An Overview of the Production of Oxygenated Fuel Additives by Glycerol Etherification, Either with Isobutene or tert-Butyl Alcohol, over Heterogeneous Catalysts
Abstract
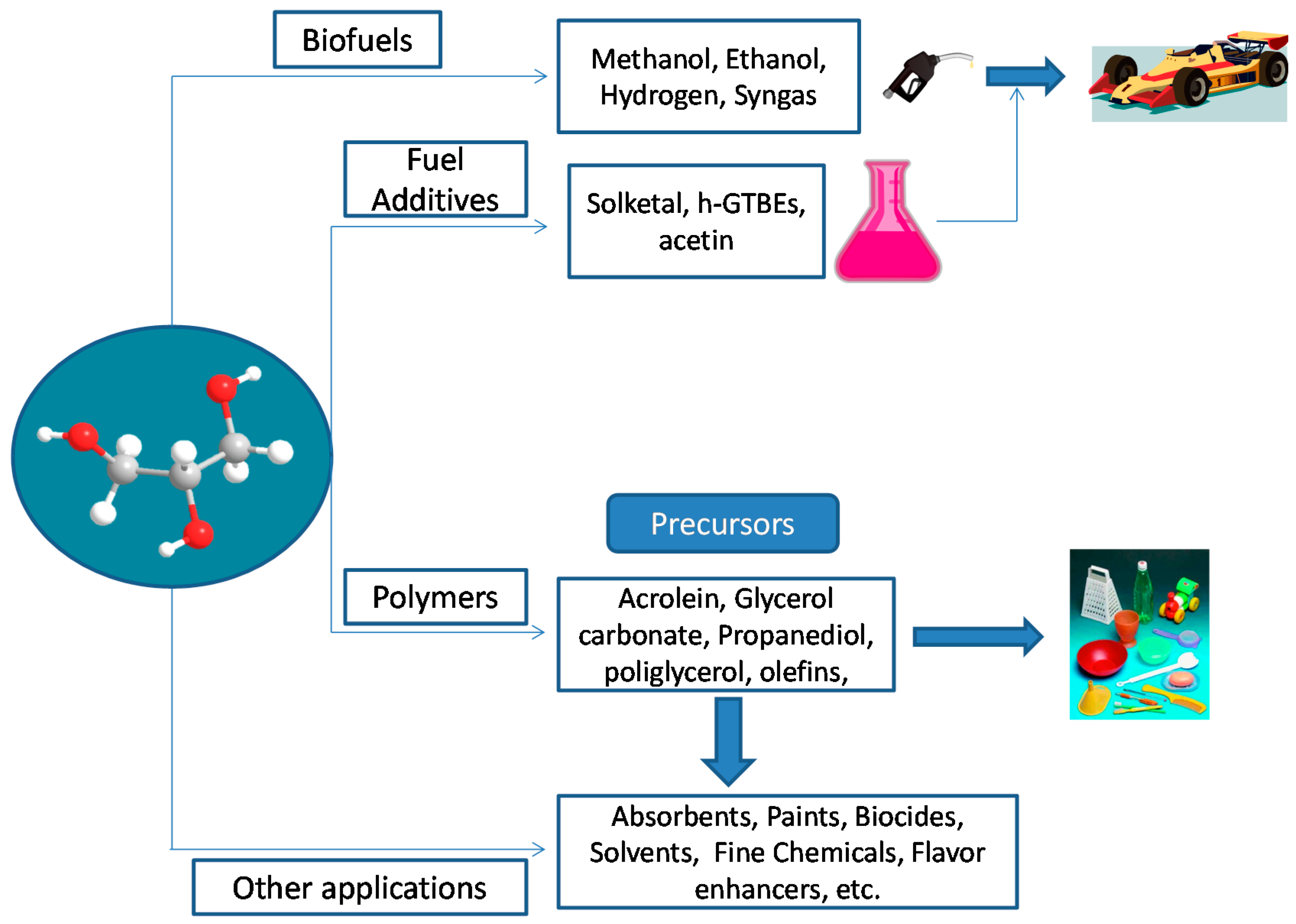
1. Introduction
2. Etherification of Glycerol to Produce Oxygenated Fuel Additives
2.1. Etherification of Glycerol with Isobutene
Experimental Designs Reported to Date for Glycerol Etherification with IB
2.2. Etherification of Glycerol with tert-Butyl Alcohol
Experimental Designs Reported to Date for Glycerol Etherification with TBA
3. Blends of Glycerol Ethers Additives and (Bio)Fuel
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A-15 | Amberlyst-15 |
| DIB | Di-isobutene |
| DOE | United States Department of Energy |
| DTBGs | Di-tert-Butyl glycerol ethers |
| EGR | Exhaust gas recirculation |
| EIA | U.S. Energy Information Administration |
| ETBE | Ethyl tert-Butyl ether |
| EN 14214 | Standard published by the European Committee for Standardization that describes the requirements and test methods for FAME |
| FAEE | Fatty acid ethyl ester |
| FAME | Fatty acid methyl esters, components of conventional biodiesel |
| G | Glycerol |
| h-GTBE | “High” glycerol tert-Butyl ethers (i.e., DTBGs + TTBG) |
| IB | Isobutene |
| MTBE | Methyl tert-Butyl ether |
| MTBGs | Methyl tert-Butyl glycerol ethers |
| OPEC | Organization of the Petroleum Exporting Countries |
| TBA | tert-Butyl alcohol |
| TTBG | Tri-tert-Butyl glycerol ether |
| VLE | Vapor liquid equilibrium |
References
- Available online: https://countryeconomy.com/raw-materials/opec (accessed on 17 June 2019).
