Environmental Performance of Effluent Conditioning Systems for Reuse in Oil Refining Plants: A Case Study in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Effluent Specification and Destination of Recovered Water
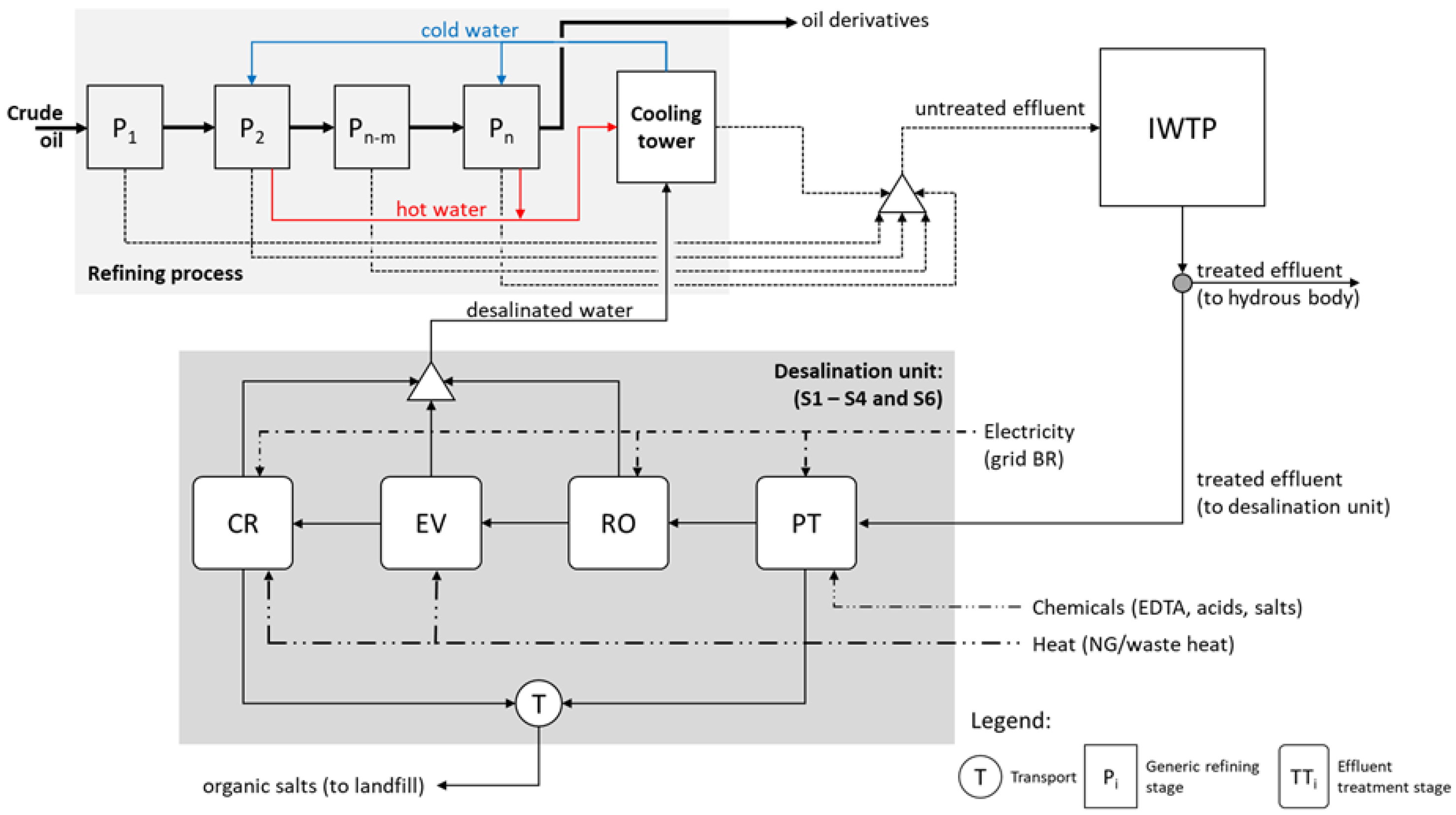
2.2. Setting the Analysis Scenarios and Description of the Recovery Systems
2.3. Mathematical Model Design
2.4. Life-Cycle Modeling
2.4.1. Scope Definition
2.4.2. Life Cycle Inventory (LCI)
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency (IEA). Key World Energy Statistics 2018; IEA: Paris, France, 2018; 51p. [Google Scholar]
- Chang, J.; Lee, W.; Yoon, S. Energy consumptions and associated greenhouse gas emissions in operation phases of urban water reuse systems in Korea. J. Clean. Prod. 2017, 141, 728–736. [Google Scholar] [CrossRef]
- Hendrickson, T.P.; Nguyen, M.T.; Sukardi, M.; Miot, A.; Horvath, A.; Nelson, K.L. Life-Cycle Energy Use and Greenhouse Gas Emissions of a Building-Scale Wastewater Treatment and Nonportable Reuse System. Environ. Sci. Technol. 2015, 49, 10303–10311. [Google Scholar] [CrossRef] [PubMed]
- Morera, S.; Corominas, L.; Poch, M.; Aldaya, M.M.; Comas, J. Water footprint assessment in wastewater treatment plants. J. Clean. Prod. 2016, 112, 4741–4748. [Google Scholar] [CrossRef]
- Cornejo, P.K.; Santana, M.V.E.; Hokanson, D.R.; Mihelcic, J.R.; Zhang, Q. Carbon footprint of water reuse and desalination: A review of greenhouse gas emissions and estimation tools. J. Water Reuse Desal. 2014, 4, 238–252. [Google Scholar] [CrossRef]
- Meneses, M.; Concepción, H.; Vrecko, D.; Vilanova, R. Life Cycle Assessment as an environmental evaluation tool for control strategies in wastewater treatment plants. J. Clean. Prod. 2015, 107, 653–661. [Google Scholar] [CrossRef]
- McNamara, G.; Horrigan, M.; Phelan, T.; Fitzsimons, L.; Delaure, Y.; Corcoran, B.; Doherty, E.; Clifford, E. Life Cycle Assessment of Wastewater Treatment Plants in Ireland. J. Sustain. Dev. Energy Water Environ. Syst. 2016, 4, 216–233. [Google Scholar] [CrossRef]
- Garfí, M.; Flores, L.; Ferrer, I. Life Cycle Assessment of wastewater treatment systems for small communities: Activated sludge, constructed wetlands and high rate algal ponds. J. Clean. Prod. 2017, 161, 211–219. [Google Scholar] [CrossRef]
- Opher, T.; Friedler, E. Comparative LCA of decentralized wastewater treatment alternatives for non-potable urban reuse. J. Environ. Manag. 2016, 182, 464–476. [Google Scholar] [CrossRef]
- García-Montoya, M.; Sengupta, D.; Nápoles-Rivera, F.; Ponce-Ortega, J.M.; El-Halwagi, M.M. Environmental and economic analysis for the optimal reuse of water in a residential complex. J. Clean. Prod. 2016, 130, 82–91. [Google Scholar] [CrossRef]
