The Effect of Mixed Wastewaters on the Biomass Production and Biochemical Content of Microalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Microalgal Suspension, Strain, and Culture Medium
2.2. Wastewater Sampling and Analysis
2.3. Experimental Method
2.4. Analysis of Lipid and ß-carotene
3. Results and Discussion
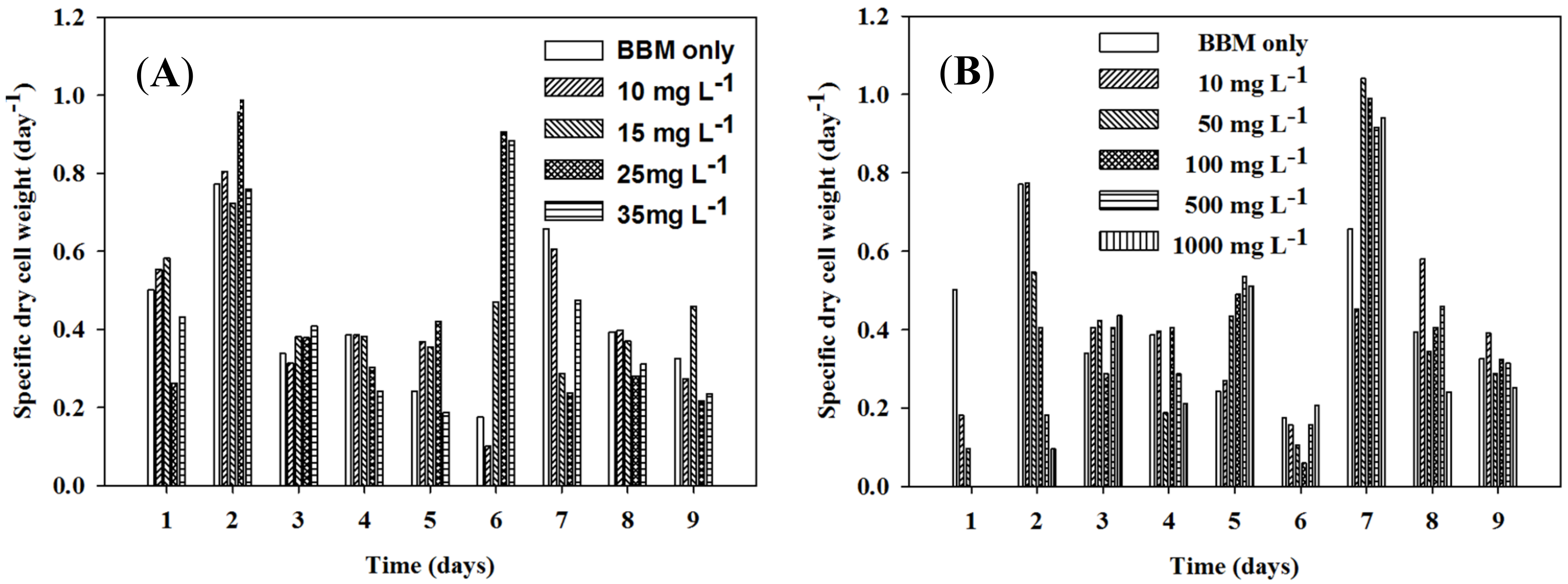
3.1. The Influence of Iron and Ammonium on the Microalgal Growth
3.2. Effect of Pretreated Piggery Wastewater by Membrane (PPWM) and Acid Mine Drainage (AMD) on the Microalgal Growth
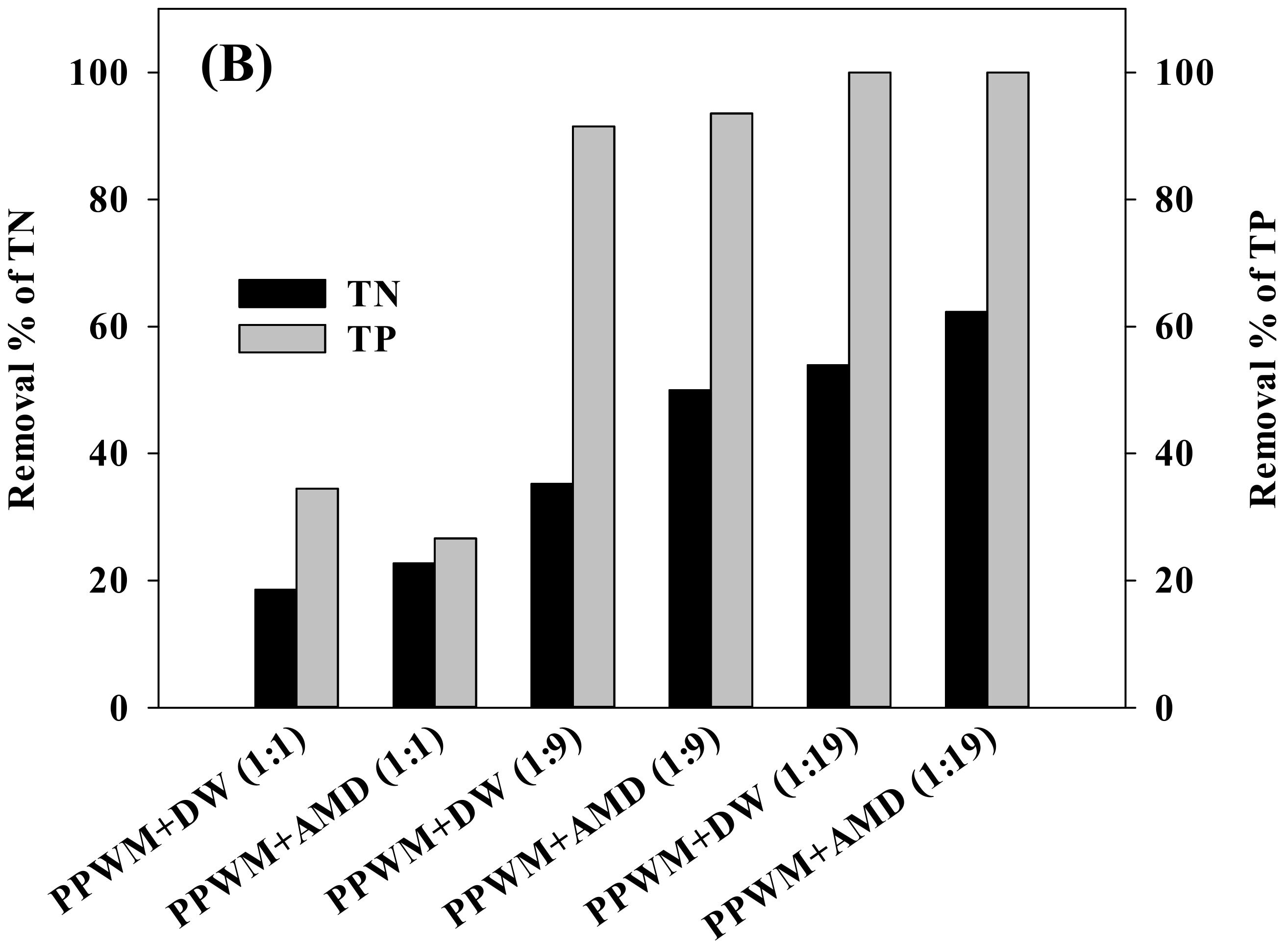
3.3. Effect of Microalgal Growth on Nutrient Removal
3.4. The Production of Lipid and ß-carotene after Cultivation in Pretreated Piggery Wastewater by Membrane (PPWM) Supplemented with Acid Mine Drainage (AMD)
3.5. Comparison of Other Researches for Lipid and ß-carotene Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sigamani, S.; Ramamurthy, D.; Natarajan, H. A review on potential biotechnological applications of microalgae. J. Appl. Pharm. Sci. 2016, 6, 179–184. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Garcia-Gonzalez, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Crutzen, P.J.; Mosier, A.R.; Smith, K.A.; Winiwarter, W. N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos. Chem. Phys. 2008, 8, 389–395. [Google Scholar] [CrossRef]
- Xiong, W.; Li, X.; Xiang, J.; Wu, Q. High-density fermentation of microalga Chlorella protothecoides in bioreactor for micobio-diesel production. Appl. Microbiol. Biotechnol. 2008, 78, 29–36. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.Q.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Duangmanee, P.; Juntawong, N.; Ma, C. The effects of light, temperature, and nutrition on growth and pigment accumulation of three Dunalliella salina strains isolated from saline soil. Jundishapur J. Microbiol. 2016, 9, e26732. [Google Scholar] [CrossRef]
- Benmalek, Y.; Halouane, A.; Hacene, H.; Fardeau, M.L. Resistance to heavy metals and bioaccumulation of lead and zinc by Chryseobacterium solincola strain 1YB–R12T isolated from soil. Int. J. Environ. Eng. 2014, 6, 68–77. [Google Scholar] [CrossRef]
