Microencapsulation of Paraffin with Poly (Urea Methacrylate) Shell for Solar Water Heater
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
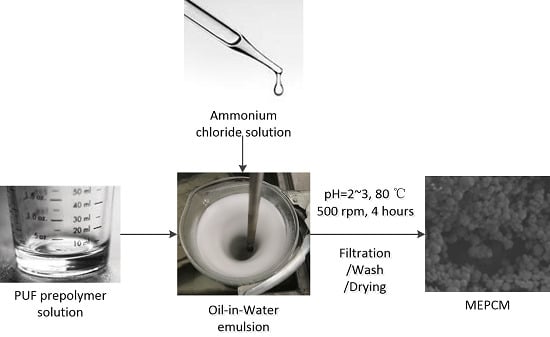
2.2. Fabrication Process of the MEPCM
3. Results and Discussion
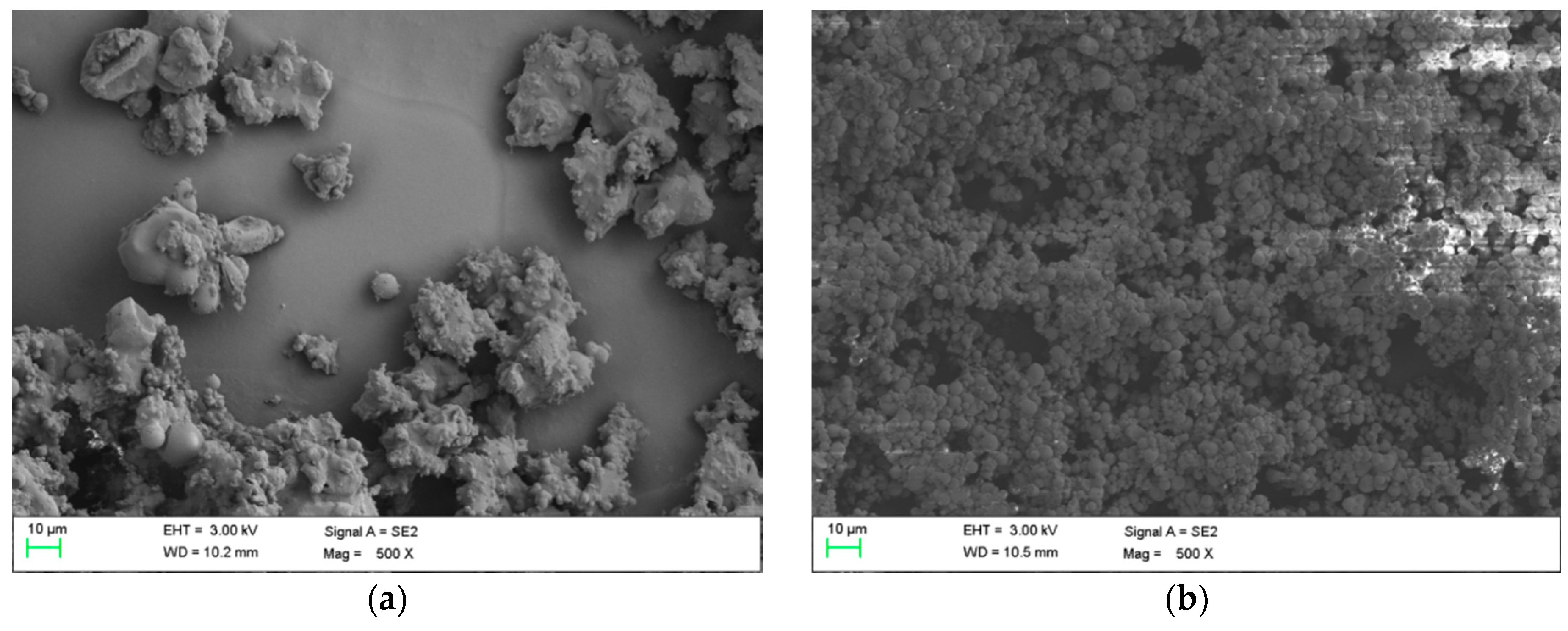
3.1. Morphology Analysis
3.2. Particle Size Distribution
3.3. Energy Storage Capacity
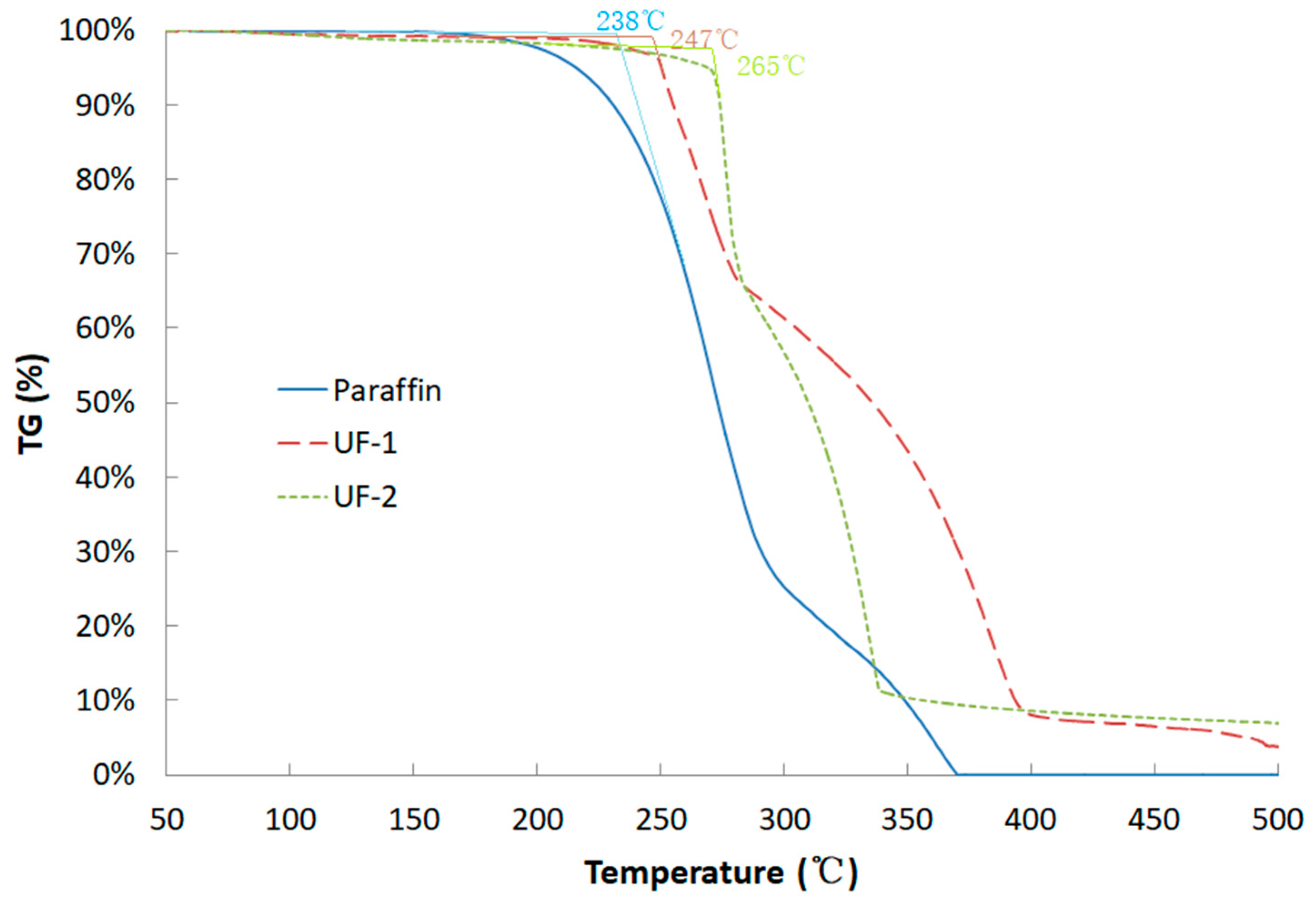
3.4. Thermal Stability
4. Conclusions
- (a)
- Agglomeration of MEPCM particles did occur during the encapsulation process which affected the uniformity of the PSD profile, especially for the sample with the emulsifier of SDS.
- (b)
- Compared with the original paraffin, there was a slight increase of 0.14–0.72 °C in the melting temperature of the MEPCM samples.
- (c)
- The dosage of 5 wt% nucleating agent (ammonium chloride) and the binary emulsifies (Brij 35 and Brij 30) resulted in higher core material content of 68.55% and encapsulation efficiency of 97.93% for the developed MEPCM.
