Energy Performance of an Encapsulated Phase Change Material PV/T System
Abstract
1. Introduction
2. System Description
3. Simulation Models of the PV/T-PCM System
- Owning to the high-quality insulation at the back of the PV/T panel and on the surface of storage tank and surrounding condition (wind speed is 0), the heat loss to the surroundings is ignored.
- The temperature gradient of the glass and PV cell in the thickness direction is ignored.
- When the circulating water enters the tank, it is mixed fully with the water in the tank, and there is no temperature stratification in the water tank.
- The water is a single-phase liquid and cannot be compressed.
- The viscous dissipation during the flow of the PCM fluid is ignored.
3.1. Solar Radiation Model
3.1.1. Simulation Model of Sunlight Incidence
3.1.2. Solar Direct Radiation
3.1.3. Scattered Radiation
3.1.4. Ground Reflected Radiation
3.1.5. Total Solar Radiation
3.2. Simulation Model of the PV/T-PCM Panel
3.2.1. Simulation Model of the Glass Cover
3.2.2. Simulation Model of a PV Cell Layer
3.2.3. Simulation Model of the PCM
3.3. Simulation Model of the Storage Tank
4. Method and Process of Solving the Simulation Model
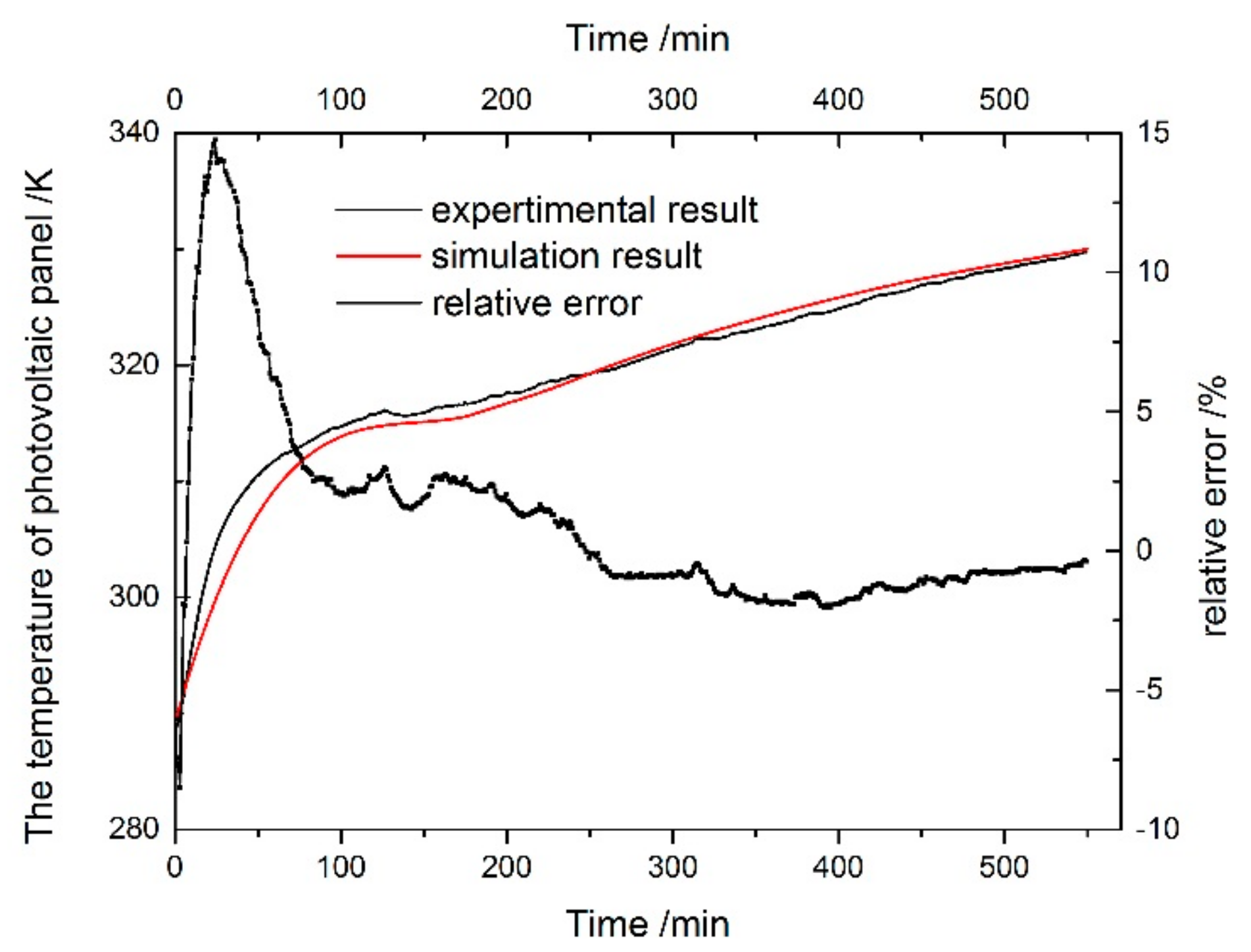
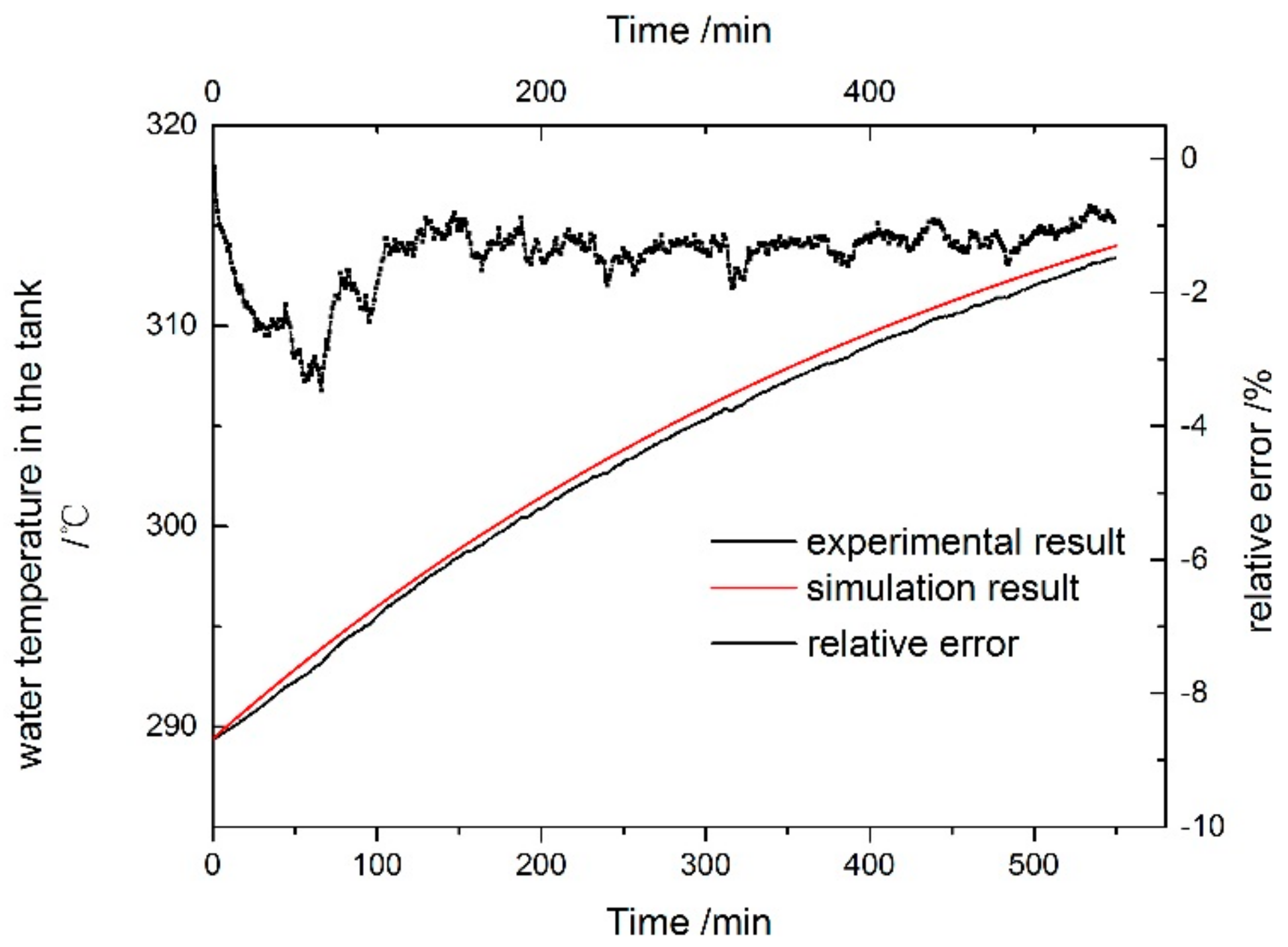
5. Experimental Verification of the Simulation Model
5.1. Error Analysis
5.2. Simulation Model Verification
6. Impact of Phase Change Temperature on the Performance of the System
