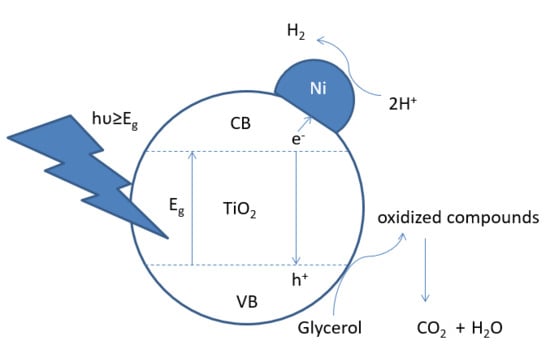
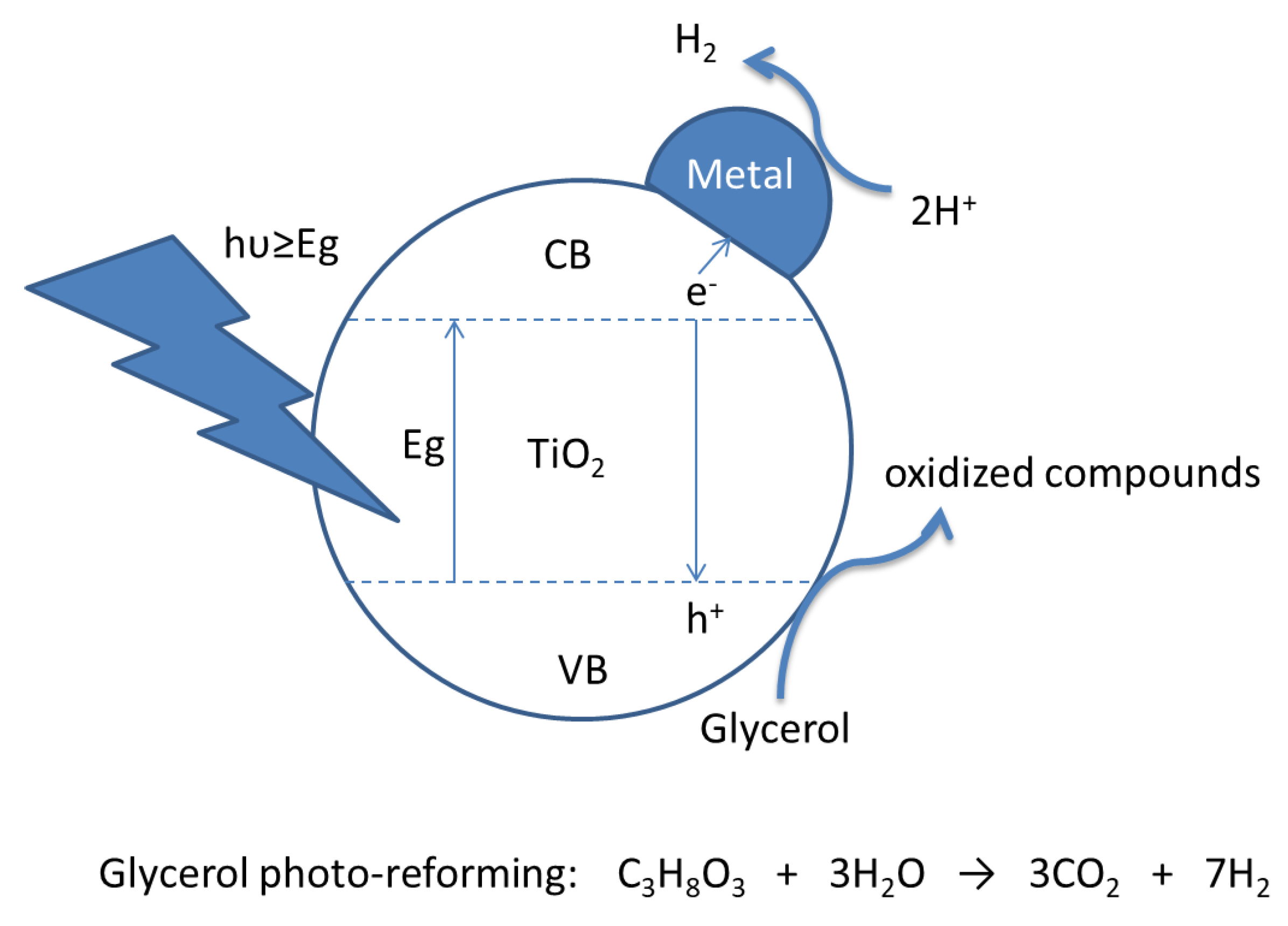
Hydrogen Photo-Production from Glycerol Using Nickel-Doped TiO2 Catalysts: Effect of Catalyst Pre-Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalysts Synthesis
2.1.1. Deposition–precipitation Method
2.1.2. Impregnation Method
2.1.3. Catalyst Pre-Reduction
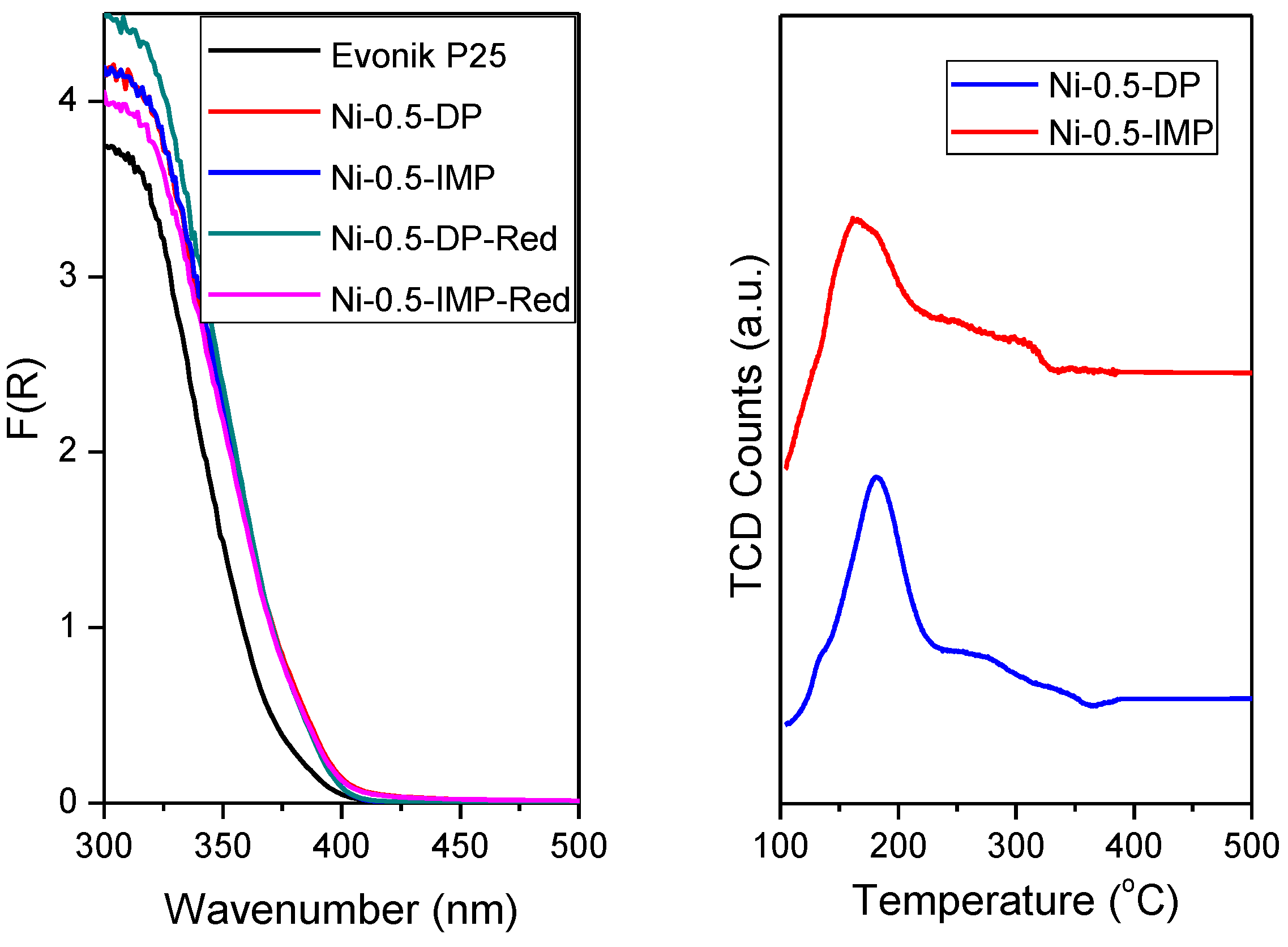
2.2. Characterization of the Solids
2.3. Photocatalytic Experiments
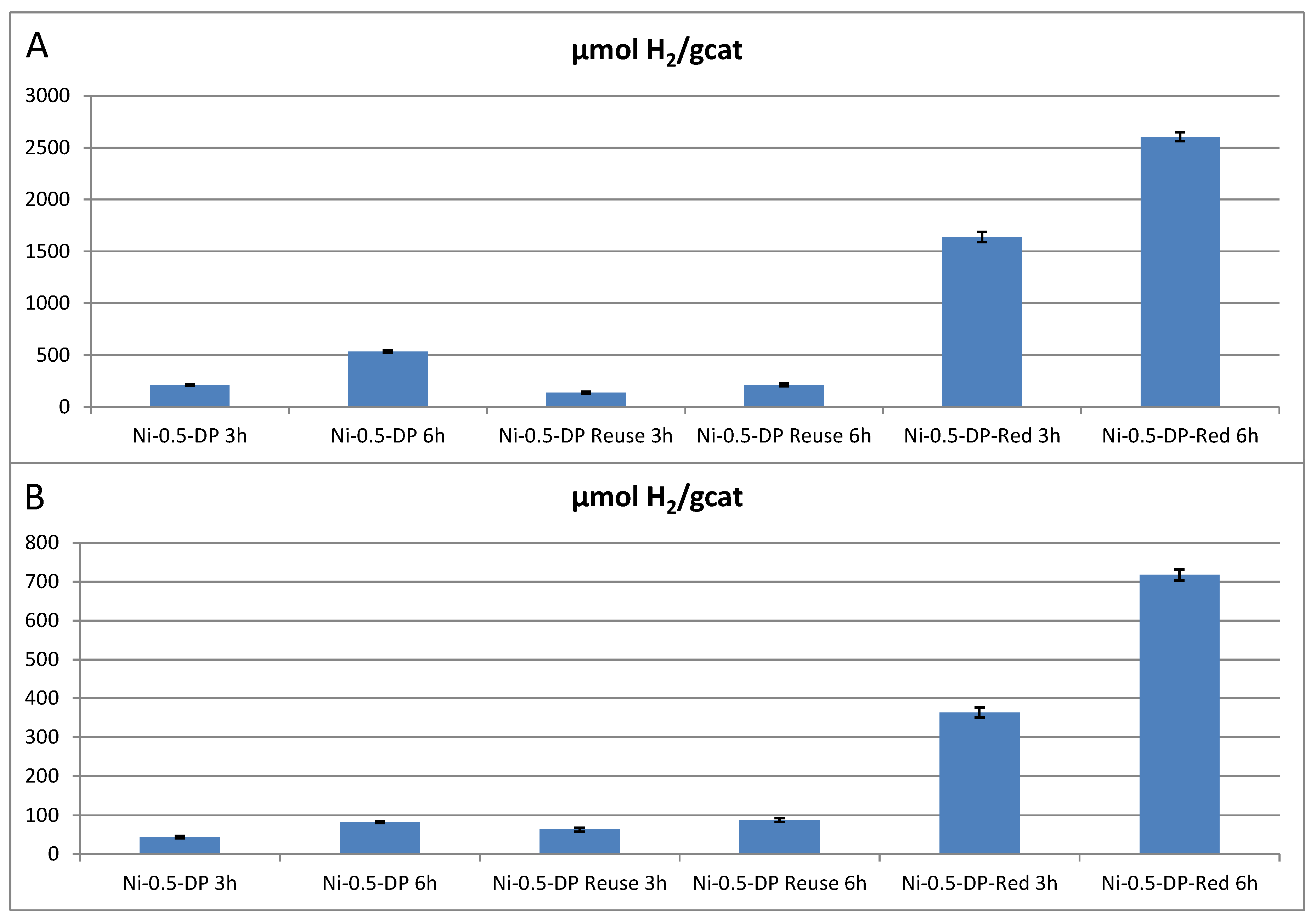
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melián, E.P.; López, C.R.; Santiago, D.E.; Quesada-Cabrera, R.; Méndez, J.A.O.; Rodríguez, J.M.D.; Díaz, O.G. Study of the photocatalytic activity of Pt-modified commercial TiO2 for hydrogen production in the presence of common organic sacrificial agents. Appl. Catal. A Gen. 2016, 518, 189–197. [Google Scholar] [CrossRef]
- Granada Ramírez, O.; Gutierrez Arzaluz, M.; Ávila Jiménez, M.; Fernández Sánchez, L.; Aguilar Pliego, J.; Mugica Álvarez, V.; Torres Rodríguez, M. Oxidación de glicerol en fase acuosa con platino soportado en óxido de titanio. Superf. Vacío 2016, 29, 9–13. [Google Scholar]
- Avgouropoulos, G.; Ioannides, T.; Kallitsis, J.K.; Neophytides, S. Development of an internal reforming alcohol fuel cell: Concept, challenges and opportunities. Chem. Eng. J. 2011, 176, 95–101. [Google Scholar] [CrossRef]
