Nanostructured Ni Based Anode and Cathode for Alkaline Water Electrolyzers
Abstract
1. Introduction
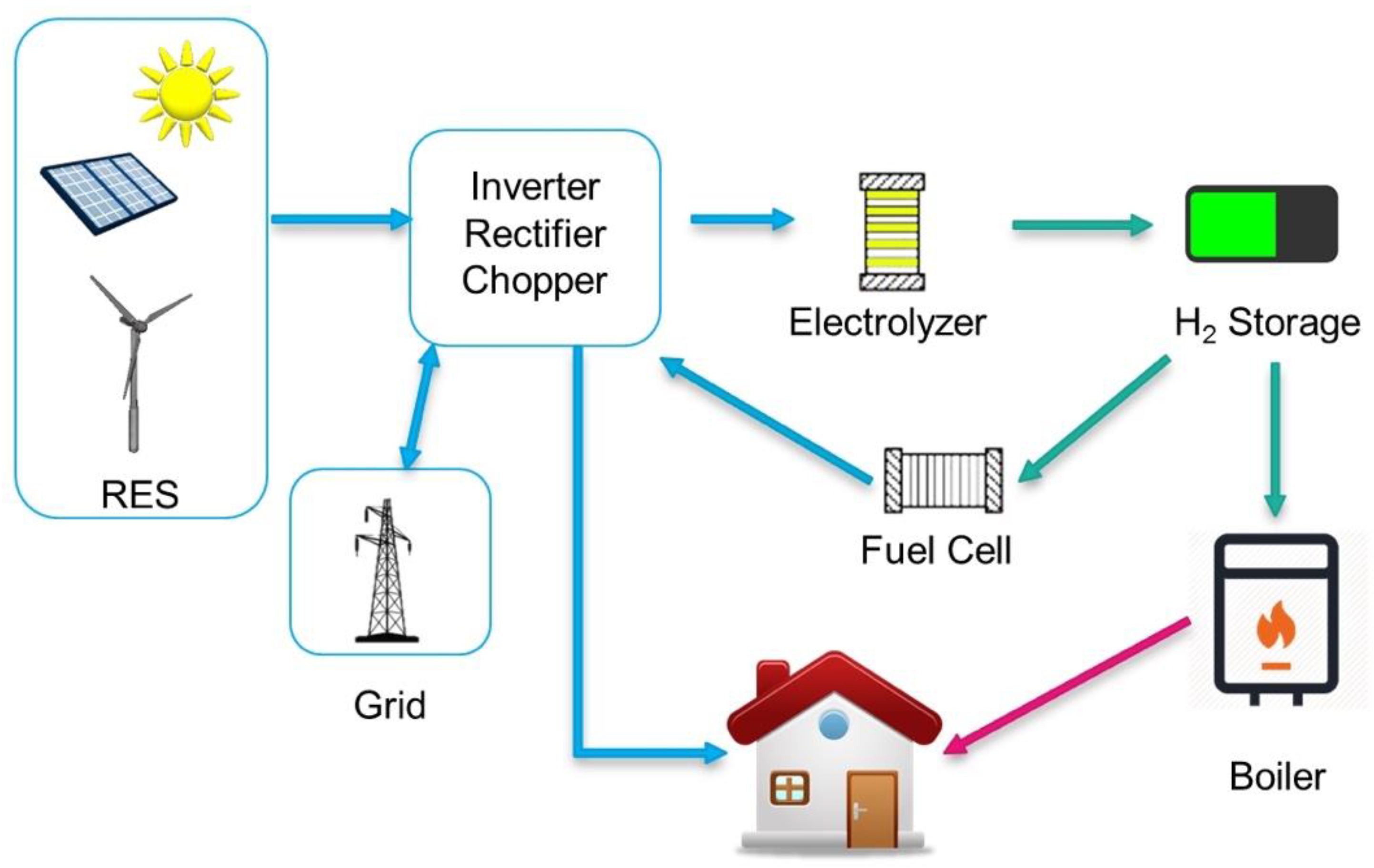
- The first phase is the production of hydrogen through electrochemical cells (electrolyzers) powered by renewable sources.
- The post-production phase is the hydrogen storage. Hydrogen storage methods can be classified into physical methods, such as compression, and chemical methods, such as metal hydrides.