- Lam, C.-M.; Leng, L.; Chen, P.-C.; Lee, P.-H.; Hsu, S.-C. Eco-efficiency analysis of non-potable water systems in domestic buildings. Appl. Energy 2017, 202, 293–307. [Google Scholar] [CrossRef]
- Baresel, C.; Dalgren, L.; Almemark, M.; Lazic, A. Environmental performance of wastewater reuse systems: Impact of system boundaries and external conditions. Water Sci. Technol. 2016, 73, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.; Garnier, G.; Batchelor, W. Life cycle assessment comparison of industrial effluent management strategies. J. Clean. Prod. 2014, 79, 168–181. [Google Scholar] [CrossRef]
- Pintilie, L.; Torres, C.M.; Teodosiu, C.; Castells, F. Urban wastewater reclamation for industrial reuse: An LCA case study. J. Clean. Prod. 2016, 139, 1–14. [Google Scholar] [CrossRef]
- American Petroleum Institute (API). Design and Operation of Oil-Water Separators; Monographs on Refinery Environmental Control—Management of Water Discharges: Washington, DC, USA, 1990; 54p. [Google Scholar]
- Diya’uddeen, B.H.; Daud, W.M.A.W.; Aziz, A.R.A. Treatment technologies for petroleum refinery effluents: A review. Process Saf. Environ. Prot. 2011, 89, 95–105. [Google Scholar] [CrossRef]
- Gripp, V.S. Environmental, Energetic and Economic Analysis of a Process Design for Water Reuse in Petroleum Refinery. Master’s Thesis, University of São Paulo, São Paulo, Brazil, 2013; 166p. (In Portuguese). [Google Scholar]
- Moreira, R.H. Desenvolvimento de um Processo de Reúso do Efluente de Refinaria Baseado em um Sistema de Osmose Reversa Combinado com Precipitação. Master’s Thesis, University of São Paulo, São Paulo, Brazil, 2017; 112p. (In Portuguese). [Google Scholar]
- Pantoja, C.E. Cristalização Assistida por Destilação por Membranas Aplicada ao Reuso de água: Comparação com Outros Métodos de Reuso, Análise do Processo e Projeto Hierárquico de Processo. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2015; 162p. (In Portuguese). [Google Scholar]
- Diepolder, P. Is zero discharge realistic? Hydrocarb. Process. 1992, 71, 129–160. [Google Scholar]
- Anze, M.; Alves, R.M.B.; Nascimento, C.A.O. Optimization of Water Use in Oil Refinery. In Proceedings of the 2010 Annual AIChE Meeting, Salt Lake City, UT, USA, 7–12 November 2010. [Google Scholar]
- Pombo, F.R. Management of Water Demand in the Oil Refining Industry: Challenges and Opportunities for Rationalization. Ph.D. Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2011; 169p. (In Portuguese). [Google Scholar]
