Self-Heating Ability of Geopolymers Enhanced by Carbon Black Admixtures at Different Voltage Loads
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Basic Physical Properties
3.2. Mechanical Properties
3.3. Thermal and Electrical Properties
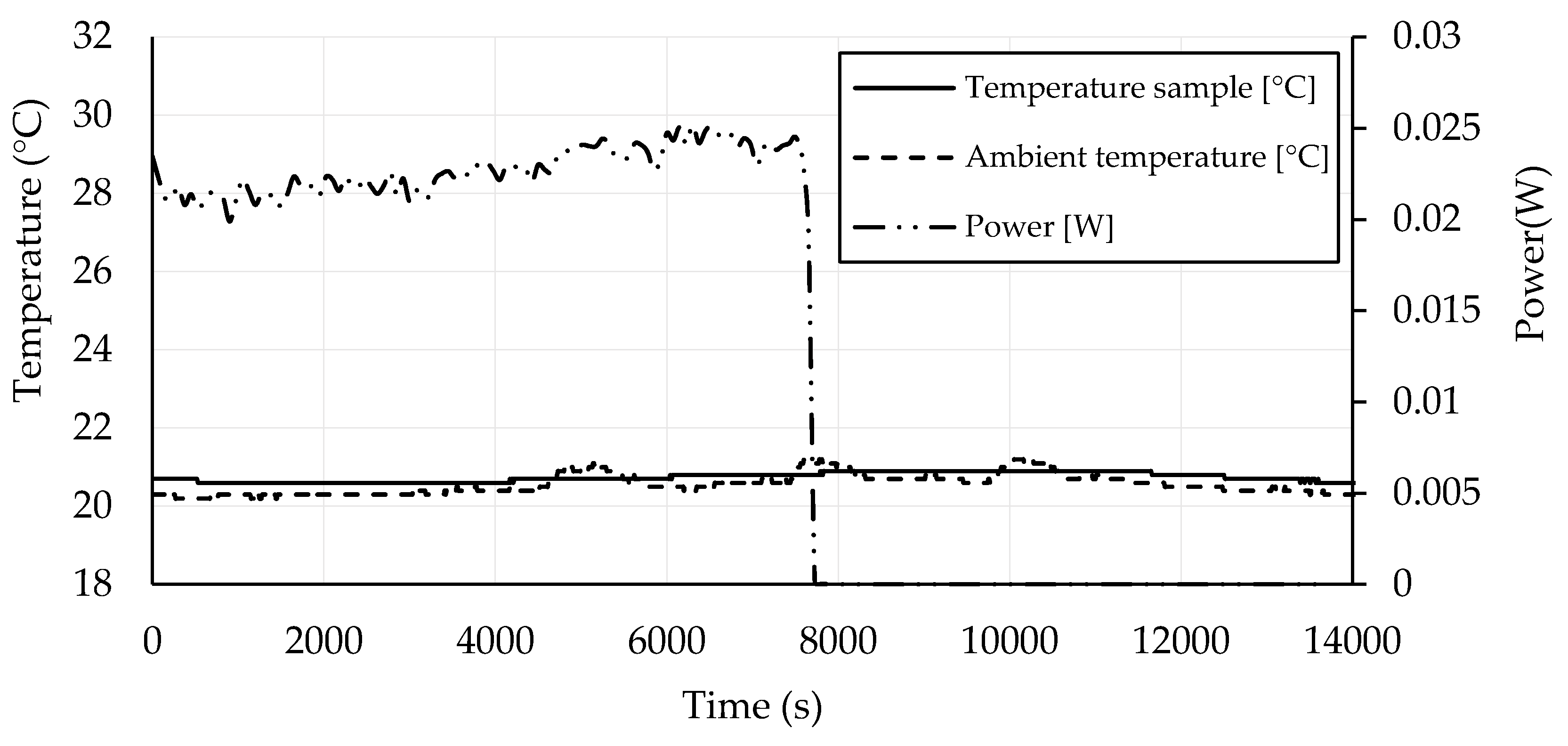
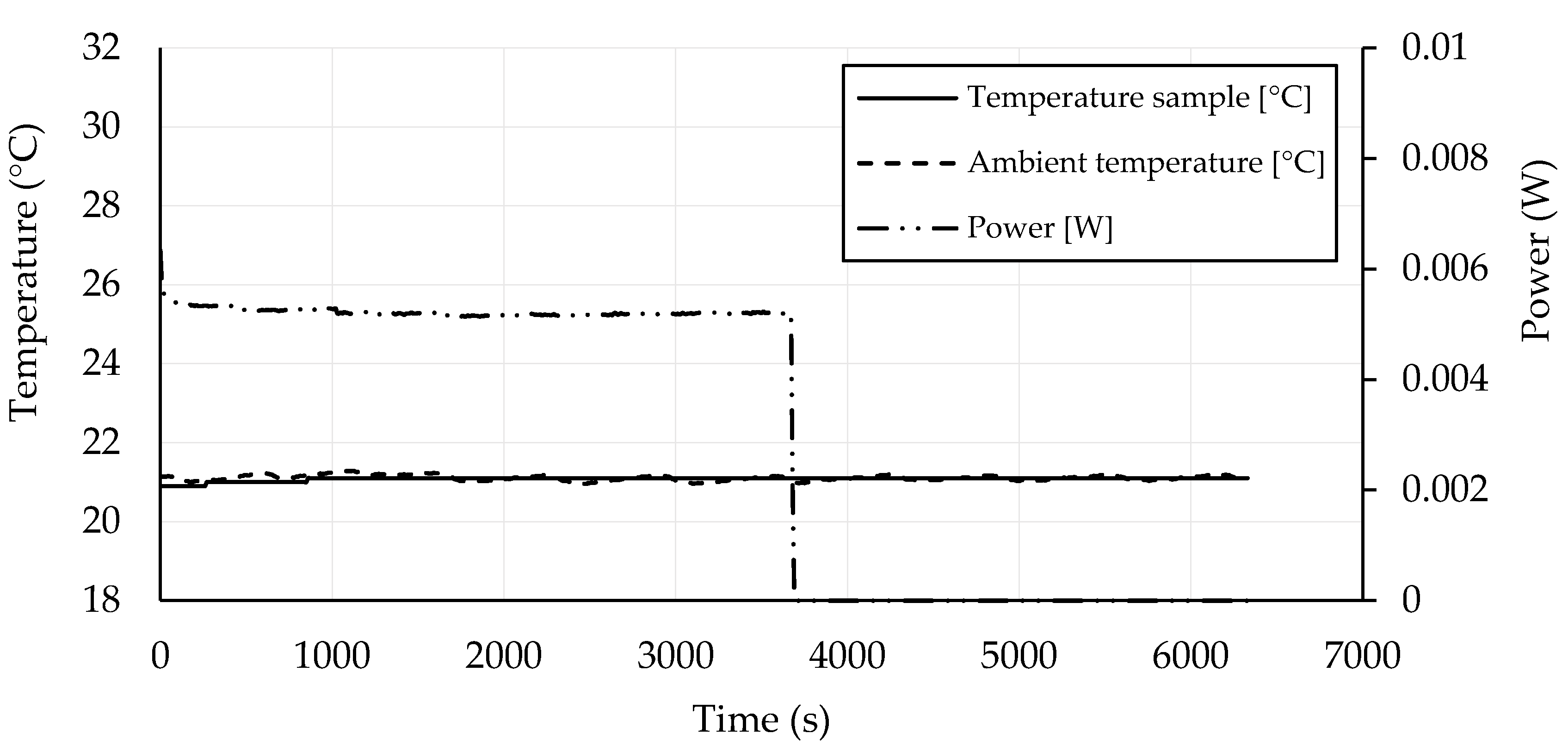
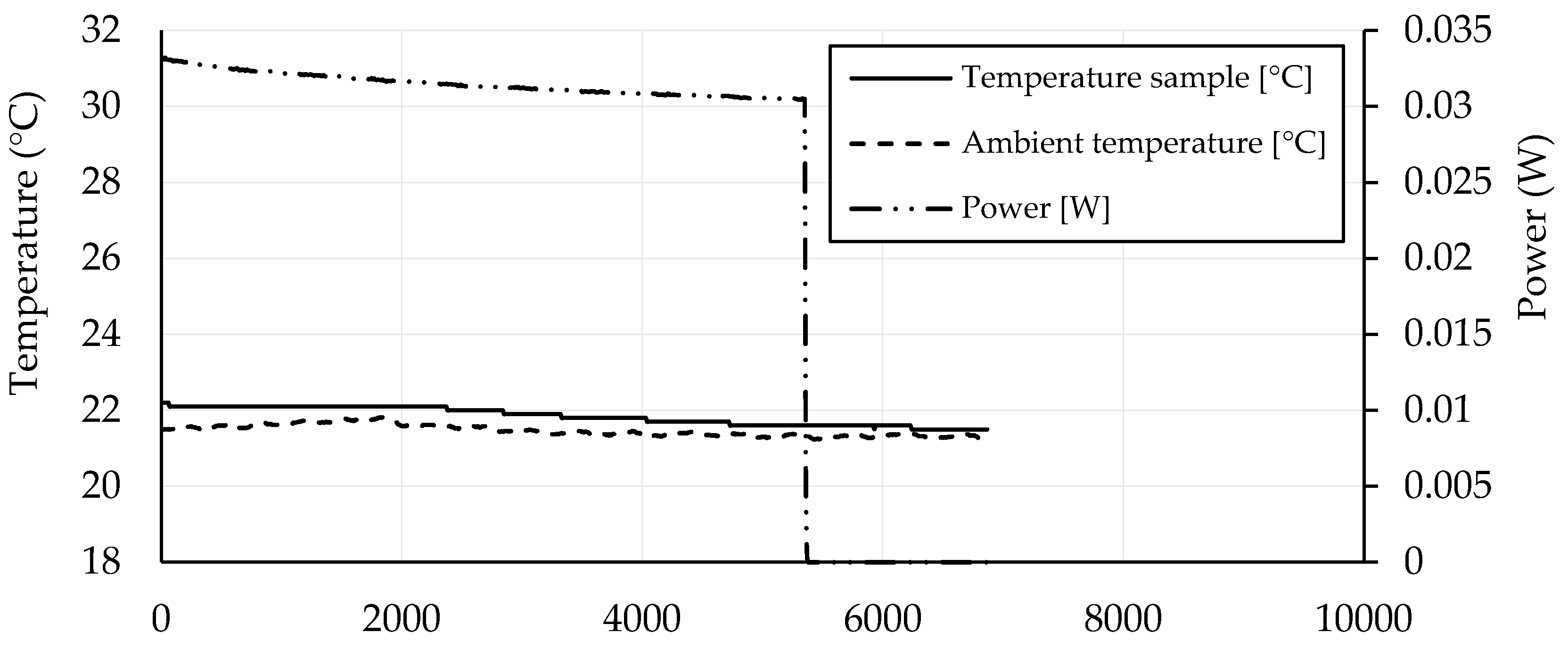