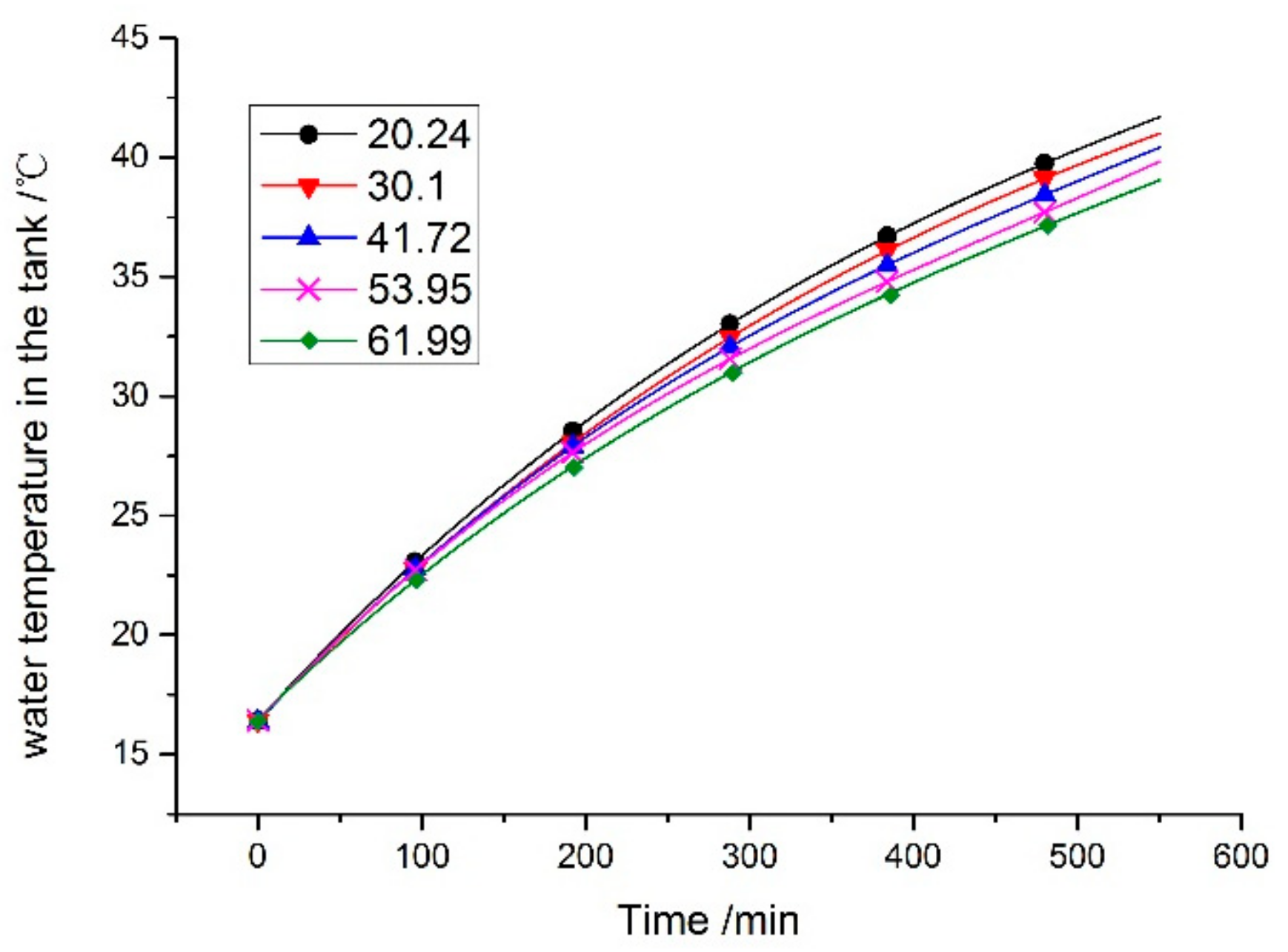
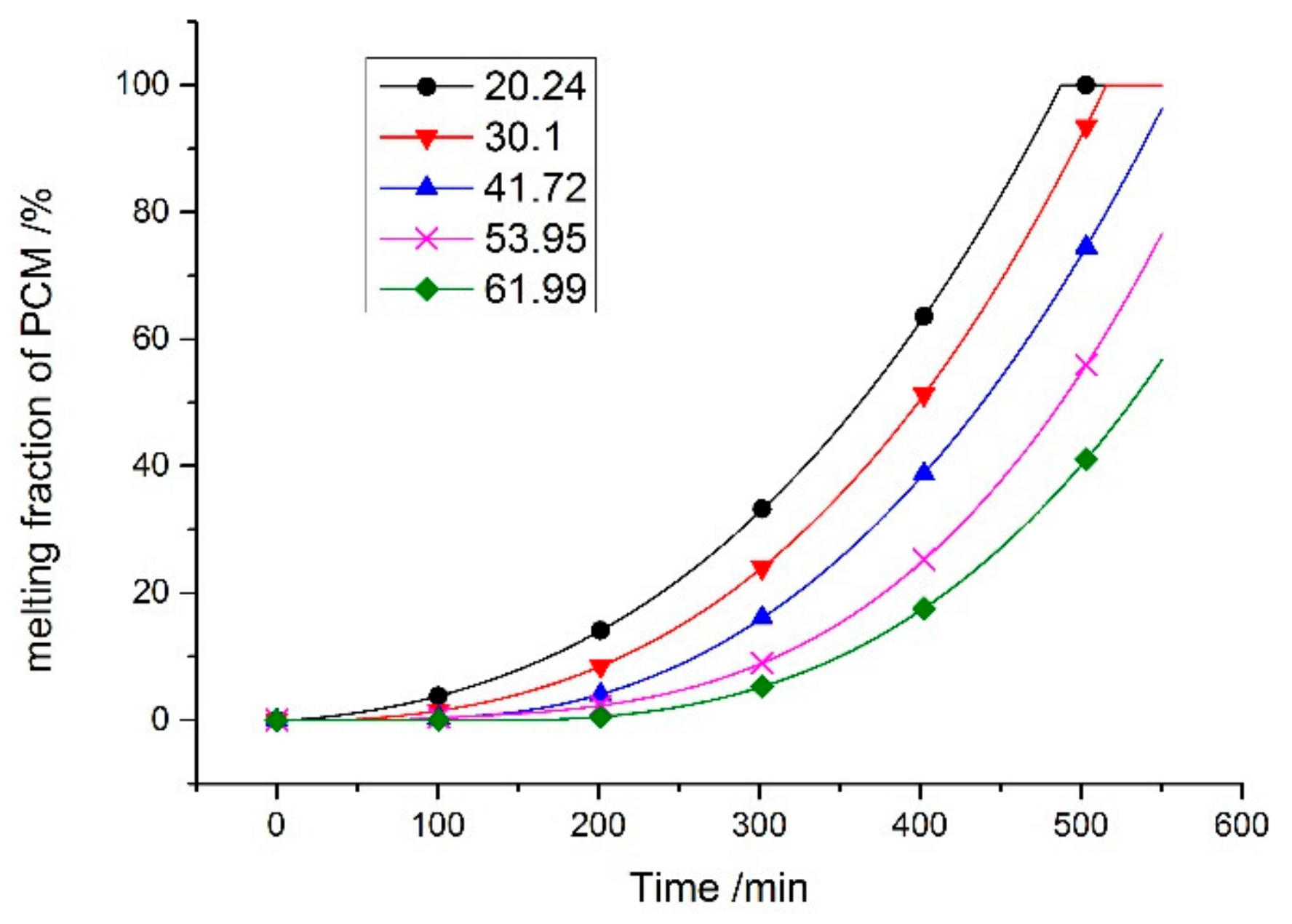
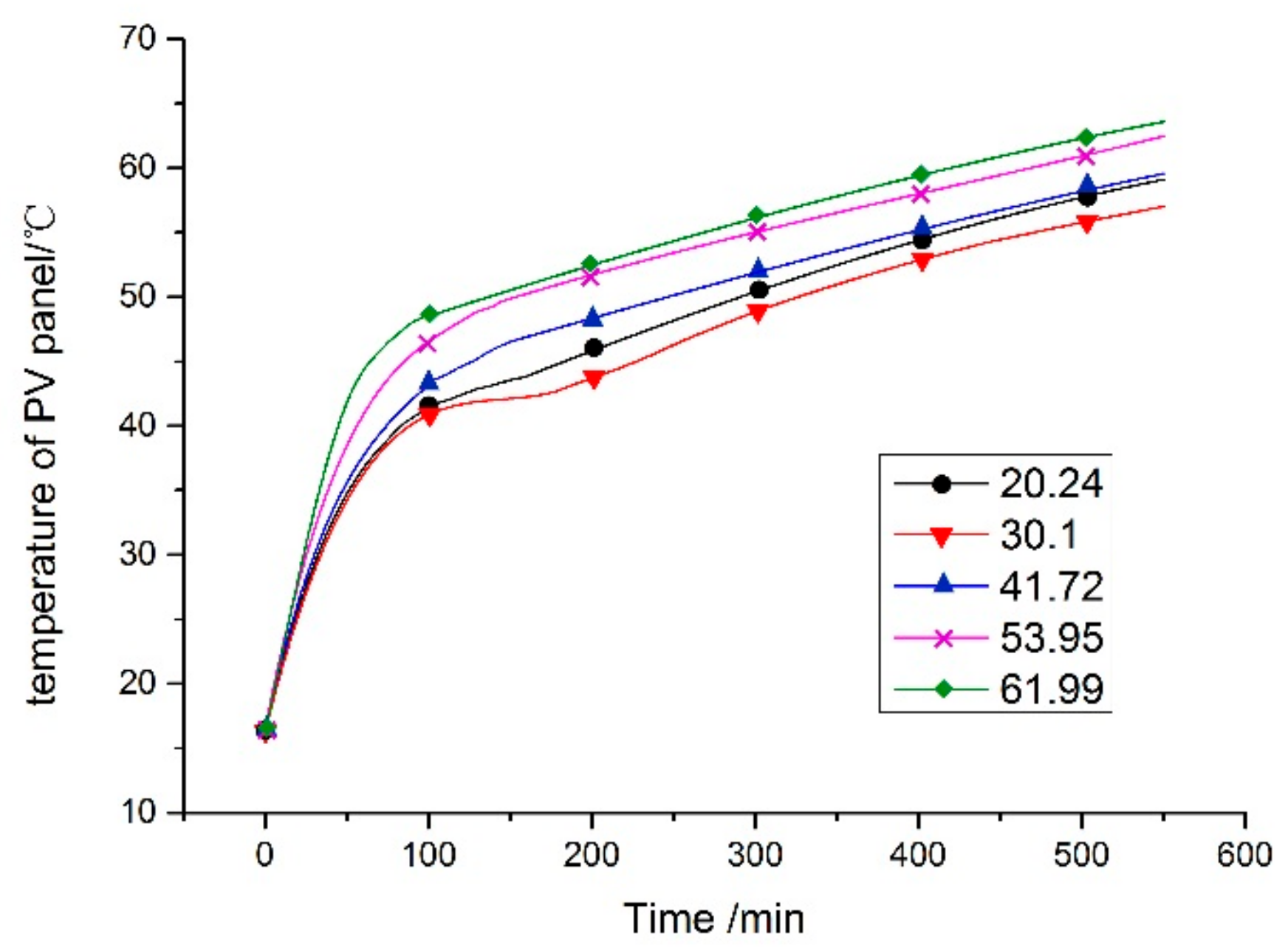
6.1. Impact of Phase Change Temperature on the Operating Temperature of the Components
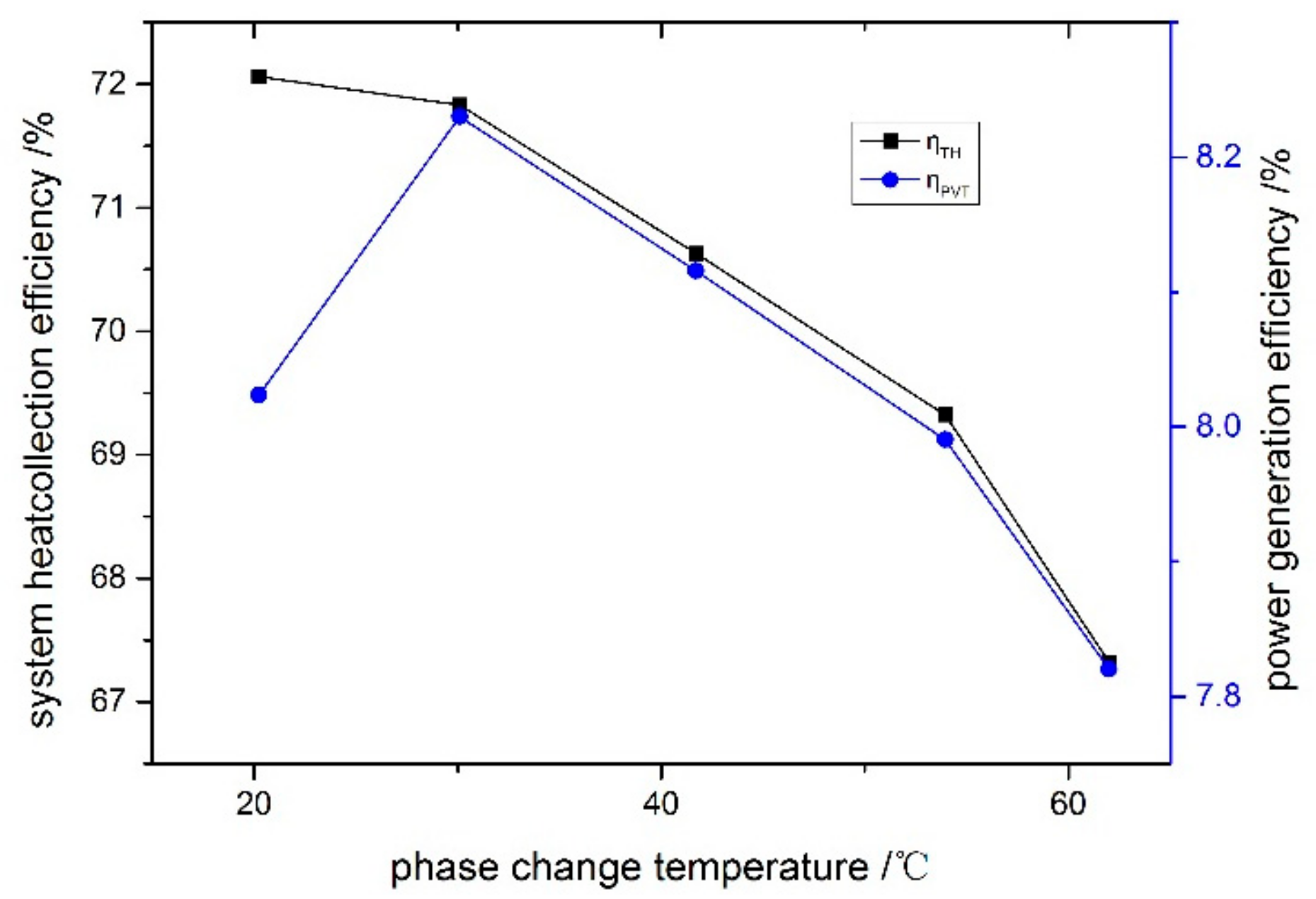
6.2. Impact of Phase Change Temperature on the Efficiency of System
7. Conclusions
- The experimental and simulation temperatures of the PV cell showed a good agreement, and the final temperature was approximately 57.0 °C.
- The relative error of the water temperature in the tank ranged from −0.1 to 3.4%, and the mean error is 4.7%. This is due to the heat loss in the practical test. The comparison results indicated that the simulation model of the system is reasonable and can be used to predict and optimize the performance of the system.
- If the overall efficiency is set as the main objective, the system with a phase change temperature of 30.1 °C exhibits the maximum value (electrical efficiency: 8.2%, thermal efficiency: 71.8%). If the exergy efficiency is set as the main objective, the system with a phase change temperature of 20.2 °C exhibits the maximum value.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Constant for the solid–liquid fuzzy region | |
| Expansion coefficient, 1/K | |
| Thermal capacity, kJ/(kg·K) | |
| Solar ridation, W/m2 | |
| Direct radiation value on the horizontal surface, W/m2 | |
| Direct radiation value on the inclined surface, W/m2 | |