- International Petroleum Industry Environmental Conservation Association (IPIECA). Petroleum Refining Water/Wastewater Use and Management; IPIECA Operations Best Practice Series: London, UK, 2010; 60p. [Google Scholar]
- Rahardianto, A.; Gao, J.; Gabelich, C.J.; Williams, M.D.; Cohen, Y. High recovery membrane desalting of low-salinity brackish water: Integration of accelerated precipitation softening with membrane RO. J. Memb. Sci. 2007, 289, 123–137. [Google Scholar] [CrossRef]
- Bremere, I.; Kennedy, M.D.; Johnson, A.; van Emmerick, R.; Witkamp, G.-J.; Schippers, J.C. Increasing conversion in membrane filtration systems using a desupersaturation unit to prevent scaling. Desalination 1998, 119, 199–204. [Google Scholar] [CrossRef]
- Bremere, I.; Kennedy, M.; Michel, P.; van Emmerick, R.; Witkamp, G.J.; Schippers, J. Controlling scaling in membrane filtration systems using a desupersaturation unit. Desalination 1999, 124, 51–62. [Google Scholar] [CrossRef]
- Astilleros, J.M.; Pina, C.M.; Fernández-Díaz, L.; Prieto, M.; Putnis, A. Nanoscale phenomena during the growth of solid solutions on calcite {101−4} surfaces. Chem. Geol. 2006, 225, 322–335. [Google Scholar] [CrossRef]
- Heins, W.; Schooley, K. Achieving Zero Liquid Discharge in SAGD heavy oil recovery. J. Can. Petrol. Technol. 2004, 43, 37–42. [Google Scholar] [CrossRef]
- Mickley, M. Survey of High-Recovery and Zero Liquid Discharge Technologies for Water Utilities; WateReuse Foundation: Alexandria, VA, USA, 2008; 180p. [Google Scholar]
- Al-Karaghouli, A.; Kazmerski, L. Energy consumption and water production cost of conventional and renewable-energy-powered desalination processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356. [Google Scholar] [CrossRef]
- Althaus, H.-J.; Chudacoff, M.; Hischier, R.; Jungbluth, N.; Osses, M.; Primas, A. Life Cycle Inventories of Chemicals; Ecoinvent Report No. 8, v2.0; EMPA Dübendorf, Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007; 957p. [Google Scholar]
- Veza, J.M. Mechanical vapour compression desalination plants—A case study. Desalination 1995, 101, 1–10. [Google Scholar] [CrossRef]
- Jongema, P. Optimization of the Fuel Consumption of an Evaporation Salt Plat with the Aid of the Exergy Concept. In Proceedings of the Sixth International Symposium on Salt, Toronto, ON, Canada, 24–28 May 1983; Salt Institute: Alexandria, VA, USA, 1983; pp. 463–469. [Google Scholar]
- International Organization for Standardization. ISO 14044, Environmental Management—Life Cycle Assessment—Requirements and Guidelines, 1st ed.; International Organization for Standardization: Genève, Switzerland, 2006; 46p. [Google Scholar]