- Nda-Umar, U.; Ramli, I.; Taufiq-Yap, Y.; Muhamad, E. An Overview of Recent Research in the Conversion of Glycerol into Biofuels, Fuel Additives and other Bio-Based Chemicals. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef]
- Hurtado, B.; Posadillo, A.; Luna, D.; Bautista, F.; Hidalgo, J.; Luna, C.; Calero, J.; Romero, A.; Estevez, R. Synthesis, Performance and Emission Quality Assessment of Ecodiesel from Castor Oil in Diesel/Biofuel/Alcohol Triple Blends in a Diesel Engine. Catalysts 2019, 9, 40. [Google Scholar] [CrossRef]
- Chum, H.L.; Overend, R.P. Biomass and bioenergy in the United States. Adv. Sol. Energy 2003, 15, 83–148. [Google Scholar]
- Akia, M.; Yazdani, F.; Motaee, E.; Han, D.; Arandiyan, H. A review on conversion of biomass to biofuel by nanocatalysts. Biofuel Res. J. 2014, 1, 16–25. [Google Scholar] [CrossRef]
- Huber, G.W.; Corma, A. Synergien zwischen Bio-und Ölraffinerien bei der Herstellung von Biomassetreibstoffen. Angew. Chem. 2007, 119, 7320–7338. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From Glycerol to Value-Added Products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Mohan, S.V.; Nikhil, G.; Chiranjeevi, P.; Reddy, C.N.; Rohit, M.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- Monthly Biodiesel Production Report. Available online: https://www.eia.gov/biofuels/biodiesel/production/ (accessed on 17 June 2019).
- REN21. Renewable Energy Policy Network for the 21st Century. Renewables 2018 Global Status Report. Available online: http://www.ren21.net/wp-content/uploads/2018/06/17-8652_GSR2018_FullReport_web_final_.pdf (accessed on 17 June 2019).