- Harguinteguy, C.A.; Cirelli, A.F.; Pignata, M.L. Heavy metal accumulation in leaves of aquatic plant Stuckenia filiformis and its relationship with sediment and water in the Suquia river (Argentina). Microchem. J. 2014, 114, 111–118. [Google Scholar] [CrossRef]
- Das, B.K.; Roy, A.; Koschorreck, M.; Mandal, S.M.; Wendt-Potthoff, K.; Bhattacharya, J. Occurrence and role of algae and fungi in acid mine drainage environment with special reference to metals and sulfate immobilization. Water Res. 2009, 43, 883–894. [Google Scholar] [CrossRef]
- Park, Y.T.; Lee, H.; Yun, H.S.; Song, K.G.; Yeom, S.H.; Choi, J. Removal of metal from acid mine drainage using a hybrid system including a pipes inserted microalgae reactor. Bioresour. Technol. 2013, 150, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Chinnasamy, S.; Singh, M.; Das, K.C. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl. Energ. 2011, 88, 3425–3431. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Ledda, C.; Schievano, A.; Scaglia, B.; Rossoni, M.; Fernandez, F.G.A.; Adani, F. Integration of microalgae production with anaerobic digestion of dairy cattle manure: An overall mass and energy balance of the process. J. Clean. Prod. 2016, 112, 103–112. [Google Scholar] [CrossRef]
- Su, Y.Y.; Mennerich, A.; Urban, B. The long-term effects of wall attached microalgal biofilm on algae-based wastewater treatment. Bioresour. Technol. 2016, 218, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Matamoros, V.; Uggetti, E.; Garcia, J.; Bayona, J.M. Assessment of the mechanisms involved in the removal of emerging contaminants by microalgae from wastewater: A laboratory scale study. J. Hazard. Mater. 2016, 301, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, H.W.; Bold, H.C. Phycological studies IV. Some soil algae from enchanted rock and related algal species. Univ. Tex. Publ. 1963, 6318, 1–95. [Google Scholar]
- Ji, M.K.; Kabra, A.N.; Salama, E.S.; Roh, H.S.; Kim, J.R.; Lee, D.S.; Jeon, B.H. Effect of mine wastewater on nutrient removal and lipid production by a green microalga Micratinium reisseri from concentrated municipal wastewater. Bioresour. Technol. 2014, 157, 84–90. [Google Scholar] [CrossRef]
