Abstract
Several methods for chemical energy storage have been discussed recently in the context of fluctuating energy sources, such as wind and solar energy conversion. Here a compression–expansion process, as also used in piston engines or compressors, is investigated to evaluate its potential for the conversion of mechanical energy to chemical energy, or more correctly, exergy. A thermodynamically limiting adiabatic compression–chemical equilibration–expansion cycle is modeled and optimized for the amount of stored energy with realistic parameter bounds of initial temperature, pressure, compression ratio and composition. As an example of the method, initial mixture compositions of methane, ethane, hydrogen and argon are optimized and the results discussed. In addition to the stored exergy, the main products (acetylene, benzene, and hydrogen) and exergetic losses of this thermodynamically limiting cycle are also analyzed, and the volumetric and specific work are discussed as objective functions. It was found that the optimal mixtures are binary methane argon mixtures with high argon content. The predicted exergy losses due to chemical equilibration are generally below 10%, and the chemical exergy of the initial mixture can be increased or chemically up-converted due to the work input by approximately 11% in such a thermodynamically limiting process, which appears promising.
1. Introduction
There is a strong demand for energy storage currently, because fluctuating weather-dependent energy sources, such as wind and solar energy, do not meet the demands of modern society. Several energy storage methods are already known, and a number of reviews are available discussing many aspects of these methods. Some of the storage methods are well established, such as pumped hydro storage or electro-chemical batteries, as reviewed in Reference [1]. Others, such as pumped thermal energy storage (PTES) [2] or water electrolysis, where electrical energy is converted to thermal or chemical energy, are subject to intense research. Although some methods are already in use, they have well-known drawbacks. For instance, pumped hydro storage requires high lying areas suitable for storing huge amounts of water, which are often not available, or a large amount of scarce and expensive materials required for batteries. Moreover, both of these approaches also only have a moderate specific energy density. From a fundamental point of view, the specific energy stored should be much higher for chemical storage schemes compared to physical schemes; however, significant exergetic losses occur if hydrogen or hydrocarbons are used for storage. A discussion of this issue was undertaken by Schüth [3] for the five most promising chemical energy carriers (hydrogen, methane, liquid hydrocarbons, methanol and ethanol). These were mainly discussed with respect to storage density, carbon dioxide emissions and total efficiency from storage to usage. For electrochemical hydrogen production and re-conversion, a total efficiency of 30% was mentioned to be optimistic, which is also consistent with Reference [4]. For all other chemicals discussed in Reference [3], the total efficiencies are lower. The production steps, which are chemical up-conversions with work input, generally have energetic efficiencies of below 60%, which could be regarded as a realistic target. Although no single preference was identified, some alternatives appear less viable as a fuel carrier, e.g., methanol.
A holistic view of combined energy conversion and chemical conversion has been recently applied [5,6,7] for conversions which are often used for energy supply and not for storage. If energy is to be stored chemically using an endothermal reaction, isobaric flow reactors with heat supply are generally considered, e.g., Reference [8]. The energy to be stored is most often electrical energy, ideally coming from renewables, supplied by some resistive heaters to the reactants. Although the energy efficiencies of such a process may be quite high, the exergetic efficiencies generally are not as favorable, because several heat transfer processes through finite temperature differences are needed for preheating the fresh reactants. This occurs in part by the products and the remaining heat is often transferred from resistive heaters. In addition, there may be chemical reactions involved, which can be influenced by a pressure change, or which change either pressure or flow rate, due to the formation or consumption of further gaseous species. Part of this additional exergy often remains unused or is totally wasted due to the absence of expanders. Thus, the question arises, whether it may be worth considering processes that are capable of using such pressure changes. This could be in piston–cylinder systems or in combined turbo machinery. The former will be addressed here from a fundamental point of view, because many questions arise about how to choose favorable conditions and whether the concept is favorable at all.
For hydrocarbon conversion, there may be several aims, either to produce more valuable chemicals, or, recently, decarburization of methane to produce hydrogen and carbon [9]. With respect to the methods for methane or hydrocarbon conversion, catalytic routes have mainly been investigated [10,11], which can have the important advantage of being very selective, but also have the disadvantages of catalyst degradation and relatively high costs. Recently, plasma-assisted methods have mainly been investigated for methane conversion mainly for chemical applications, as reviewed in Reference [12]. This seems to be promising at the laboratory scale, but has not yet been established in industry. Therefore, for the conversion of simpler chemicals, direct routes at higher temperatures may be worth re-considering. Most interestingly, reactants to be converted are relatively simple gases of high abundance, like methane or ethane, which are components of natural gas and biogas, and thus will be available for some time into the future. Thermodynamic investigations of methane pyrolysis are available as a function of temperature and pressure [13,14], which will be used as a starting point here. Experimentally, a methane conversion between 9.3% and 37% was found at temperatures between 1300 and 1400 °C at ambient pressure and with short residence times of 100 ms [15]. In previous work [13,15] it was shown for methane/hydrogen mixtures of different ratios that only a few product species are important in a homogeneous gas phase equilibrium, in addition to the starting compounds; acetylene, at higher temperatures, and ethylene and benzene at intermediate temperatures, are formed in mole fractions of at least a percent, always together with hydrogen.
Increasing the pressure, from 0.1 to 20 bar, or the initial hydrogen content shift the conversion temperature and the relative yield of products [13]. Thus, the produced higher energy species would be hydrogen, acetylene, ethene or benzene. As is known, methane is thermodynamically unstable at higher temperatures, tending toward decomposition into solid carbon (graphite) and hydrogen, and is kinetically relatively inert, meaning that high temperatures are needed for fast conversion. As summarized in Reference [14], at temperatures above 1500 °C chemical conversion takes place on the millisecond timescale, while coke formation takes place on longer time scales and can be reduced with hydrogen addition, which has the drawback that it also reduces the total conversion at moderate temperatures. At lower temperatures, reaction times increase or catalysts are needed. Guo et al. found 48% methane conversion at 1090 K using iron embedded in silica as a catalyst; the main products were ethylene, benzene, and naphthalene [10]. Wang et al. [16] also showed that the reactor material is important for the rate and amount of carbon deposition at longer reaction times. Due to the endothermal nature of these reactions, relative to the initial mixture at room temperature, an energetic upgrading takes place. This was not investigated in the previous study, but is carried out here. Also, in addition to methane, natural gas contains a considerable amount of ethane, and one may wonder whether a thermal conversion to ethylene or acetylene may be a good alternative to methane conversion, which may take place at lower temperatures. This will also be considered here; due to the lower stability of ethane compared to methane, the following reactions could take place:
Ethane pyrolysis has been investigated in many kinetic studies. Tranter et al. found in shock-tube experiments that the ethylene yield at very high pressures (up to 1000 bar) has a maximum of nearly 70% at temperatures around 1000 °C, while acetylene dominated at higher temperatures [17]. Pratt and Rogers found up to 13% ethylene from ethane at 800 K and 0.8 bar [18]. Using nickel catalysts at 750 °C, Lee and Li found that the ethylene to methane yields from ethane were between 50% and 80%, depending on the flow rate [19].
In recent years, we investigated the possibility of using either gas-turbine cycles or compression–expansion engine cycles for polygeneration of work, heat and useful chemicals like synthesis gas (CO/H2) [20,21,22,23]. It was found that such a process can be very advantageous in partial oxidation of small hydrocarbons like methane. The exergetic efficiencies are above 80% if only the engine or the conversion cycle is considered, or above 60% if gas separation and recycling is also included. These processes, where the engine can also be considered as a chemical reactor, were investigated theoretically [22] but also validated experimentally in internal combustion (IC) engines [20] and in rapid compression machines (RCM) [21]. Mathematical optimization can find suitable operating conditions, as shown in Reference [24]. One advantage of compression–expansion cycles is that the temperature can be increased rapidly in the compression stroke with work addition, initiating chemical conversion. The reactions can be stopped quickly in the expansion, due to the rapid temperature drop, and work is transferred from the system in these partial oxidation systems. Thus, due to the temperature drop, chemical species formed at high temperature are conserved, at least partially, to the end and can be exhausted for further usage. In total, in these systems, net work was transferred from the system, together with useful chemicals. In addition, compared to steady state flow processes, the temperature will rise only for a small period and wall materials can persist such harsh conditions much longer than in continuous processes. The maximum gas phase combustion temperatures in piston engines can easily exceed 2500 °C without destroying the engine, while in gas turbine processes, the temperatures are generally restricted to values below 1100 °C. Therefore, processes at high maximum temperatures can be considered to be realistic in piston–cylinder systems.
