Modeling and Simulation Studies Analyzing the Pressure-Retarded Osmosis (PRO) and PRO-Hybridized Processes
Abstract
1. Introduction
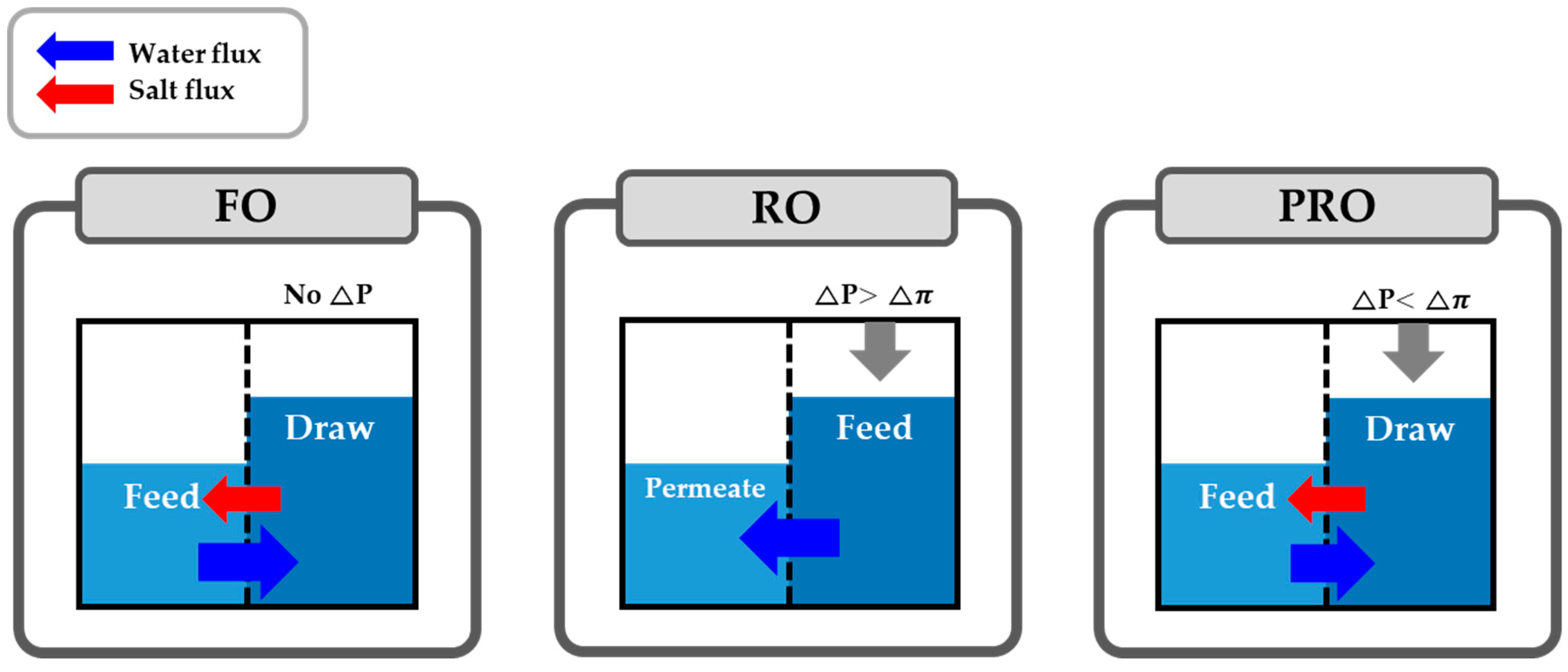
2. Fundamental Theories for a PRO Process
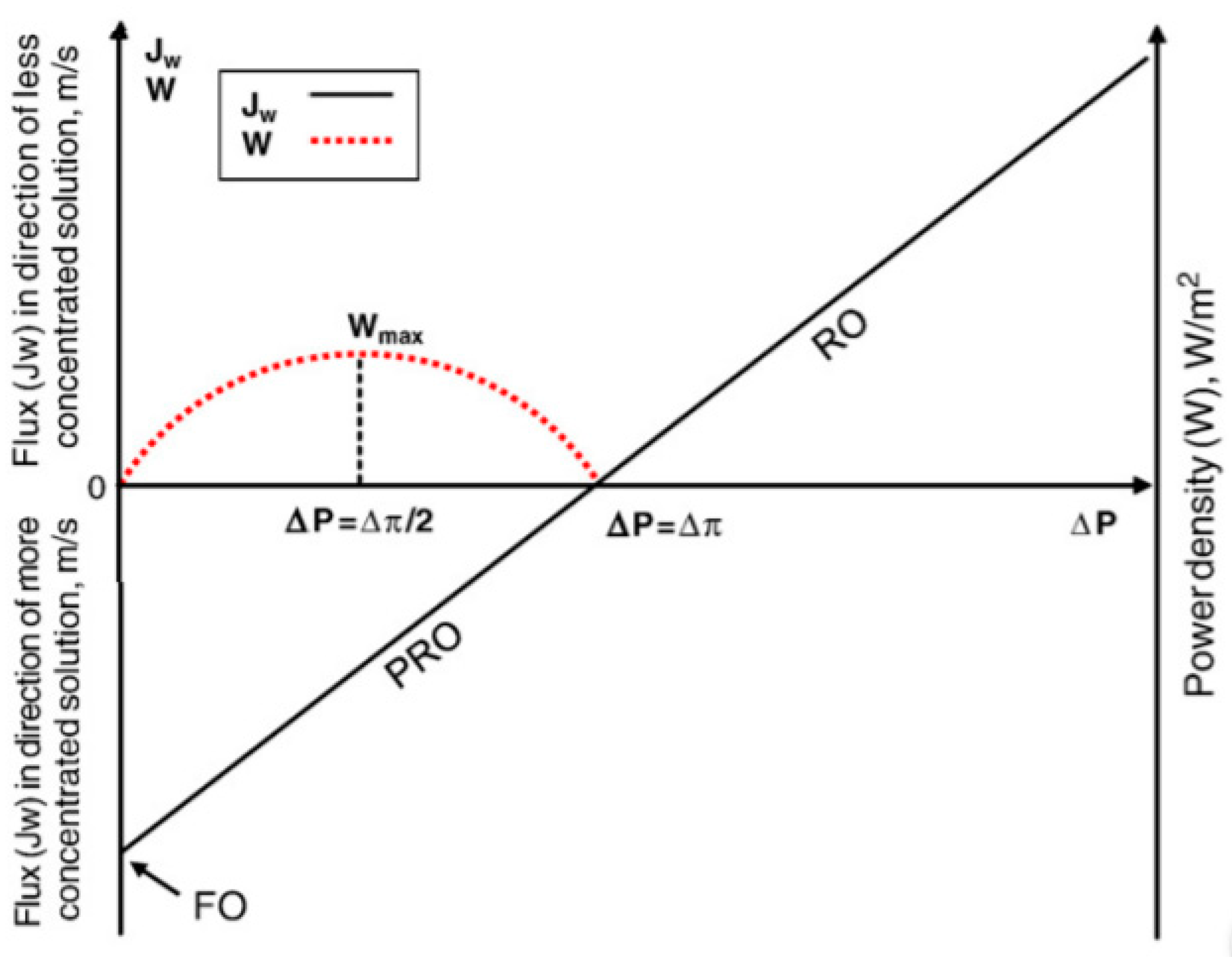
2.1. Water Flux and Power Density
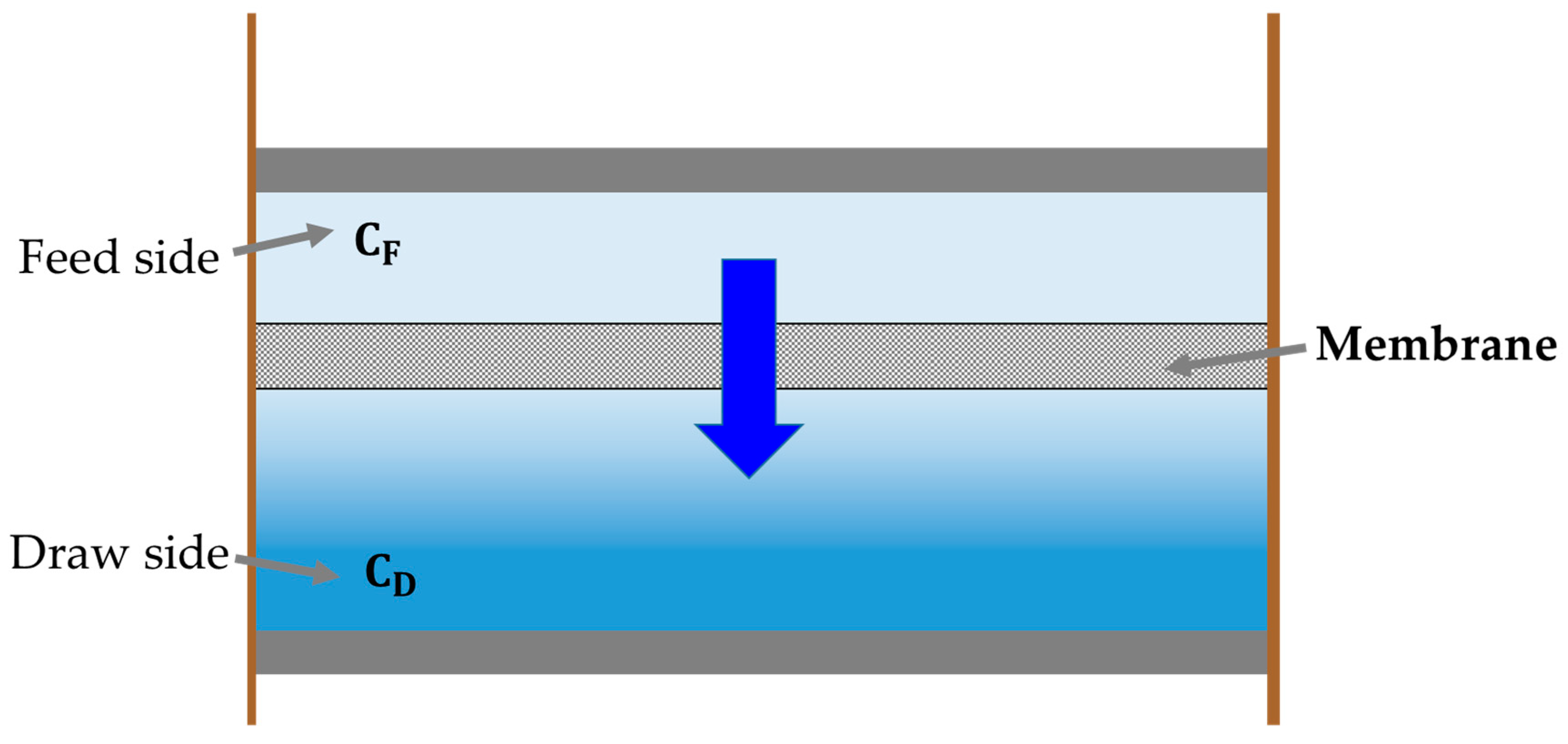
2.2. Water Flux with Concentration Polarization Phenomena
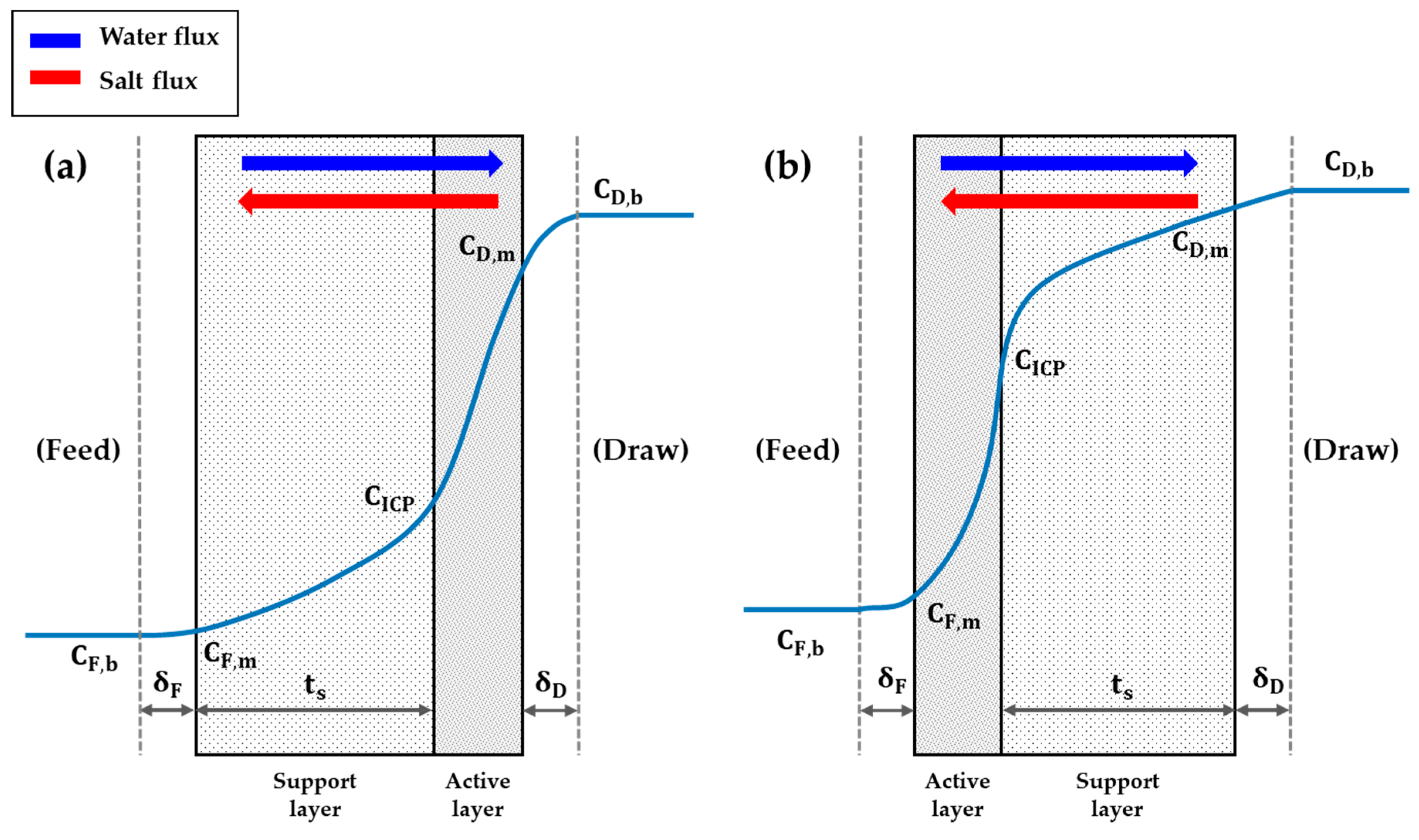
2.2.1. Internal Concentration Polarization