- Du, C.; Mo, J.; Li, H. Renewable Hydrogen Production by Alcohols Reforming Using Plasma and Plasma-Catalytic Technologies: Challenges and Opportunities. Chem. Rev. 2015, 115, 1503–1542. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; White, D.; Demirel, Y.; Kelly, R.; Noll, K.; Blum, P. Uncoupling Fermentative Synthesis of Molecular Hydrogen from Biomass Formation in Thermotoga maritima. Appl. Environ. Microbiol. 2018, 84, e00998-18. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Tevatia, R.; White, D.; Demirel, Y.; Blum, P. Comparative kinetic modeling of growth and molecular hydrogen overproduction by engineered strains of Thermotoga maritima. Int. J. Hydrogen Energy 2019, 44, 7125–7136. [Google Scholar] [CrossRef]
- Bednarczyk, K.; Stelmachowski, M.; Gmurek, M. The Influence of Process Parameters on Photocatalytic Hydrogen Production. Environ. Prog. Sustain. Energy 2018, 38, 680–687. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.; Chen, Y.; Cheng, Z.; Luo, X.; Mo, S.; Jia, L.; Lin, P.; Yang, Z. Improved hydrogen production from glycerol photoreforming over sol-gel derived TiO2 coupled with metal oxides. Chem. Eng. J. 2017, 317, 522–532. [Google Scholar] [CrossRef]