| Scattered radiation on a horizontal plane, W/m2 | |
| Ground feflected rdiation, W/m2 | |
| Total solar radiation on an inclined surface, W/m2 | |
| Acceleration due to gravity, m/s2 | |
| Total enthalpy of the PCM, kJ/kg | |
| Latent heat, kJ/kg | |
| Heat transfer rate, W/m2/K | |
| Sensible heat, kJ/kg | |
| Diffusivity, m2/s | |
| Standard latent heat, kJ/kg | |
| Thickness, m | |
| Mass flow rate, kg/s | |
| Prandtl number | |
| Pressure, Pa | |
| Rayleigh number | |
| Thermal resistance of the thermally conductive silicon grease, m2·K/W | |
| Source item | |
| Temperature, K | |
| Time, s | |
| Speed vector, m/s | |
| Flow rate, m/s | |
| Greek Symbols | |
| Absorption factor, | |
| Inclined plane inclination angle, | |
| Inclined plane azimuth angle, | |
| Solar azimuth angle, | |
| Solar declination angle, | |
| Emissivity of the glass cover | |
| Ground reflectivity | |
| Efficiency | |
| Sunlight incidence, | |
| Solar zenith angle, | |
| Thermal conductivity, W/(m·K) | |
| Dynamic viscosity, N·S/m2 | |
| Wind speed, m/s | |
| Liquid phase rate | |
| Density, kg/m3 | |
| Stefan–Boltzmann constant | |
| Transmissivity | |
| Incidence angle, | |
| Auxiliary value | |
| Time angle, | |
| Subscripts | |
| Air gap | |
| Glass cover to solar direct radiation | |
| Glass cover and the PV cell (heat transfer rate) | |
| Glass cover to scattered radiation | |
| Epermental | |
| Glass cover | |
