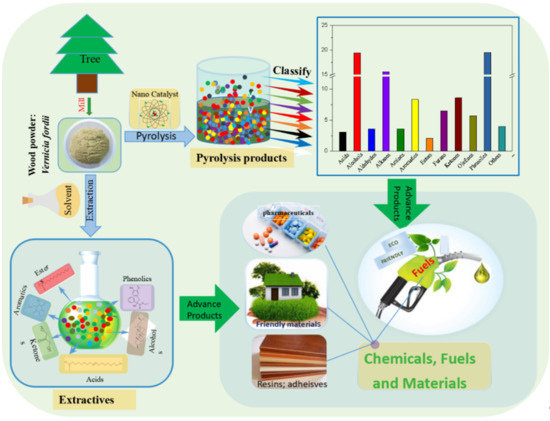
Catalytic Fast Pyrolysis of Forestry Wood Waste for Bio-Energy Recovery Using Nano-Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
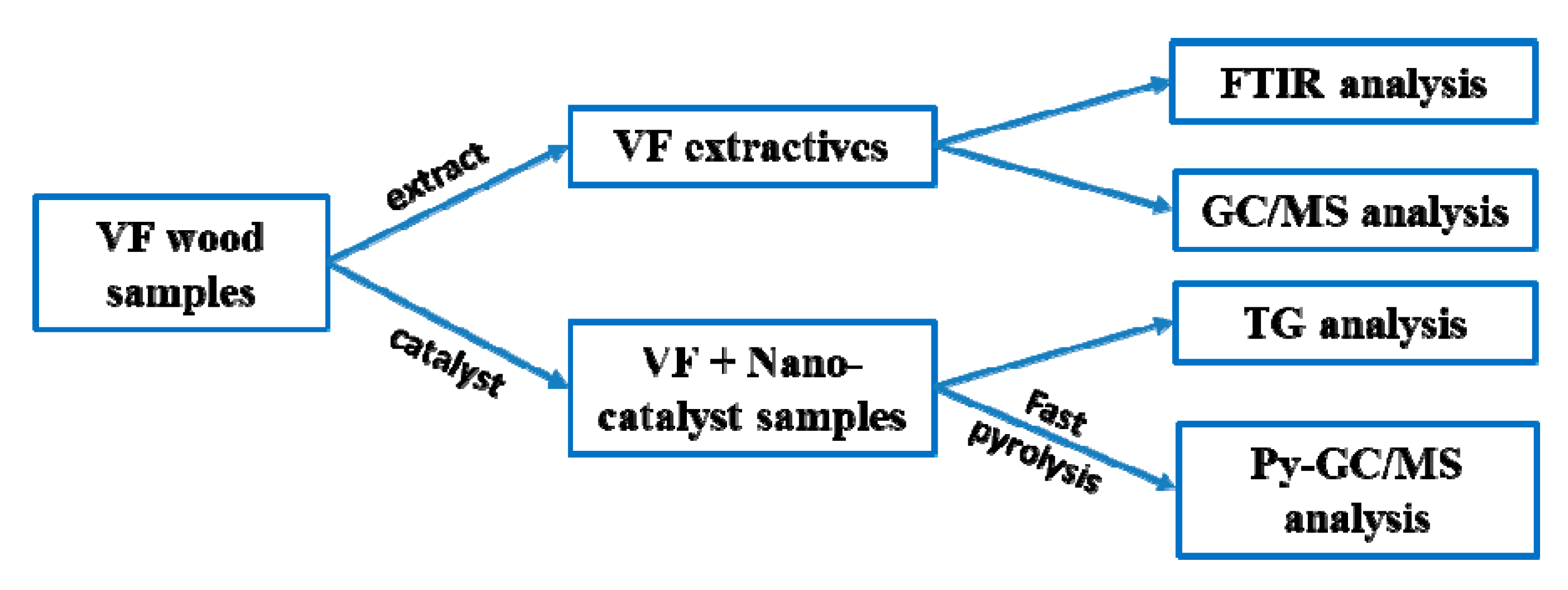
2.2. Characterization of Forestry Wood Waste (Vernicia fordii Wood)
2.3. Catalytic Fast Pyrolysis of Forestry Wood Waste (Vernicia fordii Wood) Using Nano-Catalyst
3. Results and Discussion
3.1. Characterization of Forestry Wood Waste (Vernicia fordii Wood) Extracts
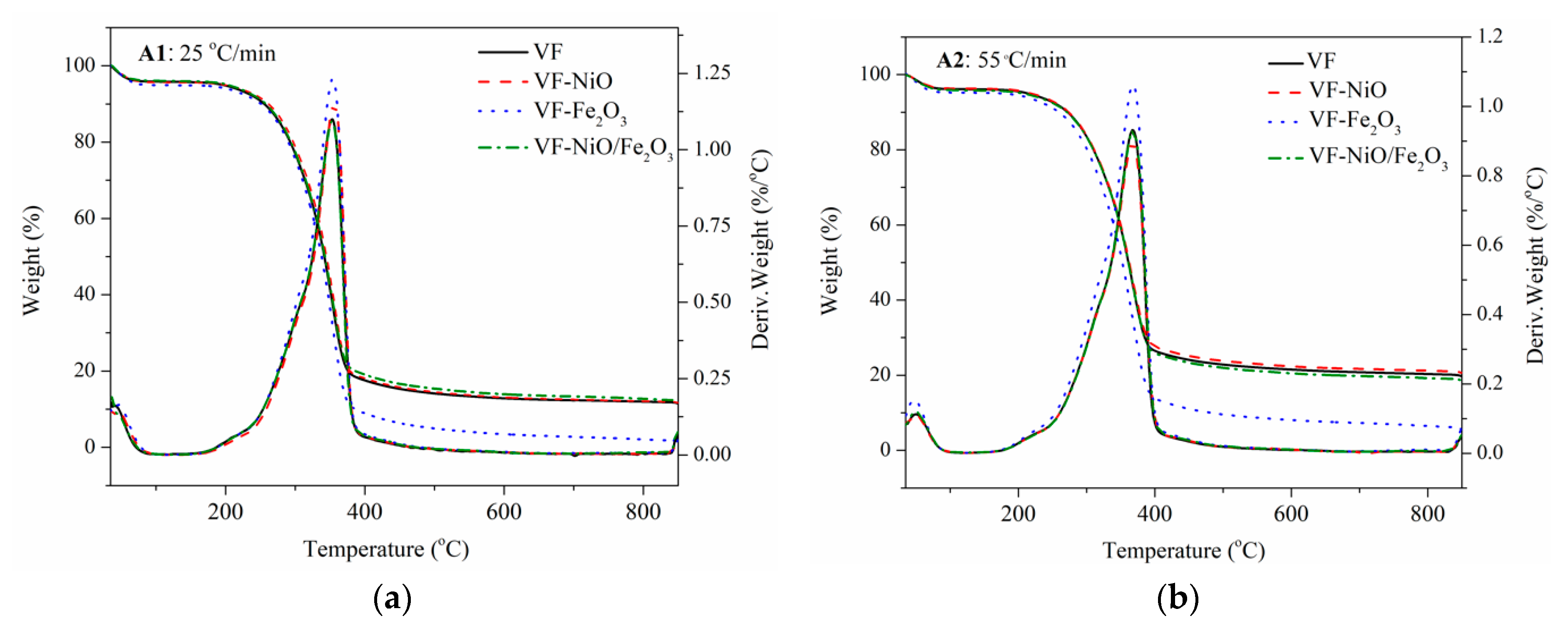
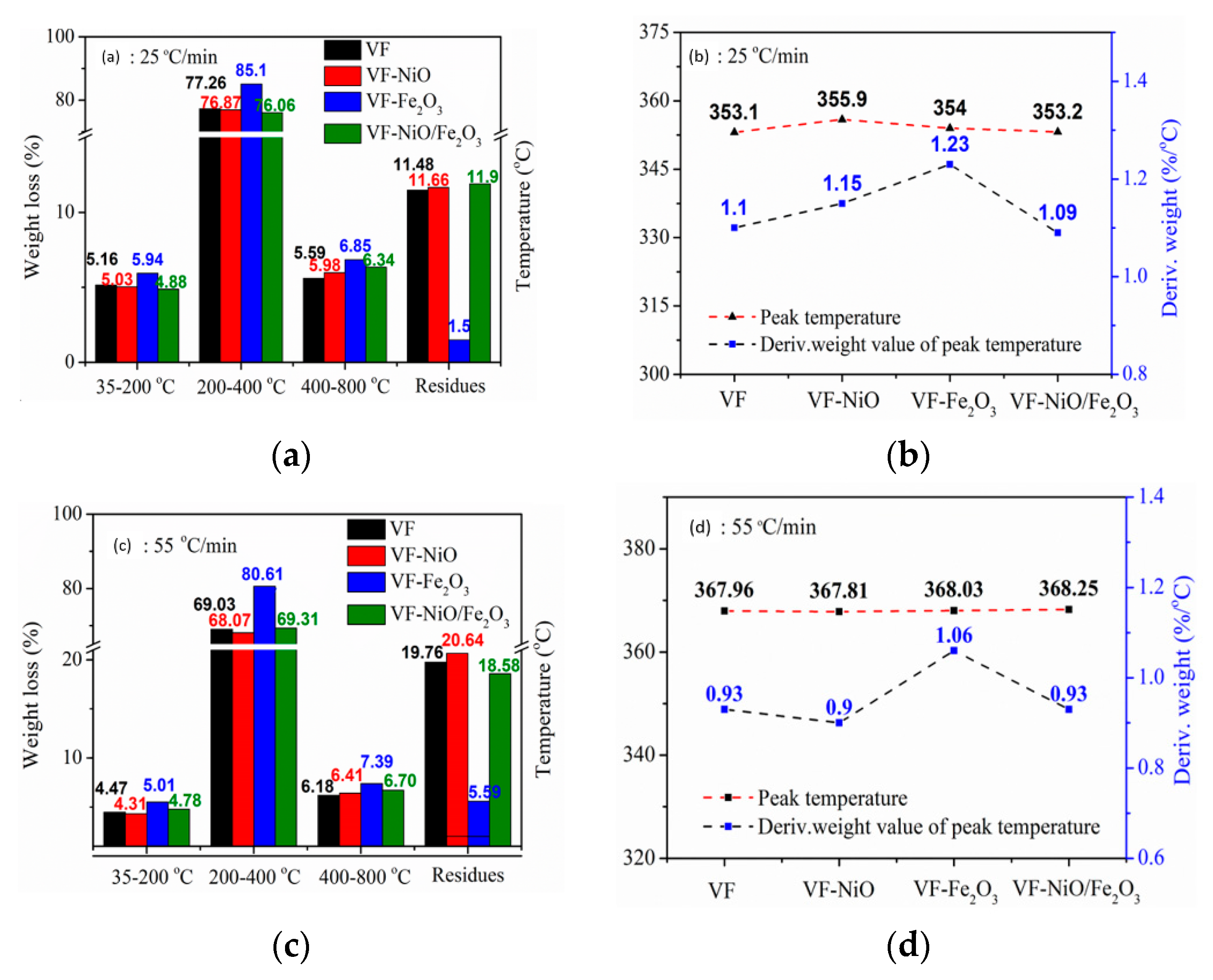
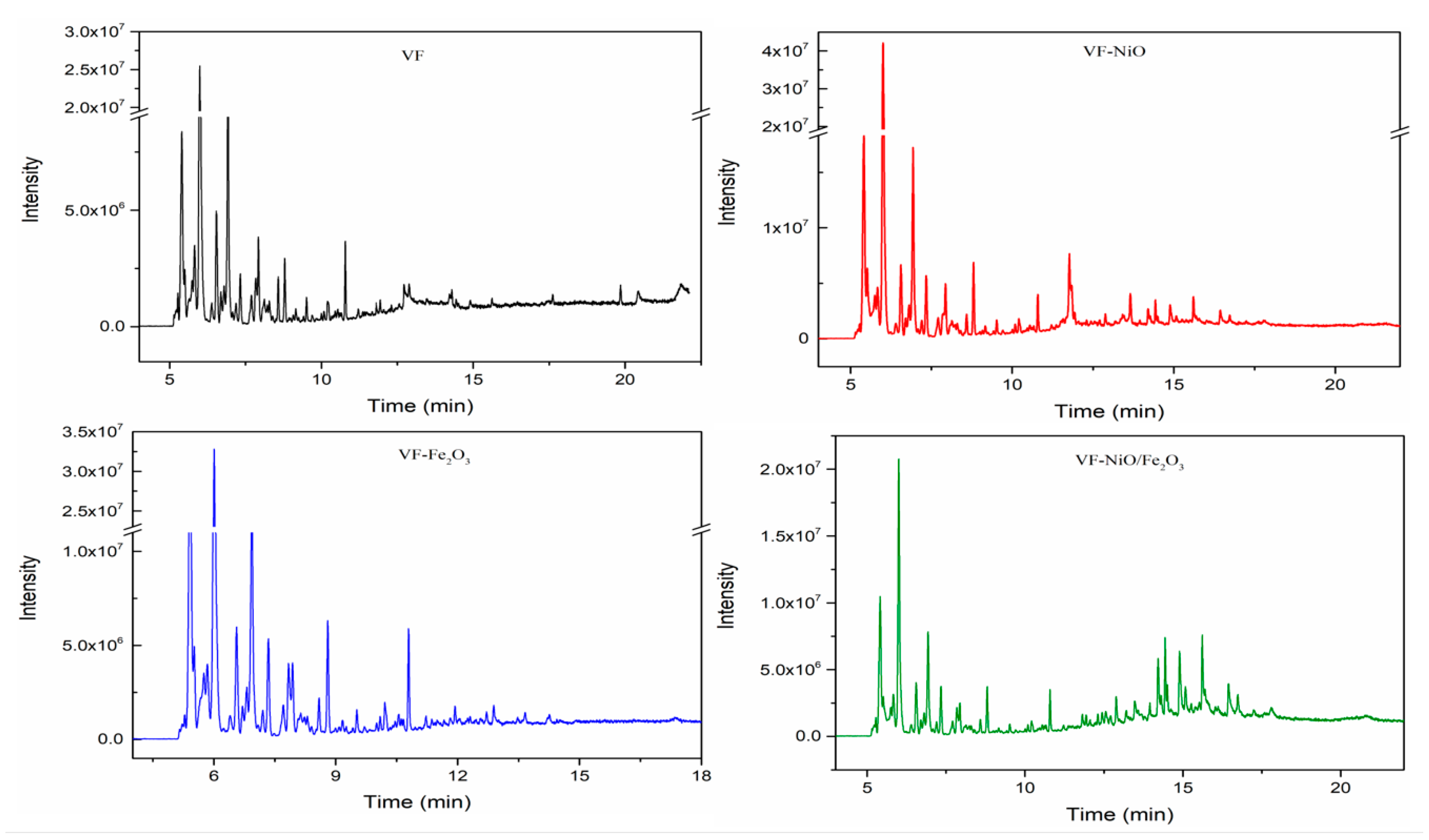
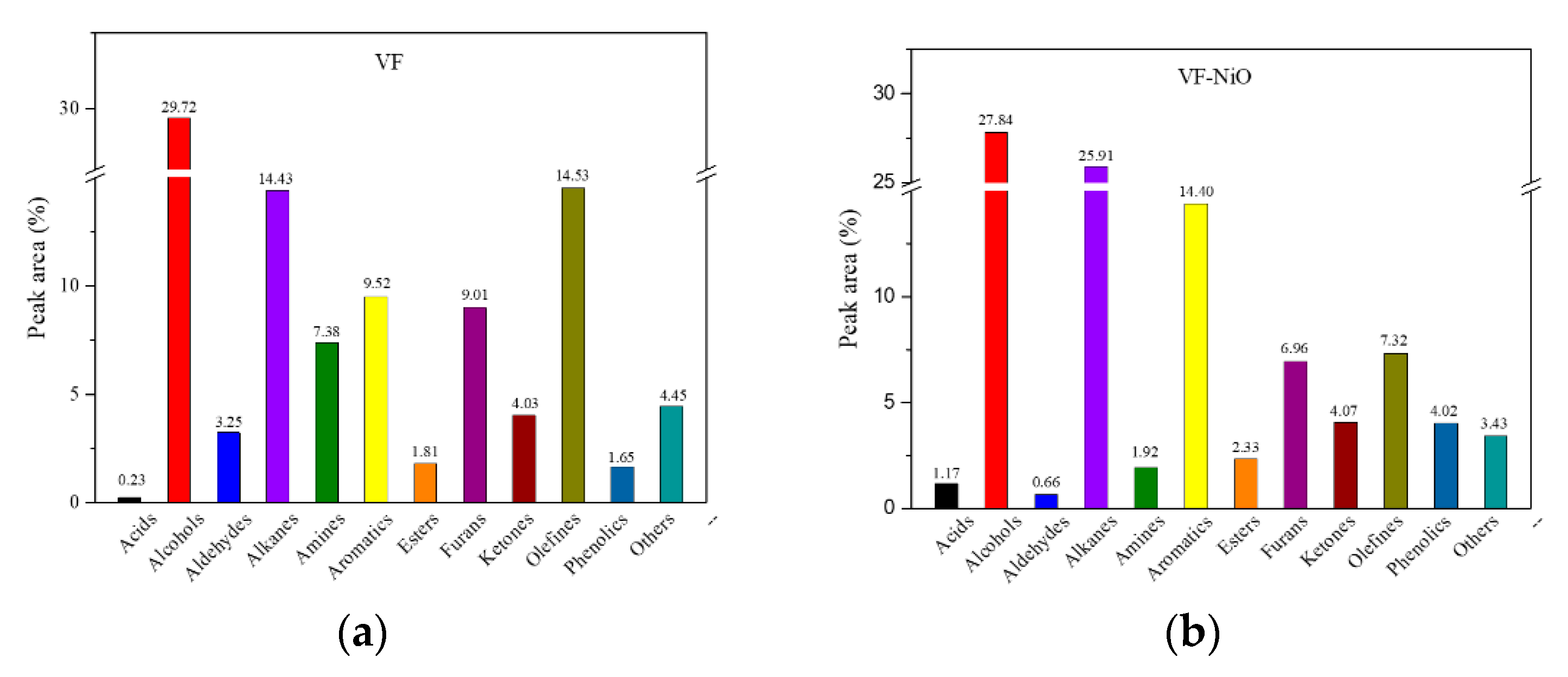
3.2. Catalytic Fast Pyrolysis of Forestry Wood Waste (Vernicia fordii Wood)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morales, M.; Ataman, M.; Badr, S.; Linster, S.; Kourlimpinis, I.; Papadokonstantakis, S.; Hatzimanikatis, V.; Hungerbühler, K. Sustainability assessment of succinic acid production technologies from biomass using metabolic engineering. Energy Environ. Sci. 2016, 9, 2794–2805. [Google Scholar] [CrossRef]
- Grams, J.; Niewiadomski, M.; Ruppert, A.M.; Kwapiński, W. Influence of Ni catalyst support on the product distribution of cellulose fast pyrolysis vapors upgrading. J. Anal. Appl. Pyrolysis 2015, 113, 557–563. [Google Scholar] [CrossRef]
- Laurent, A.; Espinosa, N. Environmental impacts of electricity generation at global, regional and national scales in 1980–2011: What can we learn for future energy planning? Energy Environ. Sci. 2015, 8, 689–701. [Google Scholar] [CrossRef]
- Morales, M.; Dapsens, P.Y.; Giovinazzo, I.; Witte, J.; Mondelli, C.; Papadokonstantakis, S.; Hungerbühler, K.; Pérez-Ramírez, J. Environmental and economic assessment of lactic acid production from glycerol using cascade bio- and chemocatalysis. Energy Environ. Sci. 2015, 8, 558–567. [Google Scholar] [CrossRef]