- Ridjan, I.; Mathiesen, B.V.; Connolly, D.; Duić, N. The feasibility of synthetic fuels in renewable energy systems. Energy 2013, 57, 76–84. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. Potential non-edible oil resources as biodiesel feedstock: An Indian perspective. Renew. Sustain. Energy Rev. 2011, 15, 1791–1800. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Avhad, M.; Marchetti, J. A review on recent advancement in catalytic materials for biodiesel production. Renew. Sustain. Energy Rev. 2015, 50, 696–718. [Google Scholar] [CrossRef]
- Ramachandran, K.; Suganya, T.; Gandhi, N.N.; Renganathan, S. Recent developments for biodiesel production by ultrasonic assist transesterification using different heterogeneous catalyst: A review. Renew. Sustain. Energy Rev. 2013, 22, 410–418. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Comelli, R.A. Glycerol, the co-product of biodiesel: One key for the future bio-refinery. In Biodiesel-Quality, Emissions and By-Products; IntechOpen: London, UK, 2011. [Google Scholar]
- Luo, X.; Ge, X.; Cui, S.; Li, Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016, 215, 144–154. [Google Scholar] [CrossRef]
- Len, C.; Delbecq, F.; Corpas, C.C.; Ramos, E.R. Continuous flow conversion of glycerol into chemicals: An overview. Synthesis 2018, 50, 723–741. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Yehye, W.A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy Rev. 2015, 41, 113–127. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C.C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Bozkurt, Ö.D.; Tunc, F.M.; Bağlar, N.; Celebi, S.; Günbaş, İ.D.; Uzun, A. Alternative fuel additives from glycerol by etherification with isobutene: Structure–performance relationships in solid catalysts. Fuel Process. Technol. 2015, 138, 780–804. [Google Scholar] [CrossRef]
- Izquierdo, J.; Montiel, M.; Palés, I.; Outón, P.; Galán, M.; Jutglar, L.; Villarrubia, M.; Izquierdo, M.; Hermo, M.; Ariza, X. Fuel additives from glycerol etherification with light olefins: State of the art. Renew. Sustain. Energy Rev. 2012, 16, 6717–6724. [Google Scholar] [CrossRef]
- Yee, K.F.; Mohamed, A.R.; Tan, S.H. A review on the evolution of ethyl tert-Butyl ether (ETBE) and its future prospects. Renew. Sustain. Energy Rev. 2013, 22, 604–620. [Google Scholar] [CrossRef]
- Garcia, E.; Laca, M.; Pérez, E.; Garrido, A.; Peinado, J. New class of acetal derived from glycerin as a biodiesel fuel component. Energy Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- Oprescu, E.-E.; Stepan, E.; Dragomir, R.E.; Radu, A.; Rosca, P. Synthesis and testing of glycerol ketals as components for diesel fuel. Fuel Process. Technol. 2013, 110, 214–217. [Google Scholar] [CrossRef]
- Zare, A.; Nabi, M.N.; Bodisco, T.A.; Hossain, F.M.; Rahman, M.M.; Ristovski, Z.D.; Brown, R.J. The effect of triacetin as a fuel additive to waste cooking biodiesel on engine performance and exhaust emissions. Fuel 2016, 182, 640–649. [Google Scholar] [CrossRef]
- Alptekin, E. Emission, injection and combustion characteristics of biodiesel and oxygenated fuel blends in a common rail diesel engine. Energy 2017, 119, 44–52. [Google Scholar] [CrossRef]
- Jaecker-Voirol, A.; Durand, I.; Hillion, G.; Delfort, B.; Montagne, X. Glycerin for new biodiesel formulation. Oil Gas Sci. Technol. Rev. l’IFP 2008, 63, 395–404. [Google Scholar] [CrossRef]
- Behr, A.; Obendorf, L. Development of a Process for the Acid-Catalyzed Etherification of Glycerine and Isobutene Forming Glycerine Tertiary Butyl Ethers. Eng. Life Sci. 2002, 2, 185–189. [Google Scholar] [CrossRef]