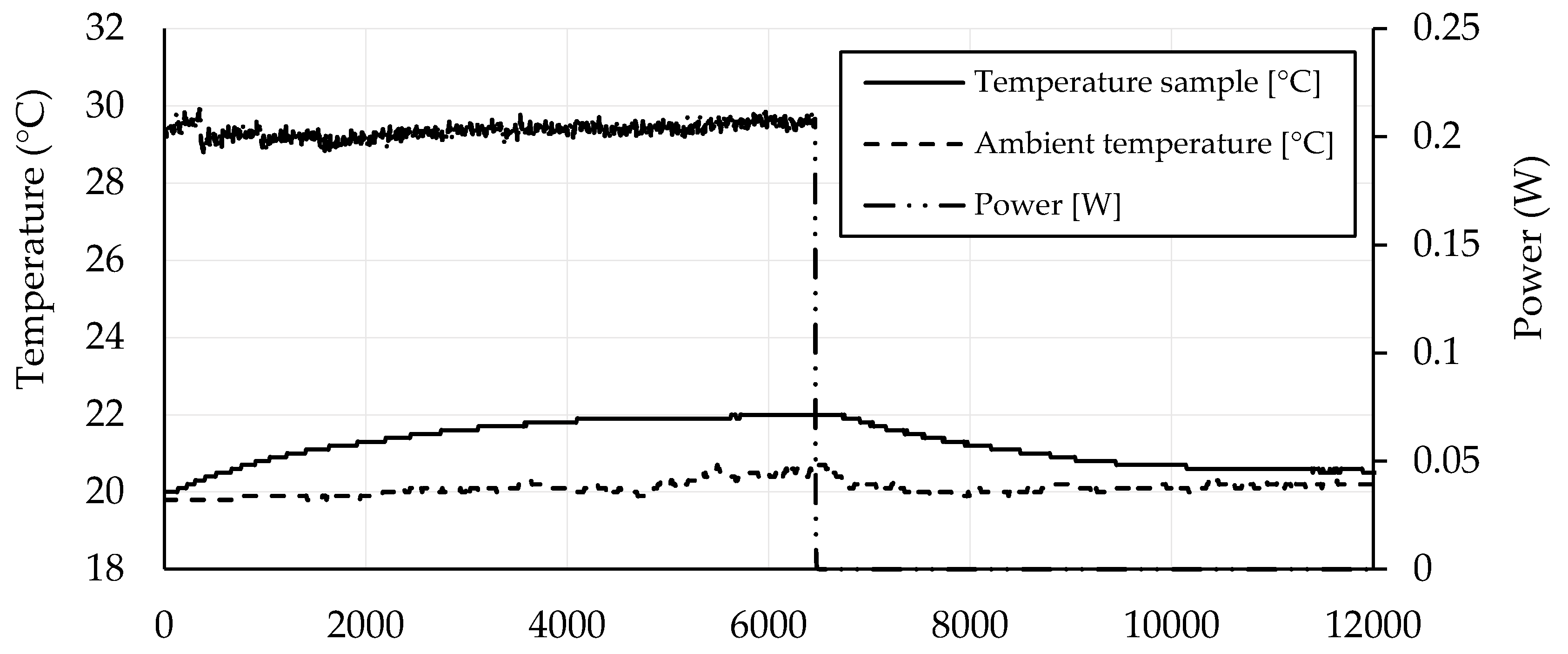
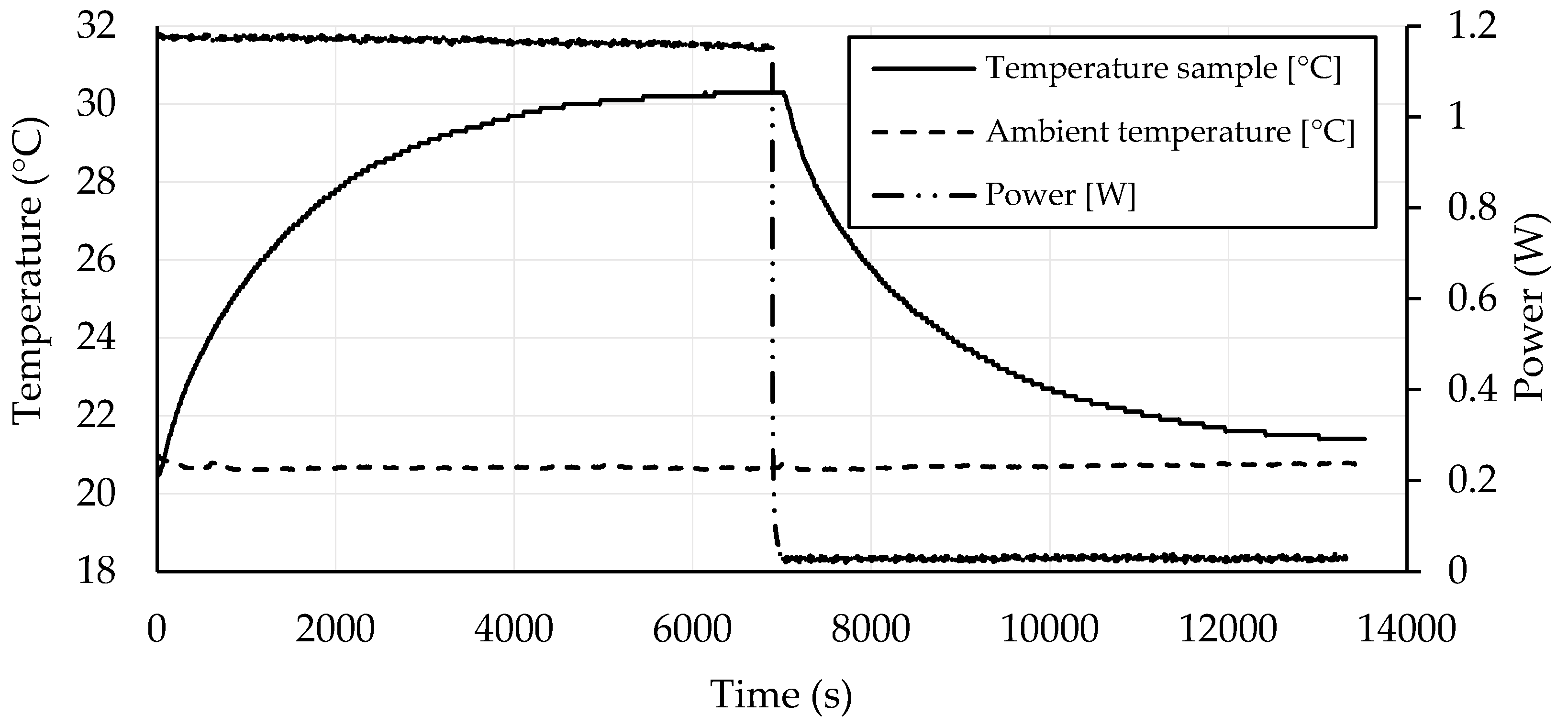
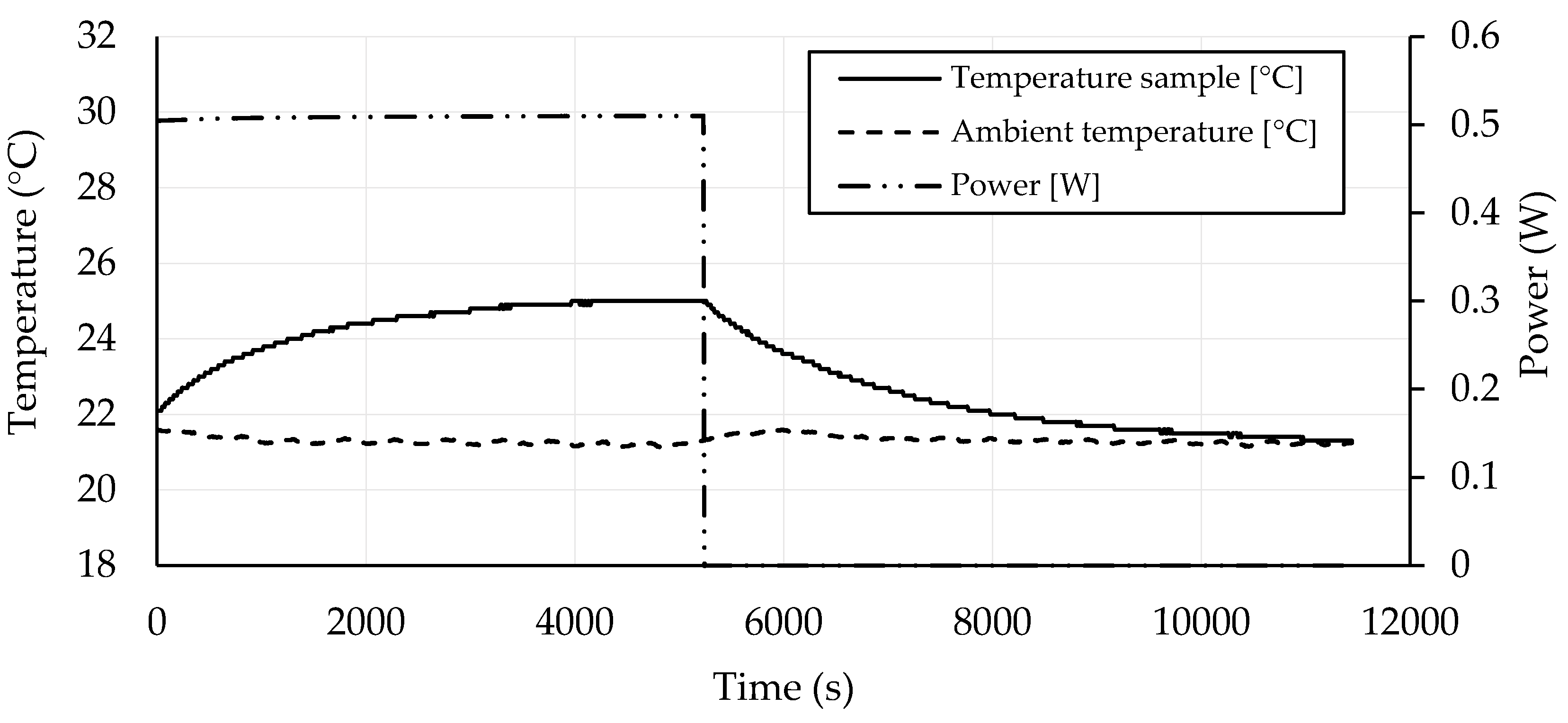
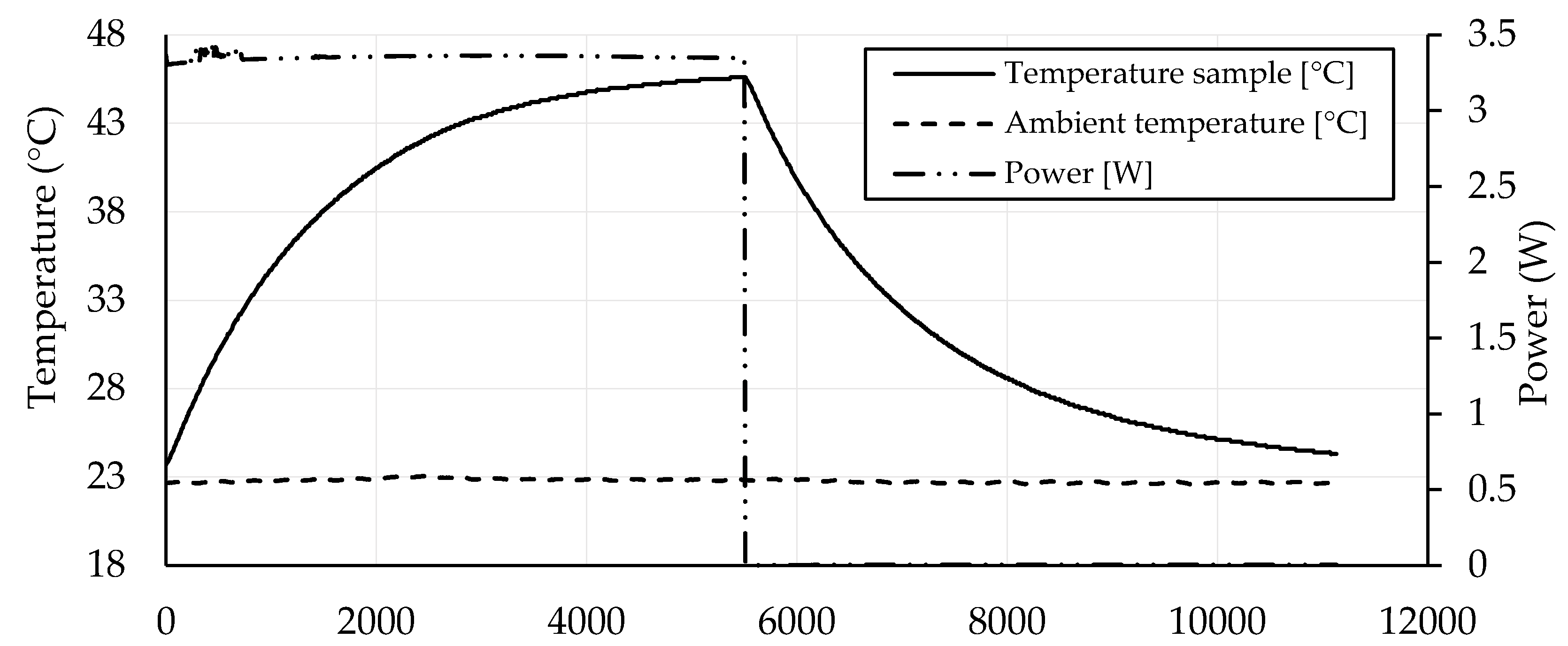
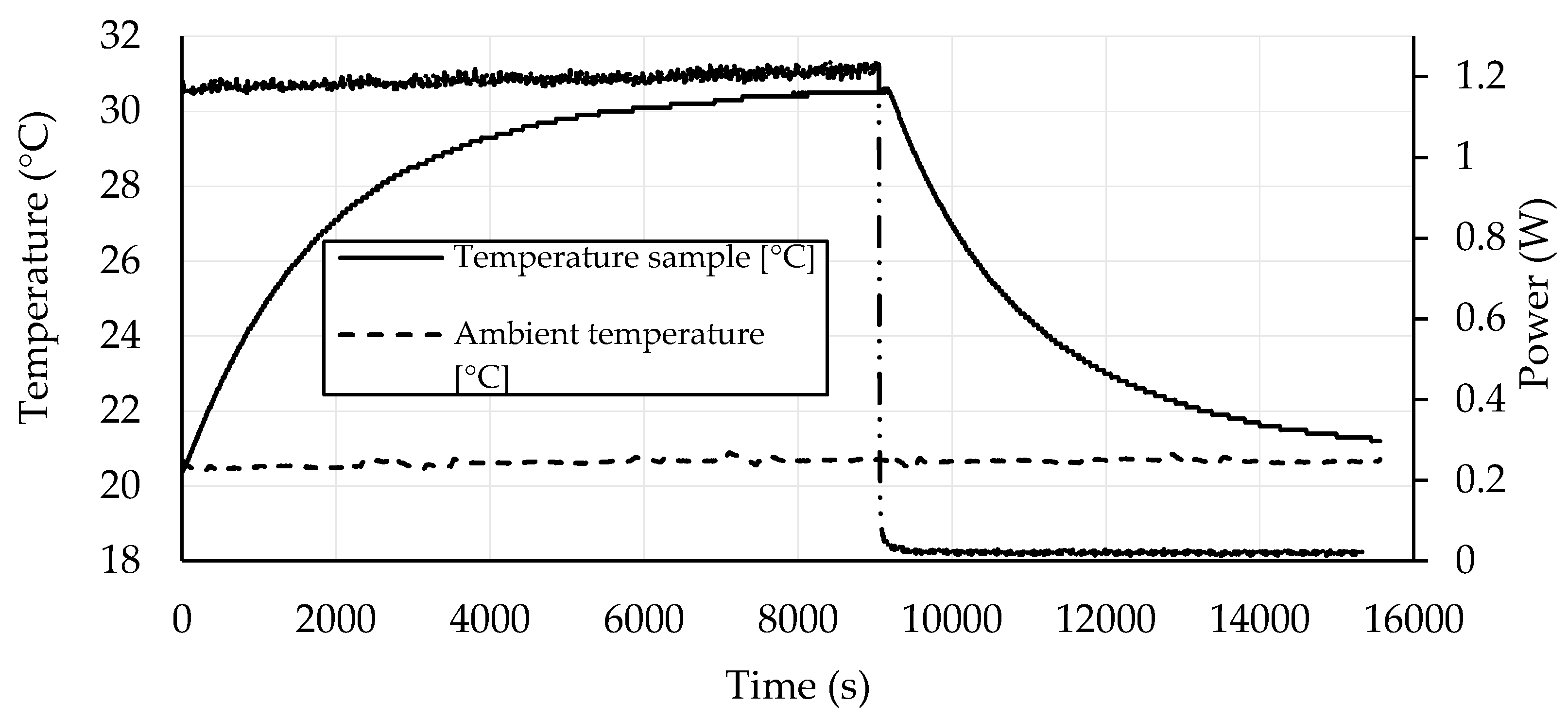