- Nel, W.P.; Cooper, C.J. Implications of fossil fuel constraints on economic growth and global warming. Energy Policy 2009, 37, 166–180. [Google Scholar] [CrossRef]
- Op de Beeck, B.; Dusselier, M.; Geboers, J.; Holsbeek, J.; Morré, E.; Oswald, S.; Giebeler, L.; Sels, B.F. Direct catalytic conversion of cellulose to liquid straight-chain alkanes. Energy Environ. Sci. 2015, 8, 230–240. [Google Scholar] [CrossRef]
- Vardon, D.R.; Franden, M.A.; Johnson, C.W.; Karp, E.M.; Guarnieri, M.T.; Linger, J.G.; Salm, M.J.; Strathmann, T.J.; Beckham, G.T. Adipic acid production from lignin. Energy Environ. Sci. 2015, 8, 617–628. [Google Scholar] [CrossRef]
- Cai, H.; Wang, J.; Feng, Y.; Wang, M.; Qin, Z.; Dunn, J.B. Consideration of land use change-induced surface albedo effects in life-cycle analysis of biofuels. Energy Environ. Sci. 2016, 9, 2855–2867. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Cui, K.; Karpuzov, D.; Tan, X.; Kohandehghan, A.; Mitlin, D. Peanut shell hybrid sodium ion capacitor with extreme energy–power rivals lithium ion capacitors. Energy Environ. Sci. 2014, 8, 941–955. [Google Scholar] [CrossRef]
- Mason, P.M.; Glover, K.; Smith, J.A.C.; Willis, K.J.; Woods, J.; Thompson, I.P. The potential of CAM crops as a globally significant bioenergy resource: Moving from ‘fuel or food’ to ‘fuel and more food’. Energy Environ. Sci. 2015, 8, 2320–2329. [Google Scholar] [CrossRef]