| Glass cover and sky | |
| Volume force source term at momentum of gravity direction | |
| Glass cover to earth surface reflection | |
| Inlet | |
| Melting | |
| Glass cover and the surroundings | |
| Ambient temperature | |
| Outlet | |
| PV cell | |
| Phase change material | |
| Glass cover and the PV cell (radiation heat transfer rate) | |
| Reference | |
| Sky temperature | |
| Simulated | |
| Solidification | |
| Tedlar-Polyester-Tellar | |
| Water | |
References
- Skoplaki, E.; Palyvos, J. Operating temperature of photovoltaic modules: A survey of pertinent correlations. Renew. Energy 2009, 34, 23–29. [Google Scholar] [CrossRef]
- Chow, T. A review on photovoltaic/thermal hybrid solar technology. Appl. Energy 2010, 87, 365–379. [Google Scholar] [CrossRef]
- Slimani, M.E.A.; Amirat, M.; Bahria, S.; Kurucz, I.; Aouli, M.; Sellami, R. Study and modeling of energy performance of a hybrid photovoltaic/thermal solar collector: Configuration suitable for an indirect solar dryer. Energy Convers. Manag. 2016, 125, 209–221. [Google Scholar] [CrossRef]
- Sun, L.; Li, M.; Yuan, Y.; Cao, X.; Lei, B.; Yu, N. Effect of tilt angle and connection mode of PVT modules on the energy efficiency of a hot water system for high-rise residential buildings. Renew. Energy 2016, 93, 291–301. [Google Scholar] [CrossRef]
- Su, D.; Jia, Y.; Huang, X.; Alva, G.; Tang, Y.; Fang, G. Dynamic performance analysis of photovoltaic–thermal solar collector with dual channels for different fluids. Energy Convers. Manag. 2016, 120, 13–24. [Google Scholar] [CrossRef]
