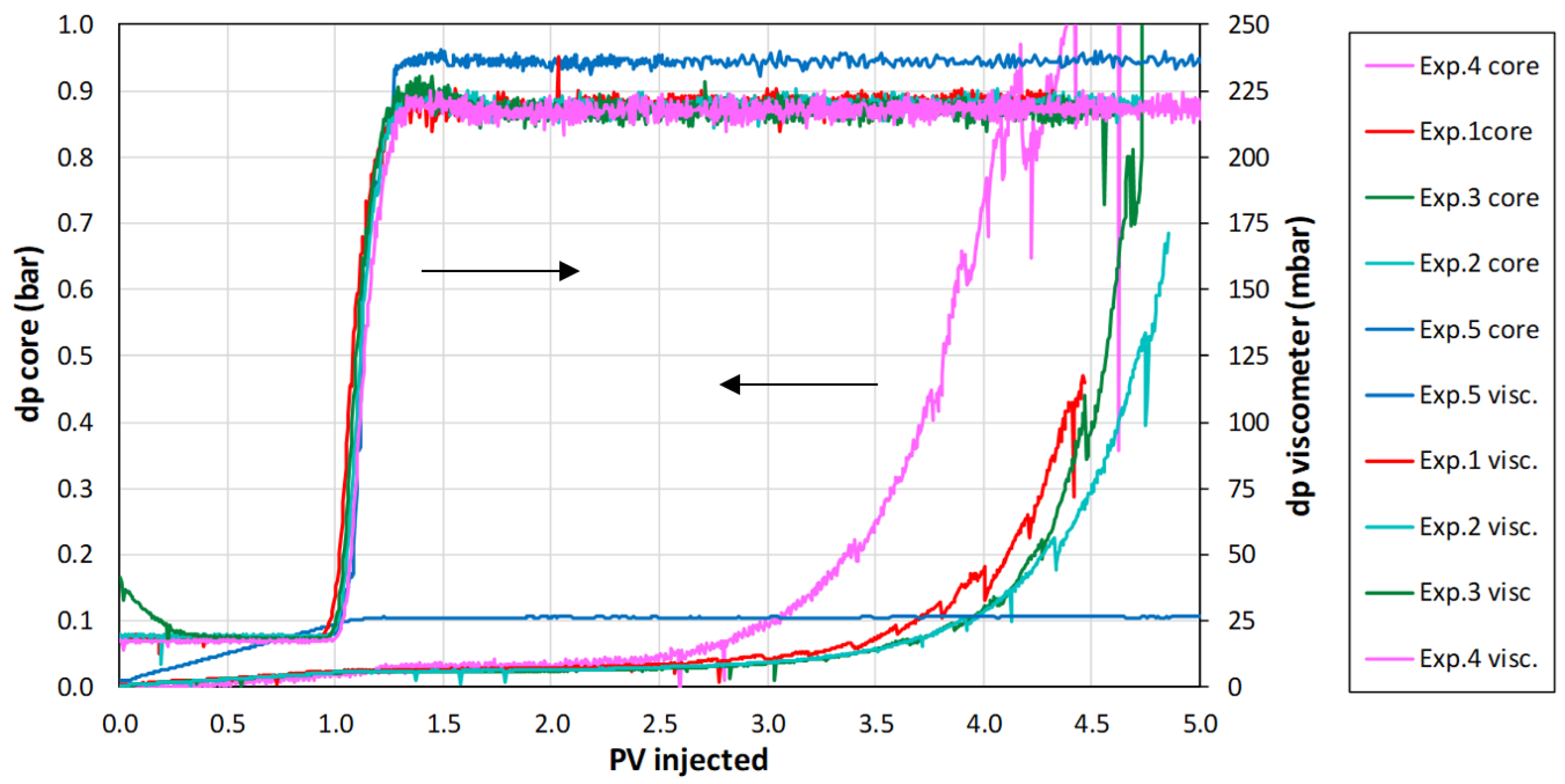
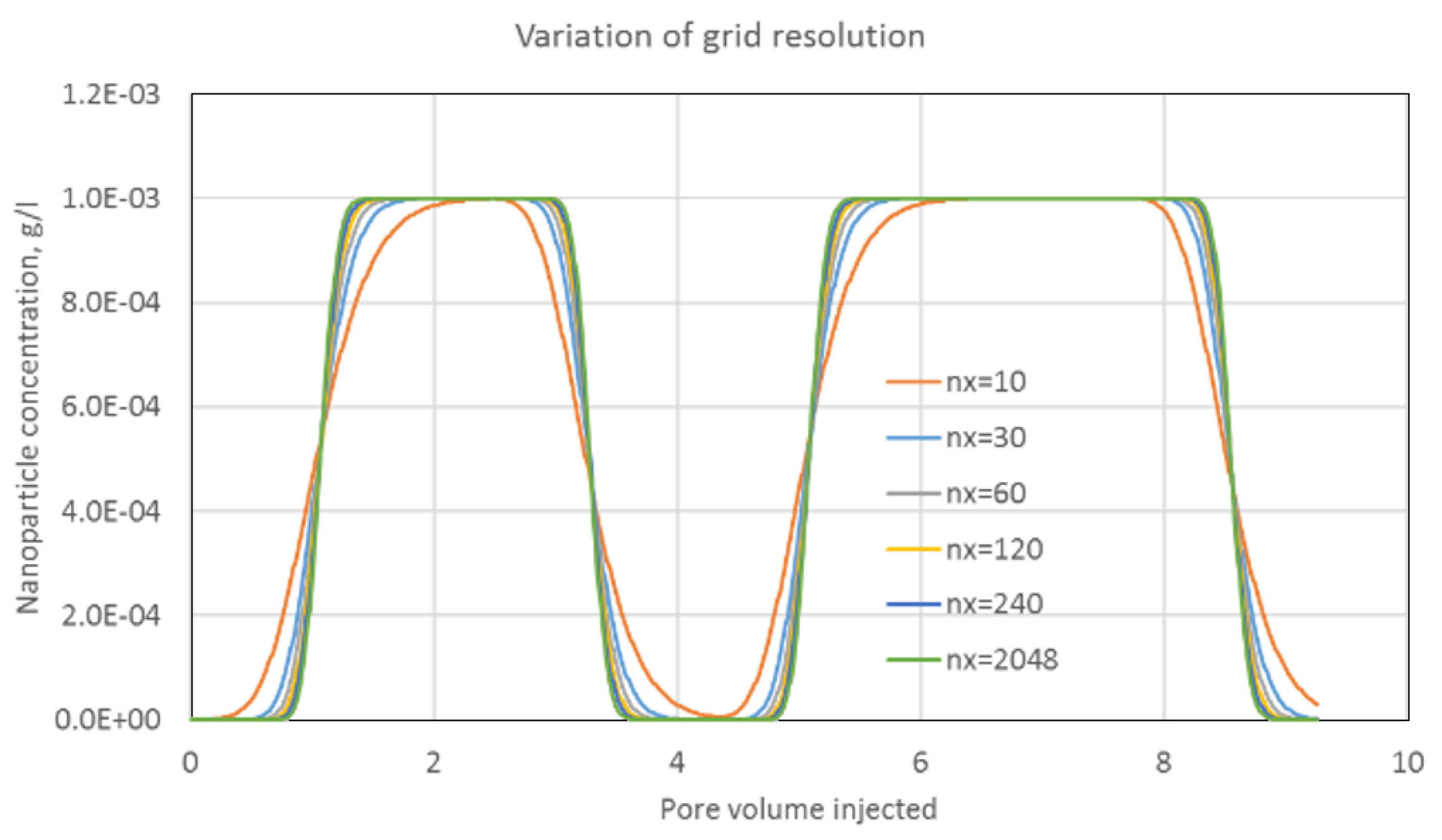
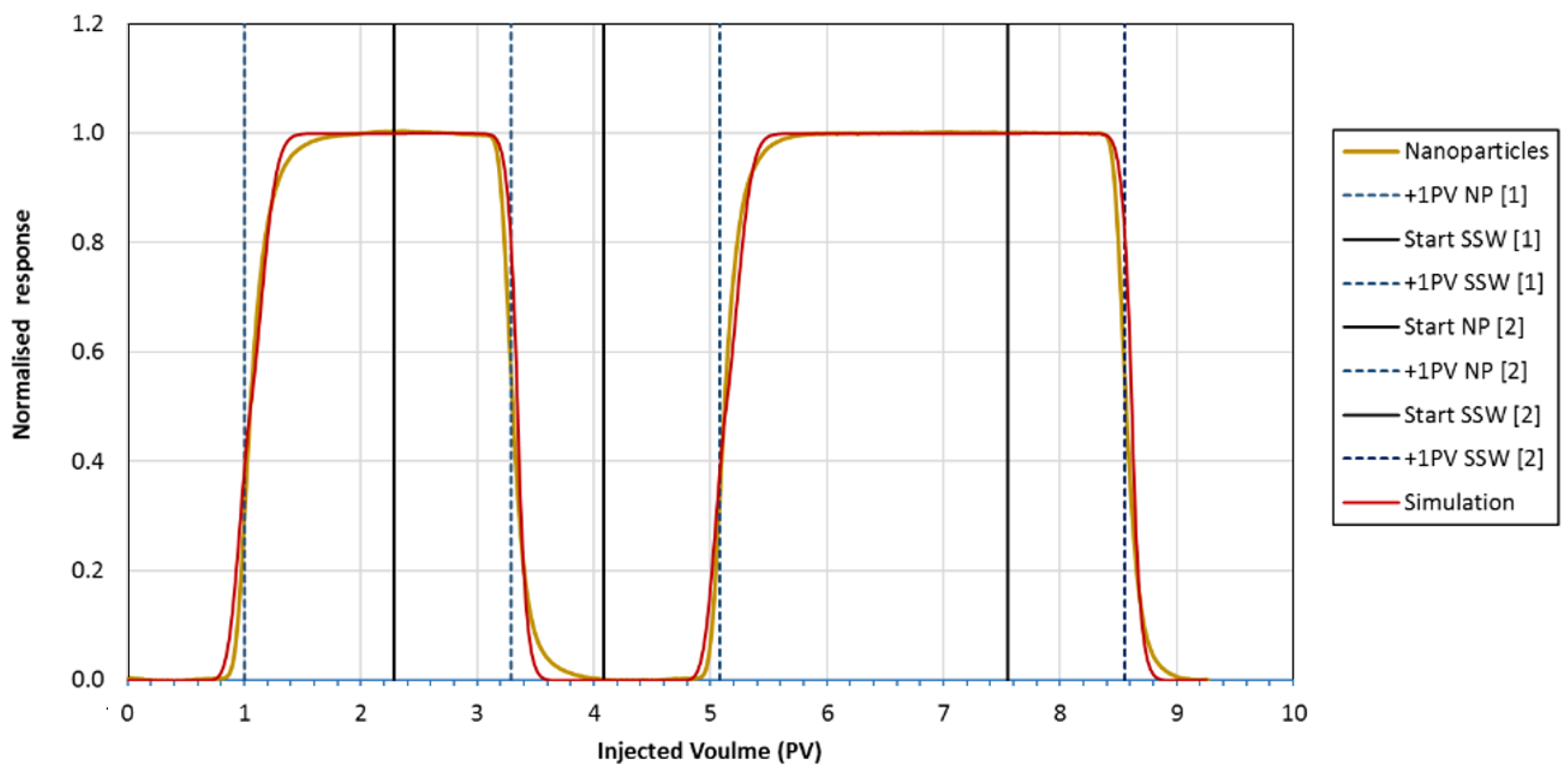
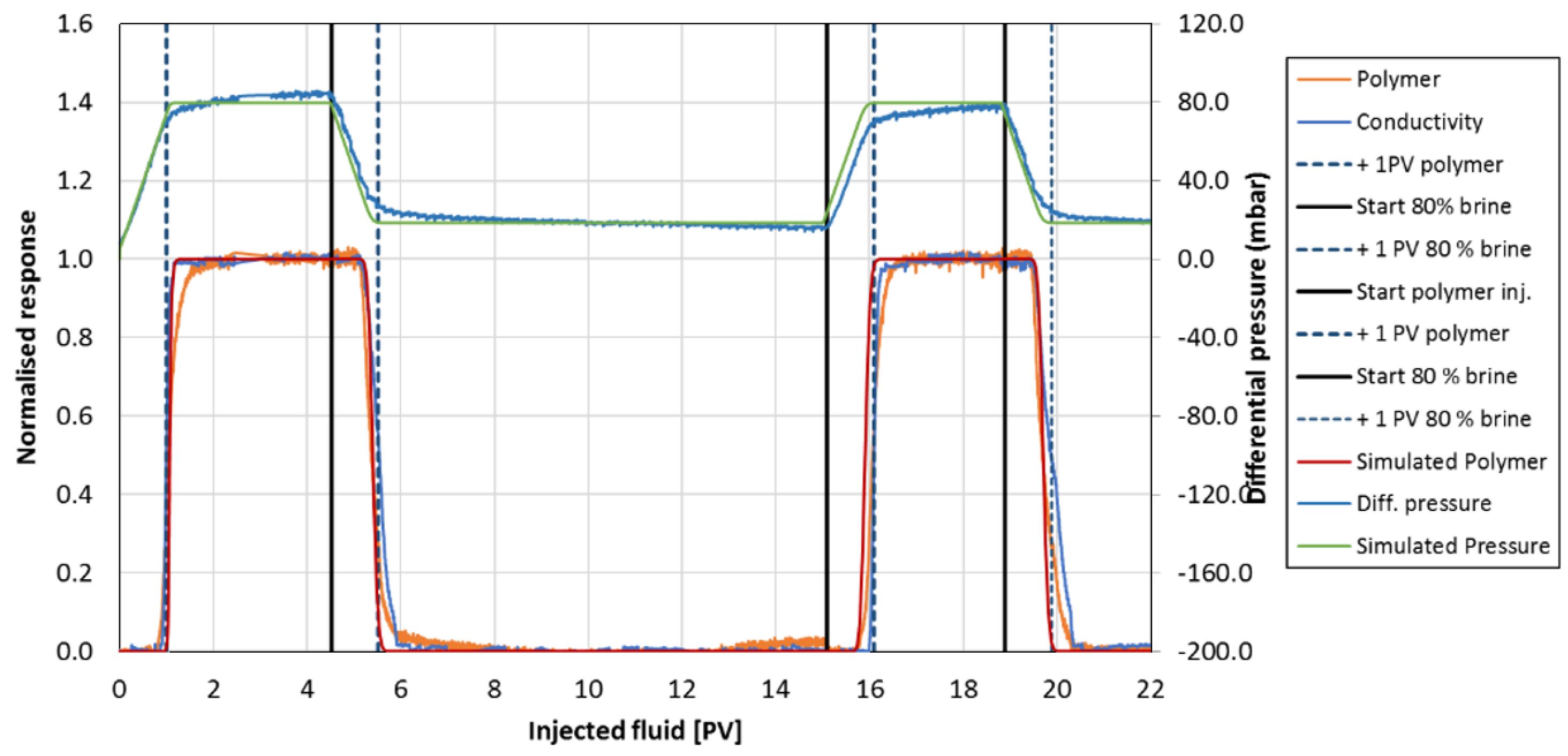
Appendix A.2. Conservation Laws
Mass conservation for oil and water [
45].
where
is the constant porosity (porosity for oil and water);
is the phase saturations; and
is the phase volumetric fluxes. These fluxes are given by Darcy’s law [
45]
where
is the pressure and
k is the absolute permeability. Here, absolute permeability is a tabulated function of absorbed polymer concentration. Furthermore,
is the relative permeabilities to oil and water, which are functions of water saturation. In the present formulation, these relative permeabilities are of Corey type [
46]
where
. Here,
and
are the irreducible water saturation and the residual oil saturation;
is the endpoint relative permeabilities; and
and
are the Corey exponents for water and oil, respectively. The oil viscosity
is assumed constant, while the water viscosity