- Karinen, R.; Krause, A. New biocomponents from glycerol. Appl. Catal. A Gen. 2006, 306, 128–133. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Bajus, M. tert-Butylation of glycerol catalysed by ion-exchange resins. Appl. Catal. A Gen. 2005, 294, 141–147. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Kaszonyi, A.; Bajus, M. Etherification of glycerol and ethylene glycol by isoButylene. Appl. Catal. A Gen. 2007, 328, 1–13. [Google Scholar] [CrossRef]
- Lee, H.J.; Seung, D.; Jung, K.S.; Kim, H.; Filimonov, I.N. Etherification of glycerol by isoButylene: Tuning the product composition. Appl. Catal. A Gen. 2010, 390, 235–244. [Google Scholar] [CrossRef]
- Lee, H.J.; Seung, D.; Filimonov, I.N.; Kim, H. Etherification of glycerol by isoButylene. Effects of the density of acidic sites in ion-exchange resin on the distribution of products. Korean J. Chem. Eng. 2011, 28, 756–762. [Google Scholar] [CrossRef]
- Bozkurt, Ö.D.; Bağlar, N.; Çelebi, S.; Uzun, A. Assessment of acid strength in sodium-exchanged resin catalysts: Consequences on glycerol etherification with isobutene in batch and flow reactors. Mol. Catal. 2019, 466, 1–12. [Google Scholar] [CrossRef]
- Melero, J.; Vicente, G.; Morales, G.; Paniagua, M.; Moreno, J.; Roldán, R.; Ezquerro, A.; Pérez, C. Acid-catalyzed etherification of bio-glycerol and isoButylene over sulfonic mesostructured silicas. Appl. Catal. A Gen. 2008, 346, 44–51. [Google Scholar] [CrossRef]
- Zhao, W.; Yi, C.; Yang, B.; Hu, J.; Huang, X. Etherification of glycerol and isoButylene catalyzed over rare earth modified Hβ-zeolite. Fuel Process. Technol. 2013, 112, 70–75. [Google Scholar] [CrossRef]
- Xiao, L.; Mao, J.; Zhou, J.; Guo, X.; Zhang, S. Enhanced performance of HY zeolites by acid wash for glycerol etherification with isobutene. Appl. Catal. A Gen. 2011, 393, 88–95. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Taboada, E.; Llorca, J.; Cesteros, Y. Microwave-assisted synthesis of sulfonic acid-functionalized microporous materials for the catalytic etherification of glycerol with isobutene. Green Chem. 2013, 15, 2230–2239. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Llorca, J.; Salagre, P. Boosted selectivity toward high glycerol tertiary Butyl ethers by microwave-assisted sulfonic acid-functionalization of SBA-15 and beta zeolite. J. Catal. 2012, 290, 202–209. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Mokaya, R.; Cesteros, Y. Tuning the acidic and textural properties of ordered mesoporous silicas for their application as catalysts in the etherification of glycerol with isobutene. Catal. Today 2014, 227, 171–178. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Taboada, E.; Llorca, J.; Molins, E.; Cesteros, Y. Sulfonic acid-functionalized aerogels as high resistant to deactivation catalysts for the etherification of glycerol with isobutene. Appl. Catal. B Environ. 2013, 136, 287–293. [Google Scholar] [CrossRef]
- Frusteri, F.; Cannilla, C.; Bonura, G.; Spadaro, L.; Mezzapica, A.; Beatrice, C.; Di Blasio, G.; Guido, C. Glycerol ethers production and engine performance with diesel/ethers blend. Top. Catal. 2013, 56, 378–383. [Google Scholar] [CrossRef]
- Beatrice, C.; Di Blasio, G.; Lazzaro, M.; Cannilla, C.; Bonura, G.; Frusteri, F.; Asdrubali, F.; Baldinelli, G.; Presciutti, A.; Fantozzi, F. Technologies for energetic exploitation of biodiesel chain derived glycerol: Oxy-fuels production by catalytic conversion. Appl. Energy 2013, 102, 63–71. [Google Scholar] [CrossRef]