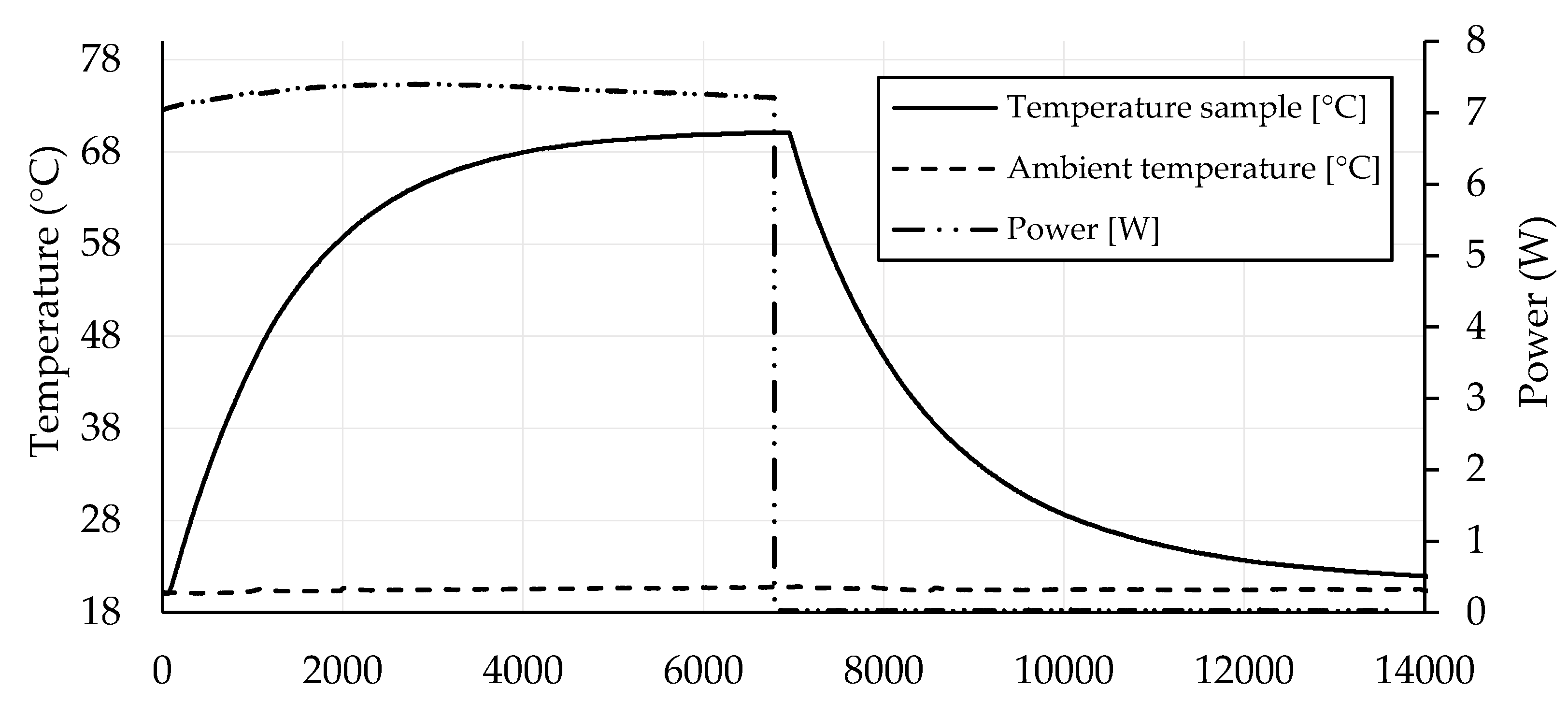
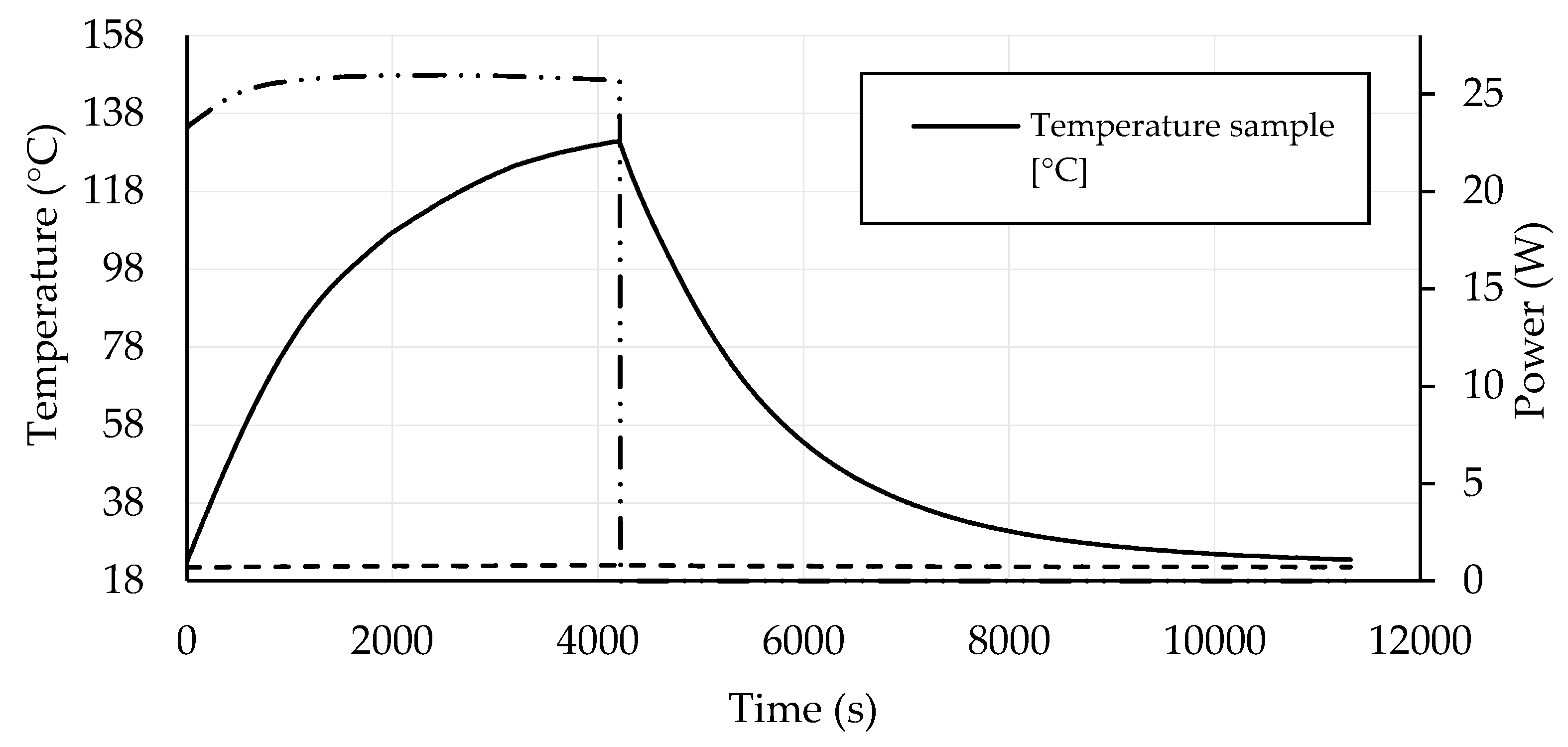
3.4. Self-Heating Ability
4. Discussion
5. Conclusions
- An increase in the amount of CB admixture in geopolymers based on GBFS activated by water glass led to the deterioration of mechanical properties, which was attributed to an increased amount of mixing water and, consequently, increased porosity.