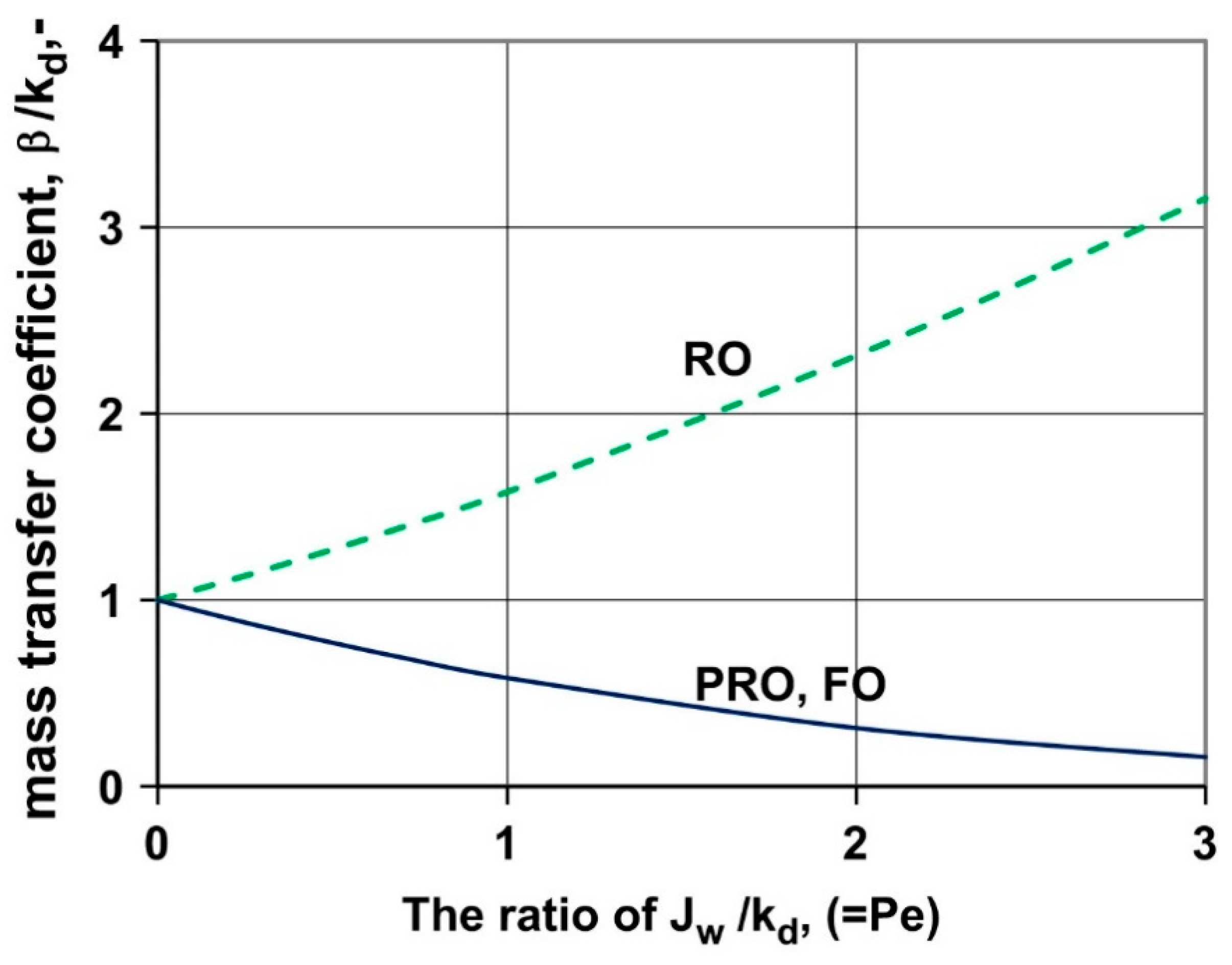
2.2.2. External Concentration Polarization
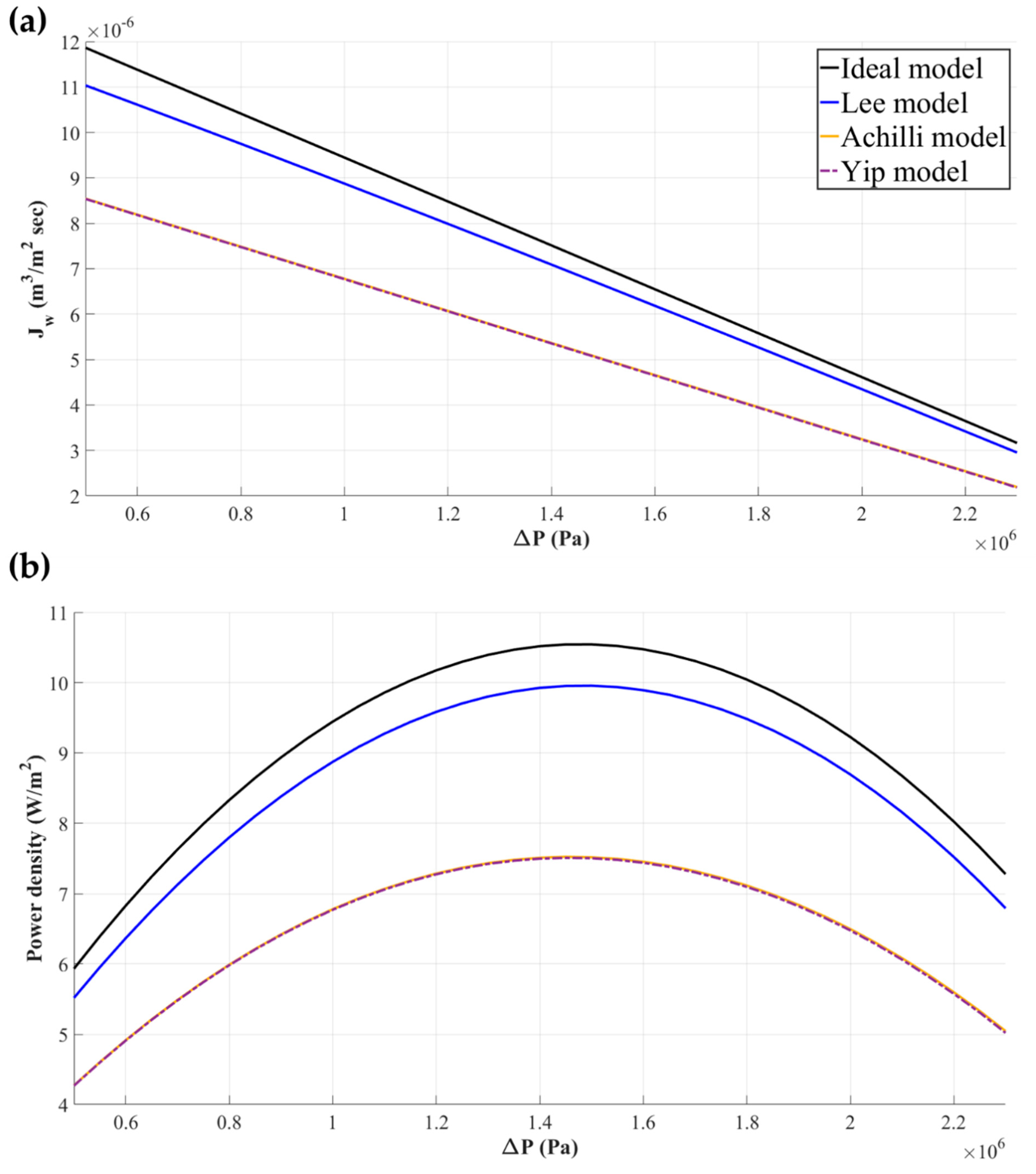
2.3. Water Flux Models Applicable to PRO
2.3.1. Water Flux Models for Flat-Sheet Membrane
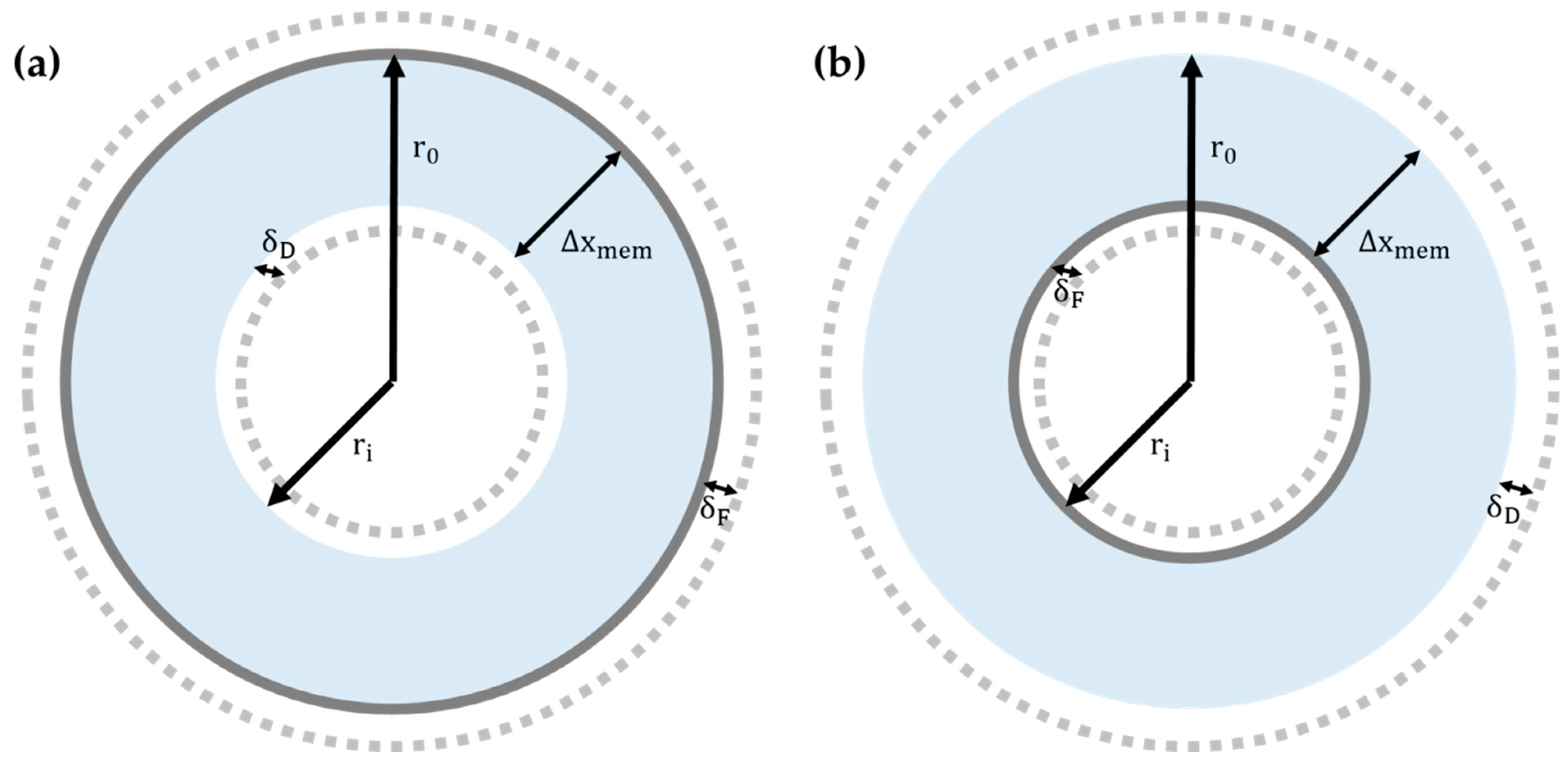
2.3.2. Water Flux Models for Hollow Fiber Membrane
3. Hybridization of a PRO Process with RO
3.1. Theoretical Energy Consumption in an RO Process
3.2. An Ideal RO-PRO Hybridized Process: Thermodynamic Approaches