- Hischier, R.; Weidema, B.; Althaus, H.-J.; Bauer, C.; Doka, G.; Dones, R.; Frischknecht, R.; Hellweg, S.; Humbert, S.; Jungbluth, N.; et al. Implementation of Life Cycle Assessment Methods; Ecoinvent report No. 3, v. 2.2; Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2010; 176p. [Google Scholar]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.D.M.; Hollander, A.; Zijp, M.; van Zelm, R. ReCiPe 2016: A Harmonized Life Cycle Impact Assessment Method at Midpoint and Endpoint Level Report I: Characterization; Department of Environmental Science, Radbound University: Nijmegen, The Netherlands, 2016; 194p. [Google Scholar]
- Empresa de Pesquisa Energética. Brazilian Energy Balance 2016—Year 2015; Empresa de Pesquisa Energética: Rio de Janeiro, Brazil, 2016; 294p. (In Portuguese)
- Empresa de Pesquisa Energética. Energia Termelétrica: Gás Natural, Biomassa, Carvão, Nuclear; Empresa de Pesquisa Energética: Rio de Janeiro, Brazil, 2016; 417p. (In Portuguese)
- Franklin Associates. Data Details for Petroleum Refining; Franklin Associates; Eastern Research Group, Inc.: Prairie Village, Kansas, 2003. [Google Scholar]
- Hischier, R. Life Cycle Inventories of Packaging and Graphical Papers; Ecoinvent report No. 11, v2.0; Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007; 17p. [Google Scholar]
- Jungbluth, N. Erdöl. In Sachbilanzen von Energiesystemen: Grundlagen fùr den ergleich von Energiesystemen und den Einbezug von Energiesystemen in Ökobilanzen für die Schweiz; Ecoinvent Report No. 6-IV, v 2.0; Dones, R., Ed.; Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007; 327p. (In German) [Google Scholar]
- Kellenberger, D.; Althaus, H.-J.; Jungbluth, N.; Künniger, T.; Lehmann, M.; Thalmann, P. Life Cycle Inventories of Building Products; Final Report Ecoinvent No. 7, v2.0; EMPA Dübendorf, Swiss Centre for Life Cycle Inventories: Dübendorf, Switzerland, 2007; 914p. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) Intergovernmental Panel on Climate Change. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar]

| Analytes | IWTP Effluent [17,18,19] | IWTP Effluent (Defined Value) |
|---|---|---|
| (Ranges of Concentration, ppm) | (ppm) | |
| Ba2+ | 0.20–0.50 | 0.30 |
| Ca2+ | 38.0–63.0 | 53.1 |
| Al3+ | 0.00–0.01 | <0.01 |
| Sr2+ | 1.10–1.83 | 1.32 |
| SiO2 | 0.80–19.4 | 7.33 |
| Fe (total) | 0.00–0.01 | <0.01 |
| Mg2+ | 4.00–7.52 | 6.56 |
| Na+ | 179–283 | 255 |
| Cl∓ | 311–425 | 385 |
| HCO3− | 55.9–308 | 216 |
| K+ | 6.00–11.5 | 7.76 |
| NH4+ | 0.50–7.60 | 4.56 |
| PO43− | 0.00–3.74 | 2.12 |
| NO3− | 22.4–207 | 101 |
| SO42− | 85.6–163 | 110 |
| F− | 0.10–0.69 | 0.20 |