- Hasan, A.; McCormack, S.; Huang, M.J.; Norton, B. Evaluation of phase change materials for thermal regulation enhancement of building integrated photovoltaics. Sol. Energy 2010, 84, 1601–1612. [Google Scholar] [CrossRef]
- Yang, X.; Sun, L.; Yuan, Y.; Zhao, X.; Cao, X. Experimental investigation on performance comparison of PV/T-PCM system and PV/T system. Renew. Energy 2018, 119, 152–159. [Google Scholar] [CrossRef]
- Navarro, L.; De Gracia, A.; Castell, A.; Cabeza, L.F. Experimental study of an active slab with PCM coupled to a solar air collector for heating purposes. Energy Build. 2016, 128, 12–21. [Google Scholar] [CrossRef]
- Serale, G.; Baronetto, S.; Goia, F.; Perino, M. Characterization and Energy Performance of a Slurry PCM-based Solar Thermal Collector: A Numerical Analysis. Energy Procedia 2014, 48, 223–232. [Google Scholar] [CrossRef]
- Sharma, S.; Tahir, A.; Reddy, K.; Mallick, T.K. Performance enhancement of a Building-Integrated Concentrating Photovoltaic system using phase change material. Sol. Energy Mater. Sol. Cells 2016, 149, 29–39. [Google Scholar] [CrossRef]
- Lin, W.; Ma, Z.; Cooper, P.; Sohel, M.I.; Yang, L. Thermal performance investigation and optimization of buildings with integrated phase change materials and solar photovoltaic thermal collectors. Energy Build. 2016, 116, 562–573. [Google Scholar] [CrossRef]