- An increase in the amount of CB admixture in geopolymers based on GBFS activated by water glass led to a decrease in thermal conductivity, which is an important parameter describing the ability to spread the evolved heat.
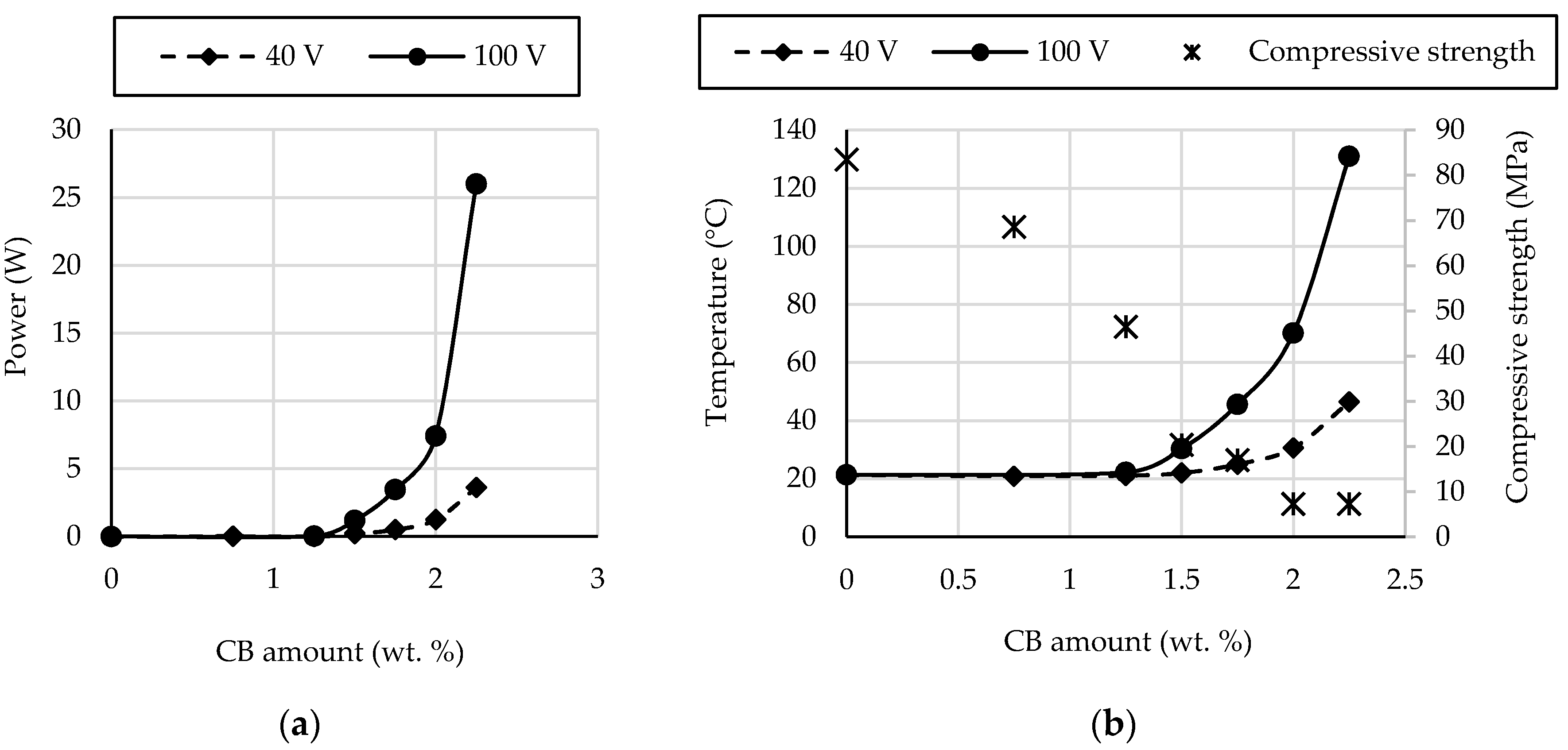
- The percolation threshold for the self-heating ability was around 1.5 wt. % of CB, where a slight self-heating ability was observed.
- Geopolymers based on GBFS activated by water glass with CB amounts in the range of 1.75–2.25 wt. % exhibited good self-heating abilities.
- The self-heating ability of geopolymers based on GBFS activated by water glass with CB can be significantly improved by increasing the voltage. The heating power of the geopolymer mortar with CB in the amount of 2.25 wt. % at 40 V was similar to the heating power of the geopolymer mortar with CB in the amount of 1.75 wt. % at 100 V (3.63 vs. 3.45 W).
