Experimental and Simulation Studies on Adsorption and Diffusion Characteristics of Coalbed Methane
Abstract
:1. Introduction
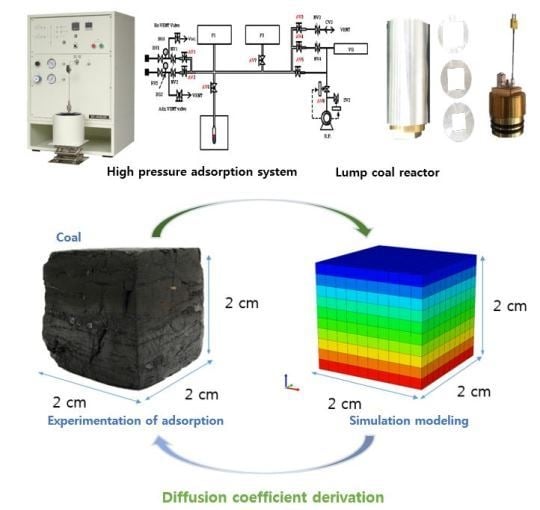
2. Materials and Methods
2.1. Gas Content Measurement
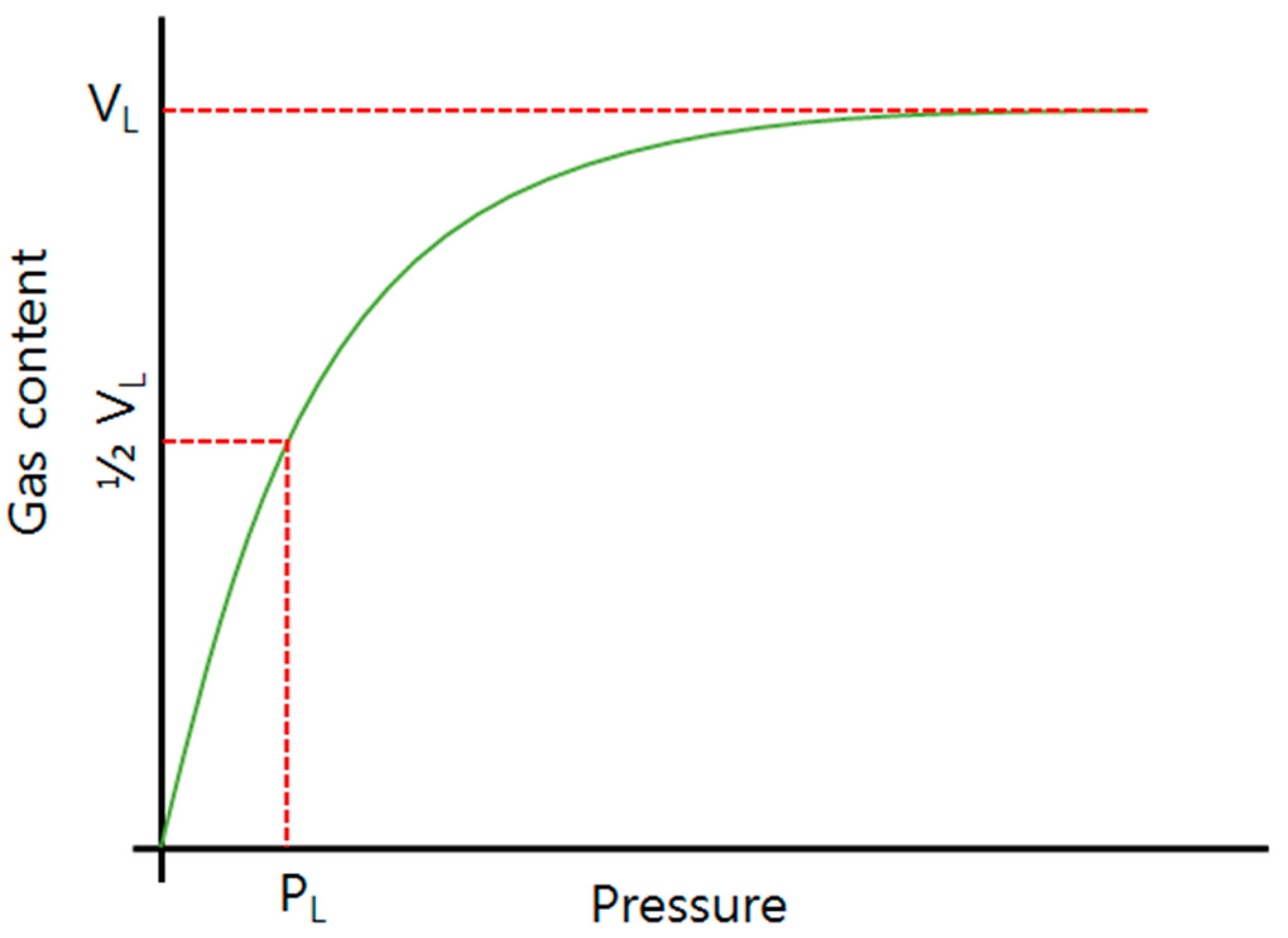
2.2. Sorption Isotherm
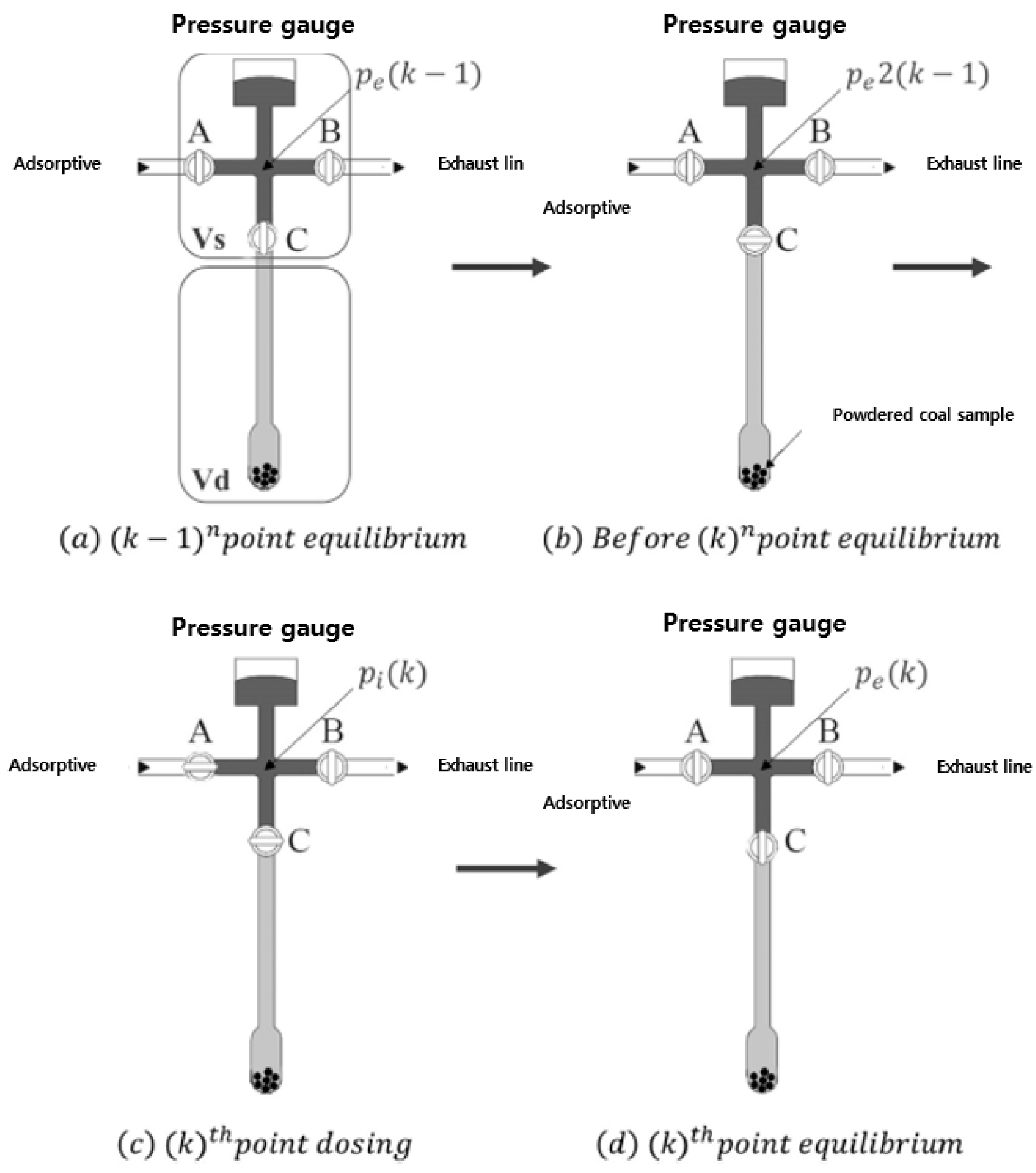
2.3. Experimental Method
3. Results and Discussion
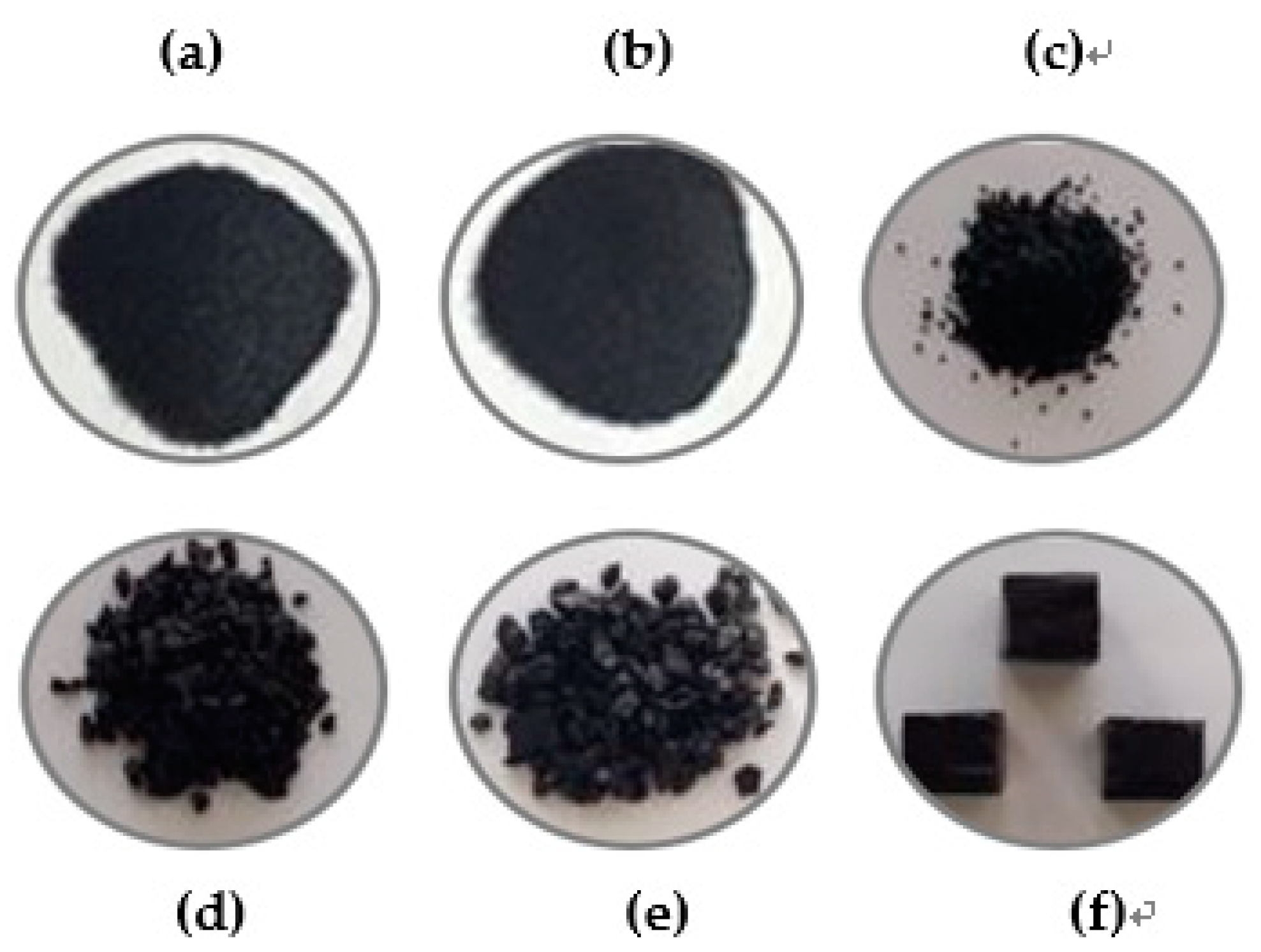
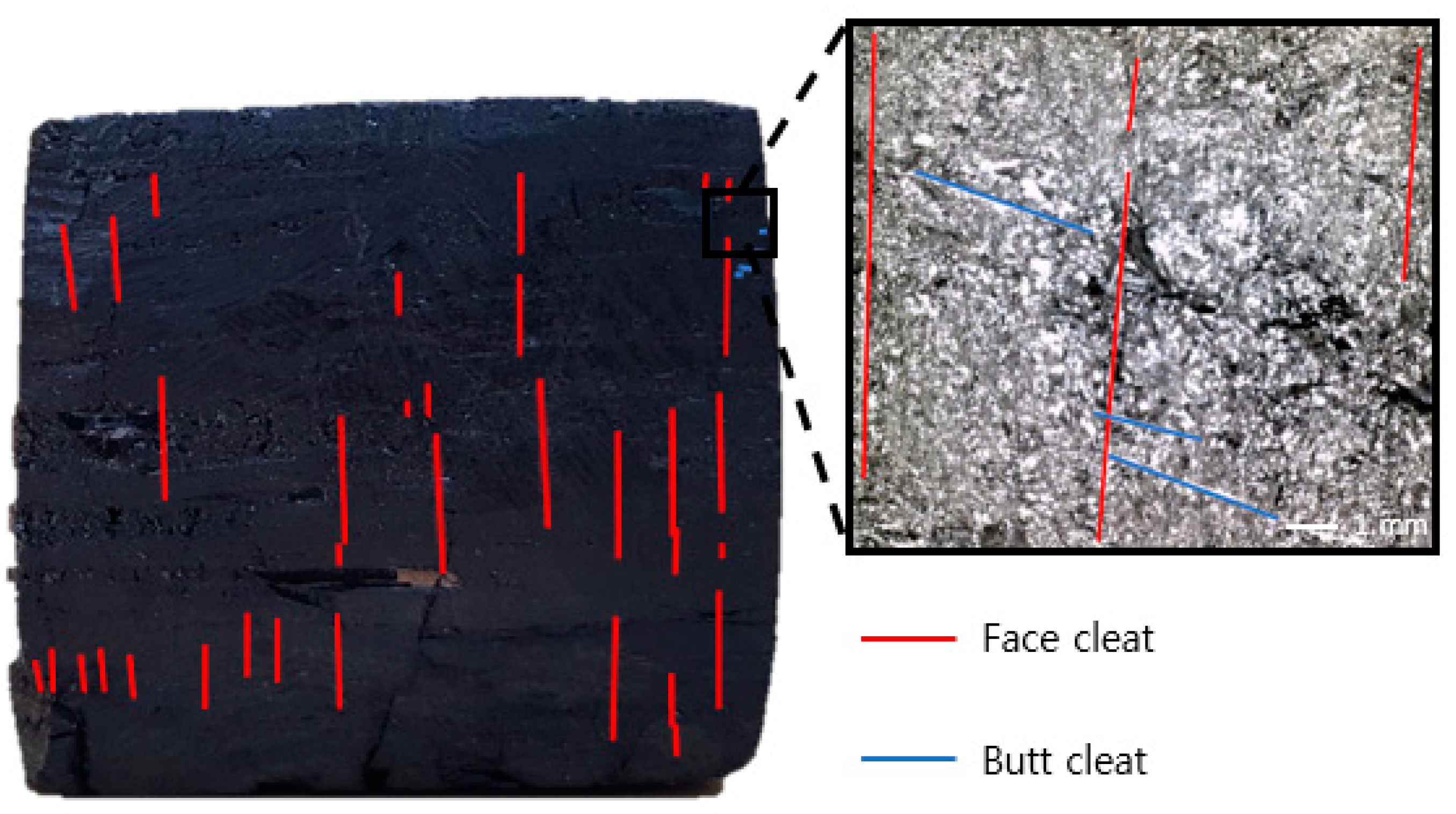
3.1. Sample Preparation
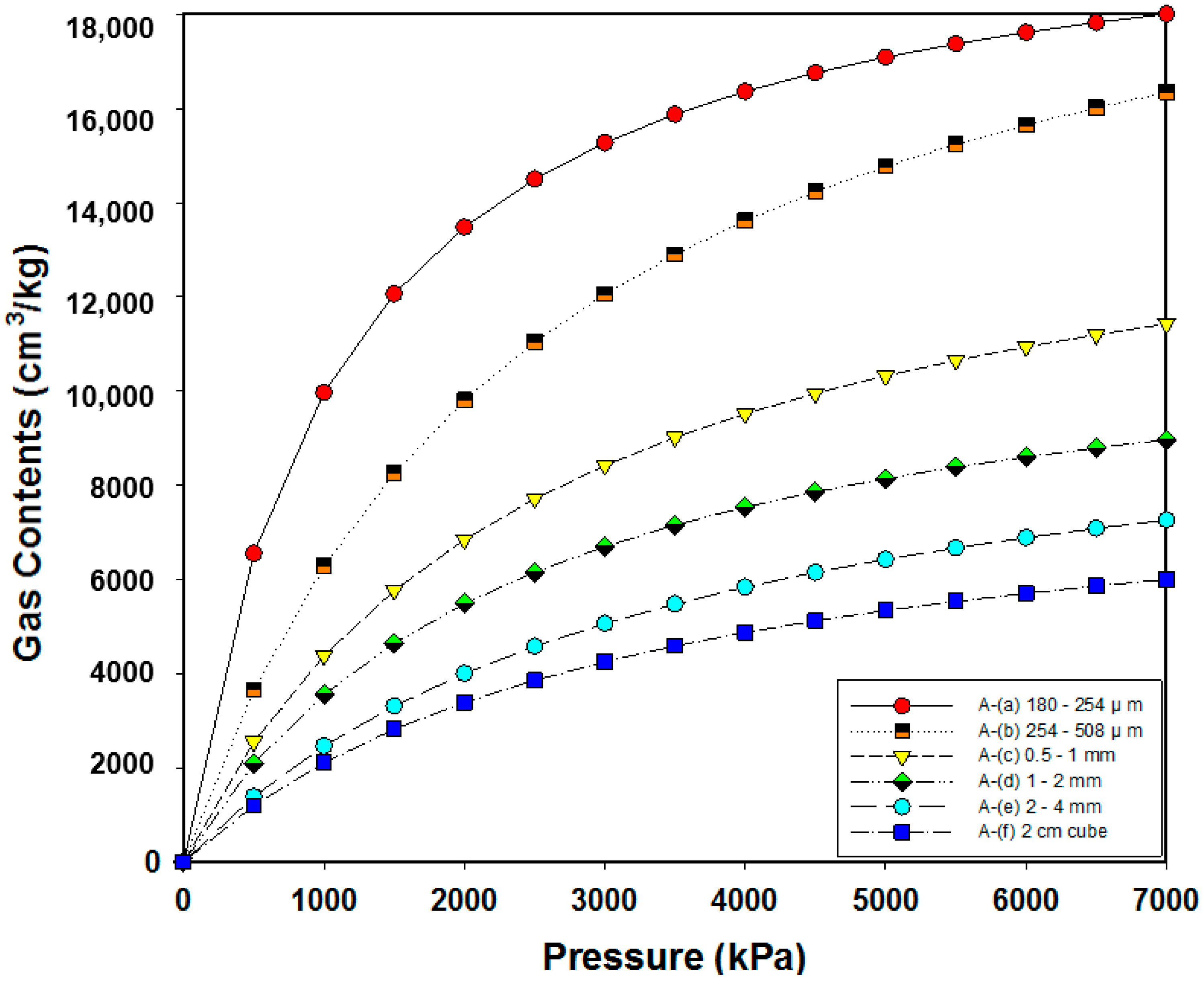
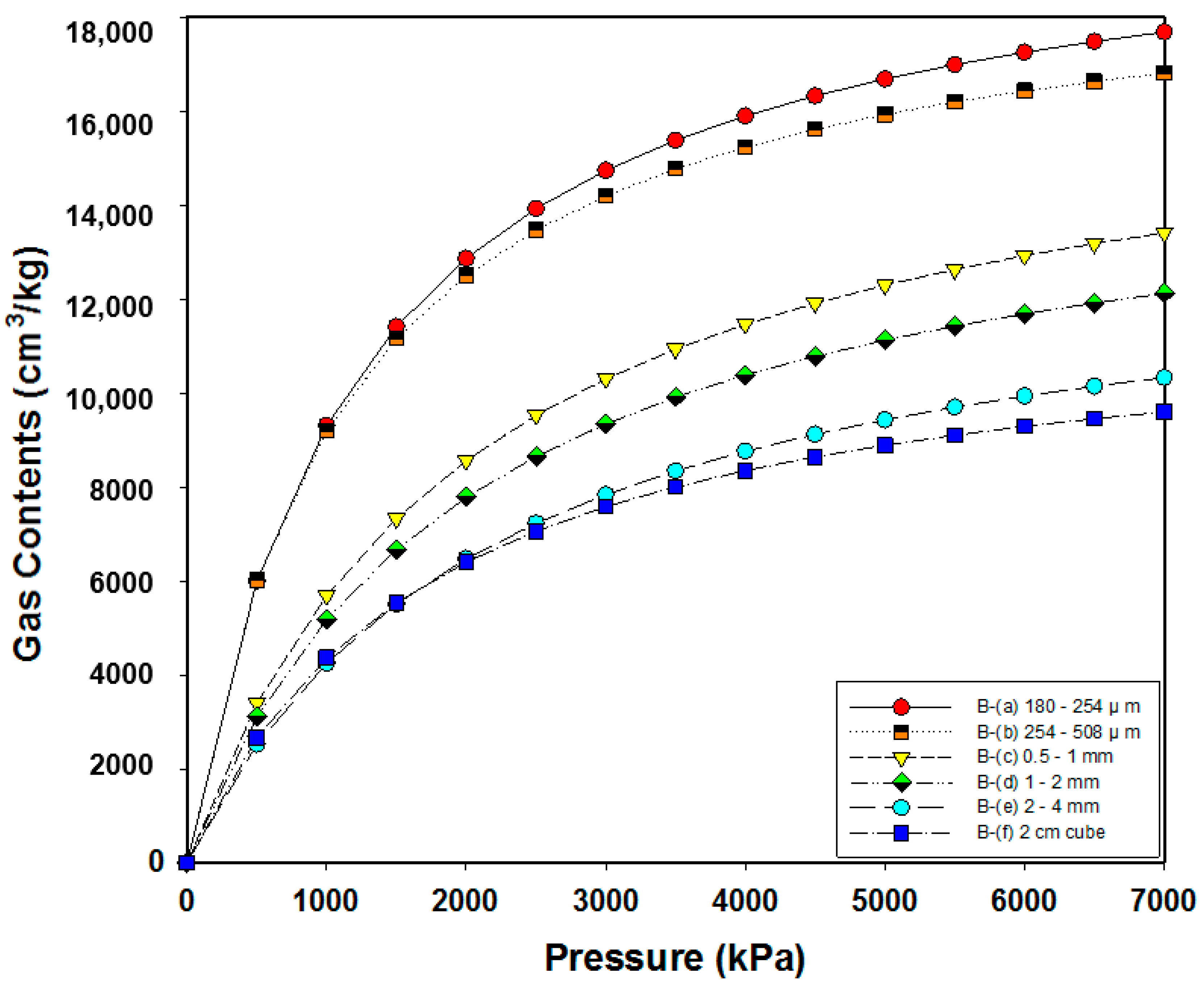
3.2. Analysis of Adsorption Characteristics According to the Particle Size
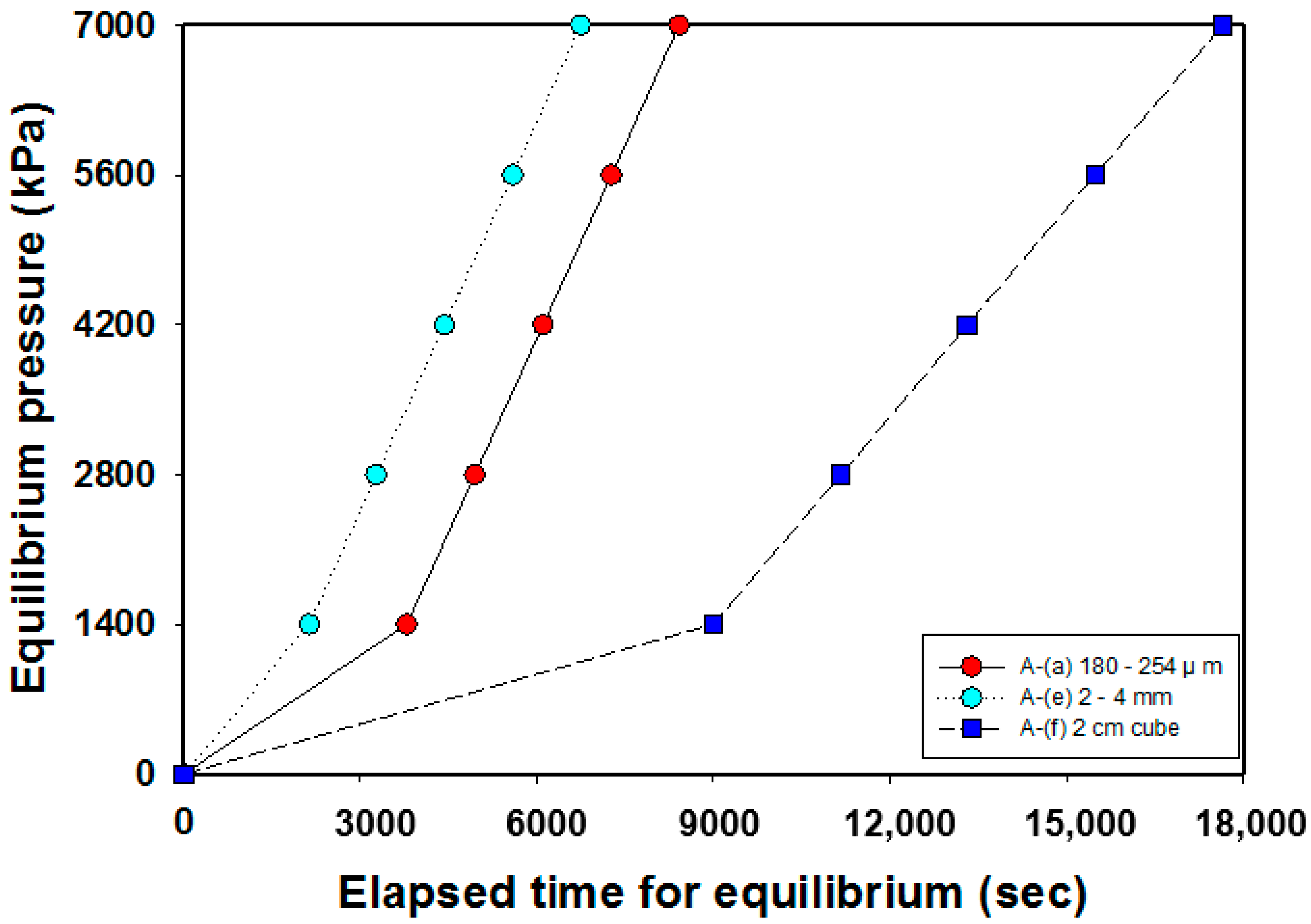
3.3. Equilibrium Time Analysis in the Adsorption Process