- Malvi, C.; Dixon-Hardy, D.; Crook, R. Energy balance model of combined photovoltaic solar-thermal system incorporating phase change material. Sol. Energy 2011, 85, 1440–1446. [Google Scholar] [CrossRef]
- Browne, M.C.; Lawlor, K.; Kelly, A.; Norton, B.; Mc Cormack, S.J. Indoor Characterisation of a Photovoltaic/Thermal Phase Change Material System. Energy Procedia 2015, 70, 163–171. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, X.; Li, P.; Zhang, X.; Ali, S.; Tan, J. Theoretical investigation of the energy performance of a novel MPCM (Microencapsulated Phase Change Material) slurry based PV/T module. Energy 2015, 87, 686–698. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, X.; Zhao, X.; Li, P.; Ali, S. Experimental investigation of the energy performance of a novel Micro-encapsulated Phase Change Material (MPCM) slurry based PV/T system. Appl. Energy 2016, 165, 260–271. [Google Scholar] [CrossRef]
- Yin, H.; Yang, D.; Kelly, G.; Garant, J. Design and performance of a novel building integrated PV/thermal system for energy efficiency of buildings. Sol. Energy 2013, 87, 184–195. [Google Scholar] [CrossRef]
- Duffie, J.A.; Beckman, W.A. Solar Engineering of Thermal Processes, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Perez, R.; Ineichen, P.; Seals, R. Modeling daylight availability and irradiance components from direct and global irradiance. Sol. Energy 1990, 44, 271–289. [Google Scholar] [CrossRef]