Author Contributions
Funding
Conflicts of Interest
References
- Han, B.G.; Wang, Y.Y.; Dong, S.F.; Zhang, L.Q.; Ding, S.Q.; Yu, X.; Ou, J.P. Smart concretes and structures: A review. J. Intell. Mater. Syst. Struct. 2015, 26, 1303–1345. [Google Scholar] [CrossRef]
- Rana, S.; Subramani, P.; Fangueiro, R.; Correia, A.G. A review on smart self-sensing composite materials for civil engineering applications. Aims Mater. Sci. 2016, 3, 357–379. [Google Scholar] [CrossRef]
- Han, B.; Sun, S.; Ding, S.; Zhang, L.; Yu, X.; Ou, J. Review of nanocarbon-engineered multifunctional cementitious composites. Compos. Part A Appl. Sci. Manuf. 2015, 70, 69–81. [Google Scholar] [CrossRef]
- Li, Z.; Ding, S.; Yu, X.; Han, B.; Ou, J. Multifunctional cementitious composites modified with nano titanium dioxide: A review. Compos. Part A: Appl. Sci. Manuf. 2018, 111, 115–137. [Google Scholar] [CrossRef]
- Pisello, A.L.; D’Alessandro, A.; Sambuco, S.; Rallini, M.; Ubertini, F.; Asdrubali, F.; Materazzi, A.L.; Cotana, F. Multipurpose experimental characterization of smart nanocomposite cement-based materials for thermal-energy efficiency and strain-sensing capability. Sol. Energy Mater. Sol. Cells 2017, 161, 77–88. [Google Scholar] [CrossRef]
- Chung, D.D.L. Self-heating structural materials. Smart Mater. Struct. 2004, 13, 562–565. [Google Scholar] [CrossRef]
- Gomis, J.; Galao, O.; Gomis, V.; Zornoza, E.; Garces, P. Self-heating and deicing conductive cement. Experimental study and modeling. Constr. Build. Mater. 2015, 75, 442–449. [Google Scholar] [CrossRef]
- Armoosh, S.R.; Oltulu, M. Self-heating of electrically conductive metal-cementitious composites. J. Intell. Mater. Syst. Struct. 2019, 30, 2234–2240. [Google Scholar] [CrossRef]
- Armoosh, S.R.; Oltulu, M. Effect of Different Micro Metal Powders on the Electrical Resistivity of Cementitious Composites. In Proceedings of the 3rd World Multidisciplinary Civil Engineering, Architecture, Urban Planning Symposium, Prague, Czech Republic, 18–22 June 2018; Volume 471. [Google Scholar]
- Wei, J.; Zhao, L.; Zhang, Q.; Nie, Z.; Hao, L. Enhanced thermoelectric properties of cement-based composites with expanded graphite for climate adaptation and large-scale energy harvesting. Energy Build. 2018, 159, 66–74. [Google Scholar] [CrossRef]
- Wei, J.; Fan, Y.; Zhao, L.; Xue, F.; Hao, L.; Zhang, Q. Thermoelectric properties of carbon nanotube reinforced cement-based composites fabricated by compression shear. Ceram. Int. 2018, 44, 5829–5833. [Google Scholar] [CrossRef]
- Wei, J.; Nie, Z.; He, G.; Hao, L.; Zhao, L.; Zhang, Q. Energy harvesting from solar irradiation in cities using the thermoelectric behavior of carbon fiber reinforced cement composites. RSC Adv. 2014, 4, 48128–48134. [Google Scholar] [CrossRef]
- Ali, M.; Saidur, R.; Hossain, M. A review on emission analysis in cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Wei, J.X.; Cen, K.; Geng, Y.B. Evaluation and mitigation of cement CO2 emissions: Projection of emission scenarios toward 2030 in China and proposal of the roadmap to a low-carbon world by 2050. Mitig. Adapt. Strateg. Glob. Chang. 2019, 24, 301–328. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2017. Earth Syst. Sci. Data 2018, 10, 2213–2239. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. An overview of geopolymers derived from industrial by-products. Constr. Build. Mater. 2016, 127, 183–198. [Google Scholar] [CrossRef]
- Ma, C.-K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Albitar, M.; Ali, M.M.; Visintin, P.; Drechsler, M. Durability evaluation of geopolymer and conventional concretes. Constr. Build. Mater. 2017, 136, 374–385. [Google Scholar] [CrossRef]
- Zuda, L.; Drchalova, J.; Rovnanik, P.; Bayer, P.; Keršner, Z.; Cerny, R. Alkali-activated aluminosilicate composite with heat-resistant lightweight aggregates exposed to high temperatures: Mechanical and water transport properties. Cem. Concr. Compos. 2010, 32, 157–163. [Google Scholar] [CrossRef]
- Nguyen, T.H.Y.; Tsuchiya, K.; Atarashi, D. Microstructure and composition of fly ash and ground granulated blast furnace slag cement pastes in 42-month cured samples. Constr. Build. Mater. 2018, 191, 114–124. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-Like Inorganic Polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Altan, E.; Erdoğan, S.T. Alkali activation of a slag at ambient and elevated temperatures. Cem. Concr. Compos. 2012, 34, 131–139. [Google Scholar] [CrossRef]
- Ben Haha, M.; Le Saoût, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review Part 2. About materials and binders manufacture. Constr. Build. Mater. 2018, 22, 1315–1322. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.; Hu, Y.; Zhou, J.L.; Tam, V.W. Review on designs and properties of multifunctional alkali-activated materials (AAMs). Constr. Build. Mater. 2019, 200, 474–489. [Google Scholar] [CrossRef]
