Solid Particles in Natural Gas from a Transportation Network: A Chemical Composition Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples for Analyses
2.2. Analytical Device and Parameters
2.3. Analysis Procedure
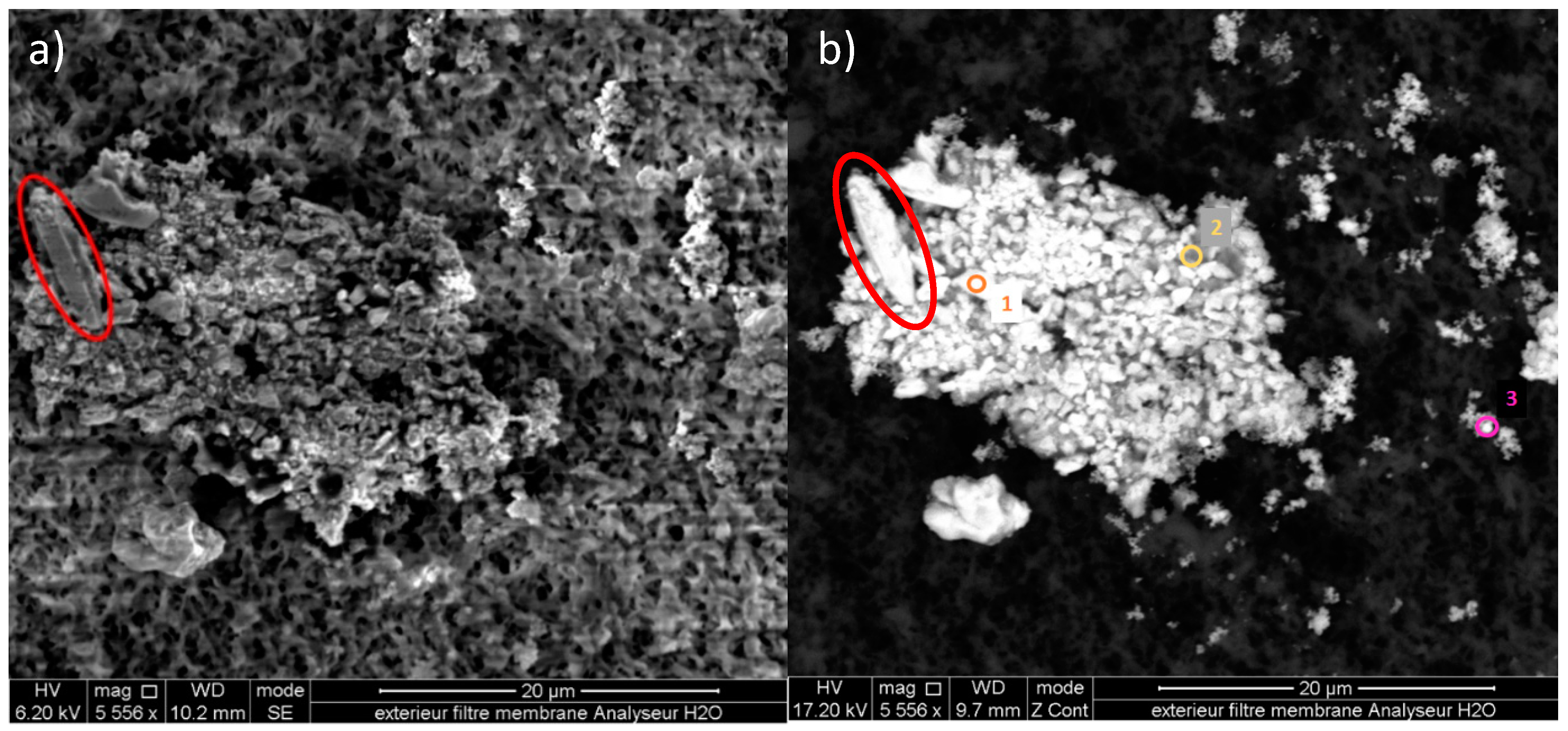
3. Results and Discussion
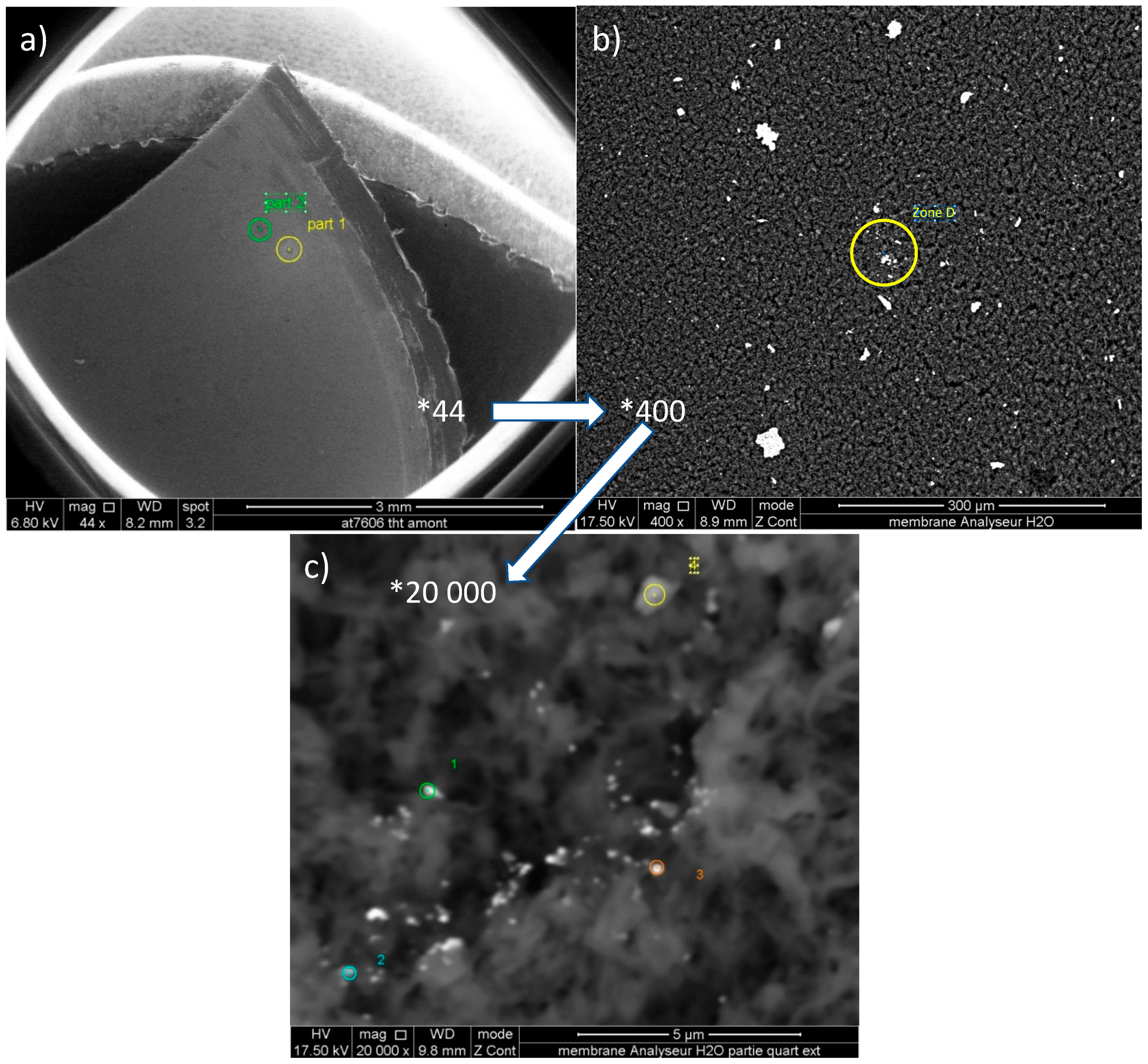
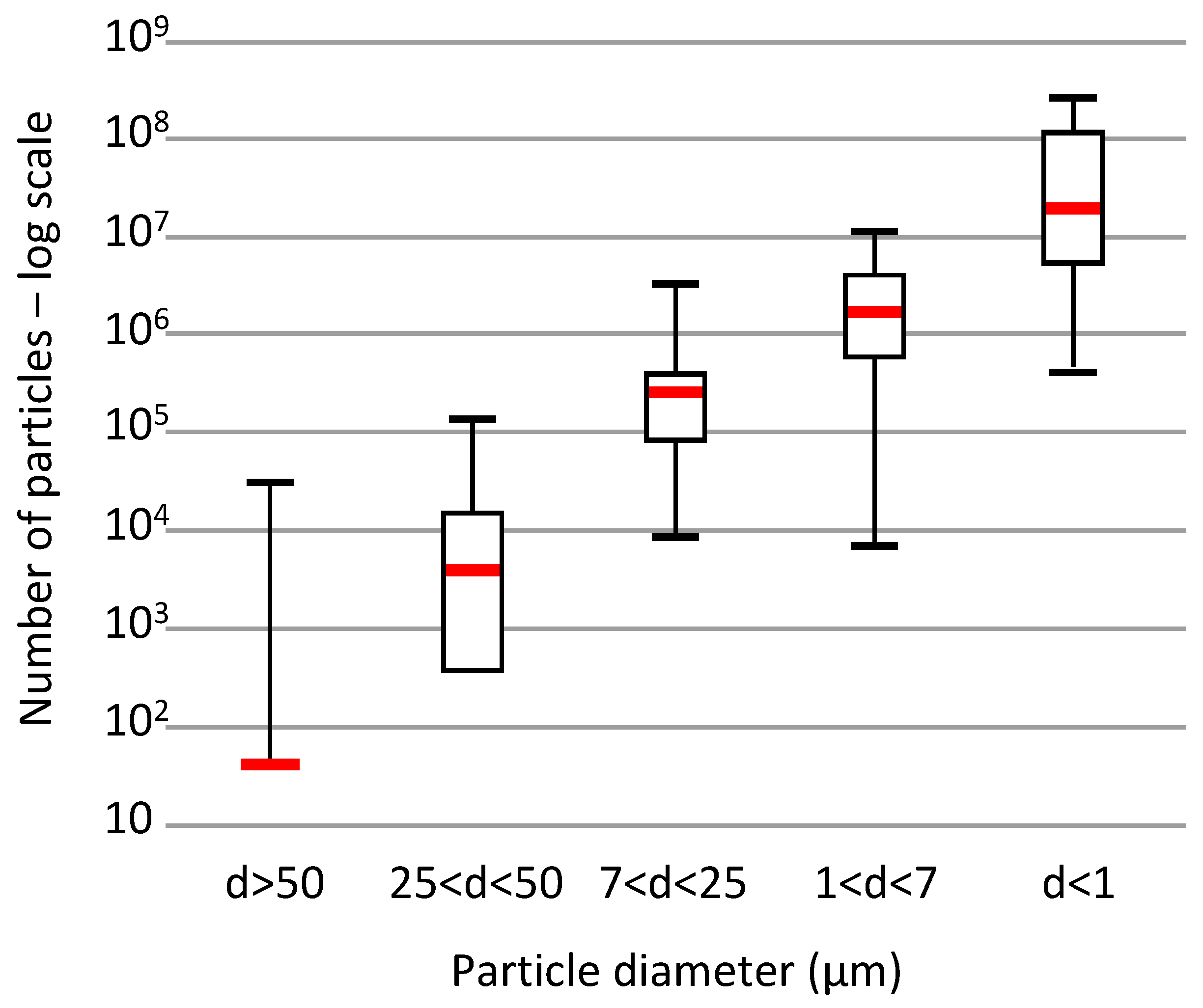
3.1. Particle Size on Cellulose Filters
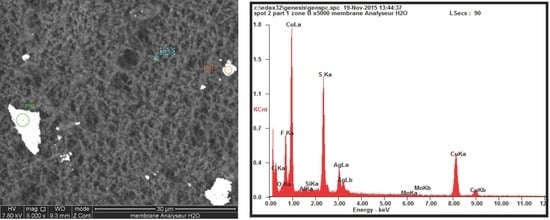
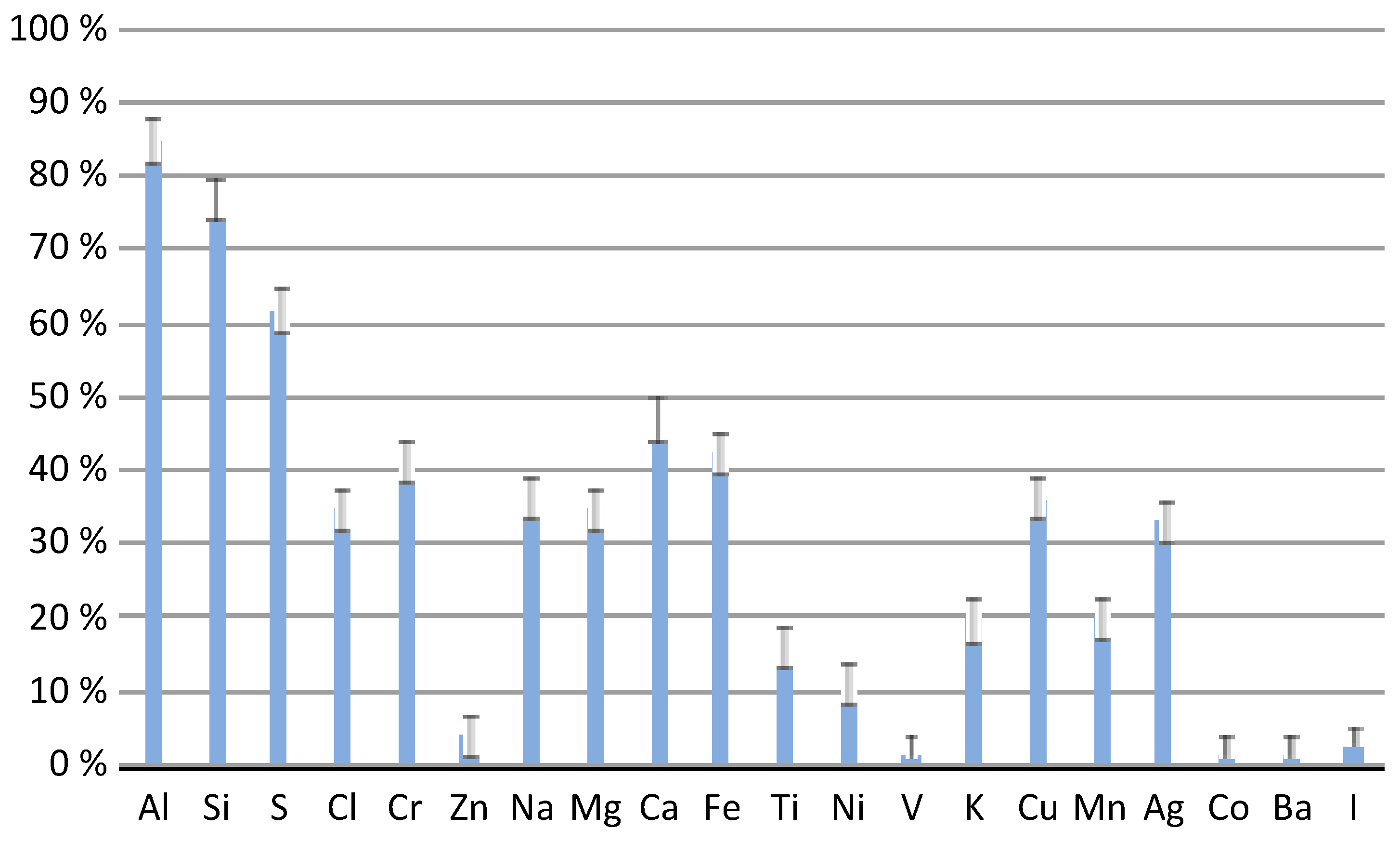
3.2. Chemical Composition of Particles
3.3. Study of Particles with Diameters >7 µm on the Cellulose Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, L.; Geng, P. A review on natural gas/diesel dual fuel combustion, emissions and performance. Fuel Process. Technol. 2016, 142, 264–278. [Google Scholar] [CrossRef]
- Dilaver, Ö.; Dilaver, Z.; Hunt, L.C. What drives natural gas consumption in Europe? Analysis and projections. J. Nat. Gas. Sci. Eng. 2014, 19, 125–136. [Google Scholar] [CrossRef]
- S EN 16726:2015+A1:2018. Gas Infrastructures. Quality of Gas; Group H. BSI: London, UK, 2017; p. 56. [Google Scholar]
