.; Le, P.L. Techno-Economic Analysis of Solar Power Plant Project in Binh Thuan, Vietnam. In Proceedings of the 2018 4th International Conference on Green Technology and Sustainable Development (GTSD), Athens, Greece, 11–15 April 2018; pp. 82–85. [Google Scholar]
- Lindquist, T.; Karlsson, J.; Wallmander, J.; Guedez, R.; Hedlund, M.L.; Jamot, J.; Gloss, D.; Lindh, J.; Hertin, A.; Nilsson, M.; et al. A novel modular and dispatchable CSP Stirling system: Design, validation, and demonstration plans. In Proceedings of the AIP Conference, Saint-Martin-d’Hères, France, 8–12 July 2019; Volume 2126, No. 1. p. 060005. [Google Scholar]
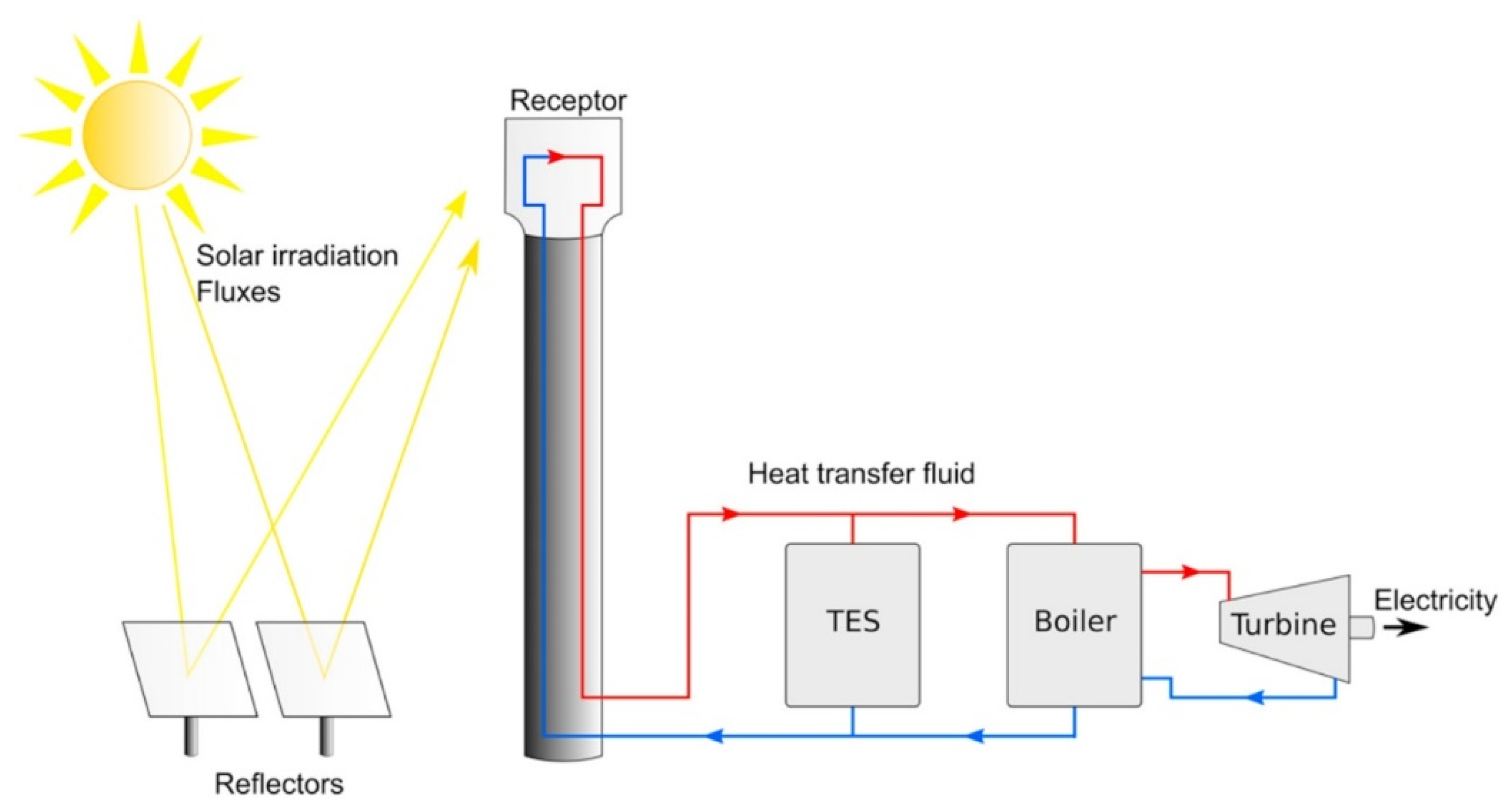
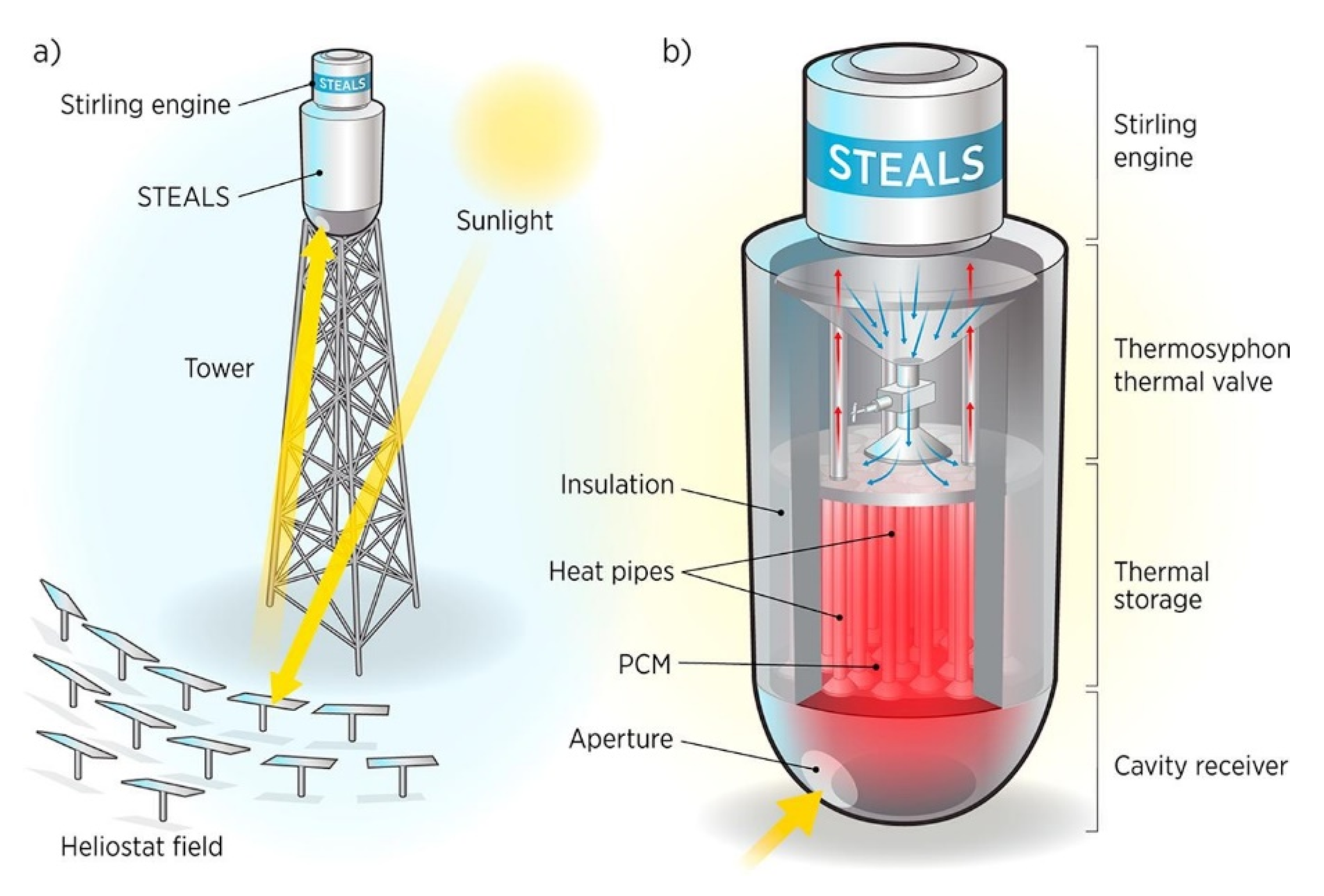

| Material | Method of Production | Melting Point (°C) | Thermal Stability | Thermal Conductivity (W/m·K) |
|---|---|---|---|---|
| KCl–KNO3–NaNO3 [24] | Static fusion method | 210 | 500 °C | 2.05–1.3 |
| Mixture [25] | Static mixing method | 550 °C | NA | |
| Nanoparticle-enhanced ionic liquids [26,27] | Dispersion | NA | Good | 0.13 |
| Nanoparticle-enhanced ionic liquids [28] | NA | NA | Good | 0.136 |
| Ca(NO3)2–KNO3–NaNO3–LiNO3 with 1% SiO2 nanoparticles [29] | High temperature melting | 85.4 | Thermally stable after long time | 0.53 |
| Ca(NO3)2–KNO3–NaNO3–LiNO3 with 1% SiO2 nanoparticles [30] | Ultrasonic dispersion | 85.4 | Poor thermal stability | 0.53 |
| Addition of LiCl to ternary salt [20] | Mixing and heating | 79 | Improved thermal stability | NA |
| Liquid tin [30] | Encapsulation | 232 | Extremely stable | 62–68 |
| Therminol VP-1 [31,32] | Organic (synthetic oil) | 12 | NA | NA |
| MgCl2–KCl–NaCl [33] | Drying/purification and mixing | 385 | 773.5 °C | NA |
| Material | Heat Capacity (kJ/kg·K) | Latent Heat (kJ/kg) | Volumetric Energy Density (MJ/m3) | Operating Temperatures (°C) | Thermal Efficiency (%) | |
|---|---|---|---|---|---|---|
| Melting | Solidification | |||||
| NaNO3:KNO3=60:40 (molar ratios) [36] | 1.24–1.5 | 107.03 | NA | 219 | NA | NA |
| NaNO3:KNO3=60:40 (molar ratios) with 1% CuO [36] | 1.68–1.93 | 122.5–178.87 | NA | 216–218.21 | NA | NA |
| Halotechnics salt stream (SS700) [16] | 0.79 | 87 | 51.85 | 700 | 300 | 95.91 |