3.2.1. A Fundamental Relationship between the Pressure and Specific Energy
3.2.2. Thermodynamic Models for RO and PRO Processes
3.3. In the Case of an RO-PRO Hybridized Process
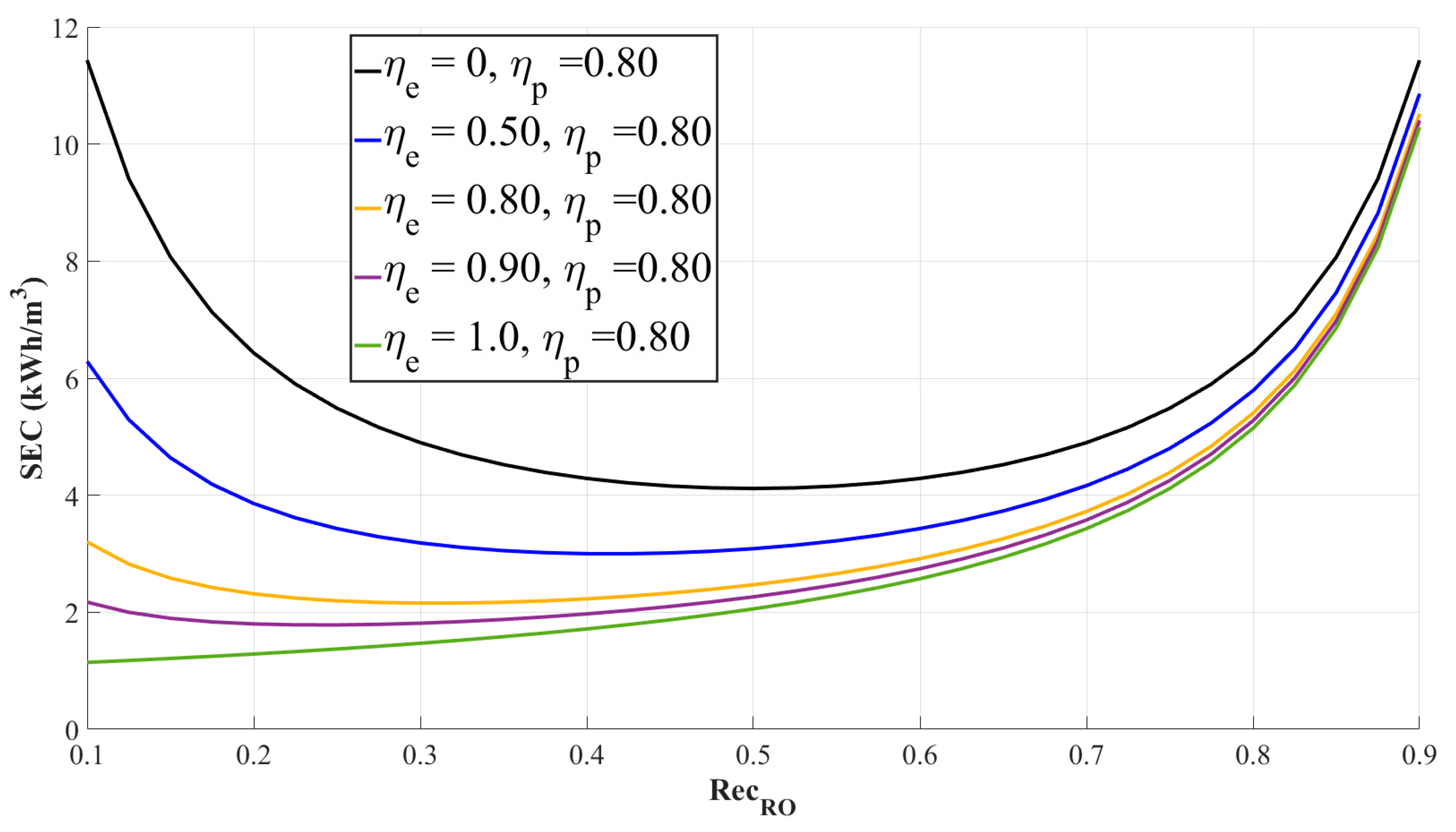
3.3.1. Specific Energy Consumption Model of RO Process with Process Efficiencies
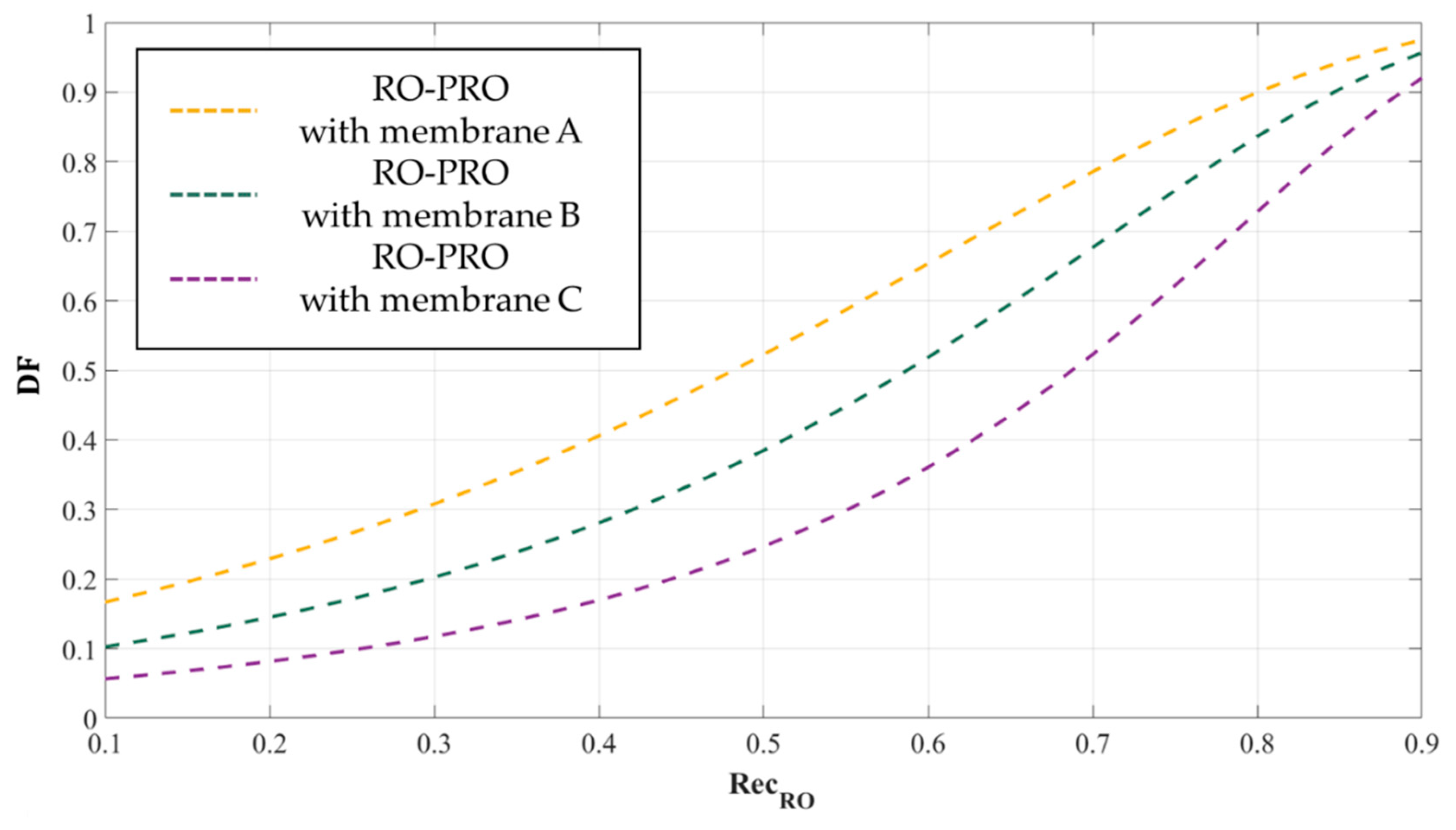
3.3.2. Dilutive Factor of the PRO Process
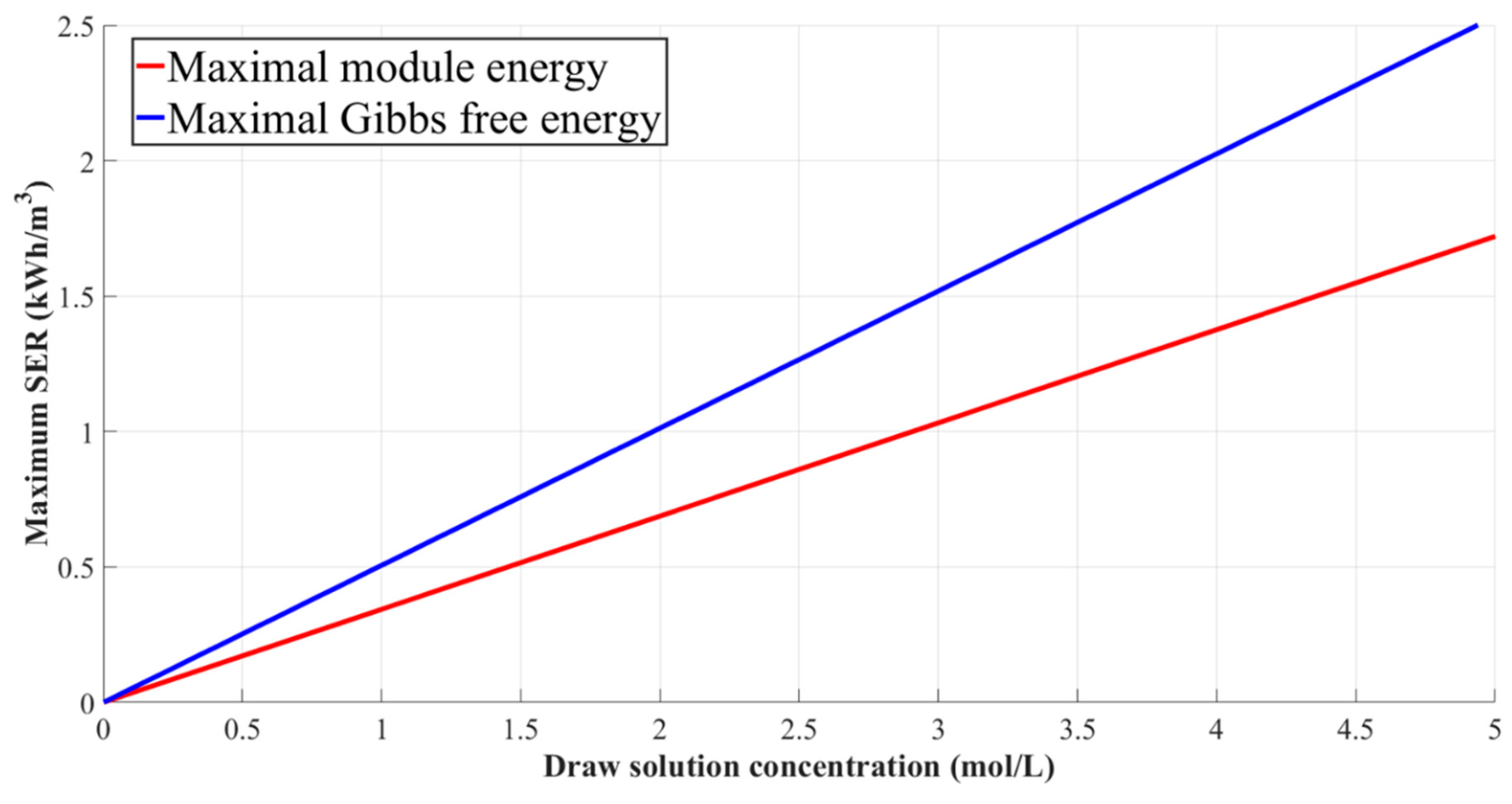
3.3.3. Specific Energy Recovery Models of the Module-Scale PRO System