- Rojey, A.; Durand, B.; Jaffret, C.; Jullian, S.; Valais, M. Le Gaz Naturel: Production, Traitement, Transport; Editions Technip: Paris, France, 1994; pp. 1–430. [Google Scholar]
- Farzaneh-Gord, M.; Niazmand, A.; Deymi-Dashtebayaz, M.; Rahbari, H.R. Effects of natural gas compositions on CNG (compressed natural gas) reciprocating compressors performance. Energy 2015, 90, 1152–1162. [Google Scholar] [CrossRef]
- Jianwen, Z.; Da, L.; Wenxing, F. An approach for estimating toxic releases of H2S-containing natural gas. J. Hazard. Mater. 2014, 264, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, L.R. Natural gas composition in a geological environment and the implications for the processes of generation and preservation. Org. Geochem. 2001, 32, 913–931. [Google Scholar] [CrossRef]
- Krupp, E.M.; Johnson, C.; Rechsteiner, C.; Moir, M.; Leong, D.; Feldmann, J. Investigation into the determination of trimethylarsine in natural gas and its partitioning into gas and condensate phases using (cryotrapping)/gas chromatography coupled to inductively coupled plasma mass spectrometry and liquid/solid sorption techniques. Spectrochim. Acta Part B At. Spectrosc. 2007, 62, 970–977. [Google Scholar] [CrossRef]