The question arises, therefore, whether an inverse process for chemical up-conversion with work input may also be reasonable and practical. An inverse process refers to a compression–expansion cycle in which net work is added to the system, leading to some increase of the chemical exergy content of the reaction mixture, which can then be stored. This mixture could then either be used energetically, as an upgraded fuel, or as base chemicals produced with a minimum amount of electrical energy. Electrical energy would be used to drive an electrical motor, which in turn would drive the compression of a gas mixture in a cylinder by a piston. A preliminary investigation for the conversion of methane [25] showed promising results from kinetic and thermodynamic calculations. With diluted mixtures of methane in Ar, with initial mole fractions of 1–15% and a compression ratio of 22, typical exergetic efficiencies between 85% and 95% were predicted. At the engine speeds and initial pressures investigated, a storage power of 25–32 kW was calculated for a four-cylinder compressor. This corresponds to a volumetric up-conversion of 800 kJ/m3 at 10 bar initial pressure. The accompanying RCM experiments confirmed the conversion and showed the same trends but, quantitatively, fuel conversion was lower than predicted theoretically, probably due to the long reaction times and heat losses.
This previous investigation lead to the question of appropriate conditions for given initial reactants in order to store energy in their conversion during such compression-ignition cycles. Also, the question arose whether such a process has any advantage compared to an isobaric pyrolysis process in a flow reactor with electrical heating, which could alternatively be used with the same products produced after energy addition. This will be addressed briefly, initially from thermodynamic and practical reasoning, before coming to the compression-expansion process and its appropriate choice of parameters.
Even with a single reactant, the choice of appropriate conditions is not simple due to the coupling of a complex thermal equilibrium with the physical compression–expansion processes. This becomes even more complicated if mixtures of several compounds are used; the choice of favorable compositions is not straightforward. A systematic approach is proposed here, including mathematical optimization. The conversion problem will be addressed in the present work from a thermodynamic point of view, in order to evaluate the limiting conversion efficiencies, and stored energy per mass of gas mixture or per volume, as will be discussed later. As a thermodynamically limiting cycle, a reversible adiabatic compression followed by a chemical equilibration and an expansion of the not further reacting (‘chemically frozen’) mixture is analyzed. The initial conditions can, in principle, be varied systematically, but the dimensionality of the problem is high, because the initial mixture composition, initial temperature, initial pressure and compression ratio all influence the process. In addition, the initial mixture properties not only influence the stored energy due to their chemical properties, but also due to their physical properties, because the temperature reached after compression depends on the temperature-dependent heat capacity of the mixture. Therefore, conditions are optimized mathematically and analyzed in the parameter regime, near the found optima. This procedure is significantly more time effective than simulating the kinetics in an engine, probably with several zones and heat transfer. Moreover, it yields important insights into promising processes and conditions that may be worth investigating with more complicated and time-consuming methods, or even experimentally. The procedure is applied here to relatively simple binary mixtures or more complex mixtures with methane, ethane, hydrogen and argon.
2. Model
First, a model for an idealized steady-state steady-flow process is described, which will be discussed for comparison with the compression–equilibration–expansion process model, described next. Then, the thermodynamic calculation methods and exergy calculations are briefly introduced and, finally, the mathematical process optimization procedure is described.
2.1. Steady-State (Flow) Process
For the subsequent comparison with the compression–equilibration–expansion process, first, an isobaric steady-state steady-flow process is analyzed. If a chemical such as methane is to be converted to an exergetically more valuable gas mixture, which is storable, it seems appropriate that the initial reactant, as well as the products, should finally be present at ambient temperature in states 0 and 3, as depicted in Figure 1. Therefore, for a first evaluation, the changes in enthalpy and entropy between these states will be considered. The initial mixture at ambient conditions must be heated to state 1. This can occur due to external heating (q01) combined with internal heat transfer (q21). If resistive heaters are used for external heating, the amount of work added (w01) equals the needed heat q01. Alternatively, one could also think about a reversible process with work addition. After reaching the high temperature T1, the mixture reacts quickly and reaches chemical equilibrium at this temperature (state 2). Then it is cooled down (q23 and q21) sufficiently quickly to ambient temperature (state 3) that this mixture does not change its composition. This process could allow the storage of energy in a flow reactor. It also provides an impression of the temperatures needed to observe high conversion in equilibrium, and the amount of storable exergy or work can be calculated.
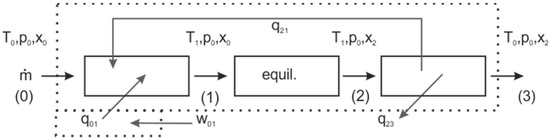
Figure 1.
Sketch of the calculation as a flow process; step-by-step within the dotted control boundary and globally, analyzing the flows passing the control boundary.
This is an isobaric open system with a constant steady-state mass flow rate of the reactant mixture and several energy transfer processes. For each step the first and second law of thermodynamics is formulated. Except for a resistive heater, which may be used, and the completely reversible overall cycle, all steps are pure heat transfer processes. The balances for energy and entropy for the overall cycle with work addition are given in Equations (6) and (7), respectively:
where q and w are the heat and work transferred to the system per mass of mixture, and hi is the specific enthalpy of state i. The European sign convention for heat and work is used, and transfer to the system is positive. Tm is the thermodynamic mean temperature for a process and sirr is the irreversibly produced entropy per mass. If the complete process (from state 0 to 3) is taken to be the system and if alternatively a reversible process is assumed, then two heat flows are transferred across the system boundary, one at high temperature, here q01, and one to the surrounding q23. The balances are given in Equations (8) and (9).
In case of equilibration at constant temperature and pressure, the composition will change, but the mass will be unchanged, thus the specific enthalpies and specific entropies are used. As an alternative total process, one can also calculate the minimum work needed to achieve the given final products, assuming that heat is only reversibly transferred at ambient temperature; this is formulated in Equations (6) and (7); Tm,23 would then be the ambient temperature and sirr would be 0. For the process without a direct work supply, the heat qh is transferred at higher thermodynamic mean temperatures . The latter would be usually followed in (nearly) isobaric reactors; the first and second law for this case is formulated in (8) and (9), the latter for a reversible process.
Although it will not be addressed here, it should be mentioned that for high volumetric exergy storage densities, it probably would be better to end with a pressurized gas mixture. Also, the reactant (like natural gas) often is already pressurized, so that an isobaric high-pressure process may be a good alternative. Since the pressure variation would add further complexity and because it is not expected that the main results will differ considerably for different constant-pressure levels, all calculations within this part were performed for atmospheric pressure.
2.2. Three-Step Process
In order to find the limits of a chemical exergy storage process in compression–expansion engines, the process is strongly idealized as an adiabatic process between states 0 and 3; see Figure 2. It consists of a control mass with a defined initial mixture (state 0), which is compressed isentropically according to a prescribed compression ratio ε to a second volume (state 1):
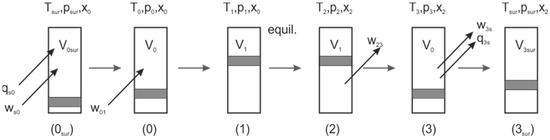
Figure 2.
Sketch of the modelled three-step process (0–3), with a possible fourth step for physical equilibration with the environment.