- Wang, M.; Kuo-Dahab, W.C.; Dolan, S.; Park, C. Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, Chlorella sp. and Micractinium sp., in wastewater treatment. Bioresour. Technol. 2014, 154, 131–137. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Wang, P.F.; Chen, B.; Hou, J.; Qian, J.; Yang, Y.Y. Effects of iron on growth, antioxidant enzyme activity, bound extracellular polymeric substances and microcystin production of Microcystis aeruginosa FACHB-905. Ecotoxicol. Environ. Saf. 2016, 132, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.M.; Song, X.S.; Wang, W.; Xiao, Y.P.; Gong, Z.J.; Wang, Y.H.; Zhao, Y.F.; Chen, Y.; Mei, M.Y. Influences of iron and calcium carbonate on wastewater treatment performances of algae based reactors. Bioresour. Technol. 2016, 216, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schowanek, D.; McAvoy, D.; Versteeg, D.; Hanstveit, A. Effects of nutrient trace metal speciation on algal growth in the presence of the chelator [S, S]-EDDS. Aquat. Toxicol. 1996, 36, 253–275. [Google Scholar] [CrossRef]
- Miazek, K.; Iwanek, W.; Remacle, C.; Richel, A.; Goffin, D. Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: A review. Int. J. Mol. Sci. 2015, 16, 23929–23969. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.B.; Zhang, Y.L.; Yang, L.B.; Chu, H.Q.; Guo, J. Outdoor cultures of Chlorella pyrenoidosa in the effluent of anaerobically digested activated sludge: The effects of pH and free ammonia. Bioresour. Technol. 2016, 200, 606–615. [Google Scholar] [CrossRef]
- Vidyashankar, S.; VenuGopal, K.S.; Swarnalatha, G.V.; Kavitha, M.D.; Chauhan, V.S.; Ravi, R.; Bansal, A.K.; Singh, R.; Pande, A.; Ravishankar, G.A.; et al. Characterization of fatty acids and hydrocarbons of Chlorophycean microalgae towards their use as biofuel source. Biomass Bioenerg. 2015, 77, 75–91. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.I.; El-Dalatony, M.M.; EL-Sheekh, M.M.; Ji, M.K.; Salama, E.S.; Kabra, A.N.; Jeon, B.H. Cultivation of a new microalga, Micractinium reisseri, in municipal wastewater for nutrient removal, biomass, lipid, and fatty acid production. Biotechnol. Bioproc. E 2014, 19, 510–518. [Google Scholar] [CrossRef]
- Yoshimura, T.; Okada, S.; Honda, M. Culture of the hydrocarbon producing microalga Botryococcus braunii strain Showa: Optimal CO2, salinity, temperature, and irradiance conditions. Bioresour. Technol. 2013, 133, 232–239. [Google Scholar] [CrossRef]