- Frusteri, F.; Frusteri, L.; Cannilla, C.; Bonura, G. Catalytic etherification of glycerol to produce biofuels over novel spherical silica supported Hyflon® catalysts. Bioresour. Technol. 2012, 118, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, B.; Yi, C.; Lei, Z.; Xu, J. Etherification of glycerol with isoButylene to produce oxygenate additive using sulfonated peanut shell catalyst. Ind. Eng. Chem. Res. 2010, 49, 12399–12404. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Guo, X.; Mao, J.; Zhang, S. Etherification of glycerol with isobutene on sulfonated graphene: Reaction and separation. Green Chem. 2014, 16, 4669–4679. [Google Scholar] [CrossRef]
- Voicu, V.; Bombos, D.; Bolocan, I.; Jang, C.R.; Ciuparu, D. The Influence of the Character of Emulsifiers on the Performance of H (4) SiW (12) O (40 center dot) 30H (2) O Heteropolyacid Catalyst in Glycerol Etherification with Isobutene. Rev. Chim. 2012, 63, 200–204. [Google Scholar]
- Liu, J.; Yuan, Y.; Pan, Y.; Huang, Z.; Yang, B. Liquid–liquid equilibrium for systems of glycerol and glycerol tert-Butyl ethers. Fluid Phase Equilibria 2014, 365, 50–57. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Yi, C. Kinetic study of glycerol etherification with isobutene. Ind. Eng. Chem. Res. 2013, 52, 3742–3751. [Google Scholar] [CrossRef]
- Di Serio, M.; Cozzolino, M.; Giordano, M.; Tesser, R.; Patrono, P.; Santacesaria, E. From homogeneous to heterogeneous catalysts in biodiesel production. Ind. Eng. Chem. Res. 2007, 46, 6379–6384. [Google Scholar] [CrossRef]
- Liu, J.; Daoutidis, P.; Yang, B. Process design and optimization for etherification of glycerol with isobutene. Chem. Eng. Sci. 2016, 144, 326–335. [Google Scholar] [CrossRef]
- Turan, A.; Hrivnák, M.; Klepáčová, K.; Kaszonyi, A.; Mravec, D. Catalytic etherification of bioglycerol with C4 fraction. Appl. Catal. A Gen. 2013, 468, 313–321. [Google Scholar] [CrossRef]
- Kiatkittipong, W.; Intaracharoen, P.; Laosiripojana, N.; Chaisuk, C.; Praserthdam, P.; Assabumrungrat, S. Glycerol ethers synthesis from glycerol etherification with tert-Butyl alcohol in reactive distillation. Comput. Chem. Eng. 2011, 35, 2034–2043. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Linares, M.; García, R.; Serrano, D.; Cesteros, Y. Effect of hierarchical porosity and fluorination on the catalytic properties of zeolite beta for glycerol etherification. Appl. Catal. A Gen. 2014, 473, 75–82. [Google Scholar] [CrossRef]
- Klepáčová, K.; Mravec, D.; Bajus, M. Etherification of glycerol with tert-Butyl alcohol catalysed by ion-exchange resins. Chem. Pap. 2006, 60, 224–230. [Google Scholar] [CrossRef]
- Chang, J.-S.; Zhang, Y.-C.; Chen, C.-C.; Ling, T.-R.; Chiou, Y.-J.; Wang, G.-B.; Chang, K.-T.; Chou, T.-C. One-step synthesis of gasoline octane booster and diesel fuel from glycerol and tert-Butyl alcohol. Ind. Eng. Chem. Res. 2014, 53, 5398–5405. [Google Scholar] [CrossRef]
- Frusteri, F.; Arena, F.; Bonura, G.; Cannilla, C.; Spadaro, L.; Di Blasi, O. Catalytic etherification of glycerol by tert-Butyl alcohol to produce oxygenated additives for diesel fuel. Appl. Catal. A Gen. 2009, 367, 77–83. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Glycerol Etherification with TBA: High Yield to Poly-Ethers Using a Membrane Assisted Batch Reactor. Environ. Sci. Technol. 2014, 48, 6019–6026. [Google Scholar] [CrossRef] [PubMed]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Catalytic production of oxygenated additives by glycerol etherification. Open Chem. 2014, 12, 1248–1254. [Google Scholar] [CrossRef]