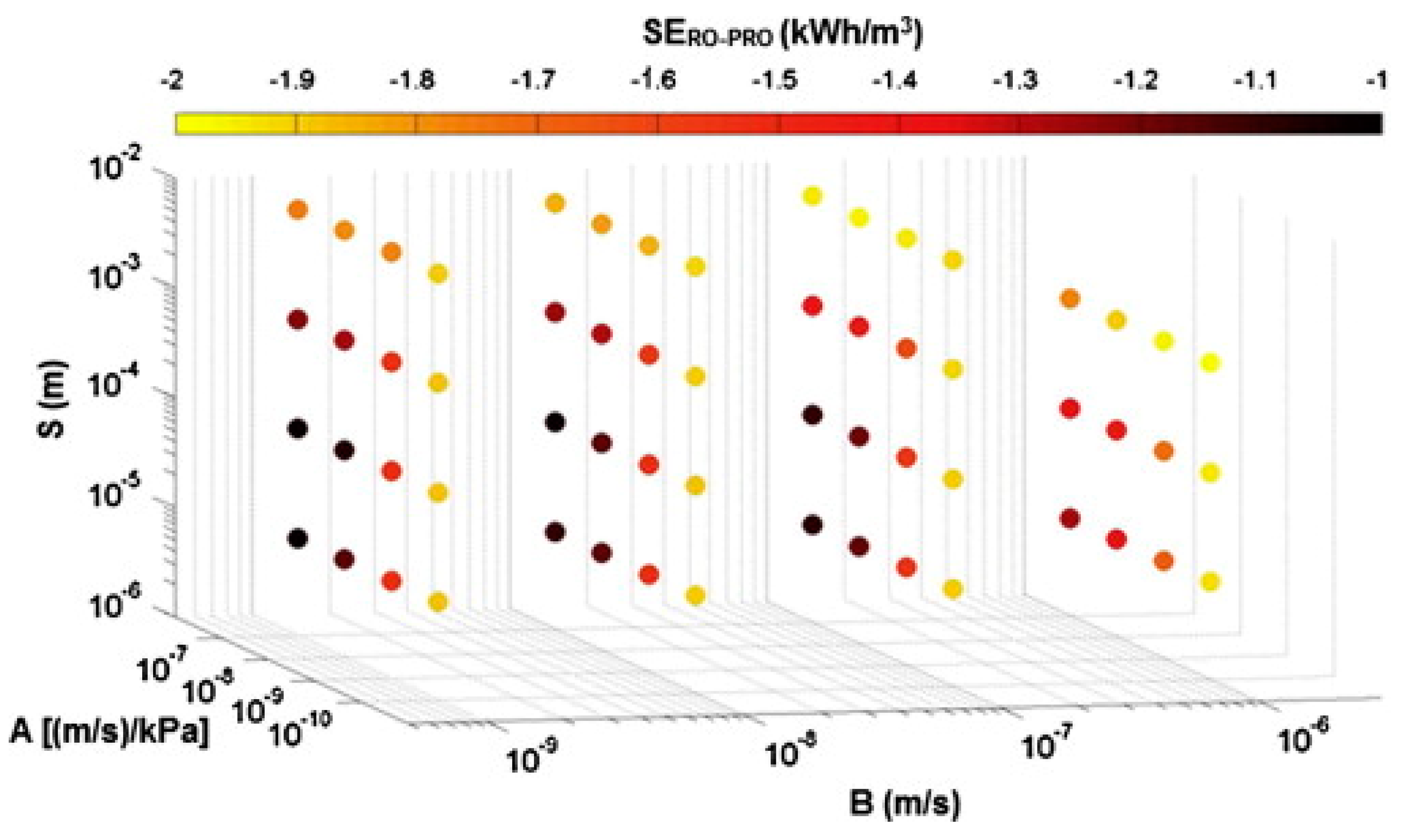
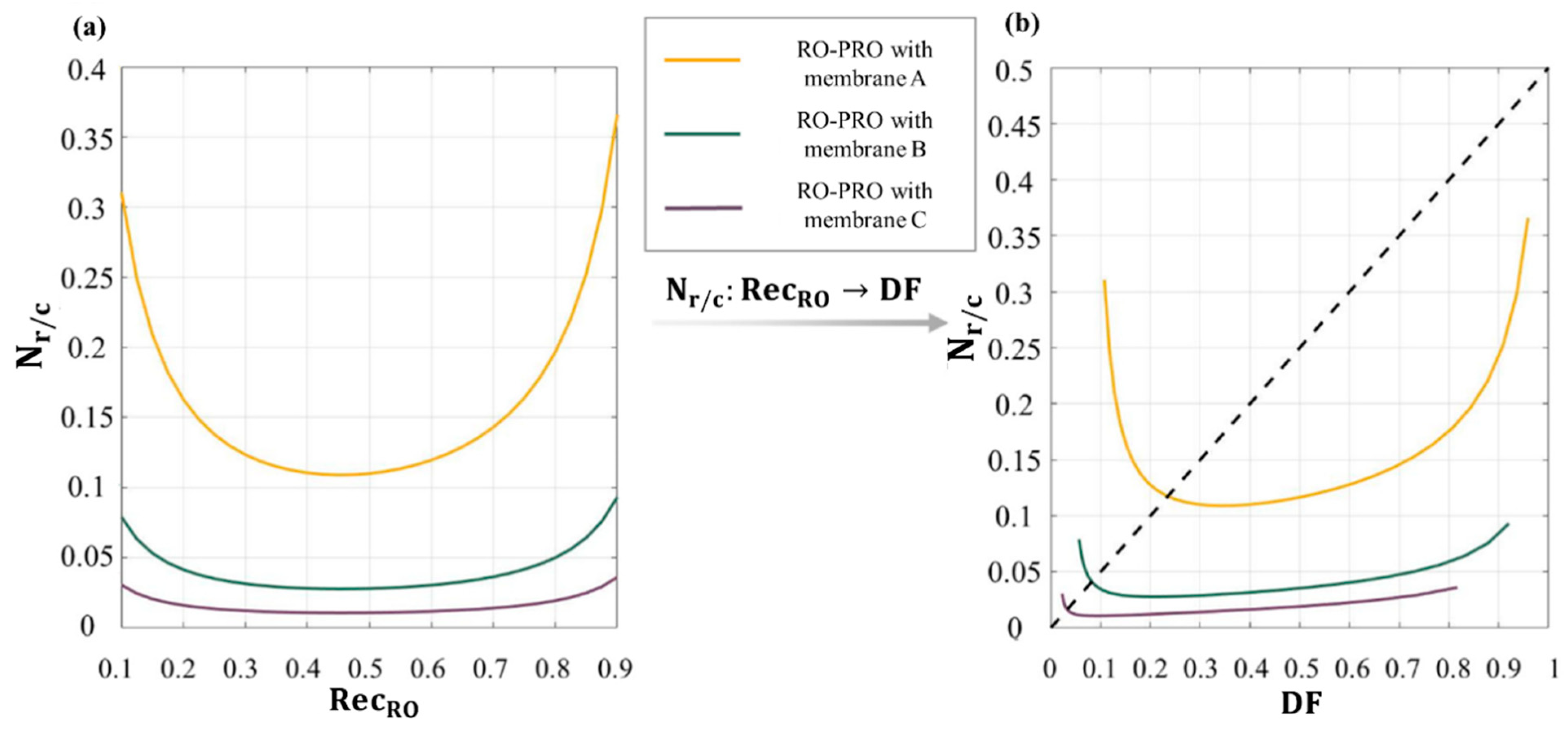
3.3.4. Performance Indices for Evaluating a Complete RO-PRO Process
4. Major Challenges and Suggested Solutions for PRO-Basis Process
4.1. Major Challenges of PRO-Basis Process
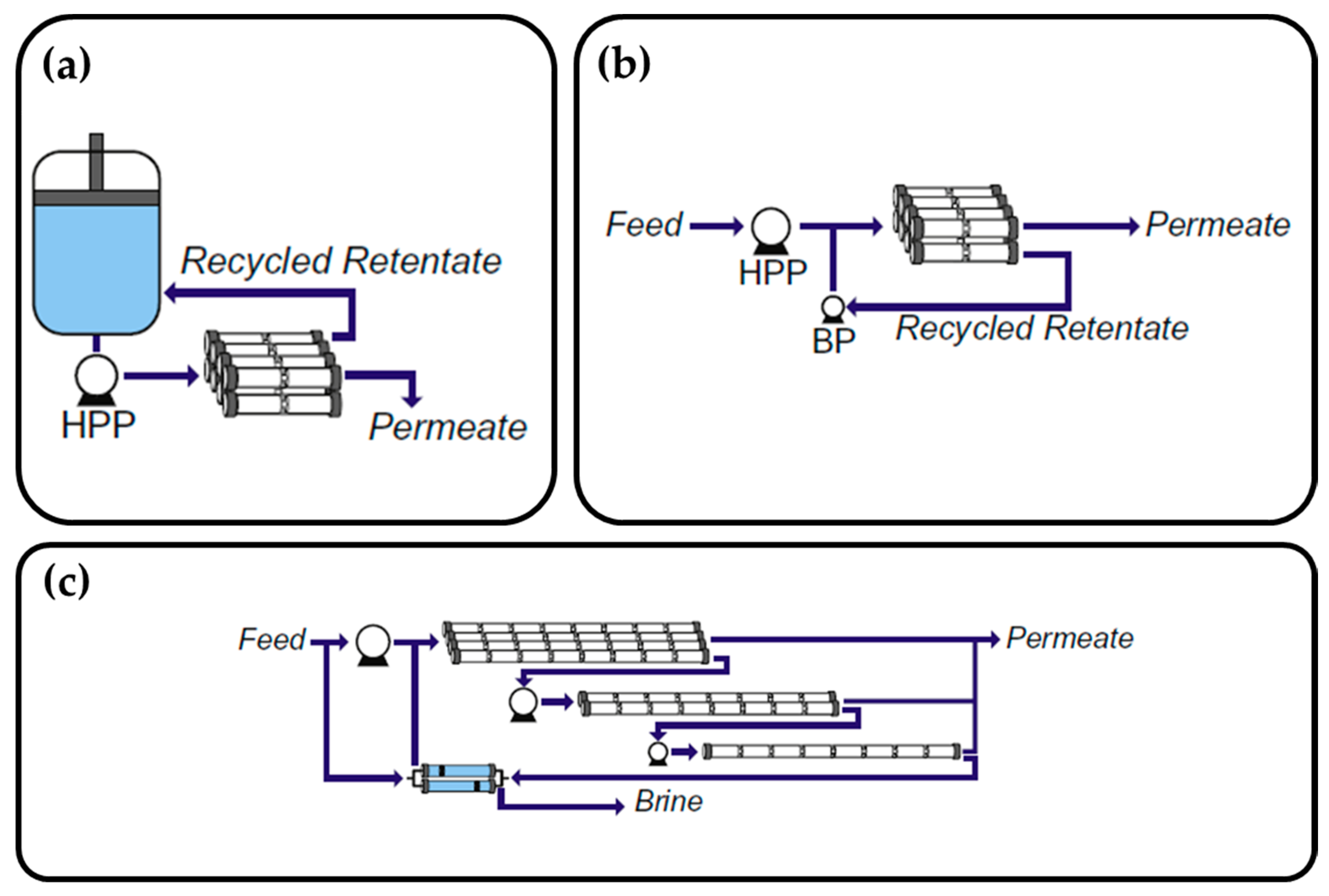
4.1.1. Challenge 1: Choosing Membrane Configuration
4.1.2. Challenge 2: Difficulty in Controlling Fouling Propensity
4.2. Suggested Solutions for the Challenges