will be a function of nanomaterial and polymer concentration, the nanomaterial age profile, as well as the flow rate. The calculation of water viscosity is presented in the next section. Mass conservation of nanomaterials and polymers are given by
where
are nanomaterial and polymer concentrations (molar or by weight);
is the adsorbed concentrations per mass of the rock for nanomaterials and polymers;
is the porosities (accessible pore spaces) for nanomaterials and polymers; and finally,
is the rock mass density. As indicated, the adsorbed concentrations are functions of their respective concentrations in the water phase, where the components can desorb or not depending on input choice. Thus, in the case of no desorption, the adsorbed concentration is a hysteretic quantity. In addition to calculating the saturations and concentrations, we need to keep track of the local age profiles of the nanomaterials, both the age profile in the water phase and the age profile of the adsorbed nanomaterials (the adsorbed age profiles are needed if nanomaterials can desorb). This age tracking is realized by formulating a conservation scheme for nanomaterials of the same age.
Let
denote the age distribution of nanomaterials in solution at a position and time.
is the fraction of nanomaterials having age in the interval
at position
x and at time
t. Thus,
and
. In case all nanomaterials at a location have aged more than a maximal age
, the age where maximal gel strength can be achieved, the age profile is given by the delta function
. The age distribution of adsorbed nanomaterials at position
x at time
t is similarly given by
. Thus, the total concentration of nanomaterials per bulk volume of age
at position
x at time
t is
The flux of nanomaterials of this age is
Thus, we can set up the conservation law for nanomaterials of age for determining the t-evolution of and . Exactly how this is realized will be described in the section describing the numerical realization of the mathematical model.
Appendix A.3. Water Viscosity
The water viscosity is expressed as
where
is the water viscosity with only the polymer present;
is the (constant) maximal possible value for water viscosity corresponding to maximal gel strength; and
is an interpolator depending on nanomaterial and polymer concentrations and the age profile
p, where
corresponds to no gelling and
signifies maximal gel strength.
The water viscosity with only polymer present is given by
where
is the water viscosity with no polymer present,
is the viscosity increase factor due to presence of polymer with no shear-thinning, while
is the viscosity decrease factor due to shear thinning. Note that the shear-thinning factor
is calculated explicitly in the numerical formulation. The two functions
and
are tabulated input parameters and can either be measured directly or calculated from some type of assumed functional form. The approach for defining water viscosity as a function of polymer concentration resembles the fully mixed case for the Todd–Longstaff formulation [
47].