- Gonçalves, M.; Souza, V.C.; Galhardo, T.S.; Mantovani, M.; Figueiredo, F.v.C.; Mandelli, D.; Carvalho, W.A. Glycerol conversion catalyzed by carbons prepared from agroindustrial wastes. Ind. Eng. Chem. Res. 2013, 52, 2832–2839. [Google Scholar] [CrossRef]
- Galhardo, T.S.; Simone, N.; Gonçalves, M.; Figueiredo, F.C.; Mandelli, D.; Carvalho, W.A. Preparation of sulfonated carbons from rice husk and their application in catalytic conversion of glycerol. ACS Sustain. Chem. Eng. 2013, 1, 1381–1389. [Google Scholar] [CrossRef]
- Gonçalves, M.; Soler, F.C.; Isoda, N.; Carvalho, W.A.; Mandelli, D.; Sepúlveda, J. Glycerol conversion into value-added products in presence of a green recyclable catalyst: Acid black carbon obtained from coffee ground wastes. J. Taiwan Inst. Chem. Eng. 2016, 60, 294–301. [Google Scholar] [CrossRef]
- Gonçalves, M.; Mantovani, M.; Carvalho, W.A.; Rodrigues, R.; Mandelli, D.; Albero, J.S. Biodiesel wastes: An abundant and promising source for the preparation of acidic catalysts for utilization in etherification reaction. Chem. Eng. J. 2014, 256, 468–474. [Google Scholar] [CrossRef]
- Pico, M.P.; Rosas, J.M.; Rodríguez, S.; Santos, A.; Romero, A. Glycerol etherification over acid ion exchange resins: Effect of catalyst concentration and reusability. J. Chem. Technol. Biotechnol. 2013, 88, 2027–2038. [Google Scholar] [CrossRef]
- Pico, M.P.; Romero, A.; Rodríguez, S.; Santos, A. Etherification of glycerol by tert-Butyl alcohol: Kinetic model. Ind. Eng. Chem. Res. 2012, 51, 9500–9509. [Google Scholar] [CrossRef]
- Magar, S.; Kamble, S.; Mohanraj, G.T.; Jana, S.K.; Rode, C. Solid-Acid-Catalyzed Etherification of Glycerol to Potential Fuel Additives. Energy Fuels 2017, 31, 12272–12277. [Google Scholar] [CrossRef]
- Celdeira, P.A.; Goncalves, M.; Figueiredo, F.C.; Dal Bosco, S.M.; Mandelli, D.; Carvalho, W.A. Sulfonated niobia and pillared clay as catalysts in etherification reaction of glycerol. Appl. Catal. A Gen. 2014, 478, 98–106. [Google Scholar] [CrossRef]
- Srinivas, M.; Raveendra, G.; Parameswaram, G.; Prasad, P.S.; Lingaiah, N. Cesium exchanged tungstophosphoric acid supported on tin oxide: An efficient solid acid catalyst for etherification of glycerol with tert-butanol to synthesize biofuel additives. J. Mol. Catal. A Chem. 2016, 413, 7–14. [Google Scholar] [CrossRef]
- González, M.D.; Cesteros, Y.; Salagre, P. Establishing the role of Brønsted acidity and porosity for the catalytic etherification of glycerol with tert-butanol by modifying zeolites. Appl. Catal. A Gen. 2013, 450, 178–188. [Google Scholar] [CrossRef]
- Simone, N.; Carvalho, W.A.; Mandelli, D.; Ryoo, R. Nanostructured MFI-type zeolites as catalysts in glycerol etherification with tert-Butyl alcohol. J. Mol. Catal. A Chem. 2016, 422, 115–121. [Google Scholar] [CrossRef]
- Estevez, R.; Iglesias, I.; Luna, D.; Bautista, F.M. Sulfonic Acid Functionalization of Different Zeolites and Their Use as Catalysts in the Microwave-Assisted Etherification of Glycerol with tert-Butyl Alcohol. Molecules 2017, 22, 2206. [Google Scholar] [CrossRef]
- Miranda, C.; Urresta, J.; Cruchade, H.; Tran, A.; Benghalem, M.; Astafan, A.; Gaudin, P.; Daou, T.; Ramírez, A.; Pouilloux, Y. Exploring the impact of zeolite porous voids in liquid phase reactions: The case of glycerol etherification by tert-Butyl alcohol. J. Catal. 2018, 365, 249–260. [Google Scholar] [CrossRef]
- Veiga, P.M.; Gomes, A.C.; Veloso, C.O.; Henriques, C.A. Acid zeolites for glycerol etherification with ethyl alcohol: Catalytic activity and catalyst properties. Appl. Catal. A Gen. 2017, 548, 2–15. [Google Scholar] [CrossRef]