| TDS (as, NaCl) 1 | 359–1103 | 762 |
| pH | 6.74–8.81 | 6.74 |
| Parameter | Technological Conditions | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|---|
| Technological approach | Reverse osmosis (RO) | + | + | + | – | + | + |
| Evaporative crystallization (EV + CR) | + | + | + | + | + | + | |
| Pre-treatment method | Barium desupersaturation (BaDs) | – | + | – | – | – | – |
| Coprecipitation (CPT) | – | – | + | – | + | + | |
| Energy source | Electricity | BR grid 1 | BR grid | BR grid | BR grid + WH | BR grid | BR grid |
| Heat | NG 2 | NG | NG | WH 3 | – | NG | |
| Yield () | Water recovery at RO (%) | 84.6 | 87.0 | 95.8 | – | 95.8 | 95.8 |
| Impact Category | S1 | S2 | S3 | S4 | S5 | S6 | |
|---|---|---|---|---|---|---|---|
| PED (MJ/RF) | Non-renewable, fossil | 44.4 | 37.6 | 15.7 | 3.49 | 5.97 | 16.8 |
| Non-renewable, nuclear | 0.51 | 0.51 | 0.57 | 0.57 | 0.88 | 0.57 | |
| Renewable, biomass | 0.78 | 0.78 | 0.78 | 0.99 | 1.32 | 0.69 | |
| Renewable, wind | 0.32 | 0.32 | 0.32 | 0.42 | 0.50 | 0.32 | |
| Renewable, water | 4.79 | 4.79 | 5.33 | 6.43 | 8.53 | 4.62 | |
| Total | 50.8 | 44.0 | 22.7 | 11.9 | 17.2 | 23.0 | |
| GW (kgCO2,eq) | 2.93 | 2.56 | 1.52 | 0.72 | 1.24 | 1.93 | |
| Stage | Energy Source | S1 | S2 | S3 | S4 | S5 | S6 |
|---|---|---|---|---|---|---|---|
| RO | Electricity | 1.12 | 1.33 | 2.12 | - | 2.12 | 2.12 |
| EV | Heat | 35.9 | 30.3 | 9.74 | - | - | 9.76 |
| Electricity | 1.40 | 1.19 | 0.40 | 9.00 | 4.86 | 0.40 | |
| Waste Heat | - | - | - | 230 | - | - | |
| CR | Electricity | 4.07 | 4.07 | 3.38 | 0.11 | 3.38 | 3.53 |
| Waste Heat | - | - | - | 3.96 | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, H.; Ronquim, F.M.; Seckler, M.M.; Kulay, L. Environmental Performance of Effluent Conditioning Systems for Reuse in Oil Refining Plants: A Case Study in Brazil. Energies 2019, 12, 326. https://doi.org/10.3390/en12020326
Sakamoto H, Ronquim FM, Seckler MM, Kulay L. Environmental Performance of Effluent Conditioning Systems for Reuse in Oil Refining Plants: A Case Study in Brazil. Energies. 2019; 12(2):326. https://doi.org/10.3390/en12020326
Chicago/Turabian StyleSakamoto, Hugo, Flávia M. Ronquim, Marcelo Martins Seckler, and Luiz Kulay. 2019. "Environmental Performance of Effluent Conditioning Systems for Reuse in Oil Refining Plants: A Case Study in Brazil" Energies 12, no. 2: 326. https://doi.org/10.3390/en12020326
APA StyleSakamoto, H., Ronquim, F. M., Seckler, M. M., & Kulay, L. (2019). Environmental Performance of Effluent Conditioning Systems for Reuse in Oil Refining Plants: A Case Study in Brazil. Energies, 12(2), 326. https://doi.org/10.3390/en12020326