- Rovnaník, P.; Kusák, I.; Bayer, P.; Schmid, P.; Fiala, L. Comparison of electrical and self-sensing properties of Portland cement and alkali-activated slag mortars. Cem. Concr. Res. 2019, 118, 84–91. [Google Scholar] [CrossRef]
- Rovnanik, P.; Kusak, I.; Bayer, P.; Schmid, P.; Fiala, L. Electrical and Self-Sensing Properties of Alkali-Activated Slag Composite with Graphite Filler. Materials 2019, 12, 1616. [Google Scholar] [CrossRef]
- Fiala, L.; Rovnanik, P.; Cerny, R. Investigation of the Joule’s effect in electrically enhanced alkali-activated aluminosilicates. Cem. Wapno Beton 2017, 22, 201–210. [Google Scholar]
- Dhoble, Y.N.; Ahmed, S. Review on the innovative uses of steel slag for waste minimization. J. Mater. Cycles Waste Manag. 2018, 20, 1373–1382. [Google Scholar] [CrossRef]
- Guo, J.; Bao, Y.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef]
- Cabot Company. Cabot launches new carbon black products for tyre applications. Addit. Polym. 2014, 2014, 2. [Google Scholar] [CrossRef]
- Li, Y.C.; Huang, X.R.; Zeng, L.J.; Li, R.F.; Tian, H.F.; Fu, X.W.; Wang, Y.; Zhong, W.H. A review of the electrical and mechanical properties of carbon nanofiller-reinforced polymer composites. J. Mater. Sci. 2019, 54, 1036–1076. [Google Scholar] [CrossRef]
- Monteiro, A.O.; Cachim, P.B.; Costa, P.M. Self-sensing piezoresistive cement composite loaded with carbon black particles. Cem. Concr. Compos. 2017, 81, 59–65. [Google Scholar] [CrossRef]
- Fiala, L.; Jerman, M.; Rovnaník, P.; Černý, R. Basic physical, mechanical and electrical properties of electrically enhanced alkali-activated aluminosilicates. Mater. Tehnol. 2017, 51, 1005–1009. [Google Scholar] [CrossRef]
- Alonso, M.; Gismera, S.; Blanco, M.; Lanzón, M.; Puertas, F. Alkali-activated mortars: Workability and rheological behaviour. Constr. Build. Mater. 2017, 145, 576–587. [Google Scholar] [CrossRef]
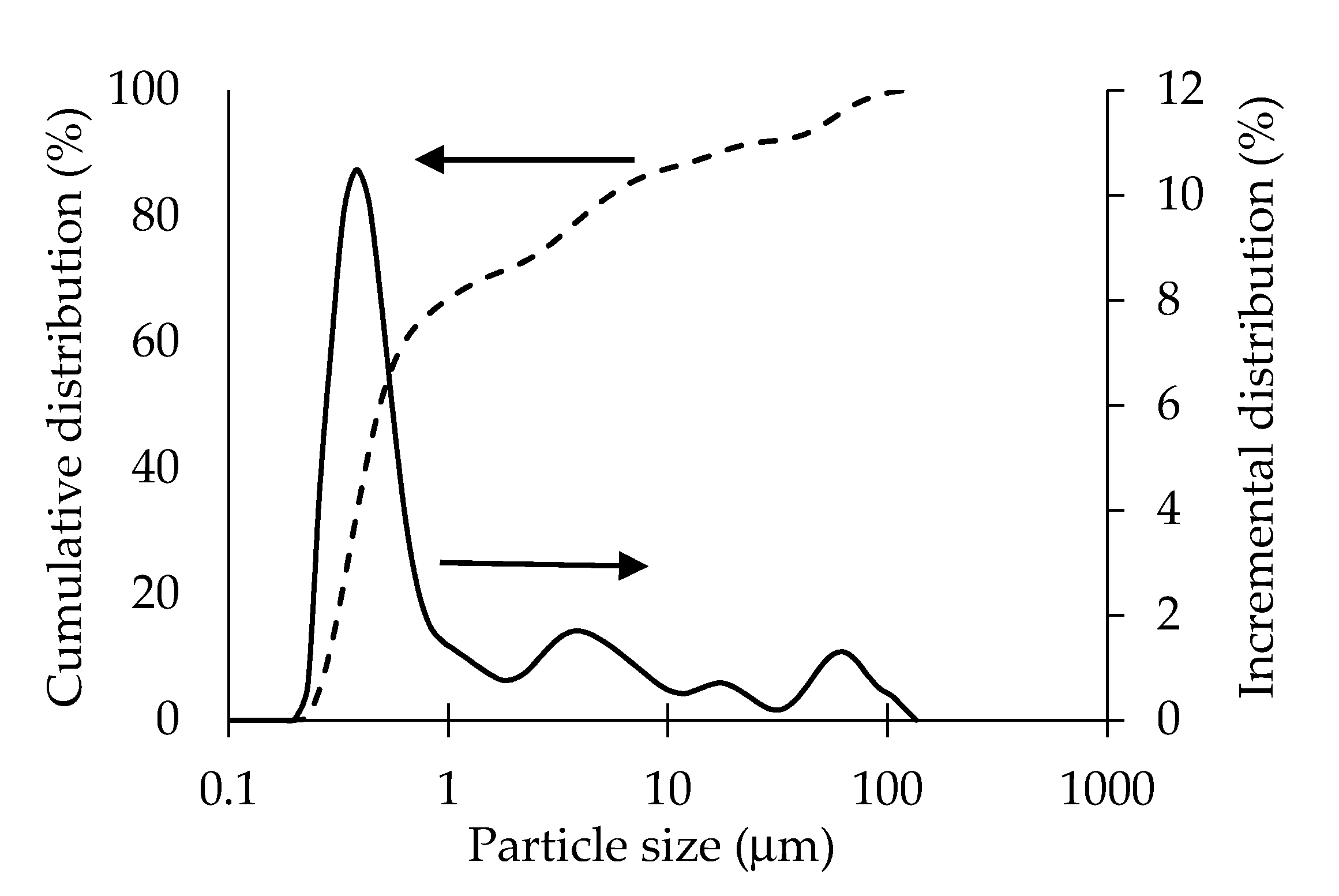



| Carbon Black (CB) 0 | CB 0.75 | CB 1.25 | CB 1.5 | CB 1.75 | CB 2 | CB 2.25 | |
|---|---|---|---|---|---|---|---|
| Granulated blast-furnace slag (GBFS) (g) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Water glass (g) | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Sand PG1 (g) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Sand PG2 (g) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Sand PG3 (g) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| CB suspension (%) | 0 | 10 | 10 | 10 | 15 | 15 | 15 |
| CB amount (g) | 0 | 3 | 5 | 6 | 7 | 8 | 9 |
| Water-to-slag ratio (–) | 0.44 | 0.60 | 0.69 | 0.74 | 0.77 | 0.81 | 0.84 |
| Bulk Density (kg∙m−3) | Matrix Density (kg∙m−3) | Total Open Porosity (%) | |
|---|---|---|---|
| CB 0 | 2111 | 2562 | 17.6 |
| CB 0.75 | 1947 | 2602 | 25.2 |
| CB 1.25 | 1914 | 2588 | 26.0 |
| CB 1.5 | 1816 | 2577 | 29.5 |
| CB 1.75 | 1773 | 2568 | 31.0 |
| CB 2 | 1749 | 2570 | 31.9 |
| CB 2.25 | 1720 | 2582 | 33.4 |
| Compressive Strength (MPa) | Flexural Strength (MPa) | |
|---|---|---|
| CB 0 | 83.45 | 8.26 |
| CB 0.75 | 68.60 | 5.23 |
| CB 1.25 | 46.49 | 5.21 |
| CB 1.5 | 20.38 | 5.04 |
| CB 1.75 | 17.00 | 3.75 |
| CB 2 | 7.31 | 3.85 |
| CB 2.25 | 7.31 | 2.40 |
| Thermal Conductivity (W∙m−1∙K−1) | Specific Heat Capacity (J∙kg−1∙K−1) | Electrical Conductivity (S∙m−1) | |
|---|---|---|---|
| CB 0 | 1.71 | 790 | 8.0 × 10−7 |
| CB 0.75 | 0.94 | 715 | 2.7 × 10−5 |
| CB 1.25 | 0.85 | 733 | 8.6 × 10−5 |
| CB 1.5 | 0.81 | 728 | 3.2 × 10−3 |
| CB 1.75 | 0.75 | 803 | 1.1 × 10−2 |
| CB 2 | 0.71 | 792 | 2.2 × 10−2 |
| CB 2.25 | 0.63 | 849 | 1.3 × 10−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiala, L.; Petříková, M.; Lin, W.-T.; Podolka, L.; Černý, R. Self-Heating Ability of Geopolymers Enhanced by Carbon Black Admixtures at Different Voltage Loads. Energies 2019, 12, 4121. https://doi.org/10.3390/en12214121
Fiala L, Petříková M, Lin W-T, Podolka L, Černý R. Self-Heating Ability of Geopolymers Enhanced by Carbon Black Admixtures at Different Voltage Loads. Energies. 2019; 12(21):4121. https://doi.org/10.3390/en12214121
Chicago/Turabian StyleFiala, Lukáš, Michaela Petříková, Wei-Ting Lin, Luboš Podolka, and Robert Černý. 2019. "Self-Heating Ability of Geopolymers Enhanced by Carbon Black Admixtures at Different Voltage Loads" Energies 12, no. 21: 4121. https://doi.org/10.3390/en12214121
APA StyleFiala, L., Petříková, M., Lin, W.-T., Podolka, L., & Černý, R. (2019). Self-Heating Ability of Geopolymers Enhanced by Carbon Black Admixtures at Different Voltage Loads. Energies, 12(21), 4121. https://doi.org/10.3390/en12214121