The interpolator (signifying degree of gelling) is given by
where
is a tabulated function, and
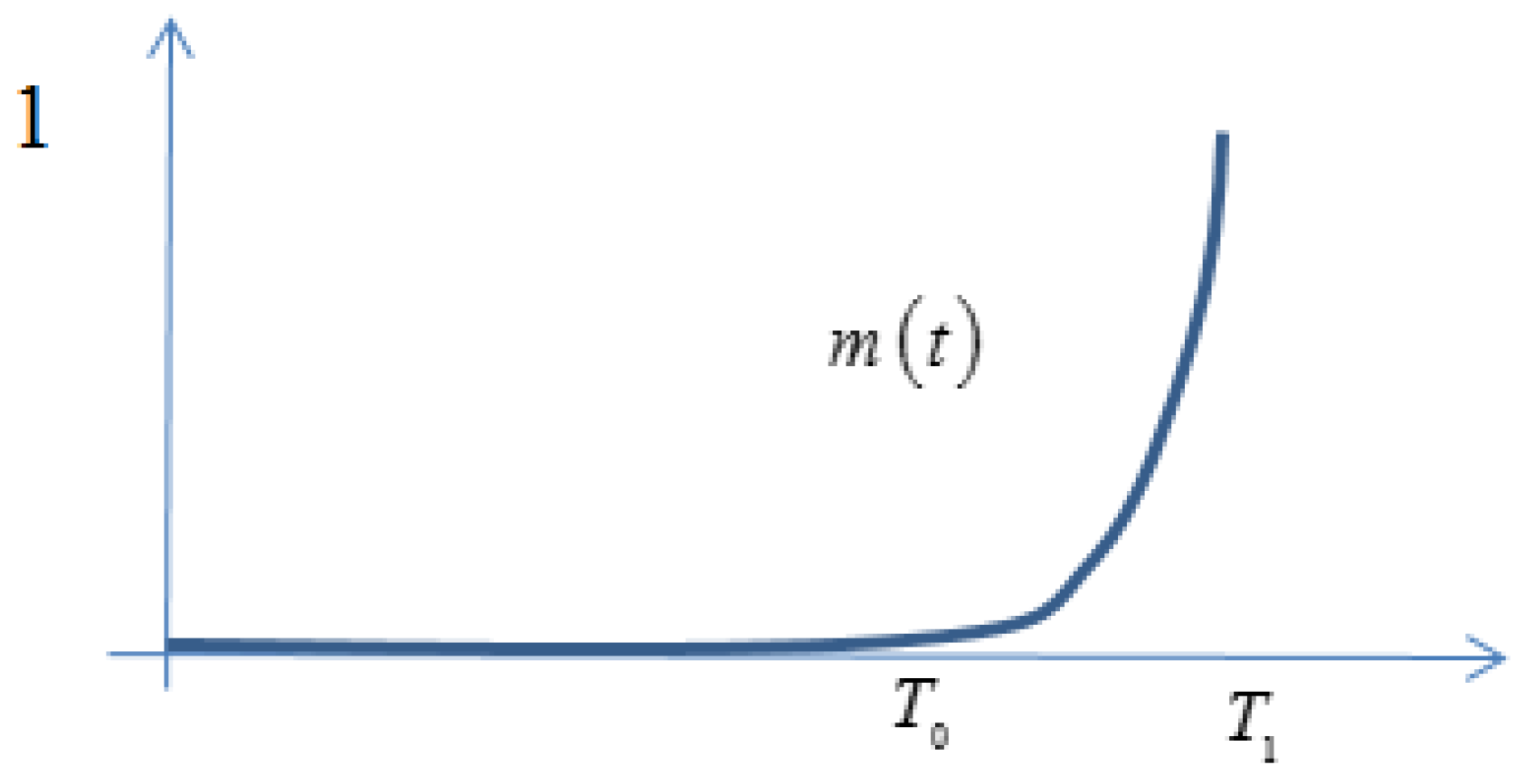
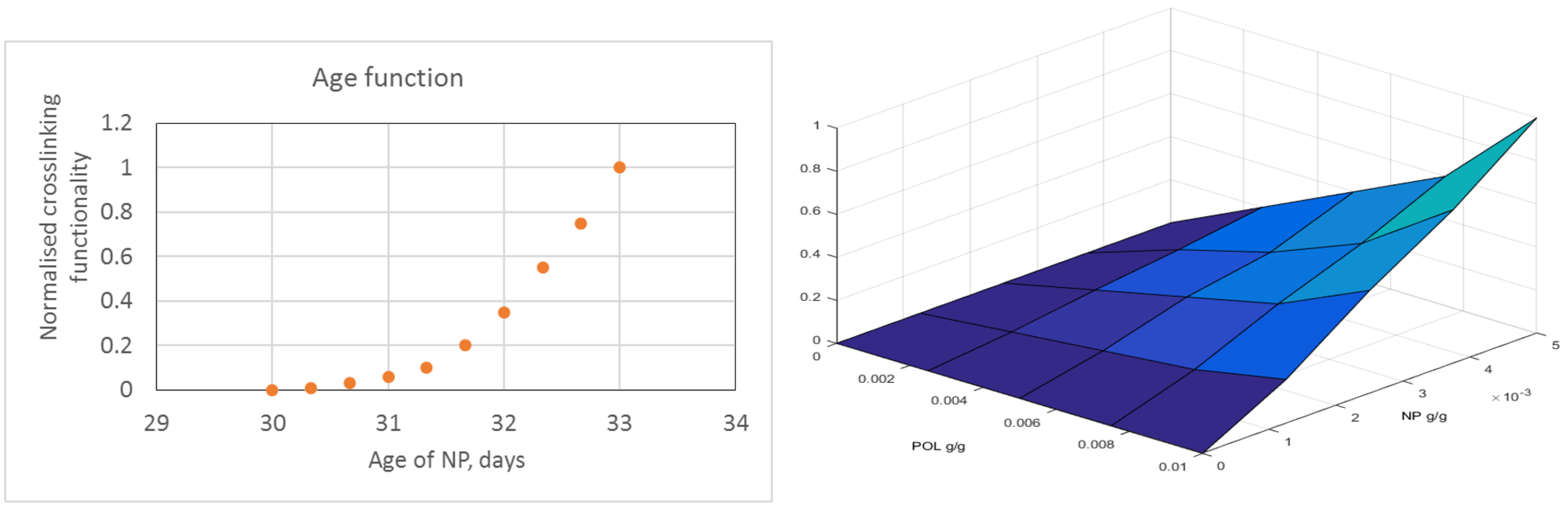
is a weight function defining how the age of the nanomaterials influences gel strength. The (normalized) function
, measures the increase in water viscosity (that is interpolator value) as a function of nanomaterial aging. Typically,
for
,
for
, and
, as illustrated in
Figure A1.
Figure A1.
Weight function signifying that gelling is initiated when nanomaterials have age and when gelling reaches maximal strength at age .
Figure A1.
Weight function signifying that gelling is initiated when nanomaterials have age and when gelling reaches maximal strength at age .
Consequently, the interpolator
is zero when all nanomaterials have aged less than
. When all nanomaterials have aged more than
, the age profile
p is set to
, meaning that the integral in Equation (
A9) satisfies
, giving the maximal value for this integral. We will refer to the integral in Equation (
A9) as
the age effect (at position
x at time
t). The function
must be measured or estimated, keeping the nanomaterial and polymer concentrations constant. At this stage, we have assumed
is independent of concentrations, but it would be straightforward to extend this function to depend on more variables. The other factor in Equation (
A9),