- The third phase consists in the transport of hydrogen from the point of production to the place of consumption—which must not necessarily coincide.
- The last phase of the supply chain is the conversion of hydrogen into electricity or into other forms of energy. In fact, hydrogen can be converted into electricity through fuel cells. However, this should not exclude the possibility of using hydrogen as a fuel in heat engines or boilers, converting hydrogen into mechanical energy and thermal energy, respectively.
2. Materials and Methods
3. Results and Discussion
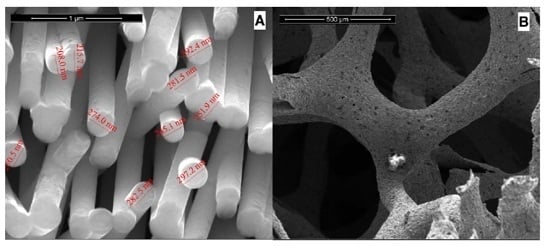
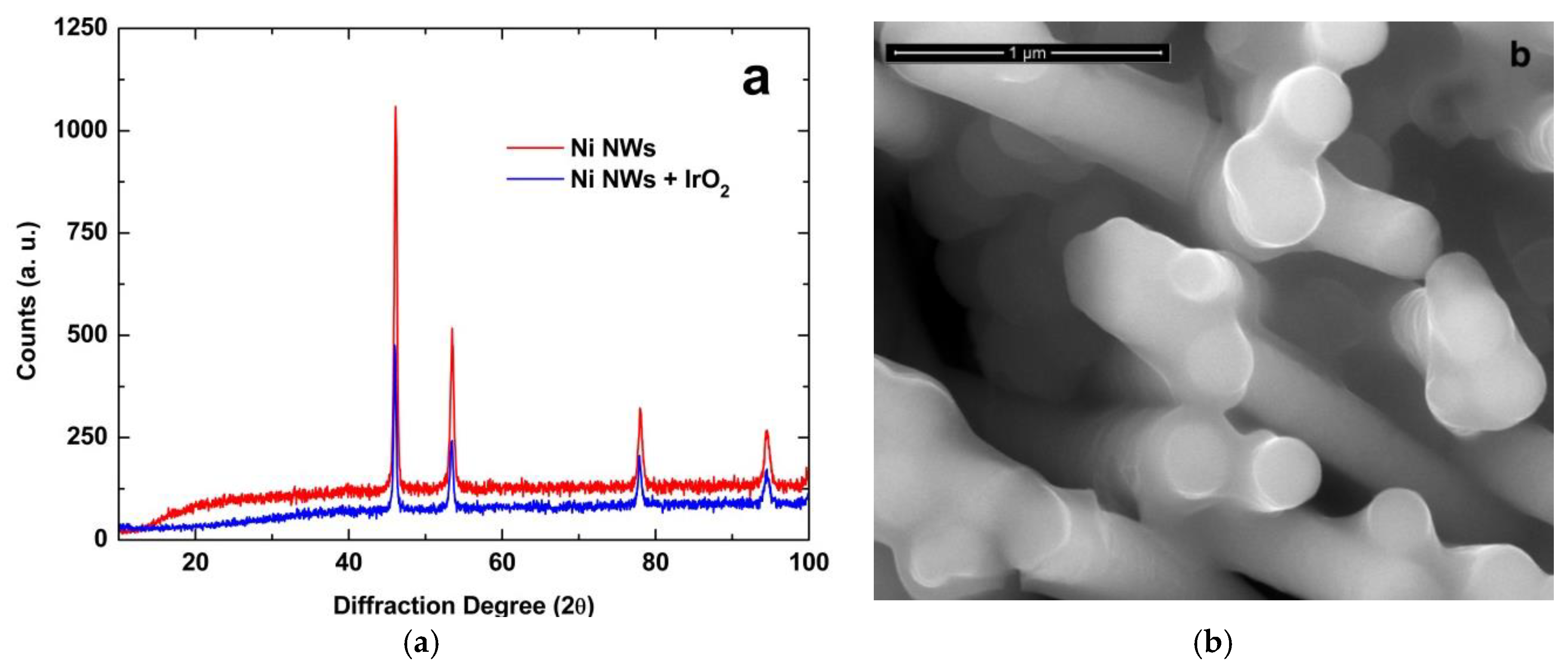
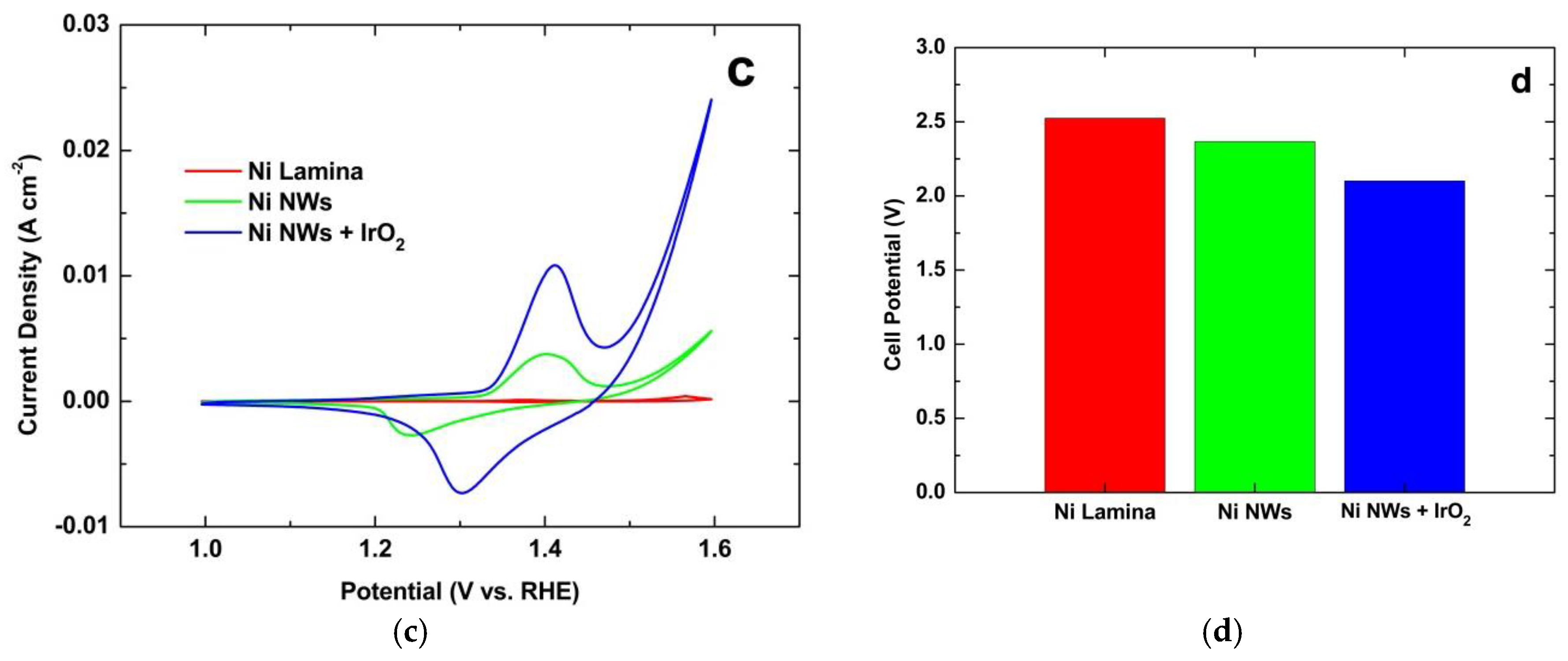
3.1. NWs Behavior with Iridium Oxide
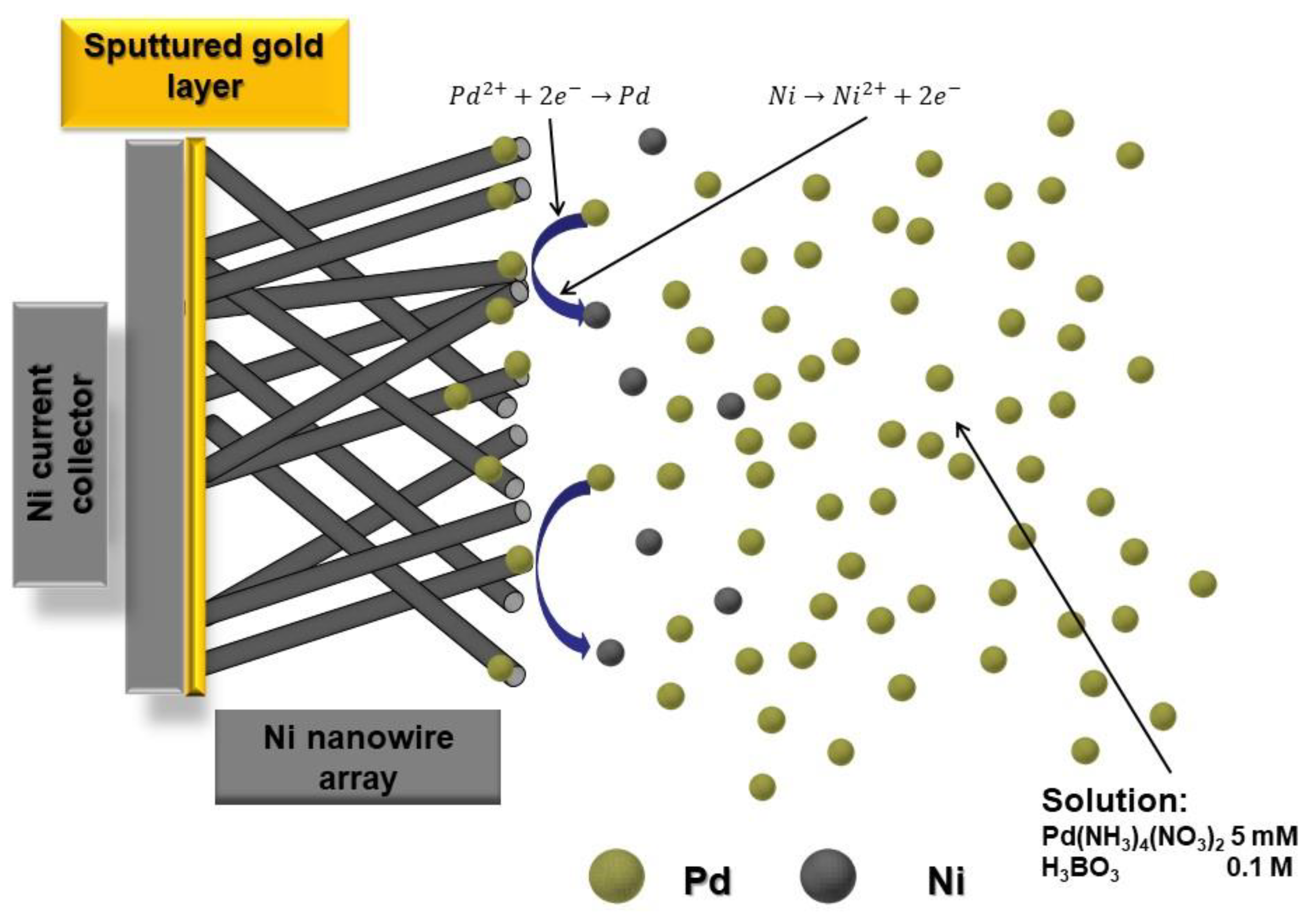