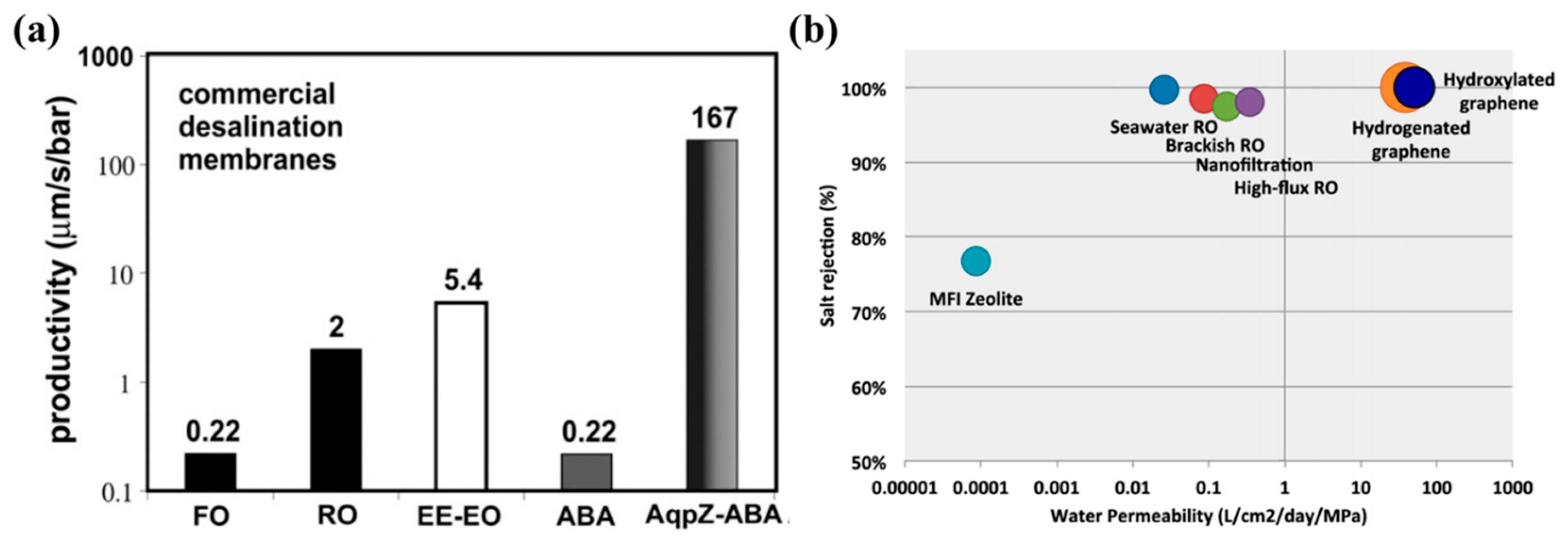
4.2.1. Suggested Solution 1: Next-Generation Membranes
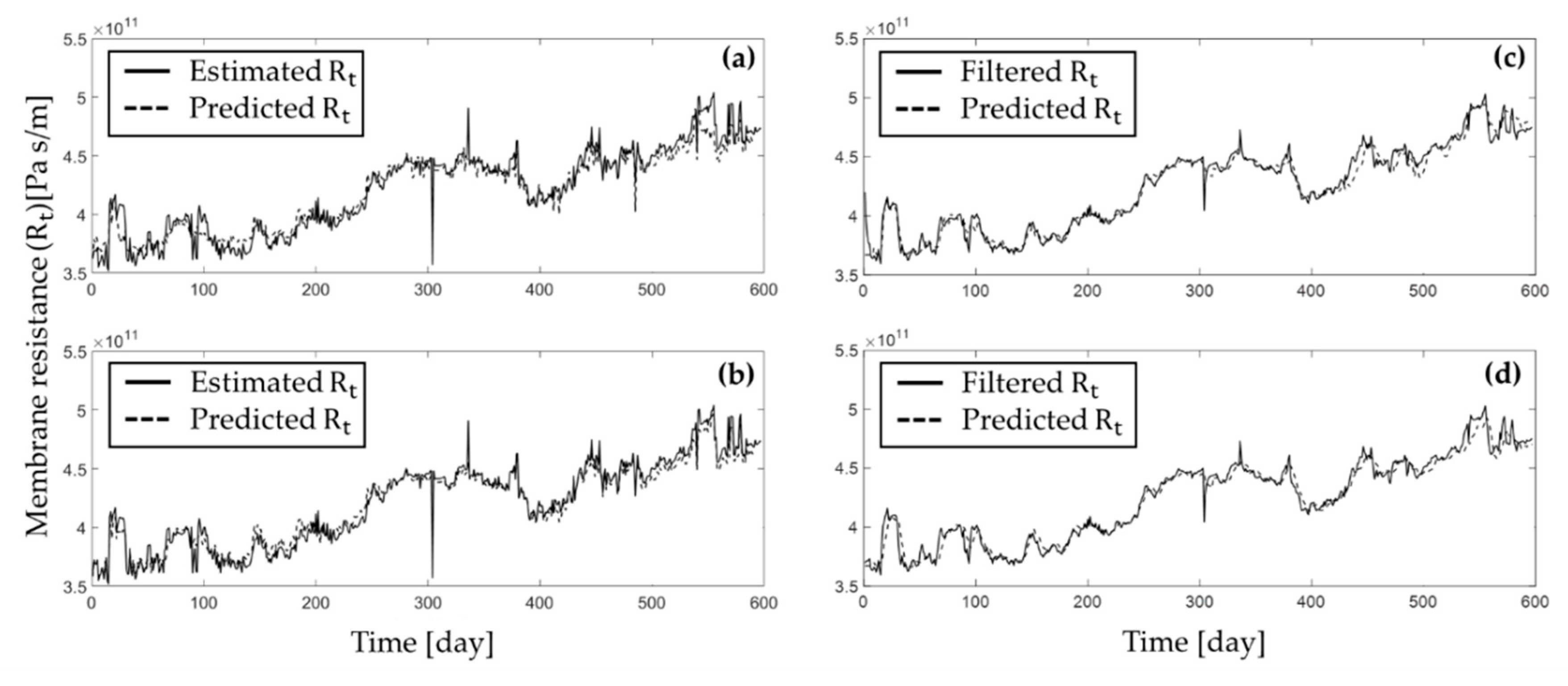
4.2.2. Suggested Solution 2: Fouling Propensity Prediction Using Machine Learning and Computational Algorithms
- Parameter (hyperparameter) setting.Parameters such as learning rate, number of hidden neurons, and the weights and bias of activation function are determined. The selected parameters contribute to the performance of modeling.