- Martínez, F.M.; Albiter, E.; Alfaro, S.; Luna, A.L.; Colbeau-Justin, C.; Barrera-Andrade, J.M.; Remita, H.; Valenzuela, M.A. Hydrogen Production from Glycerol Photoreforming on TiO2/HKUST-1 Composites: Effect of Preparation Method. Catalysts 2019, 9, 338. [Google Scholar] [CrossRef]
- Barreca, D.; Carraro, G.; Gombac, V.; Gasparotto, A.; Maccato, C.; Fornasiero, P.; Tondello, E. Supported metal oxide nanosystems for hydrogen photogeneration: Quo vadis? Adv. Funct. Mater. 2011, 21, 2611–2623. [Google Scholar] [CrossRef]
- Chen, X.; Selloni, A. Introduction: Titanium Dioxide (TiO2) Nanomaterials. Chem. Rev. 2014, 114, 9281–9282. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Dan, Y.; Jiang, L. P3HT/Ag/TiO2 ternary photocatalyst with significantly enhanced activity under both visible light and ultraviolet irradiation. Appl. Surf. Sci. 2019, 488, 228–236. [Google Scholar] [CrossRef]
- Jovic, V.; Chen, W.-T.; Sun-Waterhouse, D.; Blackford, M.G.; Idriss, H.; Waterhouse, G.I.N. Effect of gold loading and TiO2 support composition on the activity of Au/TiO2 photocatalysts for H2 production from ethanol–water mixtures. J. Catal. 2013, 305, 307–317. [Google Scholar] [CrossRef]