- (d)
- The thermal stability was only slightly enhanced by 9–27 °C after encapsulation due to the fact that PUF has a lower melting temperature than the evaporation temperature of the paraffin wax.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belmonte, J.F.; Eguía, P.; Molina, A.E.; Almendros-Ibáñez, J.A.; Salgado, R. A simplified method for modeling the thermal performance of storage tanks containing PCMs. Appl. Therm. Eng. 2016, 95, 394–410. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G.; Zhou, T.; Li, Y. Preparation of microencapsulated phase change materials (MEPCM) for thermal energy storage. Energy Procedia 2017, 121, 95–101. [Google Scholar] [CrossRef]
- Rashid, K.; Safdarnejad, S.M.; Ellingwood, K.; Powell, K.M. Techno-economic evaluation of different hybridization schemes for a solar thermal/gas power plant. Energy 2019, 181, 91–106. [Google Scholar] [CrossRef]
- Ellingwood, K.; Safdarnejad, S.M.; Rashid, K.; Powell, K. Leveraging Energy Storage in a Solar-Tower and Combined Cycle Hybrid Power Plant. Energies 2018, 12, 40. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Khan, M.M.A.; Ibrahim, N.I.; Mahbubul, I.; Ali, H.M.; Saidur, R.; Al-Sulaiman, F.A. Evaluation of solar collector designs with integrated latent heat thermal energy storage: A review. Sol. Energy 2018, 166, 334–350. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wu, D.; He, F.; Wang, R. High energy-density and power-density thermal storage prototype with hydrated salt for hot water and space heating. Appl. Energy 2019, 248, 406–414. [Google Scholar] [CrossRef]
- Şahan, N.; Paksoy, H. Novel shapeable phase change material (PCM) composites for thermal energy storage (TES) applications. Sol. Energy Mater. Sol. Cells 2018, 174, 380–387. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J. Thermal enhancement of paraffin/hydrophobic expanded perlite granular phase change composite using graphene nanoplatelets. Energy Build. 2018, 169, 206–215. [Google Scholar] [CrossRef]
- Chalco-Sandoval, W.; Fabra, M.J.; Lopez-Rubio, A.; Lagarón, J.M.; Fabra, M.J. Development of polystyrene-based films with temperature buffering capacity for smart food packaging. J. Food Eng. 2015, 164, 55–62. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Li, Y.; Lv, Y. Novel method for fabricating high thermal conductivity form-stable Phase Change Materials. In Proceedings of the 17th International Conference on Sustainable Energy Technologies—SET 2018, Wuhan, China, 21–23 August 2018. [Google Scholar]
- Lin, Y.; Zhu, C.; Alva, G.; Fang, G. Palmitic acid/polyvinyl butyral/expanded graphite composites as form-stable phase change materials for solar thermal energy storage. Appl. Energy 2018, 228, 1801–1809. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J. Effects of various carbon additives on the thermal storage performance of form-stable PCM integrated cementitious composites. Appl. Therm. Eng. 2019, 148, 491–501. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Nanosilicon dioxide hydrosol as surfactant for preparation of microencapsulated phase change materials for thermal energy storage in buildings. Int. J. Low Carbon Technol. 2018, 13, 301–310. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Bilgin, C. Micro/nano encapsulation of some paraffin eutectic mixtures with poly(methyl methacrylate) shell: Preparation, characterization and latent heat thermal energy storage properties. Appl. Energy 2014, 136, 217–227. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, R.; Peng, F.; Fang, Y.; Akiyama, T.; Wang, S. Synthesis, characterization and thermal properties of paraffin microcapsules modified with nano-Al2O3. Appl. Energy 2015, 137, 731–737. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid–liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Fang, M.-H.; Tsai, P.-S.; Yang, Y.-M. Preparation of microencapsulated phase-change materials (MCPCMs) by means of interfacial polycondensation. J. Microencapsul. 2005, 22, 37–46. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Y.; Liu, J.; Yang, Z. Synthesis and properties of paraffin capsules as phase change materials. Polymer 2008, 49, 2903–2910. [Google Scholar] [CrossRef]