- Estevez, R.; López, M.; Jiménez-Sanchidrián, C.; Luna, D.; Romero-Salguero, F.; Bautista, F. Etherification of glycerol with tert-Butyl alcohol over sulfonated hybrid silicas. Appl. Catal. A Gen. 2016, 526, 155–163. [Google Scholar] [CrossRef]
- Estevez, R.; Lopez-Pedrajas, S.; Luna, D.; Bautista, F. Microwave-assisted etherification of glycerol with tert-Butyl alcohol over amorphous organosilica-aluminum phosphates. Appl. Catal. B Environ. 2017, 213, 42–52. [Google Scholar] [CrossRef]
- Ozbay, N.; Oktar, N.; Dogu, G.; Dogu, T. Effects of sorption enhancement and isobutene formation on etherification of glycerol with tert-Butyl alcohol in a flow reactor. Ind. Eng. Chem. Res. 2011, 51, 8788–8795. [Google Scholar] [CrossRef]
- Ozbay, N.; Oktar, N.; Dogu, G.; Dogu, T. Activity comparison of different solid acid catalysts in etherification of glycerol with tert-Butyl alcohol in flow and batch reactors. Top. Catal. 2013, 56, 1790–1803. [Google Scholar] [CrossRef]
- Viswanadham, N.; Saxena, S.K. Etherification of glycerol for improved production of oxygenates. Fuel 2013, 103, 980–986. [Google Scholar] [CrossRef]
- Vlad, E.; Bildea, C.S.; Bozga, G. Design and control of glycerol-tert-Butyl alcohol etherification process. Sci. World J. 2012, 2012, 180617. [Google Scholar] [CrossRef] [PubMed]
- Vlad, E.; Bildea, C.S. Reactive Distillation-a Viable Solution for Etherification of Glycerol with tert-Butyl Alcohol. Chem. Eng. 2012, 29. [Google Scholar] [CrossRef]
- Simasatitkul, L.; Kaewwisetkul, P.; Arpornwichanop, A. Techno-economic assessment of extractive distillation for tert-Butyl alcohol recovery in fuel additive production. Chem. Eng. Process. Process Intensif. 2017, 122, 161–171. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, J.; Negi, M.; Bangwal, D.; Kaul, S.; Garg, M. Kinetics and modeling study on etherification of glycerol using isoButylene by in situ production from tert-Butyl alcohol. Ind. Eng. Chem. Res. 2015, 54, 5213–5219. [Google Scholar] [CrossRef]
- Luque, R.; Budarin, V.; Clark, J.H.; Macquarrie, D.J. Glycerol transformations on polysaccharide derived mesoporous materials. Appl. Catal. B Environ. 2008, 82, 157–162. [Google Scholar] [CrossRef]
- Noureddini, H.; Dailey, W.R.; Hunt, B.A. Production of ethers of glycerol from crude glycerol-the by-product of biodlesel production. Pap. Biomater. 1998, 18. [Google Scholar]
- Noureddini, H. Process for producing biodiesel fuel with reduced viscosity and a cloud point below thirty-two (32) degrees Fahrenheit. U.S. Patent 6,015,440, 18 January 2000. [Google Scholar]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Bustamante, J. Oxygenated compounds derived from glycerol for biodiesel formulation: Influence on EN 14214 quality parameters. Fuel 2010, 89, 2011–2018. [Google Scholar] [CrossRef]
- Beatrice, C.; Di Blasio, G.; Lazzaro, M.; Mancaruso, E.; Marialto, R.; Sequino, L.; Vaglieco, B.M. Investigation of the combustion in both metal and optical diesel engines using high-glycerol ethers/diesel blends. Int. J. Engine Res. 2015, 16, 38–51. [Google Scholar] [CrossRef]
- Samoilov, V.O.; Ramazanov, D.N.; Nekhaev, A.I.; Maximov, A.L.; Bagdasarov, L.N. Heterogeneous catalytic conversion of glycerol to oxygenated fuel additives. Fuel 2016, 172, 310–319. [Google Scholar] [CrossRef]





| Catalyst a | Reaction Conditions | XG (mol %) | Sh-GTBE (mol %) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | P b (bar) | Cat. Loading (wt.% of G) | IB/G Mol/mol | Time (h) | ||||
| p-toluenesulfonic acid | 90 | 1.4 | 2.16 | 2 | 5 | 89 | 47 | [37] |
| Amberlyst-15 (A-15) | 80 | 15 | 1 g | 4 | 7 | >95 | 97 | [38] |