It may be worth mentioning that these isentropic processes are not calculated with the well-known equation for isentropic processes of gases with constant heat capacity, but with temperature-dependent heat capacities, and thus, entropies. This can be carried out within the Python module cantera [26] (as explained later) by setting the new state of a gas mixture, by explicitly setting the entropy to the value of the state, here 1, to be equal to the entropy of the former state, here 0, and the new volume is specified. To reach this state, work must be added to the system, which can be calculated per mass of fluid according to the first law of thermodynamics for an adiabatic system from the difference in specific internal energies:
Then, at v2 the temperature is relatively high and the gas mixture is equilibrated chemically at constant volume and constant internal energy (u2 = u1), yielding state 2. Depending on the mixture, this may be an exothermal or an endothermal reaction; here, for exergy storage, only endothermally reacting mixtures are considered. In a final step, the gas mixture is expanded without change in the composition of the initial volume, again adiabatically and reversibly, and thus isentropically. The assumption here is that the gas mixture is relatively unreactive after equilibration, which is also reinforced by the temperature reduction due to the expansion, accompanied by work transfer from the system. The expansion work is thus:
The net work transferred is just the difference between the final specific internal energy and the initial specific internal energy of the control mass:
The exergy loss in this process is coupled to the entropy change in the equilibration step, which is irreversible. Due to the constant internal energy at constant volume, no work or heat is transferred:
One problem remains, namely, the final state 3 is in general not in equilibrium with the surroundings, thus some energy is either available in the final state, or sometimes needed to equilibrate the final state with the surroundings. Generally, state 3 is at a lower temperature than initially, which also holds for the pressure, but in most cases some exergy is left in state 3. If the gas is to be used at ambient conditions, a further step with heat transfer to the surroundings would be needed, as depicted in Figure 2. This issue will be neglected at first but will be discussed briefly later.
For evaluation purposes, several efficiencies can be defined. Exergies, also called availabilities, are used here. Their calculation will be explained in Section 2.3. First, the efficiency of the compression–equilibration–expansion process (0–3), here briefly indicated by cycle can be defined as:
Here the total (tot) change in exergy is compared with the net work added in compression and expansion. The difference in exergy change is just the work minus the exergy loss (el).
As a further refinement, one can include bringing the initial gas mixture from ambient temperature and pressure (0sur) to the desired initial state 0 at higher temperature and pressure. This can be achieved reversibly or irreversibly. If this is done reversibly, the (physical) exergy difference between state 0sur and state 0 is the minimum amount of work that must be added, and the remaining difference in internal energy can be transferred as heat from the surroundings. Since state 0sur is in physical equilibrium with the surroundings, its physical exergy is zero and only the physical exergy of state 0 enters the definition. If the physical exergy in the final state 3 is not used, a second efficiency can be defined indicated with rev:
Finally, in the worst case, if state 0 is reached irreversibly (subscript: irr), the whole difference in internal energy could be added as work and a third limiting efficiency can be defined as:
2.3. Thermodynamic Evaluation
The thermo–chemical model was programmed in Python using the cantera module [26] to describe the thermochemistry. An ideal gas mixture was assumed and 9 species containing hydrogen, carbon or argon were considered for the equilibrium calculation. These include the following species: CH4, C2H6, C2H4, C2H2, C3H8, C3H6, C3H4, C6H6, H2, and Ar. The inclusion of more possible product molecules did not influence the final composition in equilibrium significantly, in agreement with Reference [13]. The thermodynamic data from the NASA thermochemical tables within cantera were used, including accurate temperature-dependent data for the relevant temperature regime (generally between 200 and 6000 K). With cantera, the thermodynamic properties for all mixtures were evaluated, and equilibrium compositions were calculated using the equilibrate function, either at constant temperature and pressure, or at constant internal energy and volume, depending on the process considered, as explained below.
For a compression–equilibration–expansion process in a piston–cylinder system, the physical exergies e of a state i in a given control mass were calculated as:
where u, s, v, h, T and p have the usual meanings, namely, internal energy, entropy, volume, enthalpy, temperature and pressure, respectively, with the first four being mass-specific values. The subscript sur indicates the value of the surroundings.
For the flow process, discussed initially for comparison, the exergy is calculated as:
Chemical exergies were calculated for a mixture i after first calculating the physical exergy for a gas mixture at ambient temperature and pressure. To calculate the reversible work while equilibrating the mixture with the surrounding atmosphere, without changing the composition of the atmosphere, the following calculation is carried out. The temperature and composition of the mixture are set to ambient conditions and is equilibrated chemically, indicated as eq, with surrounding air in the ratio 1: f with f = 106 at constant Tsur, psur. The surrounding air consists of O2, N2, Ar, CO2, H2O in the molar ratio 0.209421: 0.78084: 0.00934: 0.0003: 0.013 at 298.15 K and 1.013 bar. The large dilution does not change the final air composition noticeably, and the difference in enthalpy and entropy between the separated mixtures and the equilibrated mixture can be used for chemical exergy calculations:
The resulting chemical exergies are in very good agreement (<0.1%) with values from the literature, as checked for several substances and mixtures [27,28]. According to the Gouy–Stodola theorem, the exergy loss is calculated simply by:
2.4. Optimization
The optimization of the work output was performed using the differential evolution algorithm [29], which has the advantage that bounds for the parameters can easily be set. Also, as a stochastic direct search method, the algorithm runs smoothly when there are discontinuities, where gradient-based algorithms often fail. The implementation within the scipy optimize package was used here [30]. For optimization, several objective functions seem reasonable and were considered initially: the specific work w, the work per volume wv and the work per initial fuel mass fraction. The latter becomes ambiguous when different fuel mixtures are examined, and thus was no longer followed for such cases. Optimizing the specific work can easily lead to very low densities of the mixture and thus to very large engines. A promising objective function is volumetric work; having a given engine in mind, running with a specified speed, this leads to a maximum of stored energy. The specific work is calculated per mass of the total mixture and the volumetric work as the work per volume of the initial volume in state 0.
The bounds of the variable parameters were set mainly to realistic values and are given with the results. As variables, the initial temperature, initial pressure, the compression ratio and the initial composition for the pool of specified compounds were selected. Initial compositions were given as mole fractions, which, from a chemical viewpoint, is most intuitive:
However, because the total number of moles changes in a chemical reaction, while the mass in the control mass is conserved, products are given in mass fractions:
3. Criterion for Up-Conversion Assessment
If a certain amount of exergy is stored chemically in a mixture, beyond the exergetic efficiency, a further criterion for assessment is considered: the relative exergy increase of the mixture e3 compared to the exergy of the initial mixture e0. If this was, for example, 1%, one would rather exploit efficiency improvements of the usage process of the initial mixture, instead of using an additional process for energy storage. Often an energy usage is regarded as sustainable, when the energy return on energy invested (EROI) of 3:1 is exceeded, as proposed in Reference [31]. If the primary energy came from windmills with EROIs of typically more than 15, and often exceeding 30 as given in [32], than a chemical exergy increase of 10%, driven by high-EROI windmills, would exceed the sustainability limit, even for full-time operation in exergy-storage mode. If this were reduced to part-time mode, even lower exergy gains of the initial mixture appear acceptable. For the following, exergy gains above 10% are assessed to be promising, beneath high exergetic efficiencies.
4. Results and Discussion
In the following, first a general isobaric steady-state steady-flow process with pure methane will be discussed, before the proposed compression–equilibration–expansion process for chemical up-conversion will be addressed. Methane–hydrogen, ethane–hydrogen and methane–argon mixtures are investigated first, before the pressure influence is discussed. Argon is mainly investigated because the heat capacity is low, leading to high temperatures after compression.
4.1. Storage in an Isobaric Stead-State Flow Process
Chemical conversions usually take place in nearly isobaric systems [8]. Therefore, before discussing the optimization results for piston–cylinder systems, it may be worth re-considering a few basics, with respect to the expected products, energy changes and needed temperatures for isobaric methane conversion, as well as typical temperatures that can be obtained after compressing pure methane. First, the expected conversion of pure methane, in the process depicted in Figure 1, is shown in Figure 3 as a function of the temperature in state 1. These calculations were carried out for atmospheric pressure. It is recognized that methane conversion is noticeable at temperatures slightly below 1000 K, first leading to benzene (C6H6). At higher temperatures acetylene (C2H2) is mainly formed, while ethylene remains a minor product at temperatures around 1500 K. Ethane mass fractions remain negligible throughout, and this also holds for the other possible products. With increasing methane conversion, the mass fraction of hydrogen increases. To obtain high methane conversion, temperatures between 1500 and 2500 K are needed.