- Training session.In the training session, the partial fraction of total datasets are literally trained to fit a machine learning model into specific datasets. The fraction of a dataset used for this training session is quite variant, but usually more or less than 70% of the total datasets.
- Validation session.In the validation session, a model trained during the training session is validated with the rest of the datasets. With the result of the validation session, the overall performance of the machine learning model is assessed.
5. Conclusions
- The first part (Section 2. Fundamental theories of a PRO process) of the current paper was dedicated to describing the fundamental mathematical theories which are indispensable to understanding the mechanism of PRO. In the first part, the water flux models for the salinity gradient desalting processes were mathematically derived by combining the models with concentration polarization phenomena, and the derived water flux models were used for calculating the power density of a PRO process. The roles of the membrane parameters such as water permeability, salt permeability, and structural parameters were described according to the definitions and the physical implications of those parameters.
- In the second part (Section 3. Hybridization of a PRO process with RO), the hybridization features of an RO-PRO process and the inherent limit of a stand-alone PRO process were analyzed based on thermodynamic theories and the advanced theories of a PRO process resulting from the contents of Section 2. In this part, methodologies to calculate the energy efficiency of an RO-PRO hybridized process were mainly described with a thermodynamic concept called specific energy. With the specific energy concept, many models evaluating the energy efficiency of the RO-PRO process were developed. In this review, a few such RO-PRO energy efficiency models were introduced representatively.
- The last part (Section 4. Major challenges and suggested solutions for PRO-basis process) diagnosed the critical problems facing current PRO-basis processes, namely the membrane configuration of a stand-alone PRO process and the vulnerability of membranes to fouling. Novel approaches to overcome the aforementioned problems were discussed. Next-generation membranes such as aquaporin and graphene were suggested as potential breakthroughs to the membrane configuration challenge. Molecular dynamic simulation was suggested as a means to enhance the efficiency of next-generation membranes. In response to the propensity for membrane fouling, machine learning and computational algorithm methods were highlighted as effective alternative to make rapid progress.
Funding
Conflicts of Interest
References
- United Nations Framework Convention on Climate Change (UNFCCC). Adoption of the Paris Agreement. Available online: https://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf (accessed on 12 January 2019).
- UNFCCC. Progress Tracker: Work Programme Resulting from the Relevant Requests Contained in Decision 1/CP.21. 2017. Available online: https://unfccc.int/sites/default/files/resource/pa_progress_tracker_200617.pdf (accessed on 12 January 2019).
- Yip, N.Y.; Vermaas, D.A.; Nijmeijer, K.; Elimelech, M. Thermodynamic, energy efficiency, and power density analysis of reverse electrodialysis power generation with natural salinity gradients. Environ. Sci. Technol. 2014, 48, 4925–4936. [Google Scholar] [CrossRef] [PubMed]
- Statkraft. Available online: https://www.statkraft.com/media/press-releases/Press-releases-archive/2007/statkraft-to-build-worlds-firsk-osmotic-power-plant/ (accessed on 12 January 2019).
- Skilhagen, S.E.; Dugstad, J.E.; Aaberg, R.J. Osmotic power-power production based on the osmotic pressure difference between waters with varying salt gradients. Desalination 2008, 220, 476–482. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.H.; Kim, J.H. Pressure retarded osmosis process: Current status and future. J. Korean Soc. Environ. Eng. 2014, 36, 791–802. [Google Scholar] [CrossRef]
- Saito, K.; Irie, M.; Zaitsu, S.; Sakai, H.; Hayashi, H.; Tanioka, A. Power generation with salinity gradient by pressure retarded osmosis using concentrated brine from SWRO system and treated sewage as pure water. Desalin. Water Treat. 2012, 41, 114–121. [Google Scholar] [CrossRef]
- Kurihara, M. Mega-Ton Water System including PRO. In Proceedings of the 1st International Symposium Innovative Desalination Technologies with INES Meeting, Seoul, Republic of Korea, 2 September 2014; pp. 217–223. [Google Scholar]
- Lin, S.; Straub, A.P.; Elimelech, M. Thermodynamic limits of extractable energy by pressure retarded osmosis. Energy Environ. Sci. 2014, 7, 2706–2714. [Google Scholar] [CrossRef]
- Banchik, L.C.; Sharqawy, M.H.; Lienhard V, J.H. Limits of power production due to finite membrane area in pressure retarded osmosis. J. Membr. Sci. 2014, 468, 81–89. [Google Scholar] [CrossRef]
- Reimund, K.K.; McCutcheon, J.R.; Wilson, A.D. Thermodynamic analysis of energy density in pressure retarded osmosis: The impact of solution volumes and costs. J. Membr. Sci. 2015, 487, 240–248. [Google Scholar] [CrossRef]
- Straub, A.P.; Deshmukh, A.; Elimelech, M. Pressure-retarded osmosis for power generation from salinity gradients: Is it viable? Energy Environ. Sci. 2016, 9, 31–48. [Google Scholar] [CrossRef]
- Honda, R.; Rukapan, W.; Komura, H.; Teraoka, Y.; Noguchi, M.; Hoek, E.M. Effects of membrane orientation on fouling characteristics of forward osmosis membrane in concentration of microalgae culture. Bioresour. Technol. 2015, 197, 429–433. [Google Scholar] [CrossRef]
- Touati, K.; Tadeo, F.; Chae, S.H.; Kim, J.H.; Silva, O.A. Pressure Retarded Osmosis: Renewable Energy Generation and Recovery, 1st ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Yip, N.Y.; Brogioli, D.; Hamelers, H.V.M.; Nijmeijer, K. Salinity gradients for sustainable energy: Primer, progress, and prospects. Environ. Sci. Technol. 2016, 50, 12072–12094. [Google Scholar] [CrossRef]
- Thelin, W.R.; Sivertsen, E.; Holt, T.; Brekke, G. Natural organic matter fouling in pressure retarded osmosis. J. Membr. Sci. 2013, 438, 46–56. [Google Scholar] [CrossRef]
- Yip, N.Y.; Elimelech, M. Influence of natural organic matter fouling and osmotic backwash on pressure retarded osmosis energy production from natural salinity gradients. Environ. Sci. Technol. 2013, 12607–12616. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Vigneswaran, S.; Lee, S. Evaluation of fouling potential and power density in pressure retarded osmosis (PRO) by fouling index. Desalination 2016, 389, 215–223. [Google Scholar] [CrossRef]
- Kim, J.; Park, M.J.; Park, M.; Shon, H.K.; Kim, S.H.; Kim, J.H. Influence of colloidal fouling on pressure retarded osmosis. Desalination 2016, 389, 207–214. [Google Scholar] [CrossRef]
- Touati, K.; de la Calle, A.; Tadeo, F.; Roca, L.; Schiestel, T.; Alarcón-Padilla, D.-C. Energy Recovery Using Salinity Differences in a Multi-Effect Distillation System. Desalination and Water Treatment 2014. [Google Scholar] [CrossRef]
- Touati, K.; Tadeo, F. INTEGRATION OF POWER RETARDED OSMOSIS IN SOLAR MULTIEFFECT DESTILLATION. In Proceedings of the 7th International Symposium on Design, Operation and Control of Chemical Processes (PSE ASIA 2016), Tokyo, Japan, 24–27 July 2016. [Google Scholar]
- He, W.; Wang, Y.; Shaheed, M.H. Enhanced energy generation and membrane performance by two-stage pressure retarded osmosis (PRO). Desalination 2015, 359, 186–199. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Shaheed, M.H. Energy and thermodynamic analysis of power generation using a natural salinity gradient based pressure retarded osmosis process. Desalination 2014, 350, 86–94. [Google Scholar] [CrossRef]
- Kim, J.; Park, M.J.; Park, M.; Shon, H.K.; Kim, J.H. Performance analysis of reverse osmosis, membrane distillation, and pressure-retarded osmosis hybrid processes. Desalination 2016, 380, 85–92. [Google Scholar] [CrossRef]
- Chae, S.H.; Seo, J.W.; Kim, J.; Kim, Y.M.; Kim, J.H. A simulation study with a new performance index for pressure-retarded osmosis processes hybridized with seawater reverse osmosis and membrane distillation. Desalination 2018, 444, 118–128. [Google Scholar] [CrossRef]
- Lonsdale, H.; Merten, U.; Riley, R. Transport properties of cellulose acetate osmotic membranes. J. Appl. Polym. Sci. 1965, 9, 1341–1362. [Google Scholar] [CrossRef]
- Madsen, H.T.; Nissen, S.S.; Muff, J.; Søgaard, E.G. Pressure retarded osmosis from hypersaline solutions: investigating commercial FO membranes at high pressures. Desalination 2017, 420, 183–190. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Power generation with pressure retarded osmosis: an experimental and theoretical investigation. J. Membr. Sci. 2009, 343, 42–52. [Google Scholar] [CrossRef]
- Achilli, A.; Childress, A.E. Pressure retarded osmosis: from the vision of Sidney Loeb to the first prototype installation – Review. Desalination 2010, 261, 205–211. [Google Scholar] [CrossRef]
- Kim, D.I.; Kim, J.; Hong, S. Changing membrane orientation in pressure retarded osmosis for sustainable power generation with low fouling. Desalination 2016, 389, 197–206. [Google Scholar] [CrossRef]
- Gray, G.T.; McCutcheon, J.R.; Elimelech, M. Internal concentration polarization in forward osmosis: role of membrane orientation. Desalination 2006, 197, 1–8. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. Desalination 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Kim, D.I.; Kim, J.; Shon, H.K.; Hong, S. Pressure retarded osmosis (PRO) for integrating seawater desalination and wastewater reclamation: energy consumption and fouling. J. Membr. Sci. 2015, 483, 34–41. [Google Scholar] [CrossRef]