- Zhu, L.D.; Hiltunen, E.; Shu, Q.; Zhou, W.Z.; Li, Z.H.; Wang, Z.M. Biodiesel production from algae cultivated in winter with artificial wastewater through pH regulation by acetic acid. Appl. Energ. 2014, 128, 103–110. [Google Scholar] [CrossRef]
- Yang, I.S.; Salama, E.S.; Kim, J.O.; Govindwar, S.P.; Kurade, M.B.; Lee, M.; Roh, H.S.; Jeon, B.H. Cultivation and harvesting of microalgae in photobioreactor for biodiesel production and simultaneous nutrient removal. Energ. Convers. Manag. 2016, 117, 54–62. [Google Scholar] [CrossRef]
- Papadimitriou, C.A.; Papatheodouiou, A.; Takavakoglou, V.; Zdragas, A.; Samaras, P.; Sakellaropoulos, G.P.; Lazaridou, M.; Zalidis, G. Investigation of protozoa as indicators of wastewater treatment efficiency in constructed wetlands. Desalination 2010, 250, 378–382. [Google Scholar] [CrossRef]
- Ji, M.K.; Yun, H.S.; Park, Y.T.; Kabra, A.N.; Oh, I.H.; Choi, J. Mixotrophic cultivation of a microalga Scenedesmus obliquus in municipal wastewater supplemented with food wastewater and flue gas CO2 for biomass production. J. Environ. Manag. 2015, 159, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.C.; Chen, P.; Min, M.; Chen, Y.F.; Zhu, J.; Ruan, R.R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Abou-Shanab, R.A.I.; Hwang, J.H.; Cho, Y.; Min, B.; Jeon, B.H. Characterization of microalgal species isolated from fresh water bodies as a potential source for biodiesel production. Appl. Energ. 2011, 88, 3300–3306. [Google Scholar] [CrossRef]
- Laura, G.P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Chisti, Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhöh, O.; et al. Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient-poor soil substrates for crop plants. J. Appl. Phycol. 2018, 30, 2827–2836. [Google Scholar] [CrossRef]
- Kim, M.K.; Park, J.W.; Park, C.S.; Kim, S.J.; Jeune, K.H.; Chang, M.U.; Acreman, J. Enhanced production of Scenedesmus spp. (green microalgae) using a new medium containing fermented swine wastewater. Bioresour. Technol. 2007, 98, 2220–2228. [Google Scholar] [CrossRef]
- Feng, Y.; Li, C.; Zhang, D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef]