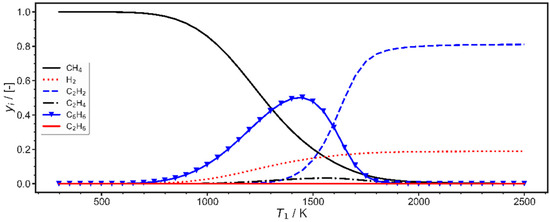
Figure 3.
Temperature dependence of methane conversion; mass fractions in equilibrium at p = 1 bar.
Since the process is investigated for energy storage purposes, the difference in energy between the initial state 0 at ambient temperature (298.15 K, 1.013 bar) and the final state is of interest. The final state used is state 3, which would involve modeling a quenching of the equilibrated state 2 without further change in composition to ambient conditions. Thus, the frozen composition of the gas mixture obtained at high temperature can be stored at ambient conditions. The difference in enthalpies is evaluated, as a measure of the net energy, which must be added to reach state 3. The enthalpy differences are plotted as a function of equilibration temperature T1 together with the heat transfer to the surroundings at 298.15 K for a reversible process, calculated from the entropy difference between states 3 and 0, multiplied with the ambient temperature according to Equations (6) and (7) with sirr set to zero.
It is seen that a large change in specific enthalpy (up to 11.74 MJ/kg) is obtained if the equilibration takes place at temperatures above 1500–2000 K. The needed heat transfer from the surroundings is moderate. After high temperature equilibration, the heat transfer remains within 2.2 MJ/kg, below 19% of the work added. Although these values seem promising, one will probably utilize this kind of a process only if the relative change in exergy is high. Therefore, the exergy difference between the final state of state 3 and state 0 is related to the initial exergy of methane (52 MJ/kg). It is seen that an exergy increase of more than 18.3% is predicted for pure methane, which is high. However, the drawback is also seen from Figure 4b (left scale): pure methane cannot be compressed to the needed high temperatures with compression ratios used in typical engines, when the initial temperature is ambient temperature. This is due to the high (molar) heat capacity of methane and the consequently small specific heat ratio k = cp/cv. Therefore, either other mixtures with lower mean k values must be considered, as can be achieved by mixing with diatomic or monoatomic gases, or pre-heating the initial mixture to higher temperatures is required. Also, a combination of both variations together could help, but this will also influence other parameters, such as the amount of stored work or the entropy production. An additional rough estimation with constant k shows that initial temperatures of 720 K are needed to reach 2000 K when compression ratios of 25 are used. The latter would be a high value, but not impossible, e.g., for diesel engines, while 720 K may be challenging to reach. Because the heat capacity of ethane is even higher than that of methane, binary methane/ethane mixtures will also not reach high enough temperatures after compression to decompose; thus, this binary system was not further investigated.
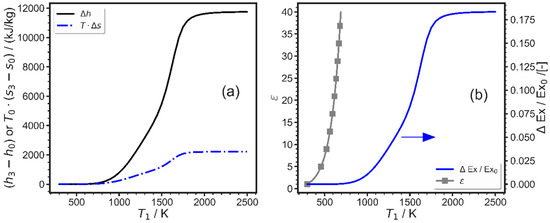
Figure 4.
Energetics of pure methane conversion. (a) The solid line is the difference in enthalpy between states 3 and 0. The dashed dotted blue line is the reversible heat transfer, when heat is only transferred at ambient temperature. (b) The blue line shows the relative increase in exergy as a function of equilibration temperature (right axis) and the grey line with the squares shows the compression ratio to obtain that temperature when starting at ambient temperature.
If the energy to be stored should be added as high temperature heat in an isobaric flow process, the needed high temperature above 1500 K is challenging for most materials. It would probably come from electrical heaters. If the energy is added reversibly at high temperatures, due to the entropy of this energy source, more energy must be provided at high temperature, compared to the work, which would have to be added directly in an alternative reversible process. To visualize this, the reversibly added heat is calculated as a function of the mean temperature of heat addition. This is shown in Figure 5 in comparison to the reversible work for the states calculated above (see Figure 4) for equilibration at 2050 K.
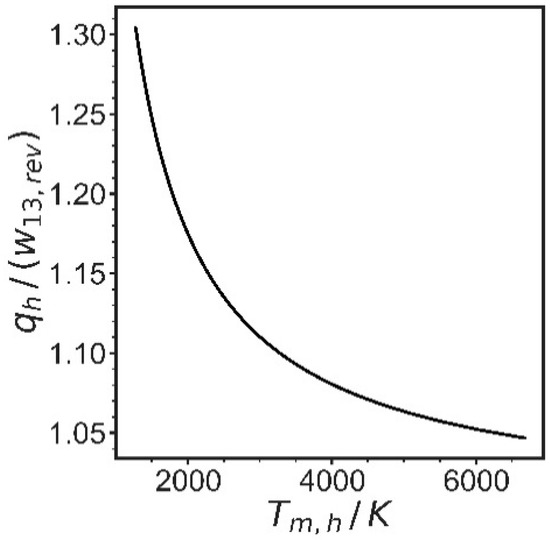
Figure 5.
The relative amount of heat added at high temperature to work addition, both reversible, as a function of the mean temperature of the heat addition. Values for h and s were used for the point at 2050 K from Figure 3.
It is seen in Figure 5 that the same final state is reached in such an internally reversible process after more than 25% additional energy is added as heat at mean temperatures of 1500 K, compared to direct work addition. Since this is the mean temperature, and the minimum temperature is ambient temperature, this means that the maximum temperature will be considerably higher, which leads to practical problems in reactor material selection. This ratio gets better with higher mean temperatures, but unrealistically high temperatures would be needed for considerable improvements. The main conclusion is that for energy addition with resistive heaters, at least 25–30% more electrical energy would be needed than for a process with direct work addition, due to heat transfer exergy losses.