| Halotechnics salt stream SS60/40 [16] | 1.53 | 120 | 64.61 | 565 | 235 | 97.23 |
| Aluminium–silicon eutectic [51] | 1.04–1.74 | 470 | NA | 577 | NA | NA |
| MgCl2/graphite foam [52] | 1.06 | 374–404 | 240 * | 720 | 715 | NA |
| Organic fatty acid [53] | NA | 184.8 | NA | 94.9–99.2 | 85.92 | NA |
| D-mannitol [54] | NA | 297 | NA | 167 | 110–120 | NA |
| Ternary carbonates [55] | 1.22–1.34 | 247 | NA | 405 | 387 | NA |
| Reactants | Operational Suitability | Volumetric Energy Density (MJm−3) | Operating Temperatures (°C) | Thermal Efficiency (%) | |
|---|---|---|---|---|---|
| Charging | Discharging | ||||
| Hydroxide looping with Ca(OH)2/CaO [16] | subcritical steam Rankine cycle | 101.97 | 700 | 505 | 4.78 |
| Hydroxide looping with Sr(OH)2/SrO [16] | subcritical steam Rankine cycle | 97.09 | 600 | 525 | 7.09 |
| Hydroxide looping with Ba(OH)2/BaO [16] | subcritical steam Rankine cycle | 77.61 | 700 | 520 | 8.28 |
| Carbonate looping with CaCO3/CaO [16] | supercritical CO2 cycle | 39.01 | 989 | 650 | 15.09 |
| Carbonate looping with SrCO3/SrO [16] | combined Brayton–Rankine cycle power block | 51.32 | 1200 | 1150 | 22.74 |
| Redox with BaO2/BaO [16] | supercritical CO2 cycle | 46.09 | 980 | 690 | 23.93 |
| Chemical looping combustion with Fe3O4/FeO [16] | combined Brayton–Rankine cycle power block | 175.54 | 1100 | 900 | 18.87 |
| Chemical looping combustion with NiO/Ni [16] | combined Brayton–Rankine cycle power block | 308.32 | 950 | 950 | 24.01 |
| CaO/SiO2 composites [57,58] | NA | NA | 950 | 650 | 95.7% |
| Criteria | Sensible Energy Storage System | Latent Energy Storage System | Thermo-Chemical Energy Storage System |
|---|---|---|---|
| Application | Easy to use | Medium complexity | Highly complex system |
| Volumetric density | Very low | Medium | High |
| Heat losses | Maximum | Medium | No heat loss |
| Maturity of technology | Commercially proven | Pilot plants, commercial projects under construction | Demonstration projects |
| Storage duration | Few hours | Few hours | Can store for seasons |
| Storage temperatures | Ranges of temperatures | Phase transitions temperatures | Room temperature |
| Reference | Findings |
|---|---|