, would reflect the gel strength as a function of the concentrations when all nanomaterials have the same age and must be measured or estimated from more basic principles. At maximal concentrations, it is assumed that
, while
when either of the concentrations are zero (i.e., no gel forms). Thus, when all nanomaterials have aged more than
and when the nanomaterial and polymer concentrations sufficiently high (i.e., when
), the interpolator is unity giving maximal value for water viscosity.
Appendix A.4. Numerical Realization, a Summary
It is important to formulate a numerical scheme that is as stable as possible. Consequently, the coupled conservation laws for water saturation, polymer concentrations, and nanomaterial concentrations are solved numerically using (mainly) an implicit scheme. As the model is spatially 1-D, not too many numerical grid blocks are needed, even for a fine spatial resolution. Few grid blocks also allow for using a small maximal time step without forbiddingly long simulation times. Therefore, the inherited numerical diffusion in implicit schemes can be limited by demanding short time steps and using a first-order implicit scheme (backward Euler) is sufficient for obtaining satisfactory precision. Also, the 1-D formulation results in a block structured Jacobi matrix (tri-block diagonal) in the Newton iterations, allowing for a generalization of the well-known explicit solution of linear equations when the coefficient matrix is tri-diagonal. Consequently, the solver for linear equations is non-iterative, very robust, and fast.