4.2. Methane/Hydrogen Mixtures in Ideal Compression–Expansion Processes
To start with the alternative piston–cylinder process depicted in Figure 2, first the system of methane with hydrogen is considered. Since methane can be converted endothermally to higher hydrocarbons and hydrogen, it is expected that the hydrogen content in the initial mixture should be zero in optimization. When the pressure bounds are restricted near atmospheric pressure (between 1.013 bar and 1.1 bar), to controllable initial temperatures T0 below 473.15 K, and to compression ratios below 22, the result is as expected. The optimization yields an initial charge of pure methane, and the maximum allowed initial temperature and compression ratio, while the pressure is at its lower bound. The process is disappointing, because the stored work would be 14.8 kJ/kg, which is 0.028% of the total exergy of the initial mixture (52.1 MJ/kg). The reason is obvious from the calculation shown in Figure 4: the compression does not end at temperatures that lead to significant methane conversion; here T1 is 825 K after compression and the methane conversion is 1%. The optimum is not a real optimum, since all physical variables are at their bounds. If the bounds chosen are less stringent, the situation changes. As a next approach, a temperature bound for T0 of 773 K is allowed, which would not exceed the thermal limit for steel. In addition, a compression ratio of 35 was set as a limit, which would be possible with larger cylinder length-to-diameter ratios. Now the optimization yields an initial methane mole fraction of 5.93%. The parameters are again at their bounds, as before, and the work stored per kg of the initial mixture is now 685.6 kJ/kg or 0.7% of the exergy of the initial gas mixture. The mass fraction of methane (y = mi/mtotal) is changed from 33.3% to 22.2%, and 2228 K is reached after compression. One-third of the methane is converted, with the high amount of hydrogen limiting the maximum conversion. The main hydrocarbon products are acetylene, with a mass fraction of 7.5%, and ethylene with, 1.7%. At first sight, the high amount of hydrogen in the optimal mixture may surprise, but the reason is mainly to be found in the lower molar heat capacity of hydrogen, which leads to higher temperatures after compression. To analyze this in more detail, the process is calculated as a function of the initial methane mole fraction (in hydrogen) for different initial temperatures at this compression ratio of 35. The results are shown in Figure 6 and Figure 7. In Figure 6 it is obvious that there are two equivalence ratio regimes where the stored work is high. One is around x0(CH4) = 0.059 and the other for pure methane. At 573.15 K, the stored specific work in both regimes is comparable, while the methane conversion is less for the low mole-fraction regime. This is rationalized if the products and maximum temperatures are considered in Figure 7. The maximum temperature, reached after compression (state 1), is much higher for high hydrogen content (lower heat capacity), and the main products here are acetylene and ethylene, as mentioned. For pure methane, the temperature hardly reaches 1000 K and benzene is formed in a less endothermal reaction, compared to acetylene formation; therefore more methane must be converted for a similar amount of work stored. At an initial temperature of 773.15 K, the amount of converted methane is now much higher at low initial mole fractions. While the product distribution remains similar, some ethylene is now also formed from pure methane pyrolysis. Benzene is known to be an important precursor for soot formation [33] and the coking tendency is expected to be higher for pure methane, as was found in previous work [13,14], but short reaction times, as normal in piston engines, can avoid coke formation. Further evaluation of the surroundings of the found optimum state, which was at the temperature bounds, shows that when the composition and compression ratio is held constant and the initial temperature is varied, the stored work could be more than doubled at initial temperatures of 1000–1200 K. However, this does not appear to be a technologically realistic option; thus, other variations are investigated.
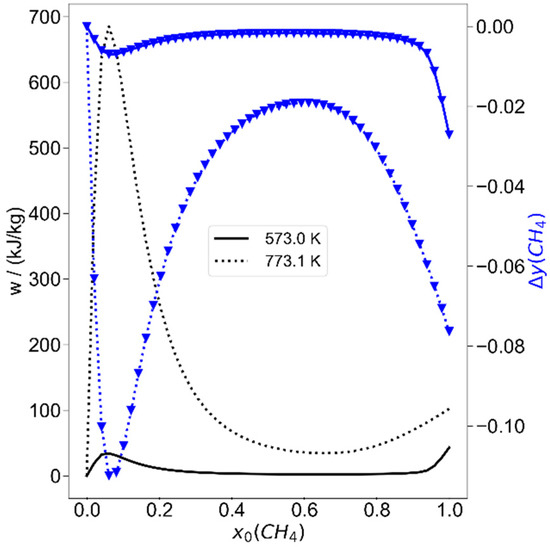
Figure 6.
The reversibly added work (black lines, left scale) and the reduction of methane mass fraction (blue lines, right scale) as a function of initial methane mole fraction for two temperatures calculated for a compression ratio of ε = 35.
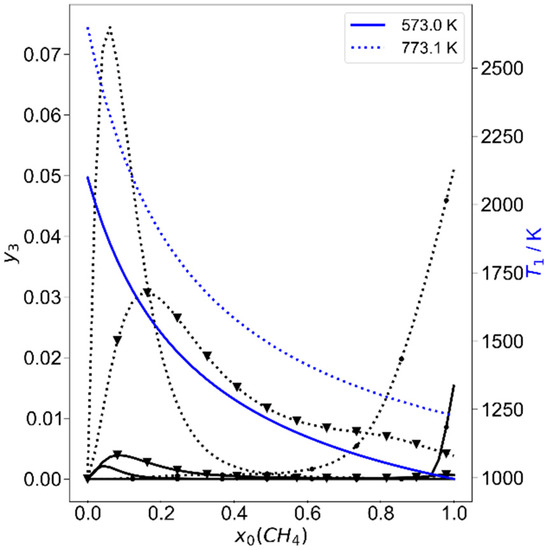
Figure 7.
Final mass fractions (state 3) of acetylene (no symbol), ethylene (triangles) and benzene (circles) as a function of initial methane mole fraction for two temperatures T0. On the right scale, the maximum temperatures after compression (state 1) are plotted in blue. All are calculated for compression ratios of ε = 35.
4.3. Ethane/Hydrogen Mixtures
Ethane is an important part of natural gas, and since it is known to be more reactive than methane, it is considered in such mixtures for exergy storage. Therefore, it seems intuitive to investigate ethane/hydrogen mixtures next. The results are shown in Figure 8 for two temperatures. They are mainly a lesson in thermodynamics: ethane has a higher molar heat capacity, thus the maximum temperatures for the same initial conditions and same mole fraction remain lower than for methane/hydrogen mixtures of the same mole fractions. Even worse is that ethane is exothermally converted to methane and acetylene, or methane and benzene at moderate temperatures. Only at very high temperatures and compression ratios can reasonably high amounts of exergy be stored. This is only shown for two initial temperatures T0 of 573.15 K and 1273.15 K and the very high compression ratio of ε = 50 in Figure 8. At the lower temperature, it is seen that there is no work added, but removed within the process; thus, work could be utilized while either acetylene and methane are produced at low initial ethane mole fractions, or benzene and methane could be produced with work transfer from the system. Only the change in methane mass fraction is shown in Figure 8 (triangles, left scale). At 1273.15 K and initial ethane mole fractions of 7.69%, 1.86 MJ/kg could be stored, meaning that the exergy would increase by 2.2% compared to the initial gas mixture. Optimizations with methane, ethane and hydrogen led to the same optimal results as with pure methane, since the optimal initial ethane mole fraction was zero throughout. The conclusion here is that small amounts of ethane will probably be acceptable within methane, as it is typical for natural gas, while large amounts of ethane or even pure ethane are not helpful for such a process. Instead, mixtures with lower heat capacities would be interesting, and this is investigated next.
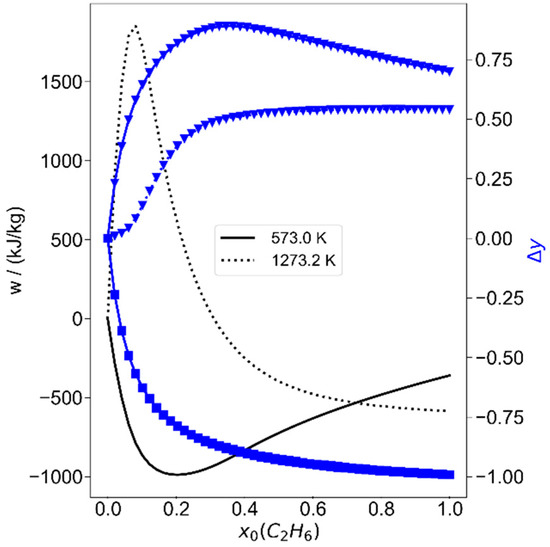
Figure 8.
Work (black lines) and changes of mass fractions as a function of initial ethane mass fraction in hydrogen, plotted for two temperatures. Left scale, blue lines: ▼ change in methane mass fraction, ■ change in ethane mass fraction. The compression ratio is ε = 50.
4.4. Methane/Argon Mixtures
Monoatomic gases are known to have the largest cp/cv ratio and the lowest molar heat capacity of all gases; this should lead to much higher temperatures after compression and increase the methane conversion. Thus, Ar-containing mixtures were investigated, as Ar is the most abundant noble gas. In our previous work, this was also investigated with respect to chemical kinetics and a process concept including gas separation [25]. With Ar addition, methane conversion is predicted to occur at lower initial temperatures and lower compression ratios. Here the lower bound for the pressure p0 was set to 1.7 bar, in order to preclude final pressures below 1 bar. The optimization again leads to initial temperatures and compression ratios at their upper bounds, here at T0 = 473.15 K and ε = 22. A total of 170.3 kJ/kg work per mass of the mixture can be stored for an optimal methane mole fraction of 5.78% (the mass fraction being y0 = 2.3%) in Ar. The exergy increase of the initial mixture is 11.4% (the ratio (e3 − e0)/e0).