- Wong, M.C.; Martinez, K.; Ramon, G.Z.; Hoek, E.M.V. Impacts of operating conditions and solution chemistry on osmotic membrane and performance. Desalination 2012, 287, 340–349. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 2017, 356, 1137–1148. [Google Scholar] [CrossRef]
- Lee, K.; Baker, R.; Lonsdale, H. Membranes for power generation by pressure-retarded osmosis. J. Membr. Sci. 1981, 8, 141–171. [Google Scholar] [CrossRef]
- Touati, K.; Hänel, C.; Tadeo, F.; Schiestel, T. Effect of the feed and draw solution temperatures on PRO performance: theoretical and experimental study. Desalination 2015, 365, 182–195. [Google Scholar] [CrossRef]
- Thorsen, T.; Holt, T. The potential for power production from salinity gradients by pressure retarded osmosis. J. Membr. Sci. 2009, 335, 103–110. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Selection of inorganic-based draw solutions for forward osmosis applications. J. Membr. Sci. 2010, 364, 233–241. [Google Scholar] [CrossRef]
- Bui, N.N.; Arena, J.T.; McCutcheon, J.R. Proper accounting of mass transfer resistances in forward osmosis: improving the accuracy of model predictions of structural parameter. J. Membr. Sci. 2015, 492, 289–302. [Google Scholar] [CrossRef]
- Brouwers, H.J.H. Stagnant film model for effect of diffusional layer thickness on heat transfer and exerted friction. AlChE Journal 1995, 41, 1821–1825. [Google Scholar] [CrossRef]
- Miranda, J.M.; Campos, J.B.L.M. Mass transfer in the vicinity of a separation membrane – the applicability of the stagnant film theory. J. Membr. Sci. 2002, 537, 137–150. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Chung, T.S. Mass transport of various membrane configurations in pressure-retarded osmosis (PRO). J. Membr. Sci. 2017, 537, 160–176. [Google Scholar] [CrossRef]
- Zydney, A.L. Stagnant film model for concentration polarization in membrane systems. J. Membr. Sci. 1997, 130, 275–281. [Google Scholar] [CrossRef]
- Kim, J.; Park, M.; Snyder, S.A.; Kim, J.H. Reverse osmosis (RO) and pressure retarded osmosis (PRO) hybrid processes: model-based scenario study. Desalination 2013, 322, 121–130. [Google Scholar] [CrossRef]
- Gekas, V.; Hallström, B. Mass transfer in the membrane concentration polarization layer under turbulent cross flow: I. critical literature review and adaptation of existing Sherwood correlations to membrane operations. J. Membr. Sci. 1987, 30, 153–170. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 40–74. [Google Scholar]
- Phillip, W.A.; Yong, J.S.; Elimelech, M. Reverse draw solute permeation in forward osmosis: Modeling and experiments. Environ. Sci. Technol. 2010, 44, 5170–5176. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E. A general, resistance-in-series, salt-and water flux models for forward osmosis and pressure-retarded osmosis for energy generation. J. Membr. Sci. 2014, 460, 71–81. [Google Scholar] [CrossRef]
- Koutsou, C.P.; Yiantsios, S.G.; Karabelas, A.G. Direct numerical simulation of flow in spacer-filled channels: Effect of spacer geometrical characteristics. J. Membr. Sci. 2007, 291, 53–69. [Google Scholar] [CrossRef]
- Koutsou, C.P.; Yiantsios, S.G.; Karabelas, A.J. A numerical and experimental study of mass transfer in spacer-filled channels: Effects of spacer geometrical characteristics and Schmidt number. J. Membr. Sci. 2009, 326, 234–251. [Google Scholar] [CrossRef]
- Li, F.; Meindersma, W.; de Haan, A.B.; Reith, T. Novel spacers for mass transfer enhancement in membrane separations. J. Membr. Sci. 2005, 253, 1–12. [Google Scholar] [CrossRef]
- Brian, P. Mass transport in reverse osmosis. In Desalination by Reverse Osmosis, 4th ed.; Merten, U., Ed.; MIT Press: Cambridge, MA, USA, 1966. [Google Scholar]
- Michaels, A.S. New separation technique for the CPI. Chem. Eng. Prog. 1968, 64, 31–43. [Google Scholar]
- He, W.; Wang, Y.; Shaheed, M.H. Modelling of osmotic energy from natural salt gradients due to pressure retarded osmosis: effects of detrimental factors and flow schemes. J. Membr. Sci. 2014, 471, 247–257. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Hoover, L.A.; Chang Kim, Y.; Elimelech, M. Thin-film composite pressure retarded osmosis membranes for sustainable power generation from salinity gradients. Environ. Sci. Technol. 2011, 45, 4360–4369. [Google Scholar] [CrossRef]
- Sivertsen, E.H.; Thelin, T.W.; Brekke, G. Modeling mass transport in hollow fiber membranes used for pressure retarded osmosis. J. Membr. Sci. 2012, 417–418, 69–79. [Google Scholar]
- Shannon, M.A. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Phillip, W.A.; Elimelech, M. The future of seawater desalination: energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar]
- Werber, J.R.; Deshmukh, A.; Elimelech, M. Can batch or semi-batch processes save energy in reverse-osmosis desalination? Desalination 2017, 402, 109–122. [Google Scholar] [CrossRef]
- Wilf, M.; Awerbuch, L.; Bartels, C.; Mickley, M.; Pearce, G.; Voutchkov, N. The Guidebook to Membrane Desalination Technology: Reverse Osmosis, Nanofiltration and Hybrid Systems: Process, Design, Applications and Economics, 1st ed.; Balaban Desalination Publications: Rome, Italy, 2007. [Google Scholar]
- Spiegler, K.S.; El-Sayed, Y.M. The energetics of desalination processes. Desalination 2001, 134, 109–128. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, D.; Lin, S. Gross vs. net energy: towards a rational framework for assessing the practical viability of pressure retarded osmosis. J. Membr. Sci. 2016, 503, 132–147. [Google Scholar] [CrossRef]
- Prante, J.L.; Ruskowitz, J.A.; Childress, A.E.; Achilli, A. RO-PRO desalination: an integrated low-energy approach to seawater desalination. Appl. Energy 2014, 120, 104–114. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, W.; Rainwater, K.; Song, L. Limiting extractable energy from pressure-retarded osmosis with different pretreatment costs for feed and draw solutions. J. Membr. Sci. 2017, 544, 208–212. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M. Introduction to Chemical Engineering Thermodynamics, 7th ed.; McGraw Hill: New York, NY, USA, 2004. [Google Scholar]
- Perrot, P. A to Z of Thermodynamics, 1st ed.; Oxford University Press: Oxford, England, UK, 1998. [Google Scholar]
- Post, J.W.; Veerman, J.; Hamelers, H.V.M.; Euverink, G.J.W.; Metz, S.J.; Nymeijer, K.; Buisman, C.J.N. Salinity-gradient power: evaluation of pressure-retarded osmosis and reverse electrodialysis. J. Membr. Sci. 2007, 288, 218–230. [Google Scholar] [CrossRef]
- Veerman, J. Reverse Electro-dialysis: Design and Experimentation by Modeling and Experimentation. Ph.D. Thesis, Rijksuniversiteit Groningen, Groningen, The Netherlands, 2010. [Google Scholar]
- Post, J.W.; Hamelers, H.V.M.; Buisman, C.J.N. Energy recovery from controlled mixing salt and fresh water with a reverse electrodialysis system. Environ. Sci. Technol. 2008, 42, 5785–5790. [Google Scholar] [CrossRef]
- Zhu, A.; Christofides, P.D.; Cohen, Y. On RO membrane and energy costs and associated incentives for future enhancement of membrane permeability. J. Membr. Sci. 2009, 344, 1–5. [Google Scholar] [CrossRef]
- Zhu, A.; Christofides, P.D.; Cohen, Y. Minimization of energy consumption for a two-pass membrane desalination: effect of energy recovery, membrane rejection and retentate recycling. J. Membr. Sci. 2009, 339, 126–137. [Google Scholar] [CrossRef]
- Stover, R.L. Energy Recovery Device Performance Analysis. In Proceedings of the Water Middle East Conference, Manama, Bahrain, 19–23 November 2005. [Google Scholar]
- Energy Recovery, Inc. Available online: www.energyrecovery.com/water/px-pressure-exchanger (accessed on 12 January 2019).
- Ye, X.Y.; Yang, S.S.; Hu, J.N.; Xiao, X.P.; Zhou, G.F. Research on Improving Efficiency of High Pressure Pump in Seawater Reverse Osmosis Desalination. In Proceedings of the ASME 2009 Fluids Engineering Division Summer Meeting, Vail, CO, USA, 2–6 August 2009; Volume 1: Symposia, Parts A, B and C. [Google Scholar]
- Papa, F.; Radulj, D. Thermodynamic method used for pump performance and efficiency testing program. Environ. Sci. Eng. Mag. 2013, 26, 44–48. [Google Scholar]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Stover, R.L. Seawater reverse osmosis with isobaric energy recovery devices. Desalination 2007, 203, 168–175. [Google Scholar] [CrossRef]
- Wan, C.F.; Chung, T.S. Energy recovery by pressure retarded osmosis (PRO) in SWRO-PRO integrated processes. Appl. Energy 2016, 162, 687–698. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Leitz, F.B. Electric power from differences in salinity: the dialytic battery. Science 1976, 191, 557–559. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Stewart, F.F. Deriving osmotic pressures of draw solutes used in osmotically driven membrane processes. J. Membr. Sci. 2013, 431, 205–211. [Google Scholar] [CrossRef]
- Yang, W.; Song, L.; Zhao, J.; Chen, Y.; Hu, B. Numerical analysis of performance of ideal counter-current flow pressure retarded osmosis. Desalination 2018, 433, 41–47. [Google Scholar] [CrossRef]
- Straub, A.P.; Lin, S.; Elimelech, M. Module-scale analysis of pressure retarded osmosis: performance limitations and implications for full-scale operation. Environ. Sci. Technol. 2014, 48, 12435–12444. [Google Scholar] [CrossRef] [PubMed]
- Yip, N.Y.; Elimelech, M. Thermodynamic and energy efficiency analysis of power generation from natural salinity gradients by pressure retarded osmosis. Environ. Sci. Technol. 2012, 46, 5230–5239. [Google Scholar] [CrossRef]
- Seawater Desalination Power Consumption—White Paper; Watereuse: Alexandria, VA, USA, November 2011.