3.4. Model Description
- The sample was homogeneous and isotropic;
- The interval of each cleat was equal;
- Gravity force and the stress of the cleat system were ignored;
- The gas diffusion coefficient was constant.
- ●
- Dual porosity formulation—matrix blocks:
- ●
- Dual porosity formulation—fracture blocks:
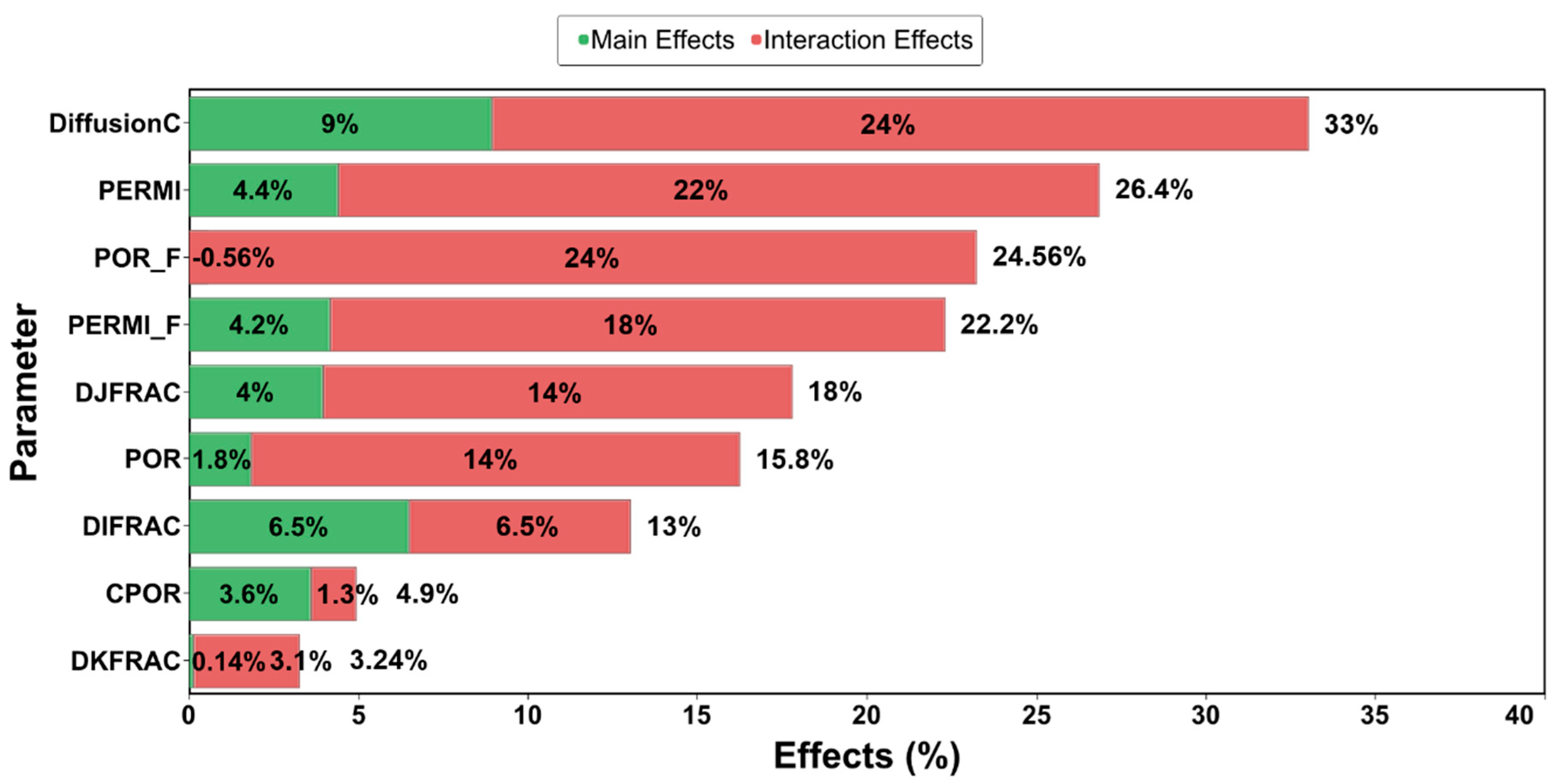
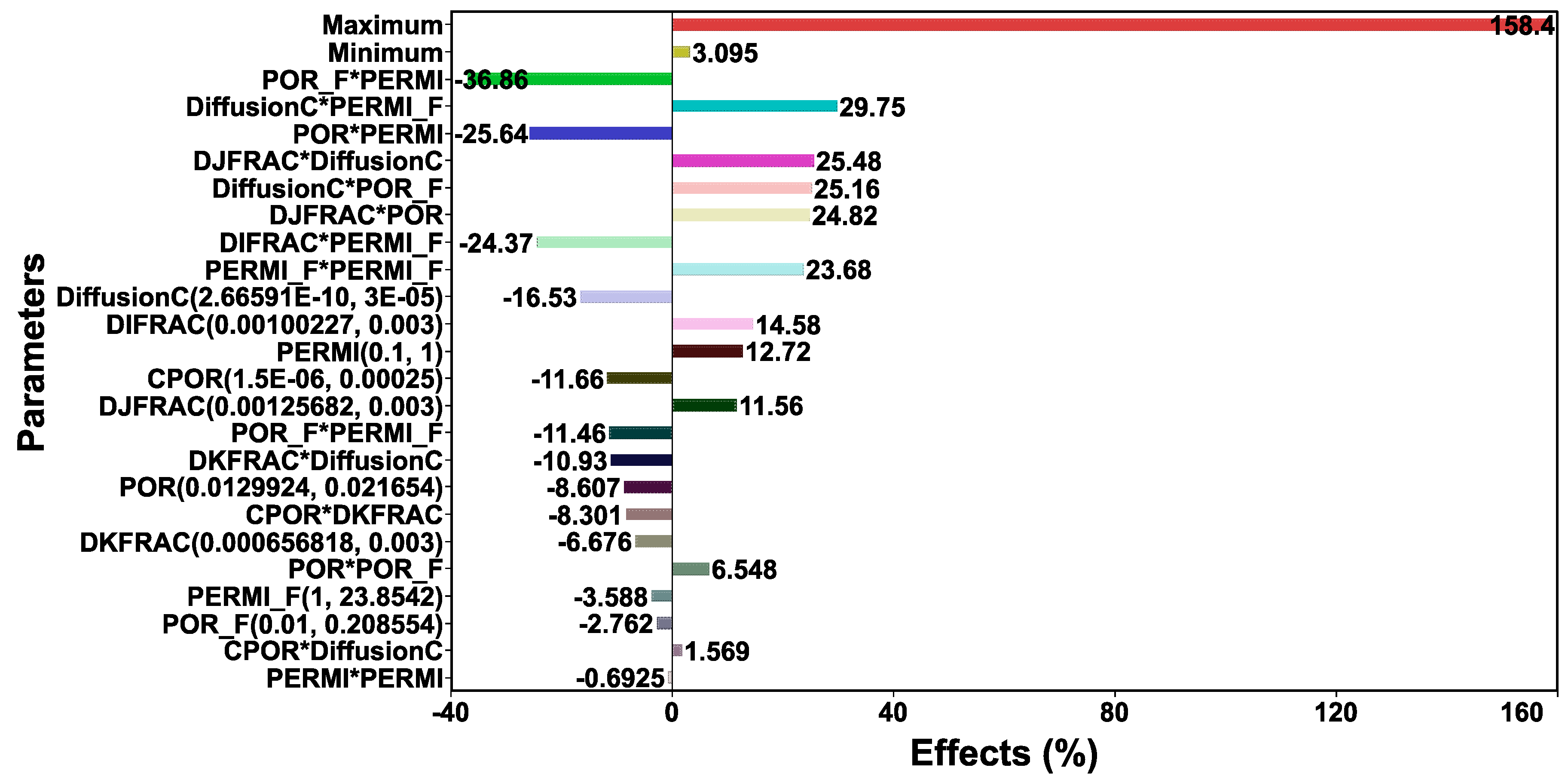
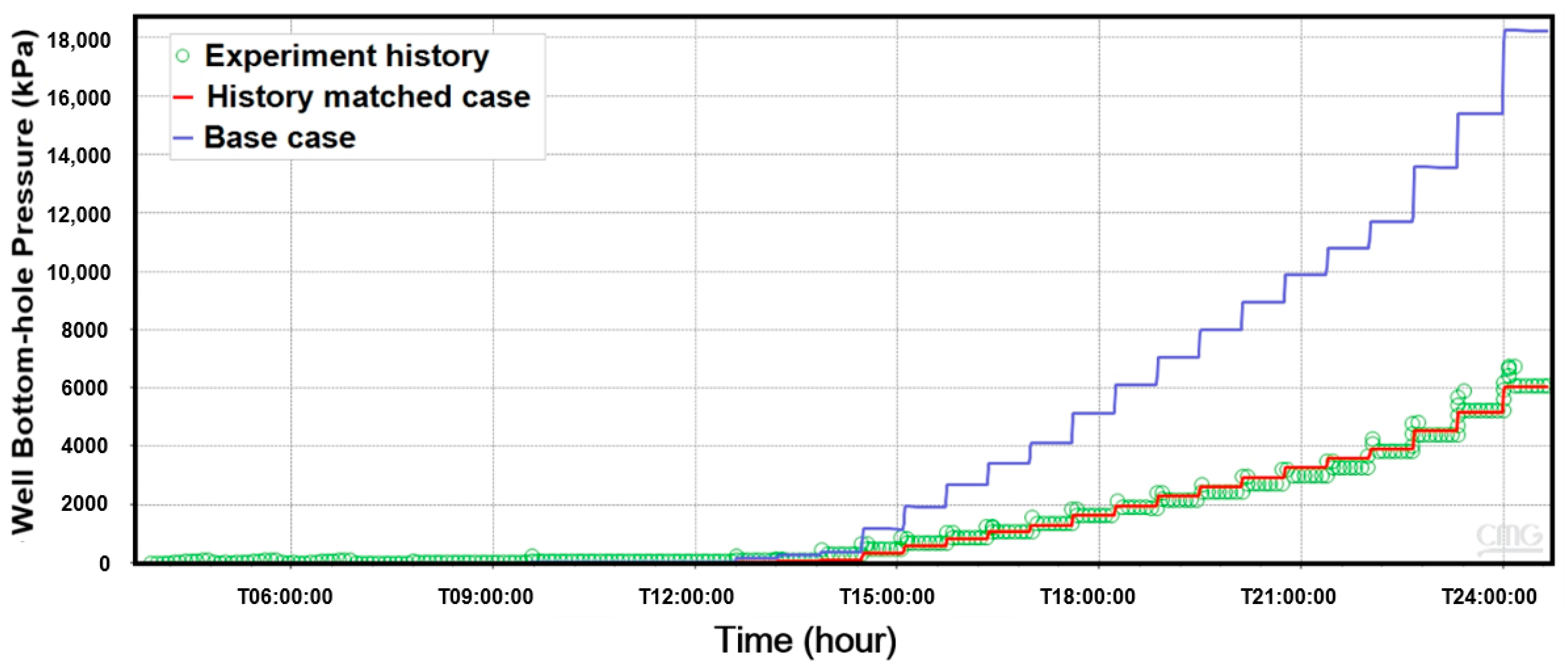
3.5. Simulation Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flores, R.M. Coal and Coalbed Gas: Fueling the Future, 1th ed.; Newnes: Waltham, MA, USA, 2013; Volume 1, pp. 210–235. [Google Scholar]