- Li, M.G.; Zhang, Y.; Xu, Y.H.; Zhang, D. Effect of different amounts of surfactant on characteristics of nanoencapsulated phase-change materials. Polym. Bull. 2011, 67, 541–552. [Google Scholar] [CrossRef]
- Xin, C.; Tian, Y.; Wang, Y.; Huang, X. Effect of curing temperature on the performance of microencapsulated low melting point paraffin using urea-formaldehyde resin as shell. Text. Res. J. 2013, 84, 831–839. [Google Scholar] [CrossRef]
- Zhang, X.; Tao, X.; Yick, K.; Wang, X. Structure and thermal stability of microencapsulated phase-change materials. Colloid Polym. Sci. 2004, 282, 330–336. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Yang, F.; Liu, X.; Wu, S. Preparation and characterization of nano-encapsulated n-tetradecane as phase change material for thermal energy storage. Chem. Eng. J. 2009, 153, 217–221. [Google Scholar] [CrossRef]
- Konuklu, Y.; Paksoy, H.Ö.; Unal, M.; Konuklu, S. Microencapsulation of a fatty acid with Poly(melamine–urea–formaldehyde). Energy Convers. Manag. 2014, 80, 382–390. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Development of microencapsulated phase change material for solar thermal energy storage. Appl. Therm. Eng. 2017, 112, 1205–1212. [Google Scholar] [CrossRef]
- Seddegh, S.; Wang, X.; Henderson, A.D.; Xing, Z. Solar domestic hot water systems using latent heat energy storage medium: A review. Renew. Sustain. Energy Rev. 2015, 49, 517–533. [Google Scholar] [CrossRef]
- International Organization for Standardization (IOS). Plastics—Differential Scanning Calorimetry (DSC) Part 1: General Principles; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- International Organization for Standardization (IOS). Plastics—Differential Scanning Calorimetry (DSC) Part 3: Determination of Temperature and Enthalpy of Melting and Crystallization; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- International Organization for Standardization (IOS). Plastics—Differential Scanning Calorimetry (DSC) Part 5: Determination of Characteristic Reaction-curve Temperatures and Times, Enthalpy of Reaction and Degree of Conversion; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]



| Sample | Urea (g) | Formaldehyde 37 wt% (g) | Emulsifier (s) | Emulsifiers (g) | Nucleating Agent (g) | Paraffin (g) |
|---|---|---|---|---|---|---|
| UF-1 | 1.54 | 6.95 | SDS | 0.25 | 0.36 | 8.40 |
| UF-2 | 2.48 | 5.03 | Brij 35 & Brij 30 (weight ratio at 1:2) | 0.25 | 0.18 | 8.40 |
| Item | Melting Point (°C) | Latent Heat (kJ/kg) | Core Material Content (wt%) | Encapsulation Efficiency (%) | Weight Loss Starting Temperature (°C) |
|---|---|---|---|---|---|
| Paraffin | 48.34 | 194.0 | - | - | 238.0 |
| UF-1 | 49.06 | 124.7 | 64.28 | 91.82 | 247.0 |
| UF-2 | 48.48 | 133.0 | 68.55 | 97.93 | 265.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.; Li, Y.; Zhou, T.; Darkwa, J.; Kokogiannakis, G.; Li, Z. Microencapsulation of Paraffin with Poly (Urea Methacrylate) Shell for Solar Water Heater. Energies 2019, 12, 3406. https://doi.org/10.3390/en12183406
Su W, Li Y, Zhou T, Darkwa J, Kokogiannakis G, Li Z. Microencapsulation of Paraffin with Poly (Urea Methacrylate) Shell for Solar Water Heater. Energies. 2019; 12(18):3406. https://doi.org/10.3390/en12183406
Chicago/Turabian StyleSu, Weiguang, Yilin Li, Tongyu Zhou, Jo Darkwa, Georgios Kokogiannakis, and Zhao Li. 2019. "Microencapsulation of Paraffin with Poly (Urea Methacrylate) Shell for Solar Water Heater" Energies 12, no. 18: 3406. https://doi.org/10.3390/en12183406
APA StyleSu, W., Li, Y., Zhou, T., Darkwa, J., Kokogiannakis, G., & Li, Z. (2019). Microencapsulation of Paraffin with Poly (Urea Methacrylate) Shell for Solar Water Heater. Energies, 12(18), 3406. https://doi.org/10.3390/en12183406