- Schock, G.; Miguel, A. Mass transfer and pressure loss in spiral wound modules. Desalination 1987, 64, 339–352. [Google Scholar] [CrossRef]
- Helfer, F.; Lemckert, C.; Anissimov, Y.G. Osmotic power with pressure retarded osmosis: Theory, performance and trends—A review. J. Membr. Sci. 2014, 453, 333–358. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, K.; Park, M.J.; Shon, H.K.; Kim, J.H. Recent advances in osmotic energy generation via pressure-retarded osmosis (PRO): A review. Energies 2015, 8, 11821–11845. [Google Scholar] [CrossRef]
- Zhang, S.; Sukitpaneenit, P.; Chung, T. Design of robust hollow fiber membranes with high power density for osmotic energy production. Chem. Eng. J. 2014, 241, 457–465. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Sun, D.D. Energy recovery from concentrated seawater brine by thin-film nanofiber composite pressure retarded osmosis membranes with high power density. Energy Environ. Sci. 2013, 6, 1199–1210. [Google Scholar] [CrossRef]
- Atarde, D.; Jain, M.; Gupta, S.K. Modeling of a forward osmosis and a pressure-retarded osmosis spiral wound module using the Spiegler-Kedem model and experimental validation. Sep. Purif. Technol. 2016, 164, 182–197. [Google Scholar] [CrossRef]
- Hong, S.S.; Ryoo, W.; Chun, M.S.; Lee, S.O.; Chung, G.Y. Numerical studies on the pressure-retarded osmosis (PRO) system with the spiral wound module for power generation. Desalination & Water Treatment 2013, 52, 6333–6341. [Google Scholar]
- Chae, S.H.; Kim, J.H. Recent issues relative to a low salinity pressure-retarded osmosis process and suggested technical solutions. In Membrane-Based Salinity Gradient Processes for Water Treatment and Power Generation, 1st ed.; Elsevier: New York, NY, USA, 2018; pp. 273–295. [Google Scholar]
- Kurihara, M.; Takeuchi, H. SWRO-PRO system in “Mega-ton Water System” for energy reduction and low environmental impact. Water 2018, 10, 48. [Google Scholar] [CrossRef]
- Wan, C.F.; Li, B.; Yang, T.; Chung, T.S. Design and fabrication of inner-selective thin-film composite (TFC) hollow fiber modules for pressure retarded osmosis (PRO). Sep. Purif. Technol. 2017, 172, 32–42. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Kunteng, D.; Veerman, J.; Saakes, M.; Nijmeijer, K. Periodic feedwater reversal and air sparging as antifouling strategies in reverse electrodialysis. Environ. Sci. Technol. 2014, 48, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- She, Q.; Wong, Y.K.W.; Zhao, S.; Tang, C.Y. Organic fouling in pressure retarded osmosis: Experiments, mechanisms and implications. J. Membr. Sci. 2013, 428, 181–189. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Bucs, S.; Li, Z.; AbuGhdeeb, M.; Amy, G.; Vrouwenvelder, J.S. Impact of spacer thickness on biofouling in forward osmosis. Water Res. 2014, 57, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Amy, G.L.; Chung, T.S. Membrane fouling and anti-fouling strategies using RO retentate from a municipal water recycling plant as the feed for osmotic power generation. Water Res. 2016, 88, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Y.; Chung, T.S. Facile preparation of antifouling hollow fiber membranes for sustainable Osmotic Power generation. ACS Sustain. Chem. Eng. 2016, 4, 1154–1160. [Google Scholar] [CrossRef]
- Yanar, N.; Son, M.; Yang, E.; Kim, Y.; Park, H.; Nam, S.E.; Choi, H. Investigation of the performance behavior of a forward osmosis membrane system using various feed spacer materials fabricated by 3D printing technique. Chemosphere 2018, 202, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Boo, C.; Lee, S.; Elimelech, M.; Meng, Z.; Hong, S. Colloidal fouling in forward osmosis: role of reverse salt diffusion. J. Membr. Sci. 2012, 390, 277–284. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Elimelech, M. Cake-enhanced concentration polarization: a new fouling mechanism for salt-rejecting membranes. Environ. Sci. Technol. 2003, 37, 5581–5588. [Google Scholar] [CrossRef]
- Mollema, P.N.; Antonellini, M.; Hubeek, A.; Van Diepenbeek, P.M.J.A. The effect of artificial recharge on hydrochemistry: a comparison of two fluvial gravel pit lakes with different post-excavation uses in the Netherlands. Water 2016, 8, 409. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Li, X.; Chou, S.; Wang, R.; Shi, L.; Fang, W.; Chaitra, G.; Tang, C.Y.; Torres, J.; Hu, X.; Fane, A.G. Nature gives the best solution for desalination: Aquaporin-based hollow fiber composite membrane with superior performance. J. Membr. Sci. 2015, 494, 68–77. [Google Scholar] [CrossRef]
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2004, 43, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhong, X.; Jiang, S.; Huang, Y.; Duan, X. Graphene nanomesh. Nat. Nanotechnol. 2010, 5, 190–194. [Google Scholar] [CrossRef]
- Grzelakowski, M.; Cherenet, M.F.; Shen, Y.; Kumar, M. A framework for accurate evaluation of the promise of aquaporin based biomimetic membranes. J. Membr. Sci. 2015, 479, 223–231. [Google Scholar] [CrossRef]
- Cohen-Tanugi, D.; McGovern, R.K.; Dave, S.H.; Lienhard, J.H.; Grossman, J.C. Quantifying the potential of ultra-permeable membranes for water desalination. Energy Environ. Sci. 2014, 7, 1134–1141. [Google Scholar] [CrossRef]
- Perrault, F.; Fonseca de Faria, A.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Tanugi, D.; Grossman, J.C. Water desalination across nanoporous graphene. Nano Lett. 2012, 12, 3602–3608. [Google Scholar] [CrossRef]
- Tang, C.Y.; Zhao, Y.; Wang, R.; Hélix-Nielsen, C.; Fane, A.G. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef]
- Ibragimova, S.; Stibius, K.B.; Szewczykowski, P.; Perry, M.; Bohr, H.; Nielsen, C.H. Hydrogels for in situ encapsulation of biomimetic membrane arrays. Polym. Adv. Technol. 2011, 23, 182–189. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, C.; Li, X.; Vararattanavech, A.; Shen, W.; Torres, J.; Hélix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G.; et al. Synthesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization—Membrane preparation and RO performance characterization. J. Membr. Sci. 2012, 423–424, 422–428. [Google Scholar] [CrossRef]
- Schlick, T. Molecular Modeling and Simulation: An Interdisciplinary Guide; Springer-Verlag: New York, NY, USA, 2002. [Google Scholar]
- Haile, J.M. Molecular Dynamics Simulation: Elementary Methods, 1st ed.; John Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Kim, Y.M.; Ebro, H.; Kim, J.H. Molecular dynamics simulation of seawater reverse osmosis desalination using carbon nanotube membranes. Desalin. Water Treat. 2016, 57, 20169–20176. [Google Scholar] [CrossRef]
- Ebro, H.; Kim, Y.M.; Kim, J.H. Molecular dynamics simulations in membrane-based water treatment processes: A systematic overview. J. Membr. Sci. 2013, 438, 112–125. [Google Scholar] [CrossRef]
- Mathai, J.C.; Tristram-Nagle, S.; Nagle, J.F.; Zeidel, M.L. Structural determinants of water permeability through the lipid membrane. J. Gen. Physiol. 2008, 131, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hovijitra, N.T.; Wuu, J.J.; Peaker, B.; Swartz, J.R. Cell-free synthesis of functional aquaporin Z in synthetic liposomes. Biotechnol. Bioeng. 2009, 104, 40–49. [Google Scholar] [CrossRef]
- Hirose, M.; Minmizki, Y.; Kamiyama, Y. The relationship between polymer molecular structure of RO membrane skin layers and their RO performances. J. Membr. Sci. 1997, 123, 151–156. [Google Scholar] [CrossRef]
- Harder, E.; Walters, D.E.; Bodnar, Y.D.; Faibish, R.S.; Roux, B. Molecular dynamics study of a polymeric reverse osmosis membrane. J. Phys. Chem. B 2009, 113, 10177–10182. [Google Scholar] [CrossRef] [PubMed]
- Dochain, D.; Couenne, F.; Jallut, C. Enthalpy based modelling and design of asymptotic observers for chemical reactors. Int. J. Control 2009, 82, 1389–1403. [Google Scholar] [CrossRef]
- Jana, A.K. A nonlinear exponential observer for a batch distillation. In Proceedings of the 11th International Conference on Control Automation Robotics & Vision, Singapore, 7–10 December 2010; pp. 1393–1396. [Google Scholar]
- Soroush, M. Nonlinear state-observer design with application to reactors. Chem. Eng. Sci. 1997, 52, 387–404. [Google Scholar] [CrossRef]
- Wang, G.B.; Peng, S.S.; Huang, H.P. A sliding observer for nonlinear process control. Chem. Eng. Sci. 1997, 52, 787–805. [Google Scholar] [CrossRef]
- Ali, J.M.; Hoang, N.H.; Hussain, M.A.; Dochain, D. Review and classification of recent observers applied in chemical process systems. Comput. Chem. Eng. 2015, 76, 27–41. [Google Scholar]
- Abbas, A.; Al-Bastaki, N. Performance decline in brackish water FilmTec spiral wound RO membranes. Desalination 2001, 136, 281–286. [Google Scholar] [CrossRef]
- Ruiz-Garcia, A.; Nuez, I. Long-term performance decline in a brackish water reverse osmosis desalination plant. Predictive model for the water permeability coefficient. Desalination 2016, 397, 101–107. [Google Scholar] [CrossRef]
- Kim, J.H. Environmental Data Analysis and Practice; Balaban Desalination Publications: Rome, Italy, 2016; pp. 345–355. [Google Scholar]
- Lim, S.J.; Jeong, K.; Kim, J.H. Membrane Fouling Prediction Algorithm Based on Machine Learning Model in Reverse Osmosis Plant. In Proceedings of the 3rd International Conference on Desalination Using Membrane Technology, Gran Canaria, Spain, 2–5 April 2017. [Google Scholar]
- Chen, K.L.; Song, L.; Ong, S.L.; Ng, W.J. The development of membrane fouling in full-scale RO processes. J. Membr. Sci. 2004, 232, 63–72. [Google Scholar]
- Lee, Y.G.; Lee, Y.S.; Kim, D.Y.; Park, M.; Yang, D.R.; Kim, J.H. A fouling model for simulating long-term performance of SWRO desalination process. J. Membr. Sci. 2012, 401–402, 282–291. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning, 1st ed.; Springer Science: Berlin, Germany, 2006; pp. 225–254. [Google Scholar]
- Welch, G.; Bishop, G. An Introduction to the Kalman Filter; TR 95-041; University of North Carolina-Chapel Hill: Chapel Hill, NC, USA, 24 July 2006. [Google Scholar]
- Sak, H.; Senior, A.; Beaufays, F. Long short-term memory recurrent neural network architectures for large scale acoustic modeling. In Proceedings of the Fifteenth Annual Conference of the International Speech Communication Association, Singapore, 14–18 September 2014. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, S.H.; Kim, Y.M.; Park, H.; Seo, J.; Lim, S.J.; Kim, J.H. Modeling and Simulation Studies Analyzing the Pressure-Retarded Osmosis (PRO) and PRO-Hybridized Processes. Energies 2019, 12, 243. https://doi.org/10.3390/en12020243
Chae SH, Kim YM, Park H, Seo J, Lim SJ, Kim JH. Modeling and Simulation Studies Analyzing the Pressure-Retarded Osmosis (PRO) and PRO-Hybridized Processes. Energies. 2019; 12(2):243. https://doi.org/10.3390/en12020243
Chicago/Turabian StyleChae, Sung Ho, Young Mi Kim, Hosik Park, Jangwon Seo, Seung Ji Lim, and Joon Ha Kim. 2019. "Modeling and Simulation Studies Analyzing the Pressure-Retarded Osmosis (PRO) and PRO-Hybridized Processes" Energies 12, no. 2: 243. https://doi.org/10.3390/en12020243
APA StyleChae, S. H., Kim, Y. M., Park, H., Seo, J., Lim, S. J., & Kim, J. H. (2019). Modeling and Simulation Studies Analyzing the Pressure-Retarded Osmosis (PRO) and PRO-Hybridized Processes. Energies, 12(2), 243. https://doi.org/10.3390/en12020243