- López-Tenllado, F.J.; Hidalgo-Carrillo, J.; Montes, V.; Marinas, A.; Urbano, F.J.; Marinas, J.M.; Ilieva, L.; Tabakova, T.; Reid, F. A comparative study of hydrogen photocatalytic production from glycerol and propan-2-ol on M/TiO2 systems (M = Au, Pt, Pd). Catal. Today 2017, 280, 58–64. [Google Scholar] [CrossRef]
- Bowker, M.; Morton, C.; Kennedy, J.; Bahruji, H.; Greves, J.; Jones, W.; Davies, P.R.; Brookes, C.; Wells, P.P.; Dimitratos, N. Hydrogen production by photoreforming of biofuels using Au, Pd and Au–Pd/TiO2 photocatalysts. J. Catal. 2014, 310, 10–15. [Google Scholar] [CrossRef]
- Carraro, G.; MacCato, C.; Gasparotto, A.; Montini, T.; Turner, S.; Lebedev, O.I.; Gombac, V.; Adami, G.; Van Tendeloo, G.; Barreca, D.; et al. Enhanced hydrogen production by photoreforming of renewable oxygenates through nanostructured Fe2O3 polymorphs. Adv. Funct. Mater. 2014, 24, 372–378. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L.; Guemez, M.B.; Sanchez-Sanchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Glycerol steam reforming over Ni catalysts supported on ceria and ceria-promoted alumina. Int. J. Hydrogen Energy 2010, 35, 11622–11633. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chan, A.; Sun-Waterhouse, D.; Llorca, J.; Idriss, H.; Waterhouse, G.I.N. Performance comparison of Ni/TiO2 and Au/TiO2 photocatalysts for H2 production in different alcohol-water mixtures. J. Catal. 2018, 367, 27–42. [Google Scholar] [CrossRef]