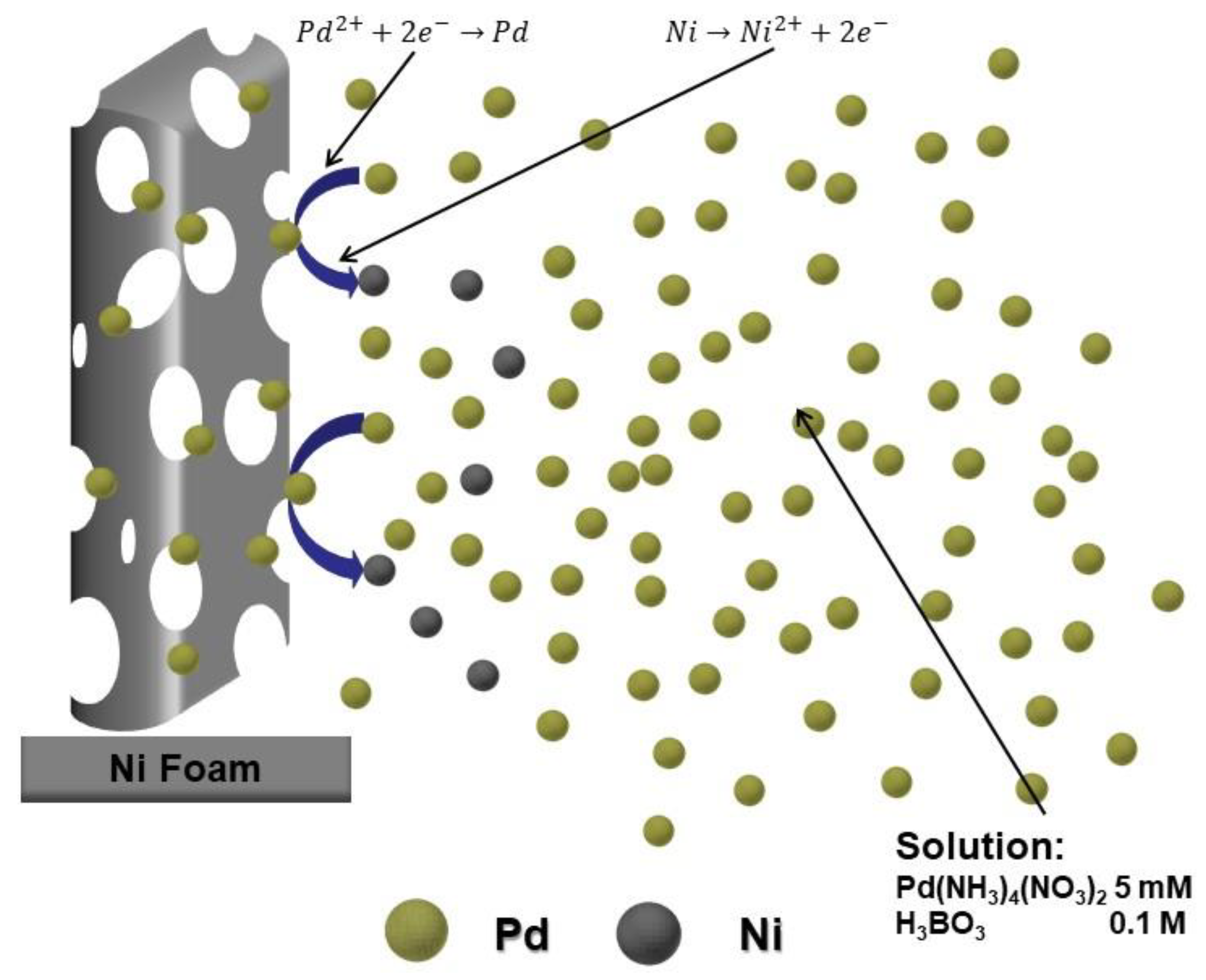
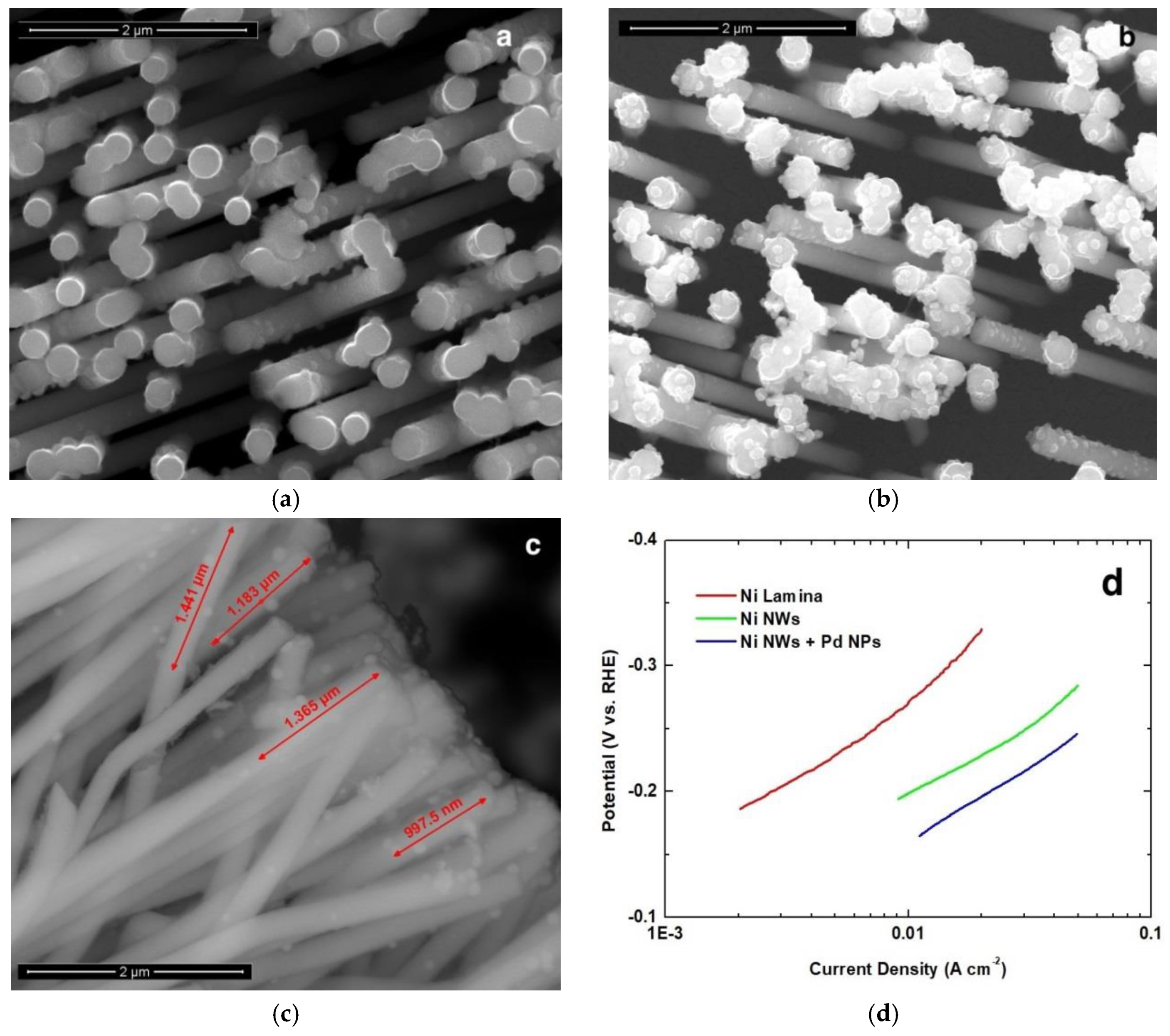
3.2. NWs Behavior with Palladium Nanoparticles
3.3. Behavior of Nanowires of Ni-Co Alloy
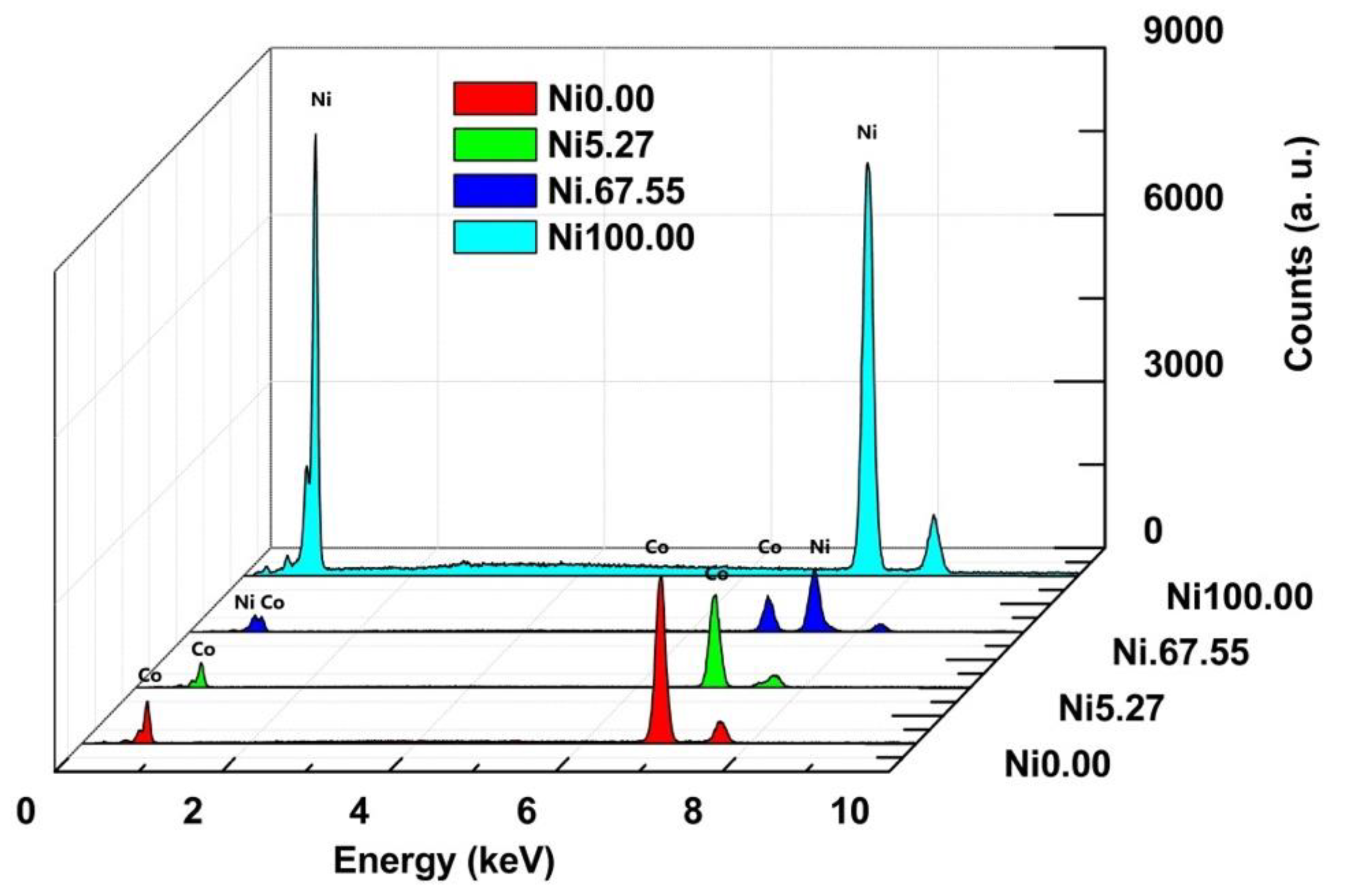
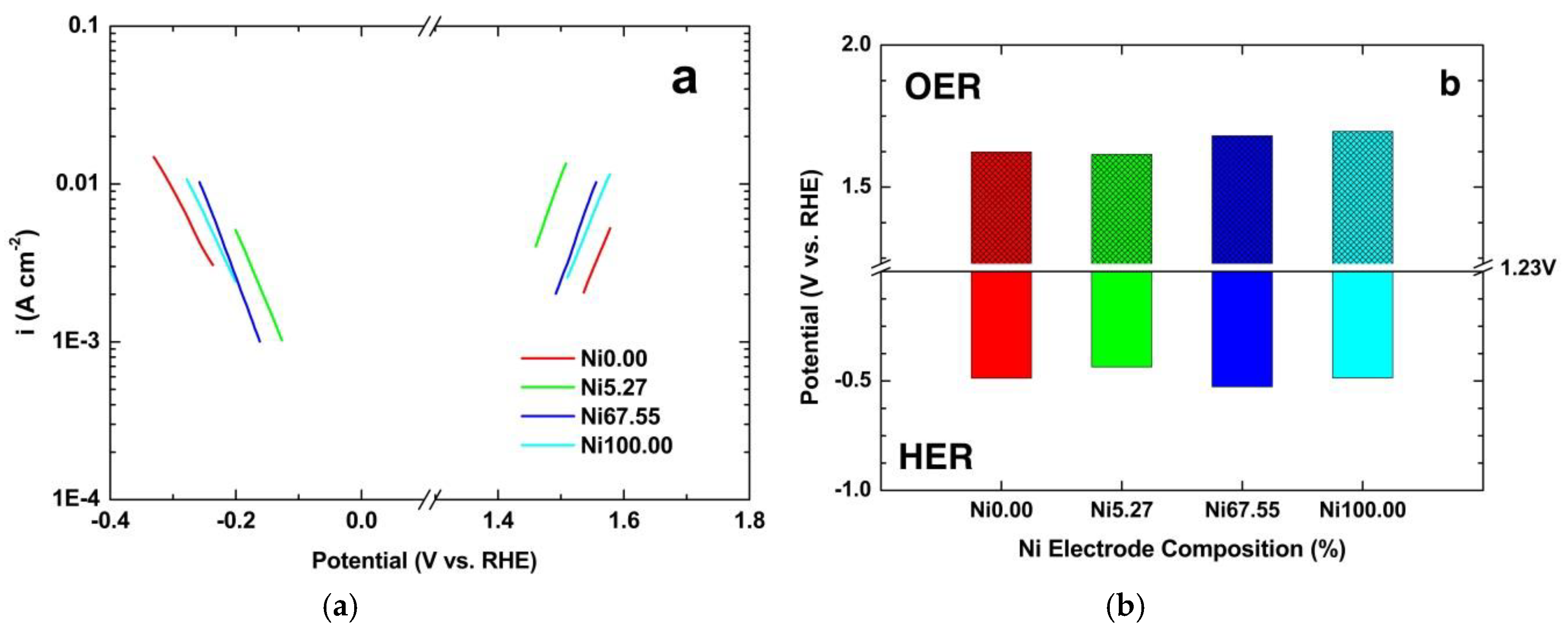
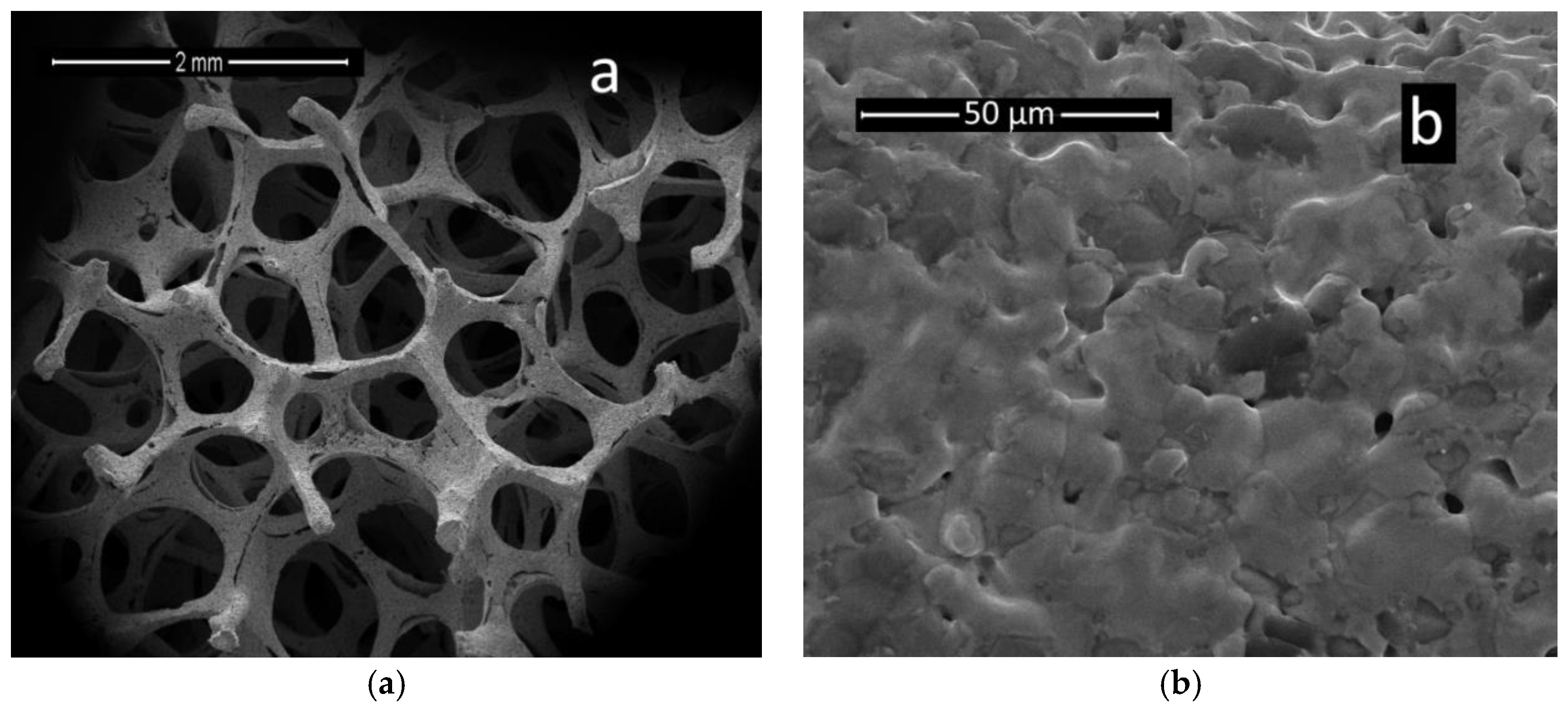
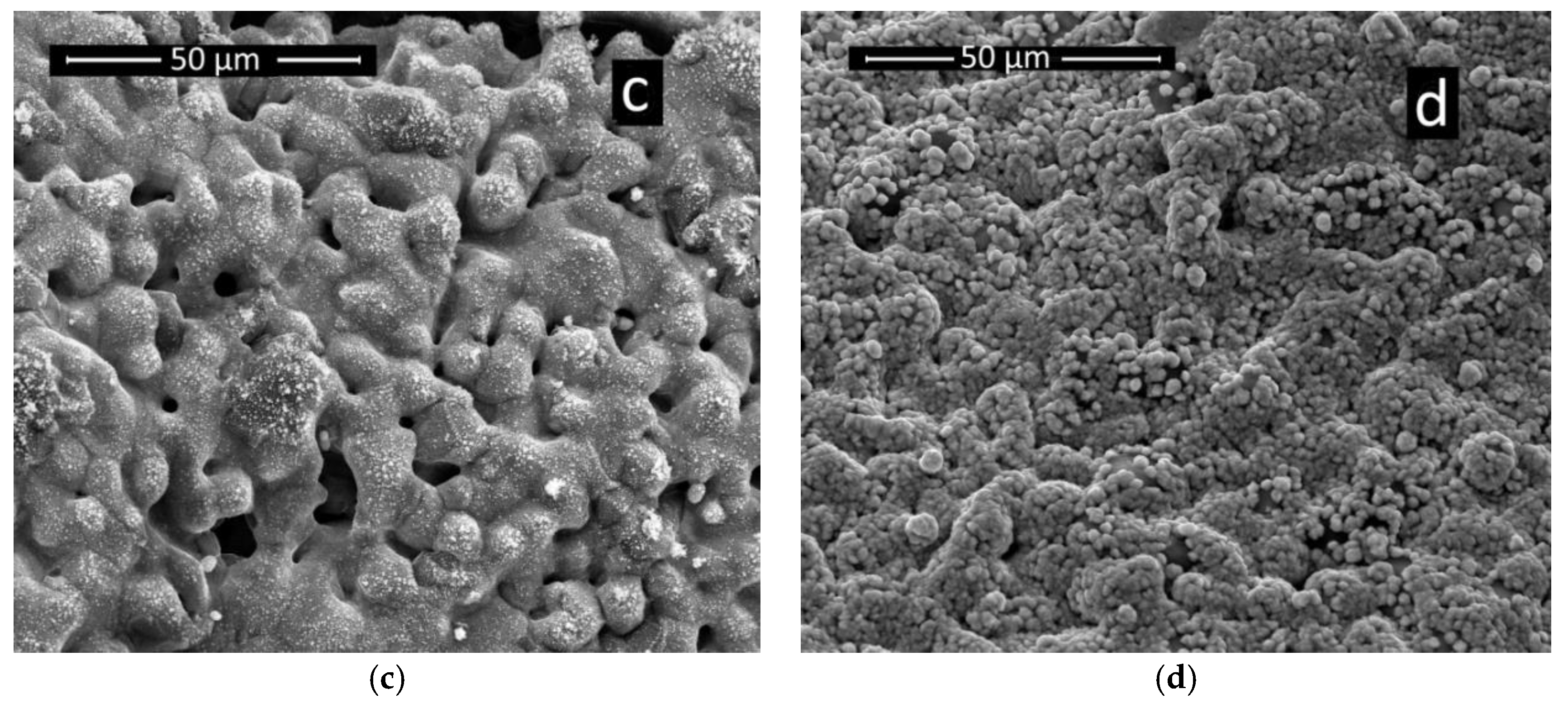
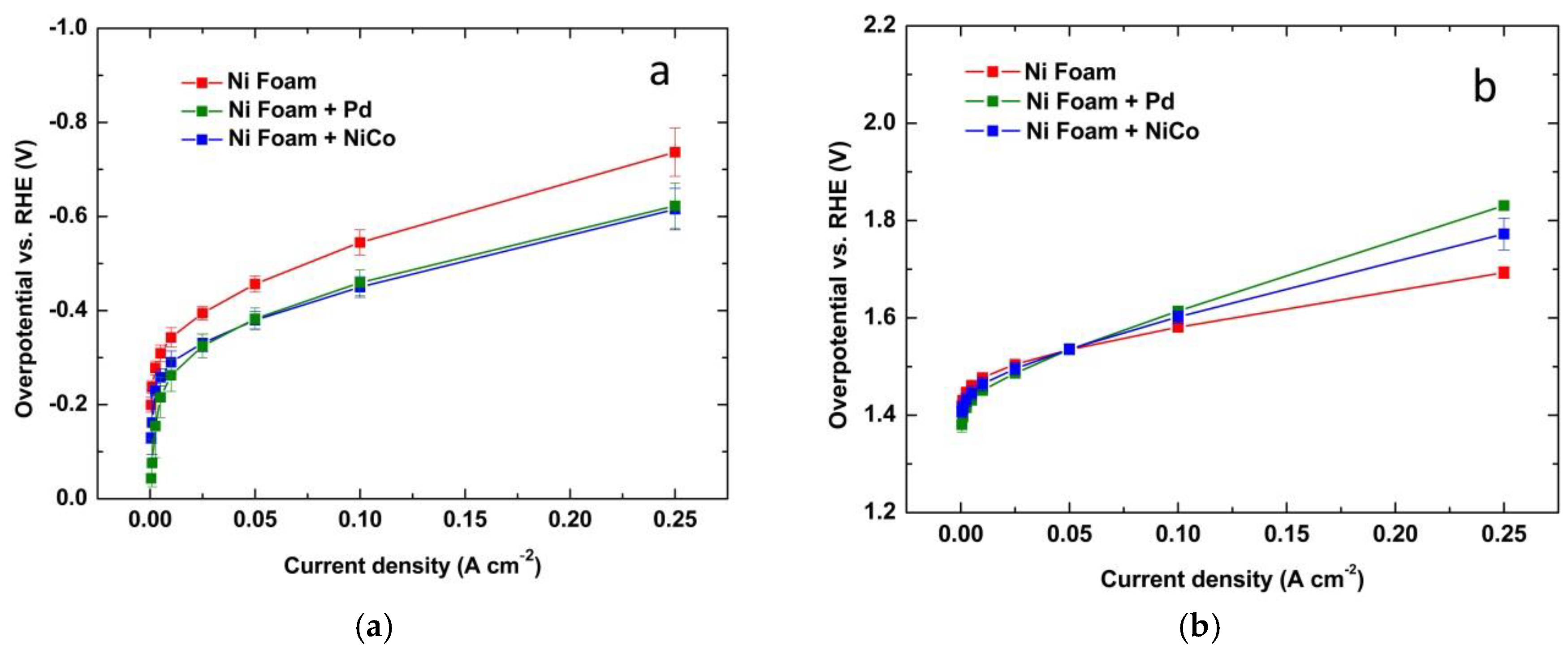
3.4. Ni-foam Behavior with Different Electrocatalysts
3.5. Performance of Lab Scale Electrolyzer
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Global Energy Demand Rose by 2.3% in 2018, Its Fastest Pace in the Last Decade. Available online: https://www.iea.org/newsroom/news/2019/march/global-energy-demand-rose-by-23-in-2018-its-fastest-pace-in-the-last-decade.html (accessed on 8 September 2019).