Not all terms are treated implicitly. The age effect
is updated at the end of the time step, meaning that only the factor
in Equation (
A9) is treated implicitly. The age distributions
p and
q are also recalculated at the end of the time step by using “mass conservation” for nanomaterials of the same age and then by adding the time step length to the age of all present nanomaterials. Incidentally, problems were encountered when solving for the “mass conservation” of the variously aged nanomaterials. An explicit scheme for solving for the transport of various aged nanomaterials was rendered unstable, while a fully implicit scheme gave rise to forbiddingly high numerical dispersion. However, by using an explicit upstream and implicit downstream seems to give excellent profiles for the age distributions. A Google search for this explicit upstream, implicit downstream approach actually revealed one single previous paper on this topic [
42].
We first describe how the conservation equations are solved numerically and then define how the age profiles are updated and how the age effect is calculated.
Appendix A.5. Conversion Equations
The phase mobilities are defined as
First, we eliminate the pressure from the system of equations to obtain the standard Buckley–Leverett formulation. Adding the two equations given by Equation (
A1) and using
, we obtain
which means that
, where
is the total volumetric flux defined by the boundary conditions. From Equation (
A2), we then solve for the pressure (-derivative)
where
is the total mobility. In particular, Equation (
A11) is used for calculating the pressure after a converged time step. Note that the absolute permeability only enters in the model through Equation (
A11) (not in the system of equations we solve) when the pressures are calculated for reporting purposes. Indeed, when using rate-controlled boundary conditions and incompressible flow, the pressure is not “an issue” as it is eliminated by Equation (
A11) from the set of equations. Also, note that total volumetric flux
in Equation (
A11) is given from the input data, not calculated. From Equation (
A1) with
, we then obtain
where
is the fractional flow of water. The mass conservation equations for nanomaterials and polymers given by Equation (
A4) are then expressed as
The three equations given by Equations (
A12) and (
A13) are coupled and nonlinear and are solved for
,
, and
using Euler’s backward method, with the explicit exceptions referred to earlier.
Given
and
(
varies in general for different time steps, while
is assumed constant) at time
, we let
and similarly for the other space and/or time dependent variables. Equation (
A12) becomes
where we have dropped the
w index for water saturation and fractional flow. In simulations, the time step is such that the total rate
is constant in the corresponding time interval. We have assumed the direction of flow is with increasing spatial index value
i and the water fractional flow is as indicated calculated using upstream weighted mobilities. As discussed, the water viscosity and, therefore, the water fractional flow
f are not entirely implicitly calculated, as the age-profiles
p and the shear-thinning effect are treated explicitly.
The flux term in Equation (
A13) is also given by upstream weighting (assuming flow in direction of increasing index
i).
The time derivative of the capacity term is approximated by
where
Here, if desorption can occur for component and if desorption does not occur.
As the model presently allows only water to be injected, we have
, where
is the given volumetric injection rate for water in the time interval
and where
A is the cross-sectional area of the porous medium. Furthermore
,
, and
, where
is the given injection concentration of component
k in the time interval
, and is constant during a time step. Let
denote the number of grid blocks. For
, we then have the
algebraic equations:
for the
unknowns
.
This set of nonlinear equations is solved using Newton’s method for systems of equations. The Jacobi matrix is sparse, and the block diagonal where the block matrices are generally matrices. Due to this structure, Gauss elimination does not destroy the sparse structure, and also the number of arithmetic operations in the Gauss-elimination only grows linearly with .
We will not write down the explicit formula for the Jacobi matrix as used in the computer code but report that the Newton iteration shows excellent second-order convergence using as the initial guess in the iteration, which actually validates the expressions obtained and used for the various partial derivatives needed for forming the Jacobi matrix.
One can also analyze the explicit expression for the matrix to conclude that the Jacobi matrix is always not near singular, and no attempt have been made at this stage for calculating the condition number for the matrix.
It is important that and are scaled in the system of equations. This gives contributions from saturations and concentrations equal footing in the calculation of the residual in each Newton iteration.
Appendix A.6. Updating Age Profiles
At each position and at each time step, the nanomaterials in solution and the adsorbed nanomaterials each have their own age profile
p and
q. These age profiles are probability distributions on the interval
, i.e.,
and
, and likewise for
q. No gelling (cross-linking) can occur before nanomaterials reach the minimum gelling age
. Maximally strong gel may form at a given position and time when all the nanomaterials in solution have reached age
(if also
) and the water reaches its maximal viscosity; see Equation (
A9)).
The numerical representations of
and
are given as follows: At time
, we let
where
is the number of ages at time
and similarly for
q. The age profile resolution
is dynamical, but the
resolution is the same for every position at a given time, both for
p and
q.