- Cho, J.; Kim, J. Global trends of unconventional CBM gas science information. J. Econ. Environ. Geol. 2013, 46, 351–358. [Google Scholar] [CrossRef]
- Yoon, Y.; Oh, S.; Kim, J. An real option application on the feasibility study of an Indonesian CBM project. J. Korean Soc. Miner. Energy Resour. Eng. 2014, 51, 631–640. [Google Scholar] [CrossRef]
- Levine, J.R. Generation, storage and migration of natural gas in coal bed reservoirs. Alta. Res. Counc. Inf. Ser. 1990, 109, 84–130. [Google Scholar]
- Stricker, G.D.; Flores, R.M.; McGarry, D.E.; Stilwell, D.P.; Hoppe, D.J.; Stilwell, K.R.; Ochs, A.M.; Ellis, M.E.; Osvald, K.S.; Taylor, S.L.; et al. Gas desorption isotherm studies in coals in the Powder River Basin and adjoining basins in Wyoming and North Dakota. US Geol. Surv. Open File Rep. 2006, 1174, 273. [Google Scholar]
- Jang, H.; Lee, J.; Shin, C.; Lee, Y.; Kwon, S.; Lee, W. A study on the development status and key technologies of coalbed methane. J. Korean Soc. Miner. Energy Resour. Eng. 2012, 49, 545–556. [Google Scholar]
- Hall, F.E.; Chunhe, Z.; Gasem, K.A.M.; Robinson, R.L.; Dan, Y. Adsorption of pure methane, nitrogen, and carbon dioxide and their binary mixtures on wet Fruitland coal. In Proceedings of the SPE Eastern Regional Conference and Exhibition 1994, Charleston, WV, USA, 8–10 November 1994; pp. 329–344. [Google Scholar] [CrossRef]
- Steidl, P.F. Coal as a reservoir. In A Guide to Coalbed Methane Reservoir Engineering; Report No. GRI-94/0397; Gas Research Institute: Chicago, IL, USA, 1996; pp. 1–16. [Google Scholar]