- Burchardt, J.; Gerbert, P.; Schonberger, S.; Herhold, P.; Brognaux, C. The Economic Case for Combating Climate Change. Available online: https://www.bcg.com/it-it/publications/2018/economic-case-combating-climate-change.aspx (accessed on 8 September 2019).
- Hoffert, M.I.; Caldeira, K.; Benford, G.; Criswell, D.R.; Green, C.; Herzog, H.; Jain, A.K.; Kheshgi, H.S.; Lackner, K.S.; Lewis, J.S.; et al. Advanced Technology Paths to Global Climate Stability: Energy for a Greenhouse Planet. Science 2002, 298, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, S.; Kainuma, M.; Masui, T.; Hasegawa, T.; Dai, H. The effectiveness of energy service demand reduction: A scenario analysis of global climate change mitigation. Energy Policy 2014, 75, 379–391. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable Hydrogen Production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Penner, S.S. Steps toward the hydrogen economy. Energy 2006, 31, 33–43. [Google Scholar] [CrossRef]
- Muradov, N.; Vezirolu, T. From hydrocarbon to hydrogen?carbon to hydrogen economy. Int. J. Hydrog. Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Tromp, T.K. Potential Environmental Impact of a Hydrogen Economy on the Stratosphere. Science 2003, 300, 1740–1742. [Google Scholar] [CrossRef]
- Ritter, J.A.; Ebner, A.D.; Wang, J.; Zidan, R. Implementing a hydrogen economy. Mater. Today 2003, 6, 18–23. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of environmental and economic aspects of various hydrogen production methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.D.; Liu, S.; Teng, C.P.; Han, M.Y. Recent Progress in Energy-Driven Water Splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef]
- Tinker, L.L.; McDaniel, N.D.; Bernhard, S. Progress towards solar-powered homogeneous water photolysis. J. Mater. Chem. 2009, 19, 3328. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrog. Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Ursua, A.; Gandia, L.M.; Sanchis, P. Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Suffredini, H. Recent developments in electrode materials for water electrolysis. Int. J. Hydrog. Energy 2000, 25, 415–423. [Google Scholar] [CrossRef]
- Fan, C.; Piron, D.L. Electrodeposition as a means of producing large-surface electrodes required in water electrolysis. Surf. Coat. Technol. 1995, 73, 91–97. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Fredriksson, H.O.; Niemantsverdriet, J.H. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Han, T.; Shi, Y.; Song, X.; Mio, A.; Valenti, L.; Hui, F.; Privitera, S.; Lombardo, S.; Lanza, M. Ageing mechanisms of highly active and stable nickel-coated silicon photoanodes for water splitting. J. Mater. Chem. A 2016, 4, 8053–8060. [Google Scholar] [CrossRef]
- Han, T.; Privitera, S.; Milazzo, R.G.; Bongiorno, C.; Di Franco, S.; La Via, F.; Song, X.; Shi, Y.; Lanza, M.; Lombardo, S. Photo-electrochemical water splitting in silicon based photocathodes enhanced by plasmonic/catalytic nanostructures. Mater. Sci. Eng. B 2017, 225, 128–133. [Google Scholar] [CrossRef]
- Godula-Jopek, A.; Stolten, D. (Eds.) Hydrogen Production: By Electrolysis, 1st ed.; Wiley-VCH: Weinheim, Germany, 2015; ISBN 978-3-527-67650-7. [Google Scholar]
- Santhanam, K.S.V.; Press, R.J.; Miri, M.J.; Bailey, A.V.; Takacs, G.A. Introduction to Hydrogen Technology, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-1-119-26557-3. [Google Scholar]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Corona-Guinto, J.L.; Cardeño-García, L.; Martínez-Casillas, D.C.; Sandoval-Pineda, J.M.; Tamayo-Meza, P.; Silva-Casarin, R.; González-Huerta, R.G. Performance of a PEM electrolyzer using RuIrCoOx electrocatalysts for the oxygen evolution electrode. Int. J. Hydrog. Energy 2013, 38, 12667–12673. [Google Scholar] [CrossRef]
- Bender, G.; Carmo, M.; Smolinka, T.; Gago, A.; Danilovic, N.; Mueller, M.; Ganci, F.; Fallisch, A.; Lettenmeier, P.; Friedrich, K.A.; et al. Initial approaches in benchmarking and round robin testing for proton exchange membrane water electrolyzers. Int. J. Hydrog. Energy 2019, 44, 9174–9187. [Google Scholar] [CrossRef]
- Sheela, G. Zinc–nickel alloy electrodeposits for water electrolysis. Int. J. Hydrog. Energy 2002, 27, 627–633. [Google Scholar] [CrossRef]
- Solmaz, R.; Salcı, A.; Yüksel, H.; Doğrubaş, M.; Kardaş, G. Preparation and characterization of Pd-modified Raney-type NiZn coatings and their application for alkaline water electrolysis. Int. J. Hydrog. Energy 2017, 42, 2464–2475. [Google Scholar] [CrossRef]
- Li, X.; Walsh, F.C.; Pletcher, D. Nickel based electrocatalysts for oxygen evolution in high current density, alkaline water electrolysers. Phys Chem Chem Phys 2011, 13, 1162–1167. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardaş, G. Fabrication and characterization of NiCoZn–M (M: Ag, Pd and Pt) electrocatalysts as cathode materials for electrochemical hydrogen production. Int. J. Hydrog. Energy 2011, 36, 12079–12087. [Google Scholar] [CrossRef]
- Milazzo, R.G.; Privitera, S.M.S.; D’Angelo, D.; Scalese, S.; Di Franco, S.; Maita, F.; Lombardo, S. Spontaneous galvanic displacement of Pt nanostructures on nickel foam: Synthesis, characterization and use for hydrogen evolution reaction. Int. J. Hydrog. Energy 2018, 43, 7903–7910. [Google Scholar] [CrossRef]
- Mayousse, E.; Maillard, F.; Fouda-Onana, F.; Sicardy, O.; Guillet, N. Synthesis and characterization of electrocatalysts for the oxygen evolution in PEM water electrolysis. Int. J. Hydrog. Energy 2011, 36, 10474–10481. [Google Scholar] [CrossRef]
- Xu, D.; Stevens, M.B.; Cosby, M.R.; Oener, S.Z.; Smith, A.M.; Enman, L.J.; Ayers, K.E.; Capuano, C.B.; Renner, J.N.; Danilovic, N.; et al. Earth-Abundant Oxygen Electrocatalysts for Alkaline Anion-Exchange-Membrane Water Electrolysis: Effects of Catalyst Conductivity and Comparison with Performance in Three-Electrode Cells. Acs Catal. 2019, 9, 7–15. [Google Scholar] [CrossRef]
- Schiller, G.; Ansar, A.; Lang, M.; Patz, O. High temperature water electrolysis using metal supported solid oxide electrolyser cells (SOEC). J. Appl. Electrochem. 2009, 39, 293–301. [Google Scholar] [CrossRef]