- Duoyi, W.; Meizhou, D.; Yinghan, L.; Yawei, L.; Xingyun, L.; Renqi, L. Discovery of the metal trace elements in natural gas and its ecological environment significance. Earth Sci. Front. 2008, 15, 124–132. [Google Scholar] [CrossRef]
- Ezzeldin, M.F.; Gajdosechova, Z.; Masod, M.B.; Zaki, T.; Feldmann, J.; Krupp, E.M. Mercury speciation and distribution in an egyptian natural gas processing plant. Energy Fuels 2016, 30, 10236–10243. [Google Scholar] [CrossRef]
- Cachia, M.; Bouyssière, B.; Carrier, H.; Garraud, H.; Caumette, G.; Le Hécho, I. Development of a high-pressure bubbling sampler for trace element quantification in natural gas. Energy Fuels 2017, 31, 4294–4300. [Google Scholar] [CrossRef]
- Azadi, M.; Mohebbi, A.; Soltaninejad, S.; Scala, F. A case study on suspended particles in a natural gas urban transmission and distribution network. Fuel Process. Technol. 2012, 93, 65–72. [Google Scholar] [CrossRef]
- Pack, D.J.; Parks, D.W.; Chesnoy, A.B. Gas pipeline preferential site selection occurrence for elemental sulphur & other particle matter formation & deposition. J. Petrol. Sci. Eng. 2012, 94–95, 12–18. [Google Scholar] [CrossRef]
- Xiong, Z.; Ji, Z.; Wu, X.; Chen, Y.; Chen, H. Experimental and numerical simulation investigations on particle sampling for high-pressure natural gas. Fuel 2008, 87, 3096–3104. [Google Scholar] [CrossRef]

| Element | % Total Weight | % Total Atomic | |
|---|---|---|---|
| Particle 1 | NaK | 22.31 ± 3.35 | 29.03 ± 4.35 |
| MgK | 3.38 ± 2.37 | 4.16 ± 2.91 | |
| AlK | 8.11 ± 0.41 | 9.00 ± 0.45 | |
| SiK | 9.60 ± 0.48 | 10.22 ± 0.51 | |
| PK | 3.71 ± 2.23 | 3.58 ± 2.15 | |
| SK | 9.42 ± 0.94 | 8,79 ± 0.88 | |
| ClK | 26.97 ± 4.05 | 22.76 ± 3.41 | |
| KK | 7.64 ± 1.91 | 5.85 ± 1.46 | |
| CaK | 8.85 ± 1.33 | 6.61 ± 0.99 | |
| Particle 2 | AlK | 6.25 ± 3.44 | 11.12 ± 6.12 |
| SiK | 4.02 ± 3.62 | 6.87 ± 6.18 | |
| CrK | 76.98 ± 26.94 | 71.05 ± 24.87 | |
| FeK | 12.75 ± 3.19 | 10.95 ± 2.74 |
| Element | % Total Weight | % Total Atomic | |
|---|---|---|---|
| Main particle | MgK | 0.54 ± 0.29 | 1.42 ± 0.78 |