If the optimization is instead carried out with the work per volume, the results are very similar, as long as the initial pressure p0 is fixed to a narrow band. The results of the specific work-optimized cycle are shown in Table 1 for all states in the cycle. The stored work per volume is 284 kJ/m3, calculated with the density of state 0, which is relevant for filling the cylinder. In addition, the properties of the initial mixture at ambient conditions (0sur) and the final mixture at ambient conditions (3sur) are also listed. The latter two have also to be considered from an energetic and exergetic point of view, because the initial conditions must be reached, and finally, the mixture will probably be used at ambient conditions. In the table, specific enthalpies, entropies and internal energies are given as a difference to the initial state in equilibrium with the surroundings (0sur). Regarding the intensive state variables, the pressure and temperature conditions at top dead center are similar to conditions in diesel engines. From the values of physical and chemical exergies, it is seen that a considerable amount of the physical exergy increase, due to work addition, is transformed into chemical exergy in the chemical equilibration step from state 1 to 2. Most of the remaining physical exergy is transferred as expansion work to reach state 3. In the adiabatic process 0–3, the specific entropy increases irreversibly by 45.7 J/(K kg) in the equilibration; the exergy loss is thus 13.7 kJ/kg. The exergetic efficiency is ηcycle = 92% and 170.2 kJ/kg work is added, while the total exergy increases by 156.6 kJ/kg. In total, this would be a very favorable standalone process. Next it is assumed that the initial increase in physical exergy from the surroundings state 0sur to state 0 would be carried out reversibly by work addition, and the final physical exergy in the final state 3, which is minor, remains unused. In this case the total efficiency, defined as exergy increase per total work added, would be ηrev = 156.6/(170.2 + 13.2) = 85.3%, which again seems acceptable. In the case that the initial energy increase is carried out irreversibly by work addition, the result, ηirr = 156.6/(170.2 + 61.4) = 67.6%, would be considerably lower, but again in the acceptable regime. However, additional losses will occur in real processes due to friction and heat transfer and in the gas separation process.

Table 1.
State variables, mass fractions and exergies for a work-optimized cycle with methane/Ar at 473 K and 1.7 bar and a compression ratio of ε = 22. The Ar mass fraction is 97.7% throughout.
The dependence of several variables on the initial methane mole fraction is shown for two temperatures in Figure 9. Nearly 90% of the initial methane is converted at the optimal conditions, reacting mainly to acetylene and hydrogen, while small amounts (y = 0.15–0.02%) of ethylene and benzene are formed. Interestingly, this optimum is neither the point with the highest methane conversion, nor that with the maximum acetylene production, although it is near the latter.
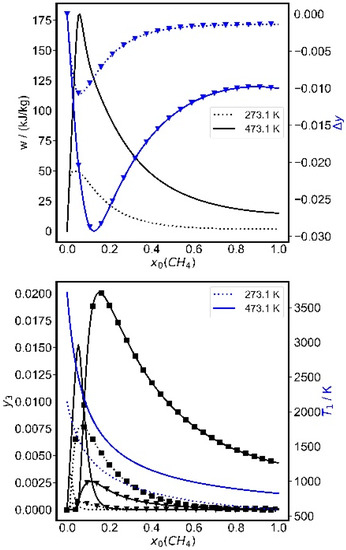
Figure 9.
Initial methane mole fraction dependence in Ar/CH4 mixtures for a compression ratio of ε = 22 and two initial temperatures. Upper graph: work per kg mixture (black lines), change in methane mass fraction (blue triangles, right scale). Lower graph: final mass fractions (black) of acetylene (lines), ethylene (triangles), benzene (squares) and the maximum temperature (state 1, blue, right scale). p0 = 1.7 bar.
The final temperature is 337 K when starting at 473.15 K, and the final pressure is 74 kPa when starting at 1 bar. The latter could be a problem because the gases would first have to be compressed before they can be exhausted; therefore the pressure bound of p0 ≥ 1.7 bar was used above. If the specific work is the objective function, the optimal pressure is generally at the lower bounds. In order to evaluate conditions with final pressures above ambient pressure, higher initial pressures are needed. For an initial pressure of 2 bar, it was found that mass fractions and the final pressure scale with pressure while the final temperatures remain unchanged. At this pressure, the stored work is reduced to 167 kJ/kg. Thus, the work is only moderately sensitive to pressure, while temperature and composition are the crucial parameters.
Another point is interesting: Upon changing the initial methane mole fraction to 15%, the product spectrum changes strongly; the acetylene yield becomes negligible, while benzene is mainly formed with minor amounts of ethylene. The reason for this strong shift in the product composition is found in the strong reduction of the maximum temperature after compression from 2400 to 1740 K at these conditions.
Due to these differences in the products, a series of initial composition optimizations were performed as a function of compression ratio and initial temperature, all for p0 = 1.7 bar, leading to final pressures of at least atmospheric pressure within the investigated parameter range. The results are summarized in Figure 10. On the left-hand side, it is seen that the largest amount of work can be stored at the highest investigated initial temperature of 673.15 K and the highest compression ratio of 35. The optimized initial methane mole fraction exceeds 10% in the upper-right corner and the specific work stored is above 400 kJ/kg. The specific chemical exergy is increased here by 12.3%. The right-hand side shows a map of the products. The mass fraction of the final product was related to the initial methane mass fraction. In the upper-right corner, m3(C2H2)/m0(CH4) is 66%, while the ratio for full stoichiometric conversion would be 81.25%, which also holds for benzene. Two peaks of benzene mass yields of more than 42 % are recognized at low compression ratios, one at low temperature, one at higher temperature. This was also our finding from kinetic modeling in our previous work [25], where benzene was found to be present in high mole fractions at moderate initial temperatures near 400 K. By selecting higher initial temperatures or higher compression ratios, acetylene can be selected as the main product. If energy storage is the only objective, high temperatures and high compression ratios are favorable; if polygeneration is aimed for, e.g., hydrogen for storage and combustion and acetylene or benzene for the chemical industry, the desired process parameters may be selected differently.
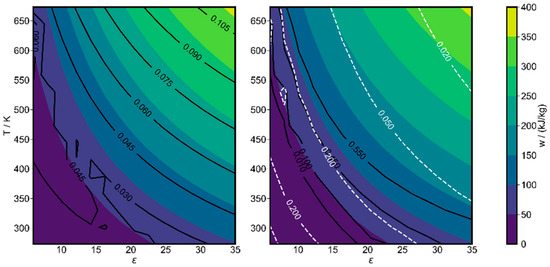
Figure 10.
Optimal work, as a function of fixed initial temperature and compression ratio at 1.7 bar initial pressure. Left figure lines: optimal initial methane mole fractions; right figure: resulting mass yield (m3,i/m0,CH4) of acetylene (black) and benzene (white), relative to initial methane mass.
4.5. Complex Mixtures
The optimization was also run with all the gases investigated so far, namely methane, ethane, hydrogen and argon. It was found that the optimal work storage throughout is without ethane and hydrogen in the mixture, for bounds set to wide ranges, that is, for temperatures between 273.15 and 673.15 K and for compression ratios between 6 and 35. Thus, addition of these compounds may be interesting for different objective functions, e.g., when benzene production shall be minimized while work is stored, but not for merely storing exergy.