- Brisse, A.; Schefold, J.; Zahid, M. High temperature water electrolysis in solid oxide cells. Int. J. Hydrog. Energy 2008, 33, 5375–5382. [Google Scholar] [CrossRef]
- Schalenbach, M.; Zeradjanin, A.R.; Kasian, O.; Cherevko, S.; Mayrhofer, K.J. A Perspective on Low-Temperature Water Electrolysis—Challenges in Alkaline and Acidic Technology. Int. J. Electrochem. Sci. 2018, 1173–1226. [Google Scholar] [CrossRef]
- Bodner, M.; Hofer, A.; Hacker, V. H2 generation from alkaline electrolyzer: H2 generation from alkaline electrolyzer. Wiley Interdiscip. Rev. Energy Environ. 2015, 4, 365–381. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions—A Review. Int. J. Hydrog. Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Amikam, G.; Nativ, P.; Gendel, Y. Chlorine-free alkaline seawater electrolysis for hydrogen production. Int. J. Hydrog. Energy 2018, 43, 6504–6514. [Google Scholar] [CrossRef]
- Ding, R.; Cui, S.; Lin, J.; Sun, Z.; Du, P.; Chen, C. Improving the water splitting performance of nickel electrodes by optimizing their pore structure using a phase inversion method. Catal. Sci. Technol. 2017, 7, 3056–3064. [Google Scholar] [CrossRef]
- Jing, S.; Zhang, L.; Luo, L.; Lu, J.; Yin, S.; Shen, P.K.; Tsiakaras, P. N-Doped Porous Molybdenum Carbide Nanobelts as Efficient Catalysts for Hydrogen Evolution Reaction. Appl. Catal. B Environ. 2018, 224, 533–540. [Google Scholar] [CrossRef]
- Mollamahale, Y.B.; Jafari, N.; Hosseini, D. Electrodeposited Ni-W nanoparticles: Enhanced catalytic activity toward hydrogen evolution reaction in acidic media. Mater. Lett. 2018, 213, 15–18. [Google Scholar] [CrossRef]
- Siwek, K.I.; Eugénio, S.; Santos, D.M.F.; Silva, M.T.; Montemor, M.F. 3D nickel foams with controlled morphologies for hydrogen evolution reaction in highly alkaline media. Int. J. Hydrog. Energy 2019, 44, 1701–1709. [Google Scholar] [CrossRef]
- Chen, S.; Thind, S.S.; Chen, A. Nanostructured materials for water splitting—State of the art and future needs: A mini-review. Electrochem. Commun. 2016, 63, 10–17. [Google Scholar] [CrossRef]
- Sunseri, C.; Cocchiara, C.; Ganci, F.; Moncada, A.; Oliveri, R.L.; Patella, B.; Piazza, S. Rosalinda Inguanta Nanostructured electrochemical devices for sensing, energy conversion and storage. Chem. Eng. Trans. 2016, 47, 43–48. [Google Scholar]
- Inguanta, R.; Piazza, S.; Sunseri, C.; Cino, A.; Di Dio, V.; Cascia, D.L.; Miceli, R.; Rando, C.; Zizzo, G. An electrochemical route towards the fabrication of nanostructured semiconductor solar cells. In Proceedings of the SPEEDAM 2010, Pisa, Italy, 14–16 June 2010; pp. 1166–1171. [Google Scholar]
- Patella, B.; Inguanta, R.; Piazza, S.; Sunseri, C. A nanostructured sensor of hydrogen peroxide. Sens. Actuators B Chem. 2017, 245, 44–54. [Google Scholar] [CrossRef]
- Silipigni, L.; Barreca, F.; Fazio, E.; Neri, F.; Spanò, T.; Piazza, S.; Sunseri, C.; Inguanta, R. Template Electrochemical Growth and Properties of Mo Oxide Nanostructures. J. Phys. Chem. C 2014, 118, 22299–22308. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C. Influence of electrodeposition techniques on Ni nanostructures. Electrochim. Acta 2008, 53, 5766–5773. [Google Scholar] [CrossRef]
- Ganci, F.; Lombardo, S.; Sunseri, C.; Inguanta, R. Nanostructured electrodes for hydrogen production in alkaline electrolyzer. Renew. Energy 2018, 123, 117–124. [Google Scholar] [CrossRef]
- Battaglia, M.; Inguanta, R.; Piazza, S.; Sunseri, C. Fabrication and characterization of nanostructured Ni–IrO2 electrodes for water electrolysis. Int. J. Hydrog. Energy 2014, 39, 16797–16805. [Google Scholar] [CrossRef]
- Ganci, F.; Cusumano, V.; Sunseri, C.; Inguanta, R. Performance Enhancement of Alkaline Water Electrolyzer Using Nanostructured Electrodes Synthetized by Template Electrosynthesis. In Proceedings of the 2018 IEEE 4th International Forum on Research and Technology for Society and Industry (RTSI), Palermo, Italy, 10–13 September 2018; pp. 1–4. [Google Scholar]
- Li, A.; Sun, Y.; Yao, T.; Han, H. Earth-Abundant Transition-Metal-Based Electrocatalysts for Water Electrolysis to Produce Renewable Hydrogen. Chem. Eur. J. 2018, 24, 18334–18355. [Google Scholar] [CrossRef] [PubMed]
- Fabrizio, G.; Valentino, C.; Carmelo, S. Inguanta Rosalinda Fabrication of Nanostructured Ni-co Electrodes for Hydrogen and Oxygen Evolution Reaction in Water-alkaline Electrolyzer. Chem. Eng. Trans. 2019, 73, 109–114. [Google Scholar]
- Ganci, F.; Inguanta, R.; Piazza, S.; Sunseri, C. Salvatore Lombardo Fabrication and characterization of nanostructured ni and pd electrodes for hydrogen evolution reaction (her) in water-alkaline electrolyzer. Chem. Eng. Trans. 2017, 57, 1591–1596. [Google Scholar]
- Moncada, A.; Piazza, S.; Sunseri, C.; Inguanta, R. Recent improvements in PbO2 nanowire electrodes for lead-acid battery. J. Power Sources 2015, 275, 181–188. [Google Scholar] [CrossRef]
- Vázquez-Gómez, L.; Cattarin, S.; Guerriero, P.; Musiani, M. Influence of deposition current density on the composition and properties of electrodeposited Ni+RuO2 and Ni+IrO2 composites. J. Electroanal. Chem. 2009, 634, 42–48. [Google Scholar] [CrossRef]
- Schmidt, T.; Wendt, H. Electrocatalysis of cathodic hydrogen and anodic oxygen evolution in alkaline water electrolysis by in situ activation procedures. Electrochim. Acta 1994, 39, 1763–1767. [Google Scholar] [CrossRef]
- Inguanta, R.; Rinaldo, E.; Piazza, S.; Sunseri, C. Lead Nanowires for Microaccumulators Obtained Through Indirect Electrochemical Template Deposition. Electrochem. Solid-State Lett. 2010, 13, K1–K4. [Google Scholar] [CrossRef]
- Battaglia, M.; Piazza, S.; Sunseri, C.; Inguanta, R. Amorphous silicon nanotubes via galvanic displacement deposition. Electrochem. Commun. 2013, 34, 134–137. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. A new route to grow oxide nanostructures based on metal displacement deposition. Lanthanides oxy/hydroxides growth. Electrochim. Acta 2012, 76, 77–87. [Google Scholar] [CrossRef]
- Yamanaka, K. Anodically Electrodeposited Iridium Oxide Films (AEIROF) from Alkaline Solutions for Electrochromic Display Devices. Jpn. J. Appl. Phys. 1989, 28, 632–637. [Google Scholar] [CrossRef]