| AlK | 0.43 ± 0.24 | 1.03 ± 0.57 | |
| SiK | 0.33 ± 0.18 | 0.78 ± 0.43 | |
| SK | 19.09 ± 10.49 | 38.02 ± 20.91 | |
| AgL | 51.47 ± 28.30 | 30.48 ± 16.76 | |
| CuK | 28.13 ± 15.47 | 28.28 ± 15.55 | |
| Smaller particles | MgK | 0.75 ± 0.23 | 2.38 ± 0.71 |
| AlK | 0.54 ± 0.16 | 1.50 ± 0.15 | |
| SiK | 0.65 ± 0.06 | 1.77 ± 0.35 | |
| SK | 11.79 ± 4.72 | 28.40 ± 12.78 | |
| AgL | 77.90 ± 15.58 | 55.78 ± 16.73 | |
| CuK | 8.37 ± 3.77 | 10.15 ± 4.57 |
| Element | % Total Weight | % Total Atomic | |
|---|---|---|---|
| Spot 1 | SK | 20.60 ± 8.24 | 21.99 ± 9.89 |
| AgL | 44.17 ± 8.83 | 14.02 ± 4.21 | |
| CuK | 35.22 ± 15.85 | 18.96 ±8.53 | |
| Spot 2 | MgK | 0.27 ± 0.08 | 0.32 ± 0.09 |
| AlK | 0.34 ± 0.10 | 0.35 ± 0.04 | |
| SiK | 0.29 ± 0.03 | 0.29 ± 0.06 | |
| SK | 21.14 ± 8.46 | 18.75 ± 8.44 | |
| AgL | 41.10 ± 8.22 | 10.84 ± 3.25 | |
| CaK | 1.89 ± 0.57 | 1.34 ± 0.40 | |
| CuK | 34.98 ± 15.74 | 15.65 ± 7.04 | |
| Spot 3 | SK | 12.89 ± 5.15 | 6.04 ± 2.71 |
| AgL | 57.05 ± 11.41 | 8.05 ± 2.42 | |