4.6. Pressure Dependence for Work per Volume Optimization
If the work per mass of the mixture is used as the objective function, only a weak pressure dependence is found and the optimum always tends to the lower initial pressure bound. However, this leads to larger cylinder volumes. If, instead, the cylinder volume is fixed, the work per cylinder volume may be more relevant, even if the work per mass is (slightly) decreasing. We also saw in our previous work that the initial pressure had an important influence on the modeled power output for a fixed cylinder volume. Thus an investigation of the pressure dependence of the work per volume seemed to be interesting [25]. The composition was optimized for a range of initial temperatures and initial pressures between 1 and 10 bar for a fixed compression ratio of 22. The results are shown in Figure 11. It is seen that the stored work per volume increases with initial pressure, but not proportionally. At 473.15 K and 1 bar initial pressure, the volumetric work is 177.1 kJ/m3, while at 10 bar it is 1319 kJ/m3. The specific volume at 1 bar is 1.02 m3/kg, and at 10 bar it is 0.1014 m3/kg, also due to the difference in optimal composition. Thus, the specific work is reduced from 180.6 to 133.7 kJ/kg if the initial pressure increases from 1 to 10 bar. Therefore, the optimizations with the specific work as the objective function always end at the lower initial pressure bound. The reason for this sub-proportional dependence of the stored work on initial pressure is due to Le Chatelier’s principle. Because the reactions taking place for exergy storage lead to an increase in the number of molecules, an increase in pressure will shift the equilibrium (slightly) towards the reactants while, at the same time, the mass and exergy content in the volume is increasing. The optimized initial mole fractions vary between 3% and 8% in the parameter regime considered here and the ratio of benzene to acetylene in the products is slightly shifted towards the latter with increasing pressure.
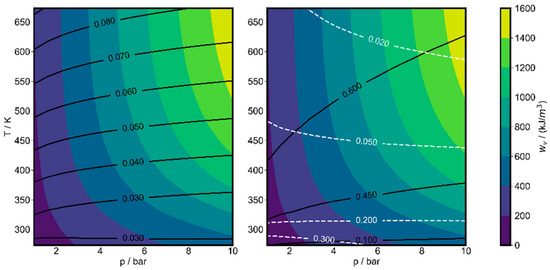
Figure 11.
Pressure dependence of optimizations of work per volume wv for methane Ar mixtures at a compression ratio of 22. Left figure lines: optimal initial methane mole fractions; right figure: resulting mass yield (m3,i/m0,CH4) of acetylene (black) and benzene (white), relative to initial methane mass.
Finally, the dependence of efficiency on pressure was investigated. For the temperature of 473.15 K in the optimization shown in Figure 11, the different efficiencies were calculated and are plotted in Figure 12.
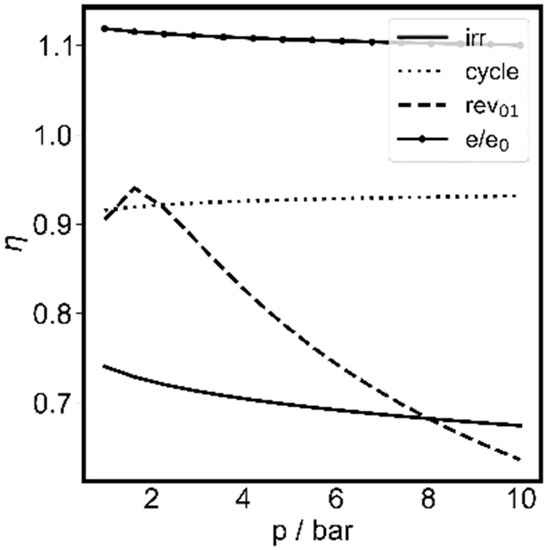
Figure 12.
The pressure dependent efficiencies for the composition optimized for volumetric work storage process at 473.15 K as a function of pressure. Three efficiencies are shown, as discussed in the text: ηirr (-), ηrev (--) and ηcycle (...), together with the relative exergy change e/e0 (●).
It is recognized that the efficiency ηcycle of the compression–equilibration–expansion process, where the total exergy difference (state 3−state 0) is compared to the net added work, is quite high throughout, with values above 90%. The efficiency ηrev starts at high values and drops at higher pressures. Here it was assumed that state 0 is reached reversibly, but the physical exergy in the final state 3 is wasted. Due to the exergy increase in state 3 with pressure, the finding is comprehensible. The third efficiency ηirr assumes that the initial state is also reached by irreversible work addition. In this case, the efficiencies vary around 70%. Therefore, it would be desirable to stay as close to the reversible process as possible, and use at least part of the energy at state 3 to heat up and pressurize the initial mixture at ambient conditions. At high process pressures, the exergy at state 3 is quite high and should not remain unused. However, in total, the losses in this limiting process appear to be reasonably low. The exergy gain e/e0 remains above 10% throughout.
It should also be mentioned that the optimization of the volumetric work input for the four compounds (methane, ethane, hydrogen and argon) ended at the same compositions, which were discussed here, meaning the optimal initial hydrogen and ethane mole fractions are zero. Again, one would add them for soot suppression or perhaps other reasons, but not for better exergy storage.
5. Summary and Conclusions
A thermodynamically limiting adiabatic compression–equilibration–expansion cycle was investigated for chemical exergy storage. Because it is a limiting cycle, all steps except for the chemical equilibration were assumed to be reversible, so the best possible outcome of such a process could be predicted as a function of process parameters. First, it was briefly shown that such a process should be intrinsically favorable compared to isobaric thermo–chemical processes for energy storage, when the heating source relies on work addition. Then, for this first thermodynamic investigation of such a process, methane and ethane were used as initial compounds together with hydrogen and argon. The first two compounds are of high abundance in natural gas and biogas. Hydrogen could be one of the energy carriers of the future, and argon or another monoatomic gas were needed to obtain high temperatures and, thus, good yields. For such a process, beyond the composition, initial temperature, volume and compression ratio also must be selected, which in this example leads to seven dimensions. This also leads to a strong coupling of physical and chemical properties, and a parameter selection from mere reasoning may lead to suboptimal results.
Therefore, mathematical optimization was applied here, which easily finds the best condition within the given bounds. The objective functions applied were the work input per mass and the work input per volume; the latter may be more appropriate for a given fixed cylinder volume. The concept can be expanded to other mixtures and other objective functions, e.g., yields of certain compounds or yields with minimum irreversibility. With different gases in the mixture, it may also be possible to find processes with a larger relative chemical exergy increase.
From the example used here, several things can be learned:
- The process is not useful for pure methane and moderately useful for methane hydrogen/mixtures, mainly due to the high temperatures needed for methane conversion and the high capacity of heat of methane.
- Ethane is exothermally converted to methane at even moderate temperatures; only at very high initial temperatures would energy storage be possible.
- Within the investigated starting species, relatively high amounts of argon or another monoatomic gas must be present to reach high conversion and a good chemical exergy storage. The addition of ethane or hydrogen will reduce the amount of stored exergy.
- In some parameter regimes, it is possible to select either acetylene or benzene as the hydrocarbon product.
- The thermodynamic losses due to chemical equilibration are small.
- It is often crucial for high efficiencies to reach the initial conditions reversibly, and at high pressures; the usage of the physical exergy in the final state is also important.
- An increase of chemical exergy of the initial mixture by more than 11% is possible under realistic conditions with reasonably high exergetic efficiencies between 67% and 92%.
The investigated process would be more complicated in practice, due to the need of gas separation for Ar recycling. Our previous investigation showed that this may be achieved with moderate losses. Also, the previously investigated system was quite near to the optimum [25], and the general approach to start with the optimized thermodynamic optimum will be crucial for more complicated mixtures. The procedure for this is now established.
Funding
The author gratefully acknowledges the generous financial support of this work by the Deutsche Forschungsgemeinschaft (DFG) within the framework of the DFG-FOR1993 research unit ‘Multifunctional conversion of species and energy’, Project GM2 (AT24/13-2), Number: 229243862.
Acknowledgments
The author thanks Sebastian Kaiser (University of Duisburg-Essen for helpful comments.
Conflicts of Interest
The author declares no conflict of interest.