- He, X.; Zhang, Z. Microscopic pore structural characteristics in coal particles. In Proceedings of the 3rd International Conference on Material, Mechanical and Manufacturing Engineering (IC3ME 2015), Guangzhou, China, 27–28 June 2015. [Google Scholar] [CrossRef]
- Beamish, B.B.; O’Donnell, G. Microbalance applications to sorption testing of coal. In Proceedings of the Symposium Coalbed Methane Research and Development, Townsville, Australia, 19–21 November 1992; pp. 31–41. [Google Scholar]
- Zhang, L.; Aziz, N.; Ren, T.; Wang, Z. Influence of temperature on the gas content of coal and sorption modeling. In Proceedings of the 11th Underground Coal Operators’ Conference, Wollongong, Australia, 10–11 February 2011; pp. 269–276. [Google Scholar]
- Owen, L.B.; Sharer, J. Method calculates gas content per foot of coalbed methane pressure core. Oil Gas J. 1992, 2, 47–49. [Google Scholar]
- Diamond, W.P.; Schatzel, S.J. Measuring the gas content of coal: A review. Int. J. Coal Geol. 1998, 35, 311–331. [Google Scholar] [CrossRef]
- Kim, A.G. Estimating methane content of bituminous coalbeds from adsorption data. US Bur. MinesRep. Investig. 1997, 8245, 22. [Google Scholar]
- Lee, S.H.; Kim, C.K.; Park, J.G.; Choi, D.K.; Ahn, J.H. Comparison of steel slag and activated carbon for phosphate removal from aqueous solution by adsorption. J. Korean Soc. Environ. Eng. 2017, 39, 303–309. [Google Scholar] [CrossRef]
- Karmakar, B. Prospective Evaluation and Prediction of Coalbed Methane Production from a Part of Raniganj and Jharia Coalfields in India. Ph.D. Thesis, Indian School of Mines, Dhanbad, India, 2013; pp. 68–116. [Google Scholar]
- Langmuir, I. the adsorption of gases on plane surfaces of glass, mica, and platinum. J. Am. Chem. Soc. 1918, 40, 1403–1461. [Google Scholar] [CrossRef]
- BELSORP. High-Pressure Gas Adsorption Measuring System Operation Manual; BEL Japan: Osaka, Japan, 2012; Volume 1, pp. 15–20. [Google Scholar]
- National Institute of Standards and Technology Chemistry Webbook. Available online: https://webbook.nist.gov/ (accessed on 23 October 2018).