- Jung, J.I.; Risch, M.; Park, S.; Kim, M.G.; Nam, G.; Jeong, H.Y.; Shao-Horn, Y.; Cho, J. Optimizing nanoparticle perovskite for bifunctional oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 176–183. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z. Catalytic Conversion of Biomass into Chemicals and Fuels over Magnetic Catalysts. ACS. Catal. 2015, 6, 326–338. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, P.; Fan, M.; Jiang, P.; Dong, Y. Influence of crystal of Fe2O3 in magnetism and activity of nanoparticle CaO/Fe2O3 for biodiesel production. Fuel 2017, 197, 343–347. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Production of high grade fuels and chemicals from catalytic pyrolysis of biomass. Catal. Today 1996, 29, 285–295. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gärtner, C.A.; Dumesic, J.A. Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science 2008, 322, 417–421. [Google Scholar] [CrossRef]
- Hara, M. Biomass conversion by a solid acid catalyst. Energy Environ. Sci. 2010, 3, 601–607. [Google Scholar] [CrossRef]
- Duan, X.F.; Zhou, Z.F.; Xu, J.M.; Tian, Y.; Wang, R. Utilization situation and suggestion of forestry residues resources in China. China Wood Panel 2017, 24, 1–5. [Google Scholar]
- Lu, Q.; Zhang, Z.F.; Dong, C.Q.; Zhu, X.F. Catalytic Upgrading of Biomass Fast Pyrolysis Vapors with Nano Metal Oxides: An Analytical Py-GC/MS Study. Energies 2010, 3, 1805–1820. [Google Scholar] [CrossRef]
- Banks, S.W.; Nowakowski, D.J.; Bridgwater, A.V. Impact of Potassium and Phosphorus in Biomass on the Properties of Fast Pyrolysis Bio-oil. Energy Fuels 2016, 30, 8009–8018. [Google Scholar] [CrossRef]
- Chen, X.; Yang, H.; Chen, Y.; Chen, W.; Lei, T.; Zhang, W.; Chen, H. Catalytic fast pyrolysis of biomass to produce furfural using heterogeneous catalysts. J. Anal. Appl. Pyrolysis 2017, 127, 292–298. [Google Scholar] [CrossRef]