- Clarizia, L.; Vitiello, G.; Pallotti, D.K.; Silvestri, B.; Nadagouda, M.; Lettieri, S.; Luciani, G.; Andreozzi, R.; Maddalena, P.; Marotta, R. Effect of surface properties of copper-modified commercial titanium dioxide photocatalysts on hydrogen production through photoreforming of alcohols. Int. J. Hydrogen Energy 2017, 42, 28349–28362. [Google Scholar] [CrossRef]
- Luciani, G.; Vitiello, G.; Clarizia, L.; Abdelraheem, W.; Esposito, S.; Bonelli, B.; Ditaranto, N.; Vergara, A.; Nadagouda, M.; Dionysiou, D.D.; et al. Near UV-irradiation of CuOx-impregnated TiO2 providing active species for H2 production through methanol photoreforming. ChemCatChem 2019, 45324, 1–14. [Google Scholar]
- Li, C.; Chen, Y.W. Temperature-programmed-reduction studies of nickel oxide/alumina catalysts: Effects of the preparation method. Thermochim. Acta 1995, 256, 457–465. [Google Scholar] [CrossRef]
- Petrik, I.S.; Krylova, G.V.; Lutsenko, L.V.; Smirnova, N.P.; Oleksenko, L.P. XPS and TPR study of sol-gel derived M/TiO2 powders (M = Co, Cu, Mn, Ni). Chem. Phys. Technol. Surf. 2015, 6, 179–189. [Google Scholar]