- Kim, J.; Kim, D.; Lee, W.; Lee, Y.; Kim, H. Impact of total organic carbon and specific surface area on the adsorption capacity in Horn River shale. J. Pet. Sci. Eng. 2017, 149, 331–339. [Google Scholar] [CrossRef]
- Parr, S.W. The Classification of Coal; Engineering Experiment Station; College of Engineering; University of Illinois: Champaign, IL, USA, 1928. [Google Scholar]
- Zhang, L.; Aziz, N.; Ren, T.; Nemcik, J.; Tu, S. Influence of coal particle size on coal adsorption and desorption characteristics. Arch. Min. Sci. 2014, 59, 807–820. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, T.; Liang, X.; Zhang, Z.; Zhu, H.; Yang, W.; Zhang, K. Influence of chemical properties on CH4 adsorption capacity of anthracite derived from southern Sichuan Basin. Mar. Pet. Geol. Chin. 2018, 89, 387–401. [Google Scholar] [CrossRef]
- King, G.; Rtekin, T.; Schwerer, F.C. Numerical simulation of the transient behavior of coal-seam degasification wells. SPE Formul. Eval. 1986, 1, 165–183. [Google Scholar] [CrossRef]
- CMG. GEM User Guide: Compositional & Unconventional Simulator; Computer Modelling Group Ltd.: Calgary, Canada, 2018. [Google Scholar]
- Nghiem, L.X.; Li, Y.K. Phase-equilibrium calculations for reservoir engineering and compositional simulation. In Proceedings of the First International Forum on Reservoir Simulation, Alpbach, Austria, 12–16 September 1988. [Google Scholar]
- Kim, Y.; Unurbayan, B.; Lee, J. A study on the estimation of CBM resources using probabilistic approach from Mongolian coal basin information. J. Korean Soc. Miner. Energy Resour. Eng. 2013, 50, 678–691. [Google Scholar] [CrossRef]
- Remner, D.J.; Ertekin, T.; Sung, W.; King, G.R. A parametric study of the effects of coal seam properties on gas drainage efficiency. SPE Reserv. Eng. 1986, 1, 633–646. [Google Scholar] [CrossRef]
- Sung, W.; Ertekin, T.; Schwerer, F.C. The development, testing, and application of a comprehensive coal seam degasification model. In Proceedings of the SPE Unconventional Gas Technology Symposium, Louisville, Kentucky, 18–21 May 1986. [Google Scholar] [CrossRef]
- Sawyer, W.K.; Paul, G.W.; Schraufnagle, R.A. Development and application of a 3D coalbed simulator. In Proceedings of the International Technical Meeting, Petroleum Society CIM, Society of Petroleum Engineers, Calgary, Alberta, Canada, 10–13 June 1990. [Google Scholar]
- Manik, J.; Ertekin, T.; Kohler, T.E. Development and validation of a compositional coalbed simulator. J. Can. Pet. Technol. 2002, 41, 39–45. [Google Scholar] [CrossRef]
- Connell, L.D.; Sander, R.; Pan, Z.; Camilleri, M.; Heryanto, D. History matching of enhanced coal bed methane laboratory core flood tests. Int. J. Coal Geol. 2011, 87, 128–138. [Google Scholar] [CrossRef]
- Dong, J.; Cheng, Y.; Liu, Q.; Zhang, H.; Zhang, K.; Hu, B. Apparent and true diffusion coefficients of methane in coal and their relationships with methane desorption capacity. Energy Fuels 2017, 31, 2643–2651. [Google Scholar] [CrossRef]
- Kumar, H.; Mishra, M.K.; Mishra, S. Laboratory investigation of gas permeability and its impact on CBM potential. J. Pet. Explor. Prod. Technol. 2018, 8, 1–15. [Google Scholar] [CrossRef]

| Title | Coal Sample Group A | Coal Sample Group B |
|---|---|---|
| Inherent moisture (air-dried basis) (wt.%) | 2.66 | 1.20 |
| Ash (air-dried basis) (wt.%) | 11.46 | 18.53 |
| Volatile matter (air-dried basis) (wt.%) | 42.68 | 25.38 |
| Fixed carbon (air-dried basis) (wt.%) | 43.20 | 54.89 |
| Volatile matter (dried basis) (wt.%) | 44.38 | 25.91 |
| Fixed carbon (dried basis) (wt.%) | 44.16 | 55.56 |
| Volatile matter (dry ash-free, DAF) (wt.%) | 49.15 | 30.33 |
| Fixed carbon (DAF) (wt.%) | 50.85 | 69.67 |
| Gross calorific value (air-dried basis) (MJ/kg) | 27.84 | 27.84 |
| Gross calorific value (dried basis) (MJ/kg) | 28.59 | 27.75 |
| Sample Group | Coal Particle Size | (a) 180–254 µm | (b) 254–508 µm | (c) 0.5–1 mm | (d) 1–2 mm | (e) 2–4 mm | (f) 2-cm Cube |
|---|---|---|---|---|---|---|---|
| A | VL (cm3/kg) | 20,809.44 | 22,295.85 | 15,607 | 12,005.53 | 10,765.40 | 8670.62 |
| PL (kPa) | 1086.76 | 2545.86 | 2556.53 | 2370.44 | 3371.62 | 3108.35 | |
| B | VL (cm3/kg) | 20,809.44 | 19,508.75 | 17,341.25 | 15,607 | 13,571.22 | 12,005.53 |
| PL (kPa) | 1233.72 | 1120.04 | 2047.85 | 2009.12 | 2191.64 | 1749.51 |
| Input Parameters | Values |
|---|---|
| Coal volume (cm3) | 8.00 |
| Grid dimension | 10 × 10 × 10 |
| Matrix porosity | 0.017 |
| Fracture porosity | 0.005 |
| Matrix permeability (mD) | 0.01 |
| Fracture permeability (mD) | 4.00 |
| Fracture spacing I (cm) | 0.20 |
| Fracture spacing J (cm) | 0.20 |
| Fracture spacing K (cm) | 0.20 |
| Cleat compressibility (1/kPa) | 2.00 × 10−6 |
| Coal Density (kg/cm3) | 1.43 × 10−3 |
| Diffusion coefficient (cm2/s) | 3.50 × 10−10 |
| Langmuir pressure (CH4) (kPa) | 3500 |
| Langmuir volume (CH4) (cm3/kg) | 12,920 |
| Matched Parameters | Values |
|---|---|
| Cleat compressibility (1/kPa) | 2.40 × 10−5 |
| Matrix porosity | 0.01 |
| Fracture porosity | 0.15 |
| Matrix permeability (mD) | 0.11 |
| Fracture permeability (mD) | 23.85 |
| Fracture spacing I (cm) | 0.24 |
| Fracture spacing J (cm) | 0.17 |
| Fracture spacing K (cm) | 0.20 |
| Diffusion coefficient (cm2/s) | 2.66 × 10−10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Seo, Y.; Kim, J.; Han, J.; Lee, Y. Experimental and Simulation Studies on Adsorption and Diffusion Characteristics of Coalbed Methane. Energies 2019, 12, 3445. https://doi.org/10.3390/en12183445
Kim D, Seo Y, Kim J, Han J, Lee Y. Experimental and Simulation Studies on Adsorption and Diffusion Characteristics of Coalbed Methane. Energies. 2019; 12(18):3445. https://doi.org/10.3390/en12183445
Chicago/Turabian StyleKim, Donghyeon, Youngjin Seo, Juhyun Kim, Jeongmin Han, and Youngsoo Lee. 2019. "Experimental and Simulation Studies on Adsorption and Diffusion Characteristics of Coalbed Methane" Energies 12, no. 18: 3445. https://doi.org/10.3390/en12183445
APA StyleKim, D., Seo, Y., Kim, J., Han, J., & Lee, Y. (2019). Experimental and Simulation Studies on Adsorption and Diffusion Characteristics of Coalbed Methane. Energies, 12(18), 3445. https://doi.org/10.3390/en12183445