- Jae, J.; Coolman, R.; Mountziaris, T.J.; Huber, G.W. Catalytic fast pyrolysis of lignocellulosic biomass in a process development unit with continual catalyst addition and removal. Chem. Eng. Sci. 2014, 108, 33–46. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Wang, S.; Luo, Z. Interactions of biomass components during pyrolysis: A TG-FTIR study. J. Anal. Appl. Pyrolysis 2011, 20, 213–218. [Google Scholar] [CrossRef]
- Wang, K.; Kim, K.H.; Brown, R.C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Li, J.; Yan, R.; Xiao, B.; Liang, D.T.; Du, L. Development of nano-NiO/Al2O3 catalyst to be used for tar removal in biomass gasification. Environ. Sci. Technol. 2008, 42, 6224–6229. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Oladipo, J.; Fu, S.L.; Al-Rahbib, A.; Yang, H.P.; Wu, C.F.; Cai, N.; Williams, P.; Chen, H.P. Hydrogen production from cellulose catalytic gasification on CeO2/Fe2O3 catalyst. Energy Conves. Mang. 2018, 171, 241–248. [Google Scholar] [CrossRef]
- Xie, Y.; Ge, S.; Jiang, S.; Liu, Z.; Chen, L.; Wang, L.; Chen, J.; Qin, L.; Peng, W. Study on biomolecules in extractives of Camellia oleifera fruit shell by GC-MS. Saudi J. Biol. Sci. 2018, 25, 234–236. [Google Scholar] [CrossRef]
- Khelfa, A.; Sharypov, V.; Finqueneisel, G.; Weber, J.V. Catalytic pyrolysis and gasification of Miscanthus Giganteus: Haematite (Fe2O3) a versatile catalyst. J. Anal. Appl. Pyrolysis 2009, 84, 84–88. [Google Scholar] [CrossRef]
- Peng, W.; Lin, Z.; Wang, L.; Chang, J.; Gu, F.; Zhu, X. Molecular characteristics of Illicium verum extractives to activate acquired immune response. Saudi J. Biol. Sci. 2016, 23, 348–352. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Yi, Z.; Yang, H.; Zhao, B.; Zhang, W.; Li, J. Preparation and characterization of a novel environmentally friendly phenol—Formaldehyde adhesive modified with tannin and urea. Int. J. Adhes. Adhes. 