- Carley, A.F.; Jackson, S.D.; O’Shea, J.N.; Roberts, M.W. The formation and characterisation of Ni3+—An X-ray photoelectron spectroscopic investigation of potassium-doped Ni(1 1 0)-O. Surf. Sci. 1999, 440, L868–L874. [Google Scholar] [CrossRef]
- Janlamool, J.; Jongsomjit, B. Characteristics and catalytic properties of Ni/Ti-Si composite oxide catalysts via CO2 hydrogenation. Eng. J. 2017, 21, 45–55. [Google Scholar]
- Ju, F.; Wang, M.; Wu, T.; Ling, H. The role of NiO in reactive adsorption desulfurization over NiO/ZnO-Al2O3-SiO2 adsorbent. Catalysts 2019, 9, 79. [Google Scholar] [CrossRef]
- Morales, J. Hydrogen Production by Glycerol Photo-Reforming, Using Noble and Transition Metals Supported on Titanium Dioxide. Master’s Thesis, University of Córdoba (SPAIN), Cordoba, Spain, 2018. [Google Scholar]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Kennedy, J.; Morgan, D.J. The importance of metal reducibility for the photo-reforming of methanol on transition metal-TiO2 photocatalysts and the use of non-precious metals. Int. J. Hydrogen Energy 2015, 40, 1465–1471. [Google Scholar] [CrossRef]
- Caravaca, A.; Jones, W.; Hardacre, C.; Bowker, M. H2 production by the photocatalytic reforming of cellulose and raw biomass using Ni, Pd, Pt and Au on titania. Proc. R. Soc. A Math. Phys. Eng. Sci. 2016, 472, 20160054. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Aramendia, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Navio, J.A. Influence of the strong metal support interaction effect (SMSI) of Pt/TiO2 and Pd/TiO2 systems in the photocatalytic biohydrogen production from glucose solution. Catal. Commun. 2011, 16, 1–6. [Google Scholar] [CrossRef]
- Szabó, F.Z.G. Solymosi in “Proceedings, 2nd International Congress on Catalysis”; Technip: Paris, France, 1961; p. 1627. [Google Scholar]

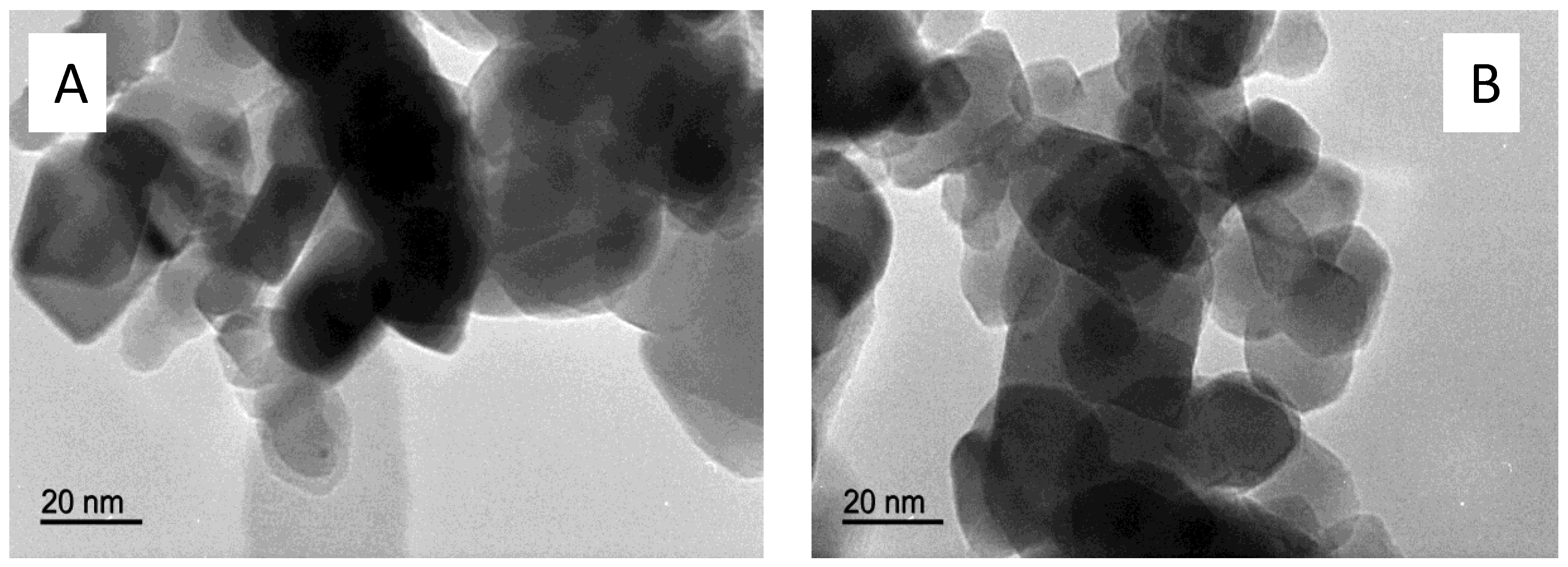
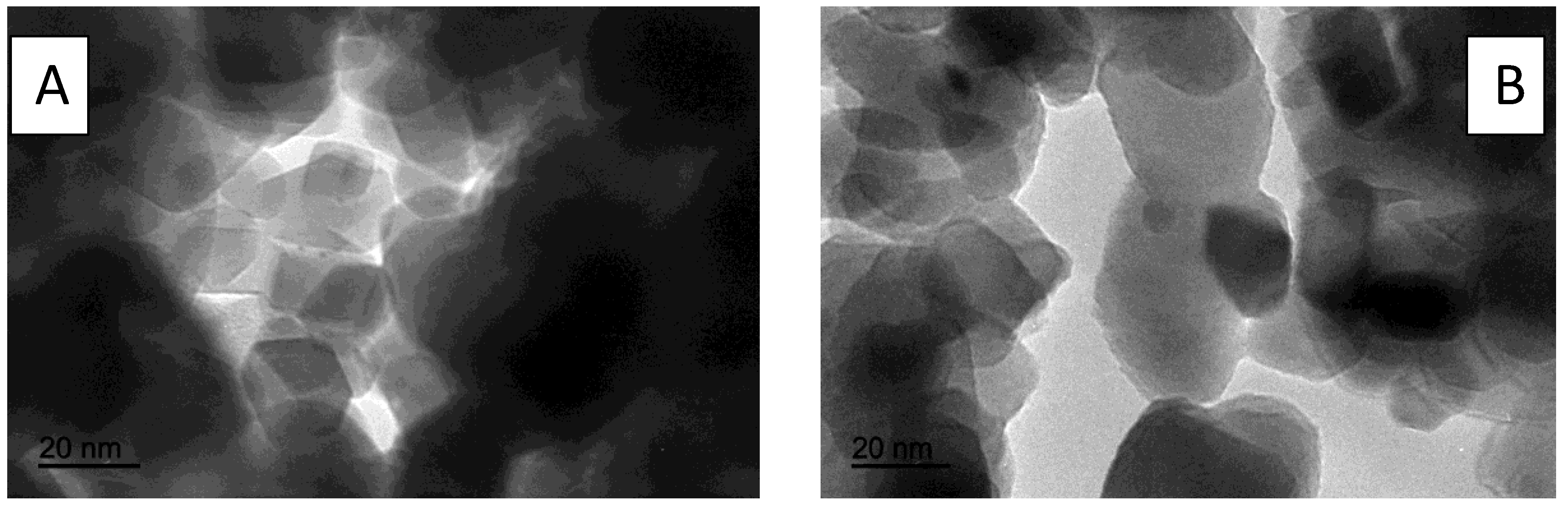
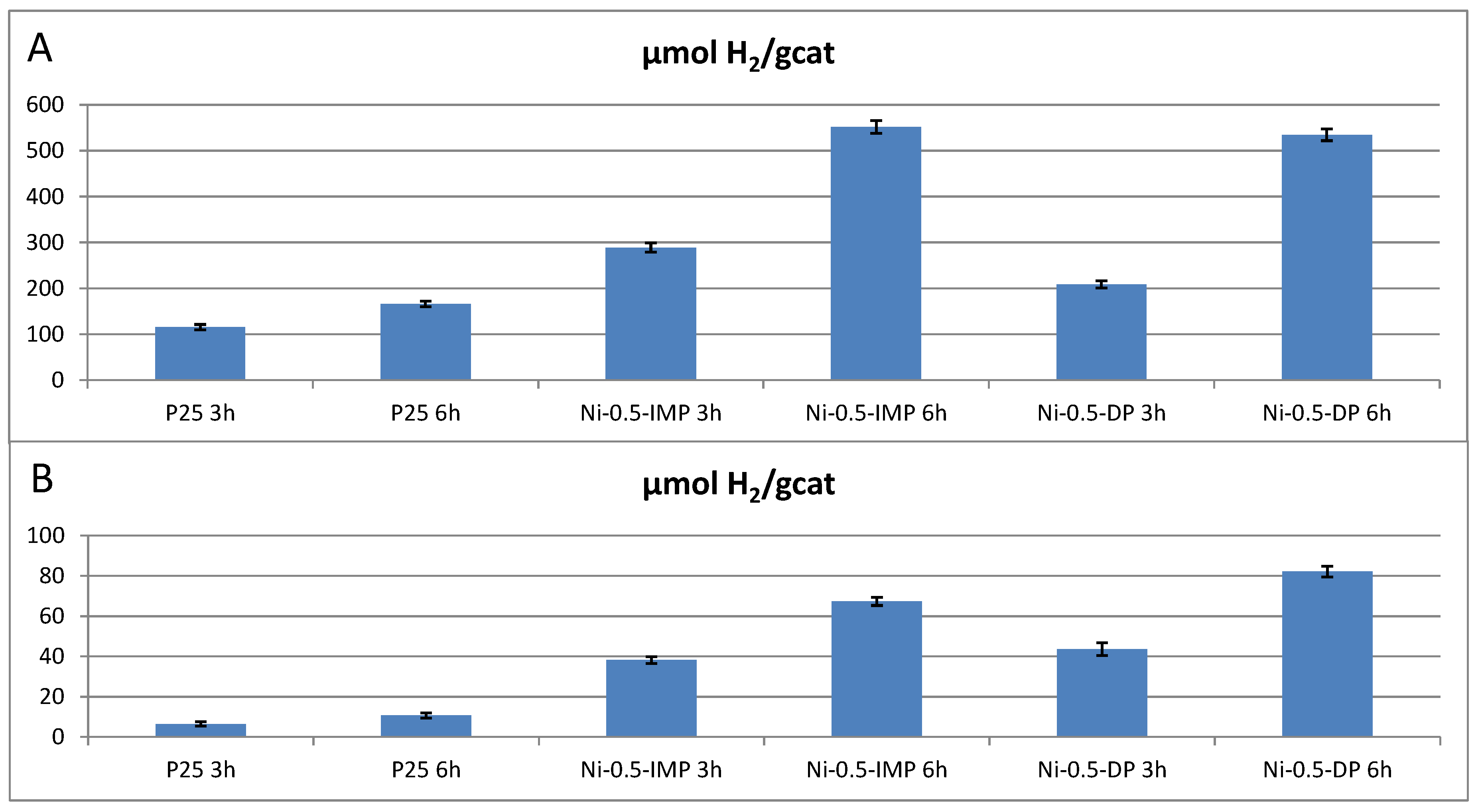
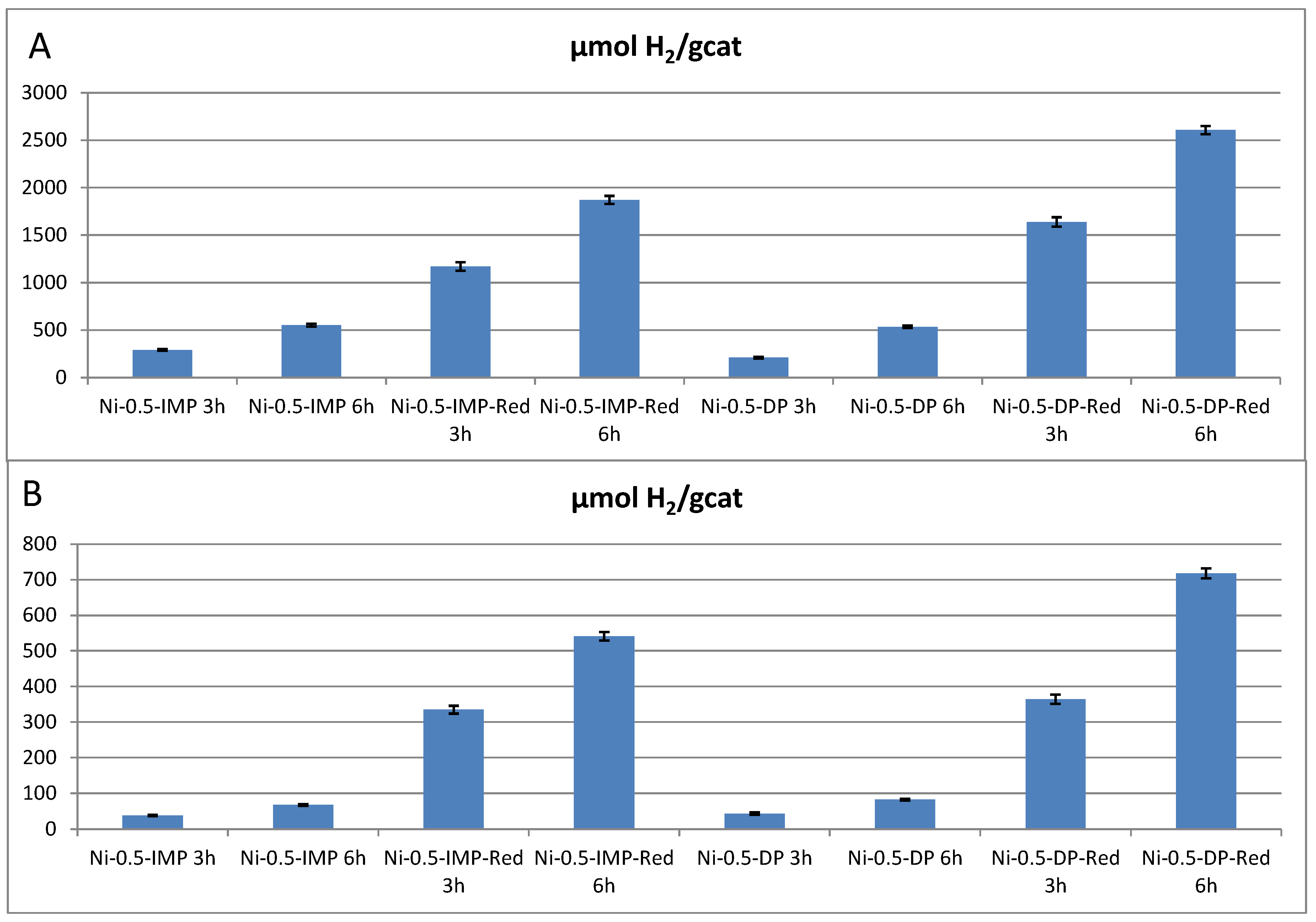

| Catalyst | Ni Nominal Content (weight%) | Experimental Ni Content (ICP-MS, weight%) |
|---|---|---|
| Ni-0.5-DP | 0.50 | 0.55 |
| Ni-0.5-IMP | 0.50 | 0.47 |
| Catalyst | Eg (eV) | Catalyst | Eg (eV) |
|---|---|---|---|
| P25 | 3.11 | ||
| Ni-0.5-DP | 3.02 | Ni-0.5-DP-Red | 3.07 |
| Ni-0.5-IMP | 3.00 | Ni-0.5-IMP-Red | 3.01 |
| Catalyst | Ni (2p3/2) | Ti (2p3/2) | Cl (2p) |
|---|---|---|---|
| Ni-0.5-DP | 855.9 | 458.8 | 199.9 |
| Ni-0.5-DP-Red | 855.0 | 458.7 | - |
| Ni-0.5-IMP | 856.1 | 458.8 | 200.0 |
| Ni-0.5-IMP-Red | 855.1 | 458.5 | - |
| Ni-0.5-DP 1 using UV | 854.9 | 458.6 | - |