| [80] | In a two-tank, indirect molten salt TES system integrated with parabolic trough plant; the impact of TES on the environment accounted for 40% of the non-operational impact of the plant. |
| [81] | Life cycle assessment (LCA) of two systems (molten salt TES and thermocline TES) revealed that the environmental impact of thermocline was almost half that of molten salt. The comparative study included only the effect of embodied greenhouse gases and considered that construction, operation, and dismantling will have an insignificant difference in both cases. |
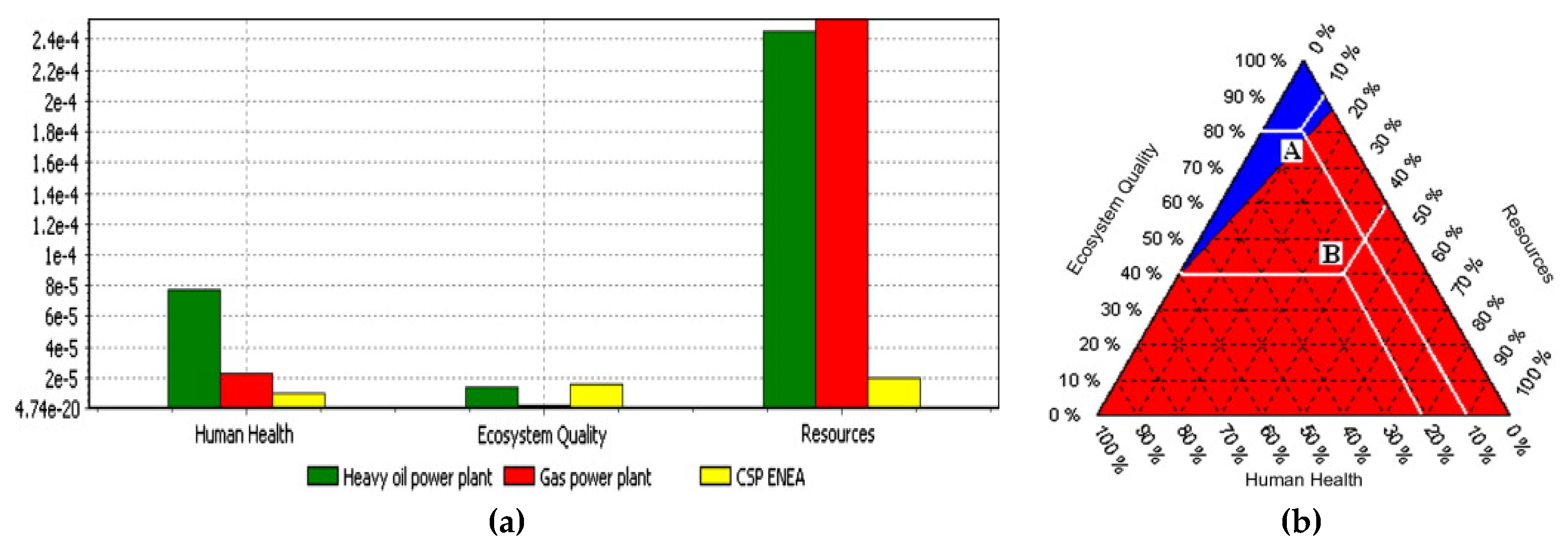
| [77] | CSP plant is preferable over oil power plant and gas power plant in terms of LCA indicators of “health” and “resource depletion”. In ecosystem quality, gas power plant fared better than CSP and gas power plant; thus, further improvements in the TES materials is needed to make CSP eco-friendly. |
| [75] | Environmental impact of solid storage media and phase change materials (PCM) storage is less than molten salt storage. Impact of molten salt is higher than PCM because it requires specific requirements to withstand high temperatures. Two-tank molten salt storage system damages the environment the most. |
| [79] | Embodied energy in the sensible storage system in concrete and molten salt, and latent storage in molten salt is investigated. In sensible storage of concrete, environmental impact is minimum in terms of the storage materials, whereas the impact of container is high, and vice-versa. |
| [82] | The study investigated LCA of all CSP plants and revealed that either of the CSP technologies are environmentally much better than conventional power plants fueled by fossil fuels. Maricopa plant (dish power plant) is the best in terms of environmental impact because of its high efficiency and lower quantity of construction materials needed. Andasol plant (parabolic trough) has the worst impact on environment because of synthetic oil as heat transfer fluid (HTF) and molten salt TES. |
| [83] | CSP plants with TES systems had twice the life cycle greenhouse gasses (GHG) than the minimal backup (MB) configuration. Plants with natural gas back-up have 4–9 times the life cycle of GHG than the MB configuration. Natural gas plants have 2–5 times higher lifetime GHG than TES counterparts. |