2016, 66, 26–32. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.B.; Yang, X.C.; Dong, C.Q.; Zhu, X.F. Catalytic fast pyrolysis of biomass impregnated with K3PO4 to produce phenolic compounds: Analytical Py-GC/MS. J. Anal. Appl. Pyrolysis 2013, 104, 139–145. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, X.; Chen, L.; Zhao, B.; Yang, S.; Xie, X. Comparision of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts. J. Anal. Appl. Pyrolysis 2016, 121, 342–346. [Google Scholar] [CrossRef]
- Peyrat-Maillard, M.N.; Cuvelier, M.E.; Berset, C. Antioxidant activity of phenolic compounds in 2, 2-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidation Synergistic and antagonistic effects. JOACS 2003, 80, 1007–1012. [Google Scholar] [CrossRef]
- Ikeda, I.; Tanabe, Y.; Sugano, M. Effects of Sitosterol and Sitostanol on Micellar solubility of Cholesterol. J. Nutr. Sci. Vitaminol. 1989, 35, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Salen, G.; Shore, V.; Tint, G.S.; Forte, T.; Shefer, S.; Horak, I.; Horak, E.; Dayal, B.; Nguyen, L.; Batta, A.K. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J. Lip. Res. 1989, 30, 1319–1330. [Google Scholar]
- Luterbacher, J.S.; Azarpira, A.; Motagamwala, A.H.; Lu, F.; Ralph, J.; Dumesic, J.A. Lignin monomer production integrated into the γ-valerolactone sugar platform. Energy Environ. Sci. 2015, 8, 2657–2663. [Google Scholar] [CrossRef]
- Rinaldi, R.; Schüth, F. Design of solid catalysts for the conversion of biomass. Energy Environ. Sci. 2009, 2, 610. [Google Scholar] [CrossRef]