| Ni-0.5-DP 1 using solar | 855.1 | 458.6 | - |
| Ni-0.5-DP 2 using UV | 855.2 | 458.4 | - |
| Ni-0.5-DP 2 using solar | 854.9 | 458.4 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Carrillo, J.; Martín-Gómez, J.; Morales, J.; Espejo, J.C.; Urbano, F.J.; Marinas, A. Hydrogen Photo-Production from Glycerol Using Nickel-Doped TiO2 Catalysts: Effect of Catalyst Pre-Treatment. Energies 2019, 12, 3351. https://doi.org/10.3390/en12173351
Hidalgo-Carrillo J, Martín-Gómez J, Morales J, Espejo JC, Urbano FJ, Marinas A. Hydrogen Photo-Production from Glycerol Using Nickel-Doped TiO2 Catalysts: Effect of Catalyst Pre-Treatment. Energies. 2019; 12(17):3351. https://doi.org/10.3390/en12173351
Chicago/Turabian StyleHidalgo-Carrillo, Jesús, Juan Martín-Gómez, Julia Morales, Juan Carlos Espejo, Francisco José Urbano, and Alberto Marinas. 2019. "Hydrogen Photo-Production from Glycerol Using Nickel-Doped TiO2 Catalysts: Effect of Catalyst Pre-Treatment" Energies 12, no. 17: 3351. https://doi.org/10.3390/en12173351
APA StyleHidalgo-Carrillo, J., Martín-Gómez, J., Morales, J., Espejo, J. C., Urbano, F. J., & Marinas, A. (2019). Hydrogen Photo-Production from Glycerol Using Nickel-Doped TiO2 Catalysts: Effect of Catalyst Pre-Treatment. Energies, 12(17), 3351. https://doi.org/10.3390/en12173351