| CuK | 30.07 ± 13.53 | 7.11 ± 3.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cachia, M.; Carrier, H.; Bouyssiere, B.; Le Coustumer, P.; Chiquet, P.; Caumette, G.; Le Hécho, I. Solid Particles in Natural Gas from a Transportation Network: A Chemical Composition Study. Energies 2019, 12, 3866. https://doi.org/10.3390/en12203866
Cachia M, Carrier H, Bouyssiere B, Le Coustumer P, Chiquet P, Caumette G, Le Hécho I. Solid Particles in Natural Gas from a Transportation Network: A Chemical Composition Study. Energies. 2019; 12(20):3866. https://doi.org/10.3390/en12203866
Chicago/Turabian StyleCachia, Maxime, Hervé Carrier, Brice Bouyssiere, Philippe Le Coustumer, Pierre Chiquet, Guilhem Caumette, and Isabelle Le Hécho. 2019. "Solid Particles in Natural Gas from a Transportation Network: A Chemical Composition Study" Energies 12, no. 20: 3866. https://doi.org/10.3390/en12203866
APA StyleCachia, M., Carrier, H., Bouyssiere, B., Le Coustumer, P., Chiquet, P., Caumette, G., & Le Hécho, I. (2019). Solid Particles in Natural Gas from a Transportation Network: A Chemical Composition Study. Energies, 12(20), 3866. https://doi.org/10.3390/en12203866