- Li, D.L.; Wu, J.Q.; Peng, W.X.; Xiao, W.F.; Wu, J.G.; Zhuo, J.Y.; Yuan, T.Q.; Sun, R.C. Effect of lignin on bamboo biomass self-bonding during hot-pressing lignin structure and characterization. Bioresources 2015, 10, 6769–6782. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef]
- Ondro, T.; Vitázek, I.; Húlan, T.K.; Lawson, M.; Csáki, Š. Non-isothermal kinetic analysis of the thermal decomposition of spruce wood in air atmosphere. Res. Agric. Eng. 2018, 64, 41–46. [Google Scholar]
- Biagini, E.; Barontini, F.; Tognotti, L. Devolatilization of biomss fuels and biomass components stutied by TG/FTIR technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. [Google Scholar] [CrossRef]
- Balat, M. Mechanisms of thermochemical biomass conversion processes. Part 1: Reactions of pyrolysis. Energy Sour. Part A 2008, 30, 620–635. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels securing the plant’s future energy needs. Energ. Convers. Manag. 2009, 50, 2239–2249. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Xing, S.; Yuan, H.; Huhetaoli Qi, Y.; Lv, P.; Yuan, Z.; Chen, Y. Characterization of the decomposition behaviors of catalytic pyrolysis of wood using copper and potassium over thermogravimetric and Py-GC/MS analysis. Energy 2016, 114, 634–646. [Google Scholar] [CrossRef]
- Svenson, J.; Pettersson, J.B.C.; Davidsson, K.O. Fast Pyrolysis of the Main Components of Birch Wood. Combust. Sci. Technol. 2004, 176, 977–990. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Blasiak, W. Thermal decomposition mechanism of levoglucosan during cellulose pyrolysis. J. Anal. Appl. Pyrolysis 2012, 96, 110–119. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Dong, C. Levoglucosan formation mechanisms during cellulose pyrolysis. J. Anal. Appl. Pyrolysis 2013, 104, 19–27. [Google Scholar] [CrossRef]