| [84] | Using natural gas as a back-up in the CSP plant causes quick disturbance in the ecosystem and quick depletion of resources. However, other impacts such as human toxicity and marine toxicity are reduced due to improved electricity outputs. |
| [85] | Environmental impact and cost are incurred at the stages of fueling and operation in solar-assisted coal power plants with and without thermal storages. Materials and transportation phases are least important in these types of power plants. |
| [86] | CSP technology is much better than fossil fuels in terms of environmental effects; however, dry-cooling is required in regions with water scarcity. Mined salts are preferable over synthetic salts, in which case, synthetic salts can increase LCA by up to 52% as compared to mined salts. |
| [87] | The average solar energy consumption through CSP is 38.35%. It is reported that global warming potential is the largest factor affecting the environment, followed by the respiratory effect potential. |
| [88] | Nitrates have a significantly adverse effect on the environment. In comparison, chlorides, hydroxides, and carbonates perform better. Embodied energy of nitrates is more than 100 times than that of halite (NaCl). Carbon footprint associated with NaOH is approximately 14 times less than KOH. Embodied energy of PCM-based TES is three times less than that of molten salt-based TES. |
| Type of TES | Findings | Reference |
|---|---|---|
| Sensible thermocline with HTF of solar salt quartzite rocks | A cost of $13,900,000 is calculated for a 688 MWh system, corresponding to a capacity cost of $20/kWh. | [91] |
| A molten salt thermocline | From $246/kWh to $34/kWh for the storage capacity ranging from 100 MWh to 3500 MWh. | [89] |
| 2165 megawatt-hour (MWh) packed-bed and structured-concrete thermocline | A packed-bed thermocline TES system is 12.5% less costly than a structured-concrete thermocline TES system ($30/kWh vs. $34/kWh). | [93] |
| Latent thermal storage system with embedded heat pipes | Minimum cost calculated is 5.37¢/kWh, less than the SunShot 2020 target of 6¢/kWh. | [94,95] |