- Ooka, H.; Yamaguchi, A.; Takashima, T.; Hashimoto, K.; Nakamura, R. Efficiency of Oxygen Evolution on Iridium Oxide Determined from the pH Dependence of Charge Accumulation. J. Phys. Chem. C 2017, 121, 17873–17881. [Google Scholar] [CrossRef]
- Abbott, D.F.; Lebedev, D.; Waltar, K.; Povia, M.; Nachtegaal, M.; Fabbri, E.; Copéret, C.; Schmidt, T.J. Iridium Oxide for the Oxygen Evolution Reaction: Correlation between Particle Size, Morphology, and the Surface Hydroxo Layer from Operando XAS. Chem. Mater. 2016, 28, 6591–6604. [Google Scholar] [CrossRef]
- Schrebler Guzmán, R.S.; Vilche, J.R.; Arvía, A.J. Rate Processes Related to the Hydrated Nickel Hydroxide Electrode in Alkaline Solutions. J. Electrochem. Soc. 1978, 125, 1578. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An overview on Pd-based electrocatalysts for the hydrogen evolution reaction. Inorg. Chem. Front. 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Naga Mahesh, K.; Balaji, R.; Dhathathreyan, K.S. Palladium nanoparticles as hydrogen evolution reaction (HER) electrocatalyst in electrochemical methanol reformer. Int. J. Hydrog. Energy 2016, 41, 46–51. [Google Scholar] [CrossRef]
- Hall, D.S.; Bock, C.; MacDougall, B.R. The Electrochemistry of Metallic Nickel: Oxides, Hydroxides, Hydrides and Alkaline Hydrogen Evolution. J. Electrochem. Soc. 2013, 160, F235–F243. [Google Scholar] [CrossRef]
- Pérez-Alonso, F.J.; Adán, C.; Rojas, S.; Peña, M.A.; Fierro, J.L.G. Ni–Co electrodes prepared by electroless-plating deposition. A study of their electrocatalytic activity for the hydrogen and oxygen evolution reactions. Int. J. Hydrog. Energy 2015, 40, 51–61. [Google Scholar] [CrossRef]
- Hong, S.H.; Ahn, S.H.; Choi, I.; Pyo, S.G.; Kim, H.J.; Jang, J.H.; Kim, S.K. Fabrication and evaluation of nickel cobalt alloy electrocatalysts for alkaline water splitting. Appl. Surf. Sci. 2014, 307, 146–152. [Google Scholar] [CrossRef]
- Lupi, C.; Dell’Era, A.; Pasquali, M. Nickel–cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int. J. Hydrog. Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- Bousher, A. Review: Unidentate complexes involving borate. J. Coord. Chem. 1995, 34, 1–11. [Google Scholar] [CrossRef]
- Cardoso, D.S.P.; Amaral, L.; Santos, D.M.F.; Šljukić, B.; Sequeira, C.A.C.; Macciò, D.; Saccone, A. Enhancement of hydrogen evolution in alkaline water electrolysis by using nickel-rare earth alloys. Int. J. Hydrog. Energy 2015, 40, 4295–4302. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, S.; Seo, B.; Hwang, J.M.; Joo, S.H.; Moon, H.R. Direct conversion of coordination compounds into Ni 2 P nanoparticles entrapped in 3D mesoporous graphene for an efficient hydrogen evolution reaction. Mater. Chem. Front. 2017, 1, 973–978. [Google Scholar] [CrossRef]
- Wang, F.; Yang, X.; Dong, B.; Yu, X.; Xue, H.; Feng, L. A FeP powder electrocatalyst for the hydrogen evolution reaction. Electrochem. Commun. 2018, 92, 33–38. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Ji, W.B.; Hu, B.C.; Liang, H.W.; Xu, X.X.; Yu, Z.L.; Li, B.Y.; Yu, S.H. Partially oxidized Ni nanoparticles supported on Ni-N co-doped carbon nanofibers as bifunctional electrocatalysts for overall water splitting. Nano Energy 2018, 51, 286–293. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, A.; Li, L.; Ai, L. Nickel–cobalt layered double hydroxide nanosheets as high-performance electrocatalyst for oxygen evolution reaction. J. Power Sources 2015, 278, 445–451. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, Y.; Deng, H.; Zhang, C.; Su, J.W.; Dong, Y.; Lin, J. Ternary nickel iron phosphide supported on nickel foam as a high-efficiency electrocatalyst for overall water splitting. Int. J. Hydrog. Energy 2018, 43, 7299–7306. [Google Scholar] [CrossRef]
- Guan, C.; Xiao, W.; Wu, H.; Liu, X.; Zang, W.; Zhang, H.; Ding, J.; Feng, Y.P.; Pennycook, S.J.; Wang, J. Hollow Mo-doped CoP nanoarrays for efficient overall water splitting. Nano Energy 2018, 48, 73–80. [Google Scholar] [CrossRef]

| Properties | Hydrogen | Methane | Gasoline | Diesel | LPG | Methanol |
|---|---|---|---|---|---|---|
| Specific energy [MJ/kg] | 141.86 | 55.6 | 46.4 | 45.6 | 49.6 | 19.87 |
| Energy density [MJ/L] | 10.044 (liquid) | 22.2 | 34.2 | 38.6 | 25.3 | 15.6 |
| Electrode | a (V) | b (V/dec) | Potential (V vs. RHE) |
|---|---|---|---|
| Ni Lamina | −0.560 | −0.142 | −0.980 |
| Ni NWs | −0.431 | −0.118 | −0.703 |
| Ni NWs + Pd NPs | −0.400 | −0.120 | −0.734 |
| Electrocatalysts | HER/OER | KOH Solution | Potential (V versus RHE) | Reference |
|---|---|---|---|---|
| Ni Lamina | HER | 30% w/w | −0.269 | This work |
| Ni Foam + Pd | HER | 30% w/w | −0.250 | This work |
| Ni0.95Dy0.05 | HER | 8 M | −0.240 | [76] |
| CoP/CC | HER | 1 M | −0.206 | [77] |
| Ni NWs | HER | 30% w/w | −0.196 | This work |
| Ni2P@mesoG | HER | 1 M | −0.188 | [78] |
| Petaloid FeP/C | HER | 1 M | −0.185 | [79] |
| Ni NWs + Pd NPs | HER | 30% w/w | −0.150 | This work |
| PO-Ni/Ni-N-CNFs | OER | 1 M | 1.69 | [80] |
| Ni NWs | OER | 30% w/w | 1.67 | This work |
| NiCo-LDH | OER | 0.1 M | 1.65 | [81] |
| Ni-FexP/NF | OER | 1 M | 1.62 | [82] |
| Mo-CoP | OER | 1 M | 1.56 | [83] |
| Ni NWs + IrO2 | OER | 1 M | 1.53 | This work |
| NiCo NWs (Ni5.27) | OER | 30% w/w | 1.49 | This work |
| Ni Foam + NiCo | OER | 30% w/w | 1.48 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganci, F.; Baguet, T.; Aiello, G.; Cusumano, V.; Mandin, P.; Sunseri, C.; Inguanta, R. Nanostructured Ni Based Anode and Cathode for Alkaline Water Electrolyzers. Energies 2019, 12, 3669. https://doi.org/10.3390/en12193669
Ganci F, Baguet T, Aiello G, Cusumano V, Mandin P, Sunseri C, Inguanta R. Nanostructured Ni Based Anode and Cathode for Alkaline Water Electrolyzers. Energies. 2019; 12(19):3669. https://doi.org/10.3390/en12193669
Chicago/Turabian StyleGanci, Fabrizio, Tracy Baguet, Giuseppe Aiello, Valentino Cusumano, Philippe Mandin, Carmelo Sunseri, and Rosalinda Inguanta. 2019. "Nanostructured Ni Based Anode and Cathode for Alkaline Water Electrolyzers" Energies 12, no. 19: 3669. https://doi.org/10.3390/en12193669
APA StyleGanci, F., Baguet, T., Aiello, G., Cusumano, V., Mandin, P., Sunseri, C., & Inguanta, R. (2019). Nanostructured Ni Based Anode and Cathode for Alkaline Water Electrolyzers. Energies, 12(19), 3669. https://doi.org/10.3390/en12193669