- Vitázek, I.; Tkáč, Z. Isothermal kinetic analysis of thermal decomposition of woody biomass: The thermogravimetric study. AIP Conf. Proc. 2019, 2118. [Google Scholar] [CrossRef]
- Wang, H.; Lee, S.J.; Olarte, M.V.; Zacher, A.H. Bio-oil Stabilization by Hydrogenation over Reduced Metal Catalysts at Low Temperatures. ACS Sustain. Chem. Eng. 2016, 4, 5533–5545. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K.; Helleur, R. Production and Characterization of Pyrolysis Oil from Sawmill Residues in an Auger Reactor. Ind. Eng. Chem. Res. 2017, 56, 1920–1925. [Google Scholar] [CrossRef]
- Fushimi, C.; Katayama, S.; Tsutsumi, A. Elucidation of interaction among cellulose, lignin and xylan during tar and gas evolution in steam gasification. J. Anal. Appl. Pyrolysis 2009, 86, 82–89. [Google Scholar] [CrossRef]
- Gani, A.; Naruse, I. Effect of cellulose and lignin content on pyrolysis and combustion characteristics for several types of biomass. Renew. Energy 2007, 32, 649–661. [Google Scholar] [CrossRef]
- Giudicianni, P.; Cardone, G.; Ragucci, R. Cellulose, hemicellulose and lignin slow steam pyrolysis: Thermal decomposition of biomass components mixtures. J. Anal. Appl. Pyrolysis 2015, 100, 213–222. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Cellulose—Hemicellulose and cellulose—Lignin interactions in wood pyrolysis at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 80, 118–125. [Google Scholar] [CrossRef]
- Caballero, J.A.; Font, R.; Marcilla, A. Comparative study of the pyrolysis of almond shells and their fractions, holocellulose and lignin: Product yields and kinetics. Thermochim. Acta 1996, 276, 57–77. [Google Scholar] [CrossRef]
- Lu, Q.; Dong, C.Q.; Zhang, X.M.; Tian, H.Y.; Yang, Y.P.; Zhu, X.F. Selective fast pyrolysis of biomass impregnated with ZnCl2 to produce furfural: Analytical Py-GC/MS study. J. Anal. Appl. Pyrolysis 2011, 90, 204–212. [Google Scholar] [CrossRef]