| Four different sensible and latent heat storage systems were investigated | Latent heat storage (LHS) using PCM with 6 h charge has the lowest cost per capacity of $101/kWh, which is about 43% less than that of sensible heat storage (SHS) using granite rock with 6 h charge. The cost is significantly high because of considering Therminol VP-1 as HTF, which can be reduced to $20.5/kWh by using solar salt as HTF. | [96] |
| Overall CSP technology | The demand of sodium or potassium-based salts for CSP technology to replace the conventional power plants is too high and cannot be met until mid-century. Authors advised investigating the use of PCMs and concrete-based storages. | [97] |
| Sensible, latent, and hybrid | Cut-off temperature has an effect on unit cost. Minimum unit cost of $21.67/kWht−1 and $29.34/kWht−1 for sensible and latent heat storage units are reported by cost optimization based on molten-salt packed-bed thermal energy storage. | [98] |
| Sensible storage | Sensible energy storage with the geothermal concentrated power plant was modeled. The CSP was based on the parabolic trough collector, whereas the production operated on the organic Rankine cycle. The energy storage system increased solar energy utilization by 19% annually. | [99] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnaimat, F.; Rashid, Y. Thermal Energy Storage in Solar Power Plants: A Review of the Materials, Associated Limitations, and Proposed Solutions. Energies 2019, 12, 4164. https://doi.org/10.3390/en12214164
Alnaimat F, Rashid Y. Thermal Energy Storage in Solar Power Plants: A Review of the Materials, Associated Limitations, and Proposed Solutions. Energies. 2019; 12(21):4164. https://doi.org/10.3390/en12214164
Chicago/Turabian StyleAlnaimat, Fadi, and Yasir Rashid. 2019. "Thermal Energy Storage in Solar Power Plants: A Review of the Materials, Associated Limitations, and Proposed Solutions" Energies 12, no. 21: 4164. https://doi.org/10.3390/en12214164
APA StyleAlnaimat, F., & Rashid, Y. (2019). Thermal Energy Storage in Solar Power Plants: A Review of the Materials, Associated Limitations, and Proposed Solutions. Energies, 12(21), 4164. https://doi.org/10.3390/en12214164