Nomenclature
| Abbreviations | |
| RCM | Rapid compression machine |
| PTES | Pumped thermal energy storage |
| Greek symbols | |
| ε | compression ratio |
| η | efficiency |
| Subscripts | |
| air | air |
| ch | chemical |
| eq | equilibrium |
| i | state number |
| irr | irreversible |
| l | loss |
| phys | physical |
| rev | reversible |
| sur | surrounding |
| tot | total |
| Symbols | |
| cp, cv | specific heat capacity, isobaric, isochoric |
| e | specific exergy |
| f | factor air: mixture |
| h | specific enthalpy |
| m | mass |
| n | number of moles |
| p | pressure |
| q | heat per mass |
| s | specific entropy |
| T | temperature |
| u | specific internal energy |
| v | specific volume |
| V | volume |
| w | work per mass |
| x | mole fraction |
| y | mass fraction |
References
- Luo, X.; Wang, J.; Dooner, M.; Clarke, J. Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl. Energy 2015, 137, 511–536. [Google Scholar] [CrossRef]
- Benato, A.; Stoppato, A. Pumped Thermal Electricity Storage: A technology overview. Therm. Sci. Eng. Prog. 2018, 6, 301–315. [Google Scholar] [CrossRef]
- Schüth, F. Chemical Compounds for Energy Storage. Chem. Ing. Tech. 2011, 83, 1984–1993. [Google Scholar] [CrossRef]
- López González, E.; Isorna Llerena, F.; Silva Pérez, M.; Rosa Iglesias, F.; Guerra Macho, J. Energy evaluation of a solar hydrogen storage facility: Comparison with other electrical energy storage technologies. Int. J. Hydrog. Energy 2015, 40, 5518–5525. [Google Scholar] [CrossRef]
- Haynes, B.S. Combustion research for chemical processing. Proc. Combust. Inst. 2018, 37, 1–32. [Google Scholar] [CrossRef]
- Adams, T.A., II; Barton, P.I. A dynamic two-dimensional heterogeneous model for water gas shift reactors. Int. J. Hydrog. Energy 2009, 34, 8877–8891. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Adams, T.A., II. Integrated petroleum coke and natural gas polygeneration process with zero carbon emissions. Energy 2015, 91, 479–490. [Google Scholar] [CrossRef]
- Lu, Y.R.; Nikrityuk, P. A fixed-bed reactor for energy storage in chemicals (E2C): Proof of concept. Appl. Energy 2018, 228, 593–607. [Google Scholar] [CrossRef]
- Abánades, A.; Rubbia, C.; Salmieri, D. Technological challenges for industrial development of hydrogen production based on methane cracking. Energy 2012, 46, 359–363. [Google Scholar] [CrossRef]
- Guo, X.; Fang, G.; Li, G.; Ma, H.; Fan, H.; Yu, L.; Ma, C.; Wu, X.; Deng, D.; Wei, M.; et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 2014, 344, 616–619. [Google Scholar] [CrossRef]
- Muradov, N. Hydrogen via methane decomposition: An application for decarbonization of fossil fuels. Int. J. Hydrog. Energy 2001, 26, 1165–1175. [Google Scholar] [CrossRef]
- Scapinello, M.; Delikonstantis, E.; Stefanidis, G.D. The panorama of plasma-assisted non-oxidative methane reforming. Chem. Eng. Process. Process Intensif. 2017, 117, 120–140. [Google Scholar] [CrossRef]
- Guéret, C.; Daroux, M.; Billaud, F. Methane pyrolysis: Thermodynamics. Chem. Eng. Sci. 1997, 52, 815–827. [Google Scholar] [CrossRef]
- Holmen, A.; Olsvik, O.; Rokstad, O.A. Pyrolysis of natural gas: Chemistry and process concepts. Fuel Process. Technol. 1995, 42, 249–267. [Google Scholar] [CrossRef]
- Billaud, F.G.; Gueret, C.P.; Baronnet, F. Thermal coupling of methane in a tubular flow reactor: Experimental setup and influence of temperature. Ind. Eng. Chem. Res. 1992, 31, 2748–2753. [Google Scholar] [CrossRef]
- Wang, S.; Lee, W.J.; Li, C.E.; Kuan, B.; Burke, N.; Patel, J. The pyrolysis of natural gas: A study of carbon deposition and the suitability of reactor materials. AIChE J. 2018, 37, 915. [Google Scholar] [CrossRef]
- Tranter, R.S.; Raman, A.; Sivaramakrishnan, R.; Brezinsky, K. Ethane oxidation and pyrolysis from 5 bar to 1000 bar: Experiments and simulation. Int. J. Chem. Kinet. 2005, 37, 306–331. [Google Scholar] [CrossRef]
- Pratt, G.; Rogers, D. Wall-less reactor studies. Part 1.—Ethane pyrolysis. J. Chem. Soc. Faraday Trans. 1 1979, 75, 1089. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.-Z. Coke formation and reaction pathways of catalyst-surface-generated radicals during the pyrolysis of ethane using Ni mesh catalyst. Appl. Catal. A Gen. 2007, 316, 90–99. [Google Scholar] [CrossRef]
- Wiemann, S.; Hegner, R.; Atakan, B.; Schulz, C.; Kaiser, S.A. Combined production of power and syngas in an internal combustion engine—Experiments and simulations in SI and HCCI mode. Fuel 2018, 215, 40–45. [Google Scholar] [CrossRef]
- Hegner, R.; Werler, M.; Schießl, R.; Maas, U.; Atakan, B. Fuel-Rich HCCI Engines as Chemical Reactors for Polygeneration: A Modeling and Experimental Study on Product Species and Thermodynamics. Energy Fuels 2017, 31, 14079–14088. [Google Scholar] [CrossRef]
- Hegner, R.; Atakan, B. A polygeneration process concept for HCCI-engines—Modeling product gas purification and exergy losses. Int. J. Hydrog. Energy 2017, 42, 1287–1297. [Google Scholar] [CrossRef]
- Atakan, B. Gas turbines for polygeneration? A thermodynamic investigation of a fuel rich gas turbine cycle. Int. J. Thermodyn. 2011, 14, 185–192. [Google Scholar] [CrossRef]
- Gossler, H.; Deutschmann, O. Numerical optimization and reaction flow analysis of syngas production via partial oxidation of natural gas in internal combustion engines. Int. J. Hydrog. Energy 2015, 40, 11046–11058. [Google Scholar] [CrossRef]
- Hegner, R.; Drost, S.; Werler, M.; Schießl, R.; Maas, U.; Atakan, B. Methane pyrolysis in piston engines—A novel approach to exergy storage. In Proceedings of the 30th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, ECOS 2017, San Diego, CA, USA, 2–6 July 2017. [Google Scholar]
- Goodwin, D.G.; Moffat, H.K.; Speth, R.L. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes; Version 2.3.0; Zenodo: Geneva, Switzerland, 2017. [Google Scholar]
- Ertesvåg, I.S. Sensitivity of chemical exergy for atmospheric gases and gaseous fuels to variations in ambient conditions. Energy Convers. Manag. 2007, 48, 1983–1995. [Google Scholar] [CrossRef]
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis, Repr. with Corr. and New Appendix G.; Krieger: Malabar, FL, USA, 1995; ISBN 0894649469. [Google Scholar]
- Storn, R.; Price, K. Differential Evolution—A Simple and Efficient Heuristic for Global Optimization over Continuous Spaces. J. Glob. Optim. 1997, 11, 341–359. [Google Scholar] [CrossRef]
- Jones, E.; Oliphant, T.; Peterson, P. SciPy: Open Source Scientific Tools for Python. Available online: http://www.scipy.org/ (accessed on 15 December 2018).
- Hall, C.; Balogh, S.; Murphy, D. What is the Minimum EROI that a Sustainable Society Must Have? Energies 2009, 2, 25–47. [Google Scholar] [CrossRef]
- Kubiszewski, I.; Cleveland, C.J.; Endres, P.K. Meta-analysis of net energy return for wind power systems. Renew. Energy 2010, 35, 218–225. [Google Scholar] [CrossRef]
- McEnally, C.S.; Pfefferle, L.D.; Atakan, B.; Kohse-Höinghaus, K. Studies of aromatic hydrocarbon formation mechanisms in flames: Progress towards closing the fuel gap. Prog. Energy Combust. Sci. 2006, 32, 247–294. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).