At early times in the simulation, the list of ages,
, is defined by the length of the time steps. When the (user-defined) maximum number of ages is obtained, the list of ages is recalculated. The maximal number of ages with
is a given input value, and the same applies for the maximal numbers of ages for
. When the maximum number of ages is reached, either ages younger than
or for ages in the interval
, the ages are redistributed evenly and the age fractions
p and
q corresponding to the new list of ages are recalculated to match the previous representation of
p and
q. The recalculation of
p and
q uses linear interpolation for obtaining new values of
and
and a renormalization to ensure
and
. When all ages of the nanomaterials in solution are older than
, the age effect entering in Equation (
A9),
is simply set to unity.
Now, assume we have obtained at time from solving the conservation equations. In addition, we know the age distributions and at time .
The flux of nanomaterials having ages in the interval
is
To compute the
x derivative, we will adopt an explicit upstream, implicit downstream method. That is, we will let
Thus,
approximates the mass (or molar) of nanomaterials in solution with ages in the interval
flowing out of grid block
during the time step
. Here,
A is the cross-sectional area of the model. As previously discussed, any other way of realizing the
x derivative in Equation (
A23) leads to nonphysical results, either highly unstable (using explicit both up- and downstream) or forbiddingly dispersive (using a full implicit formulation).
Assume first that the mass of nanomaterials flowing out of the grid block is negative. Then, the nanomaterial concentration increases locally, and consequently, no adsorbed nanomaterials will desorb at this position. Therefore, the age profile of the adsorbed nanomaterials
at time
will not affect the new age profile
of the nanomaterials in solution at time
. The mass of nanomaterials with ages in the interval
is
Thus, numerically, the change of mass for these materials during the time step
is
We can eliminate
by noting that the increased mass of adsorbed nanomaterials at this time step is
Thus, the increased mass of absorbed nanomaterials (which all come from the nanomaterials in solution) with ages in the interval
is
Writing down mass conservation for absorbed nanomaterials of this age, we find
Using Equation (
A25), we can then eliminate
in Equation (
A24).
Thus, formulating the mass conservation for nanomaterials of age
using Equations (
A23) and (
A24) (having eliminated
), we simply obtain an explicit formula
. Then, from Equation (
A25), we find the values for
.
Assume now that the mass of nanomaterials decreases at a given position during a time step, i.e., that Equation (
A23) is positive. Then, if the option for no desorption of nanomaterials is set, the age profile for the adsorbed nanomaterials stays the same (except that all the adsorbed nanomaterials age the amount of the time step) and
. Thus, Equation (
A25) is satisfied, and
. The mass conservation for
can be solved directly.
If desorption of nanomaterials can occur, then Equation (
A25) still applies and
and
can be found as previously described for the case when Equation (
A23) is negative.
We also observe that the procedure for updating the age profiles is done independently for each k.
In Equation (
A23) for
, the age profile for the injected nanomaterials,
, has
and
for
. That is, all injected nanomaterials have minimum age. Note that it is simple to modify the model to allow for a general user-defined age profile of the injected nanomaterials.
When and have been determined, the ages the distributions represent are of course increased by one time step and the k index is shifted one up. In fact, when the user-defined maximal number of ages is reached, the computer code will always recalculate the representations of the age profiles as previously described, since adding a new age will exceed the maximal number of ages.
Finally, the age profile
is used for estimating the integral
the age effect.
Since m and p generally are represented with values at different nodes, we linearly interpolate p to match the nodes where m is given and calculate the integral using the trapezoidal method. This value for the age effect is then used in the upcoming new time step, where the other parameters defining water viscosity are treated implicitly in this new next time step (except the shear thinning effect).
Appendix A.7. Table-Based Input
A number of the functions needed for the model are table based, allowing for input generality. These functions constitute part of the input for the model and must be measured in the laboratory or estimated from physical principles. All tabulated functions are either functions of one or two variables. Numerical noise may result in function calls outside the given domain, which could lead to ambiguities and possible failure of simulation. For functions of a single variable (defined on an interval), the code will both interpolate and extrapolate linearly. However, the program issues a warning when it has to extrapolate a function outside its given domain, reporting which function and which out-of-domain argument value it extrapolates to.
Two-variable functions are defined on a rectangle . Two-variable functions are evaluated by successive linear interpolation. Concerning extrapolation outside the domain of definition for an argument which both has and , the error will be fatal and the program stops. In case of membership in only one of the intervals, the code will extrapolate linearly and report a warning.
Also, the various derivatives of table-defined functions are needed for the Jacobi matrix. The derivatives are piecewise constant and are calculated consistently with the linear interpolation of function values. Regarding extrapolation of derivatives, the values of derivatives are simply extended constantly outside their domains, both the ordinary and the partial derivatives.
As mentioned so far, the Newton iterations behave excellently, showing second-order convergence. This indicates that the implemented formulation and evaluation of function values and derivatives of table-based functions is sound and robust.