- Singh, P.; Guldhe, A.; Kumari, S.; Rawat, I.; Bux, F. Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus falcatus KJ671624 using response surface methodology. Biochem. Eng. J. 2015, 94, 22–29. [Google Scholar] [CrossRef]






| Experimental Condition | Pretreated Manure + Distilled Water (v/v) | Pretreated Manure + Acid Mine Drainage (v/v) | |||||
|---|---|---|---|---|---|---|---|
| Parameters | 1/1 | 1/9 | 1/19 | 1/1 | 1/9 | 1/19 | |
| Total nitrogen (mg L−1) | 743 | 173 | 76 | 762 | 168 | 69 | |
| Ammonium (mg L−1) | 726 | 162 | 79 | 732 | 148 | 69 | |
| Nitrate (mg L−1) | 13 | 3 | 1 | 15 | 2 | 1 | |
| Total phosphorous (mg L−1) | 29 | 5.9 | 2.9 | 30 | 6.2 | 3.1 | |
| Manganese (mg L−1) | - | - | - | 2.9 | 0.6 | 0.3 | |
| Sulfate (mg L−1) | - | - | - | 160.2 | 32 | 16 | |
| Iron (mg L−1) | 4.6 | 0.9 | 0.5 | 39.6 | 69.8 | 73.7 | |
| Strain | Cultivation Condition | Lipid (g/L d) | β-carotene (mg/g d) | Reference |
|---|---|---|---|---|
| Uronema sp. KGE 3a | Iron | 0.249 | 3.69 | This study |
| Uronema sp. KGE 3a | Ammonia | 0.166 | 1.01 | This study |
| Scenedesmus spp. -Mixed cultureb | Fermented swine urine | - | 0.05 | Kim et al. (2007) [39] |
| C. vulgaris FACHB1068c | Artificial wastewater | 0.147 | - | Feng et al. (2011) [40] |
| Ankistrodesmus falcatus KJ671624 | Iron | 0.074 | - | Singh et al. (2015) [41] |
| Uronema sp. KGE 3a | AMDS+PPWM | 0.251 | 3.01 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Ahn, Y.; Park, Y.-T.; Ji, M.-K.; Choi, J. The Effect of Mixed Wastewaters on the Biomass Production and Biochemical Content of Microalgae. Energies 2019, 12, 3431. https://doi.org/10.3390/en12183431
Park S, Ahn Y, Park Y-T, Ji M-K, Choi J. The Effect of Mixed Wastewaters on the Biomass Production and Biochemical Content of Microalgae. Energies. 2019; 12(18):3431. https://doi.org/10.3390/en12183431
Chicago/Turabian StylePark, Sanghyun, Yongtae Ahn, Young-Tae Park, Min-Kyu Ji, and Jaeyoung Choi. 2019. "The Effect of Mixed Wastewaters on the Biomass Production and Biochemical Content of Microalgae" Energies 12, no. 18: 3431. https://doi.org/10.3390/en12183431
APA StylePark, S., Ahn, Y., Park, Y.-T., Ji, M.-K., & Choi, J. (2019). The Effect of Mixed Wastewaters on the Biomass Production and Biochemical Content of Microalgae. Energies, 12(18), 3431. https://doi.org/10.3390/en12183431