- Chow, T. Performance analysis of photovoltaic-thermal collector by explicit dynamic model. Sol. Energy 2003, 75, 143–152. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.; Chen, C.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Huang, B.-J.; Lin, T.; Hung, W.; Sun, F. Performance evaluation of solar photovoltaic/thermal systems. Sol. Energy 2001, 70, 443–448. [Google Scholar] [CrossRef]
- Loveday, D.; Taki, A. Convective heat transfer coefficients at a plane surface on a full-scale building facade. Int. J. Heat Mass Transf. 1996, 39, 1729–1742. [Google Scholar] [CrossRef]
- ANSYS. Ansys Fluent 14.0, Theory Guide; ANSYS Inc.: Canonsburg, PA, USA, 2013. [Google Scholar]
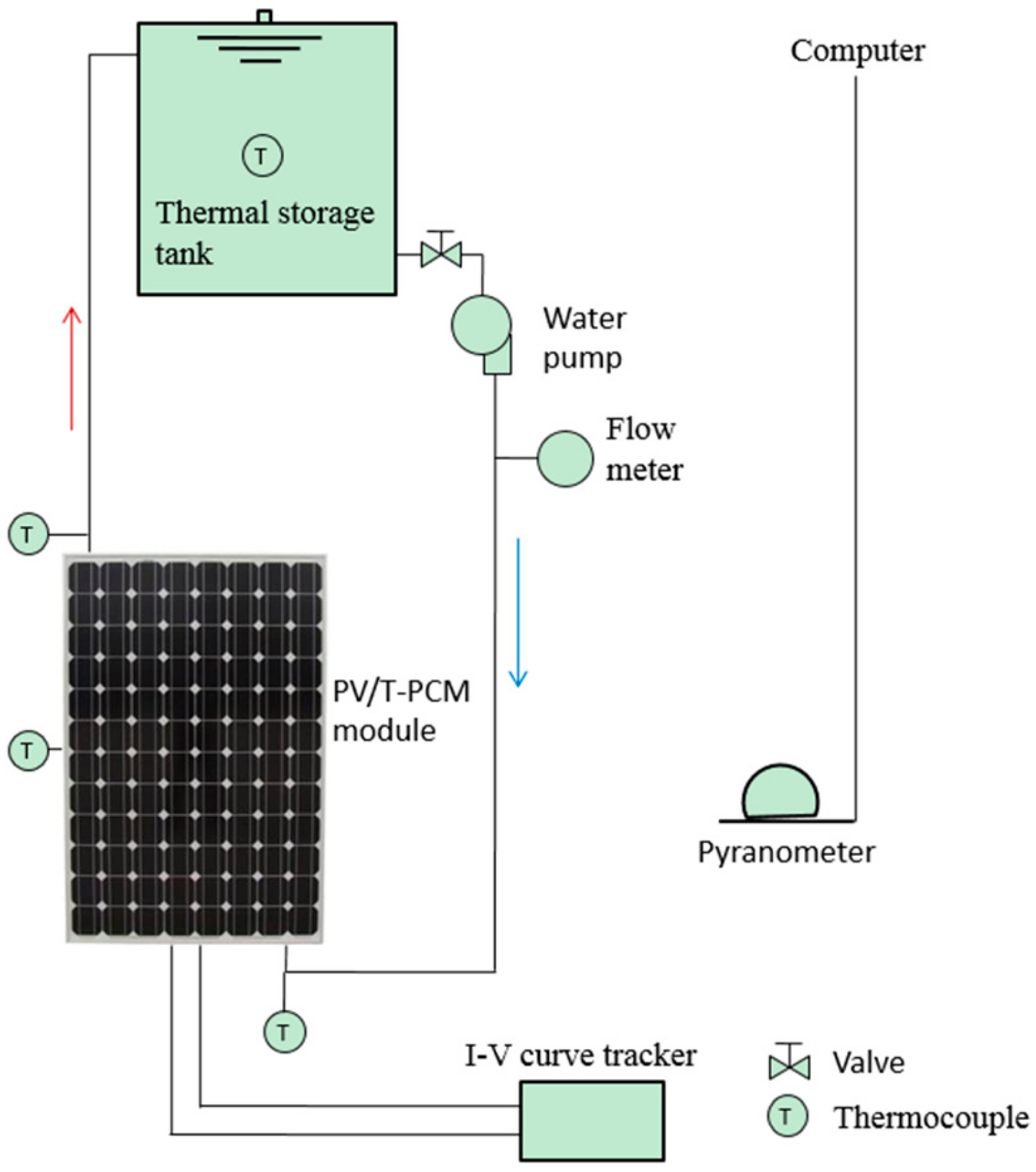

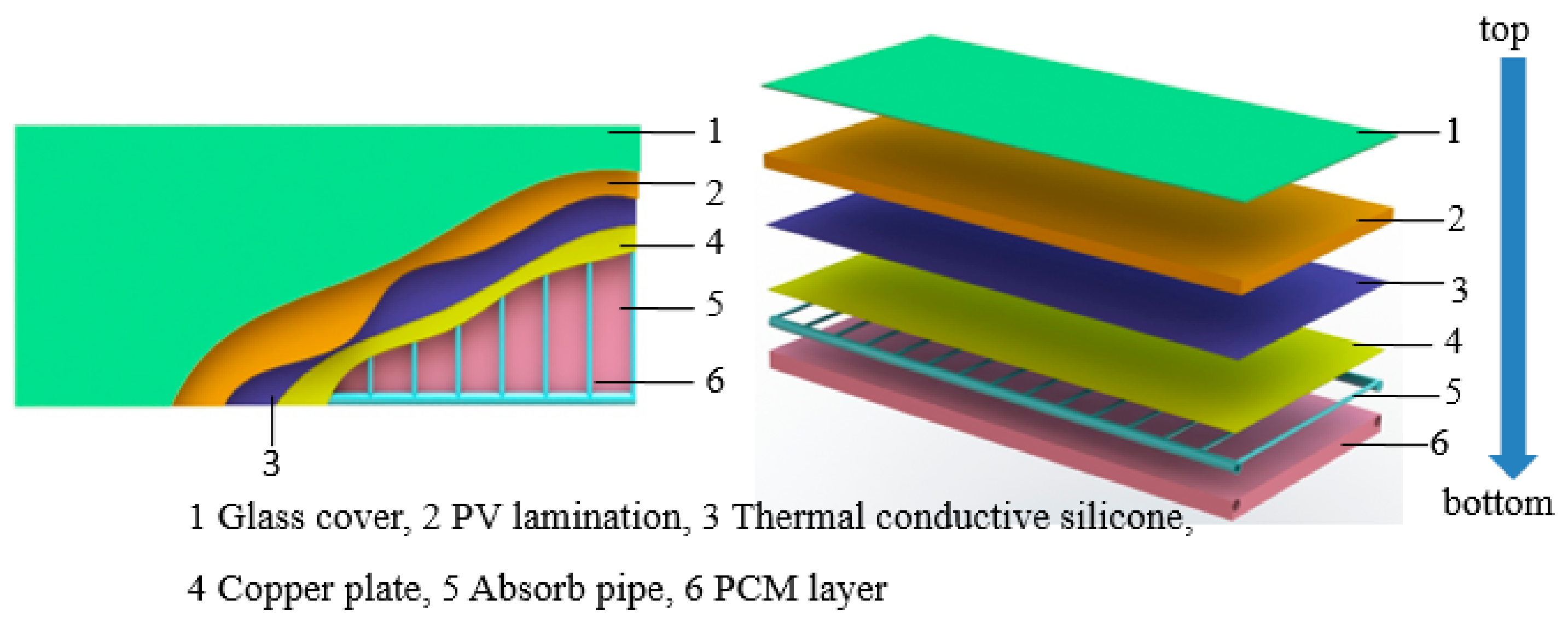

| Parameter | Measurement Equipment | Measurement Point |
|---|---|---|
| Temperature | K-type thermocouples; measuring range: −200–1300 °C, accuracy: ±0.2 °C | PCM layer at the back of the PV lamination (the middle and bottom of the layer) Thermal storage tanks (Dimensions: 0.5 × 0.5 × 0.5) |
| Flow rate | LWGY-4 turbine flowmeter; measuring range: 0.04–0.25 m3/h | Inlet of the module |
| Data logger | Agilent 34980A terminal module (Agilent Technologies) | Data recorded by computing unit |
| Electrical output | Qunling PV-8010; voltage measuring range: 10–1000 V; current measuring range: 0.1–12 A; measurement error ≤1% | Module power output |