| Amberlyst-39 wet | 60 | Autoge | 7.5 | 4 | 8 | 100 | 93 | [39] |
| A-15 in dioxane | 60 | Autoge | 7.5 | 4 | 8 | 79 | 47 | [40] |
| Ag(62)A-15 c, powder | 60 | 20 | 7.5 | 4 | 20 | >90 | 92 | [41] |
| Na(51)A-15 c | 60 | 20 | 7.5 | 4 | 20 | 99 | 90 | [42] |
| 0.3MNa-exchange A-15 | 75 | 10–15 | 7.5 | 3 | 6 | 100 | 92 | [43] |
| Ar-SBA-15 | 75 | 8 + VLE | 5 | 4 | 4 | 100 | 92 | [44] |
| Zeolite β + Nd+3 | 70 | 15 | 6 | 3 | 2 | 93 | 75 | [45] |
| Zeolite Y+1M citric acid c | 80 | Autoge | 1 | 4 | 5 | 82 | 57 | [46] |
| Zeolite β-MwS(1.4) | 75 | 10 + VLE | 0.5 g | 4 | 48 | 100 | 90 | [47,48] |
| HMS(dda)-S | 75 | 10 + VLE | 0.5 g | 4 | 24 | 100 | 84 | [49] |
| MwS-AG | 75 | 10 + VLE | 0.5 g | 4 | 24 | 99 | 75 | [50] |
| 730SS1 | 70 | Autoge | 7.5 | 3 | 17 | 100 | 89 | [51] |
| Hyflon®/ES70Y | 70 | Autoge | 7.5 | 3 | 17 | 100 | 93 | [52] |
| Sulfonated peanut shell | 70 | 15 | 6 | 4 | 2 | 100 | 92 | [54] |
| SG | 70 | 10 | 2 | 6 | 7 | 99 | 96 | [55] |
| HSiW·20H2O, in 0.7% C19 | 80 | Auto | 5 | 3 | 5 | 99 | 90 | [56] |
| Catalyst a | Reaction Conditions | XG (mol %) | Sh-GTBE (mol %) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | P b (bar) | Cat. Loading (wt.% of G) | TBA/G Mol/mol | Time (h) | ||||
| A-15-dry | 60 | Auto | 7.5 | 4 | 8 | 79 | 19 | [39] |
| Hierarchical-Beta zeolite | 75 | Auto | 5 | 4 | 24 | 77 | 35 | [63] |
| A-35-dry | 90 | Auto | 7.5 | 4 | 8 | 69 | 24 | [64] |
| A-70 | 190 | Auto | 7.5–10 | 2 | 8 | 60 | 37 * | [65] |
| A-15 | 70 | 1 | 7.5 | 4 | 6 | 94 | 30 | [66] |
| H730/ES70Y | 80 | Auto | 7.5 | 8 | 27 | 100 | 70 | [67] |
| SCC-S | 120 | Auto | 5 | 4 | 7 | 81 | 21 | [69] |
| TC-L | 120 | Auto | 5 | 4 | 8 | 53 | 25 | [70] |
| BCC-S5h | 120 | Auto | 5 | 4 | 5 | 70 | 29 | [71] |
| BC 1:3 | 120 | Auto | 5 | 4 | 6 | 75 | 29 | [72] |
| A-15 | 60 | Auto | 7.5 | 4 | 8 | 80 | 20 | [73,74] |
| Mont-KSF/O | 110 | Auto | 27 | 20 | 6 | ~100 | ~30 | [75] |
| AS-100 | 120 | Auto | 5 | 4 | 5 | 100 | 40 | [76] |
| 20C1TS | 100 | Auto | 27 | 6 | 1 | 91 | 44 | [77] |
| FHB | 75 | Auto | 5 | 4 | 24 | 75 | 37 | [78] |
| MFI-UL-100 | 120 | Auto | 5 | 4 | 12 | 82 | 24 | [79] |
| M-HY | 85 c | Auto | 5 | 4 | 15 min | 59 | 22 | [80] |
| BEANC15 | 90 | Auto | 7.5 | 4 | 10 | 57 | 29 | [81] |
| USY-650-L-2 | 90 | Auto | 7.6 | 4 | 4 | 75 | 21 | [82] |
| C(10)AlPO(1.5)-250 | 85 | Auto | 5 | 4 | 15 min | 83 | 25 | [84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, R.; Aguado-Deblas, L.; Luna, D.; Bautista, F.M. An Overview of the Production of Oxygenated Fuel Additives by Glycerol Etherification, Either with Isobutene or tert-Butyl Alcohol, over Heterogeneous Catalysts. Energies 2019, 12, 2364. https://doi.org/10.3390/en12122364
Estevez R, Aguado-Deblas L, Luna D, Bautista FM. An Overview of the Production of Oxygenated Fuel Additives by Glycerol Etherification, Either with Isobutene or tert-Butyl Alcohol, over Heterogeneous Catalysts. Energies. 2019; 12(12):2364. https://doi.org/10.3390/en12122364
Chicago/Turabian StyleEstevez, Rafael, Laura Aguado-Deblas, Diego Luna, and Felipa M. Bautista. 2019. "An Overview of the Production of Oxygenated Fuel Additives by Glycerol Etherification, Either with Isobutene or tert-Butyl Alcohol, over Heterogeneous Catalysts" Energies 12, no. 12: 2364. https://doi.org/10.3390/en12122364
APA StyleEstevez, R., Aguado-Deblas, L., Luna, D., & Bautista, F. M. (2019). An Overview of the Production of Oxygenated Fuel Additives by Glycerol Etherification, Either with Isobutene or tert-Butyl Alcohol, over Heterogeneous Catalysts. Energies, 12(12), 2364. https://doi.org/10.3390/en12122364