- Mettler, M.S.; Mushrif, S.H.; Paulsen, A.D.; Javadekar, A.D.; Vlachos, D.G.; Dauenhauer, P.J. Revealing pyrolysis chemistry for biofuels production: Conversion of cellulose to furans and small oxygenates. Energy Environ. Sci. 2012, 5, 5414–5424. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.; Ding, K.; Cao, Y.; Liu, Z. Catalytic Upgrading of Corn Stalk Fast Pyrolysis Vapors with Fresh and Hydrothermally Treated HZSM-5 Catalysts Using Py-GC/MS. Ind. Eng. Chem. Res. 2014, 53, 9979–9984. [Google Scholar] [CrossRef]
- Imrana, A.; Bramer, E.A.; Seshanb, K.; Brem, G. Catalytic flash pyrolysis of oil-impregnated-wood and jatropha cake using sodium based catalysts. J. Anal. Appl. Pyrolysis 2016, 117, 236–246. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yue, X.; Yang, J.; Yang, Y.; Gu, H.; Peng, W. Catalytic Fast Pyrolysis of Forestry Wood Waste for Bio-Energy Recovery Using Nano-Catalysts. Energies 2019, 12, 3972. https://doi.org/10.3390/en12203972
Li C, Yue X, Yang J, Yang Y, Gu H, Peng W. Catalytic Fast Pyrolysis of Forestry Wood Waste for Bio-Energy Recovery Using Nano-Catalysts. Energies. 2019; 12(20):3972. https://doi.org/10.3390/en12203972
Chicago/Turabian StyleLi, Cheng, Xiaochen Yue, Jun Yang, Yafeng Yang, Haiping Gu, and Wanxi Peng. 2019. "Catalytic Fast Pyrolysis of Forestry Wood Waste for Bio-Energy Recovery Using Nano-Catalysts" Energies 12, no. 20: 3972. https://doi.org/10.3390/en12203972
APA StyleLi, C., Yue, X., Yang, J., Yang, Y., Gu, H., & Peng, W. (2019). Catalytic Fast Pyrolysis of Forestry Wood Waste for Bio-Energy Recovery Using Nano-Catalysts. Energies, 12(20), 3972. https://doi.org/10.3390/en12203972