| Solar irradiance | Delta-T SPN1 pyranometer; measuring range: 0~2000 W/m2; total radiation and scattering accuracy: 8% ± 10 W/m2 | In parallel with modules |
| Material | ||||
|---|---|---|---|---|
| PV cell | 2330 | 700 | 100 | -- |
| Thermal Grease | 1730 | 700 | 1.3 | -- |
| Absorber | 8500 | 400 | 100 | -- |
| Heat exchange pipe | 8500 | 400 | 100 | -- |
| PCM | 878 | 1700 | 0.153 | 0.007 |
| Water | 998.2 | 4182 | 0.6 | 0.001003 |
| Fatty Acid Phase Change Material | Phase Change Temperature (°C) | Latent Heat (kJ/kg) |
|---|---|---|
| CA-MA | 20.24 | 136.7 |
| CA | 30.1 | 163 |
| MA-PA-SA | 41.72 | 159.6 |
| PA-SA | 53.95 | 177.7 |
| PA | 61.99 | 186 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhou, J.; Yuan, Y. Energy Performance of an Encapsulated Phase Change Material PV/T System. Energies 2019, 12, 3929. https://doi.org/10.3390/en12203929
Yang X, Zhou J, Yuan Y. Energy Performance of an Encapsulated Phase Change Material PV/T System. Energies. 2019; 12(20):3929. https://doi.org/10.3390/en12203929
Chicago/Turabian StyleYang, Xiaojiao, Jinzhi Zhou, and Yanping Yuan. 2019. "Energy Performance of an Encapsulated Phase Change Material PV/T System" Energies 12, no. 20: 3929. https://doi.org/10.3390/en12203929
APA StyleYang, X., Zhou, J., & Yuan, Y. (2019). Energy Performance of an Encapsulated Phase Change Material PV/T System. Energies, 12(20), 3929. https://doi.org/10.3390/en12203929




