Synthesis of Bi2O3-MnO2 Nanocomposite Electrode for Wide-Potential Window High Performance Supercapacitor
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Method of Synthesis
2.3. Fabrication of ES Electrode and Full-Cell Device Assembly
2.4. Material Characterizations
2.5. Electrochemical Measurements
3. Results and Discussion
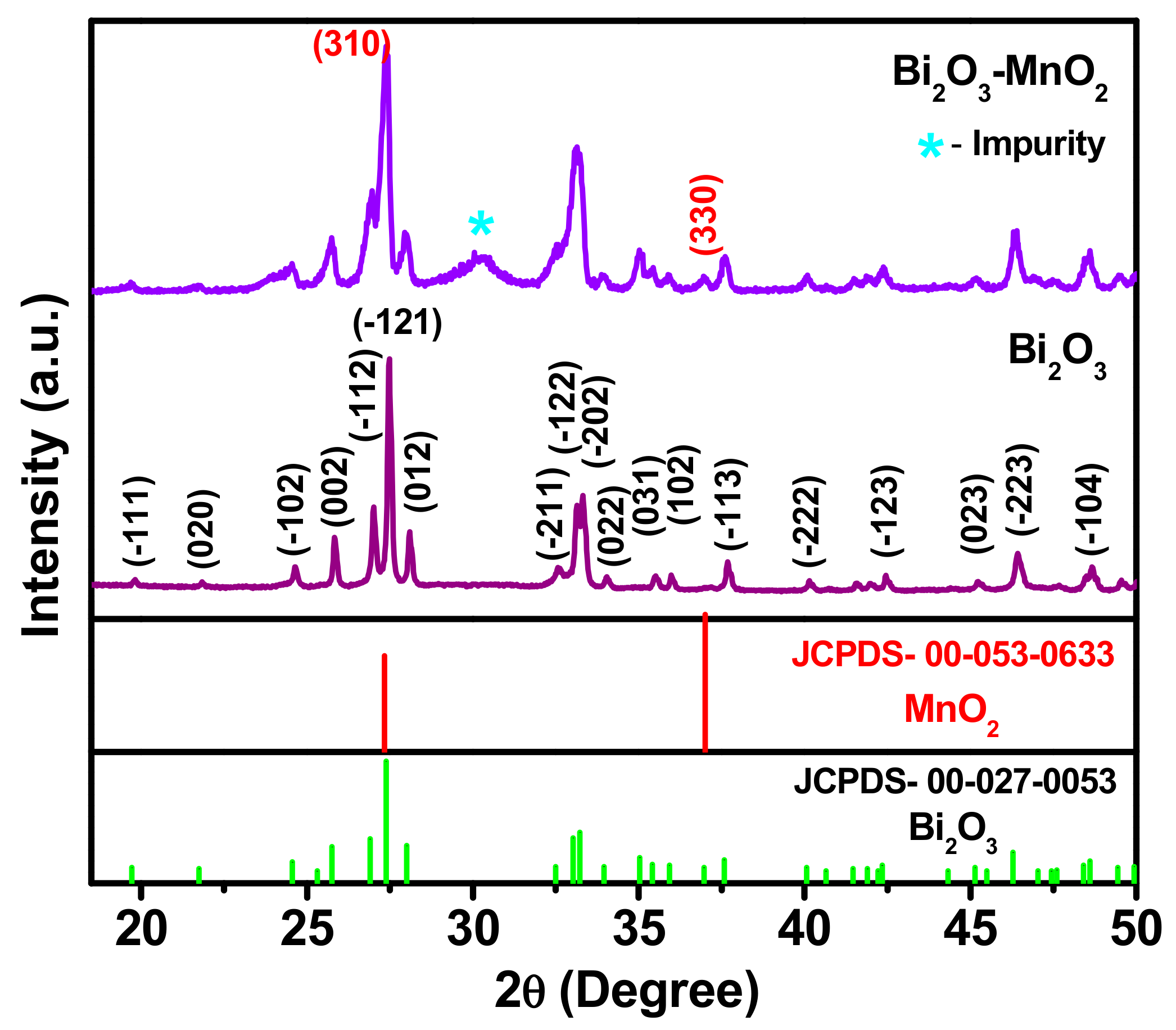
3.1. Structural Analysis
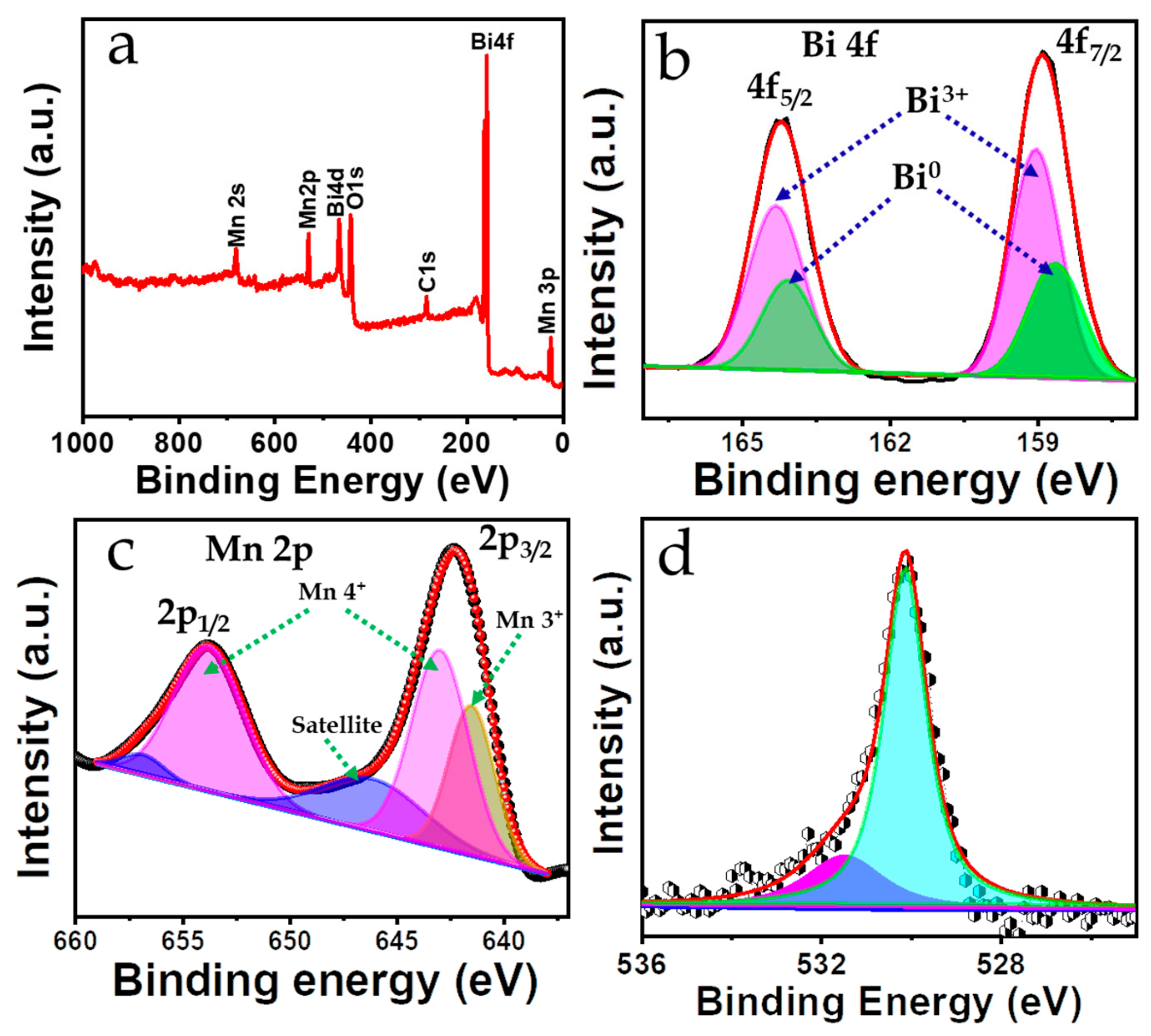
3.2. Electronic States and Chemical Composition Confirmation
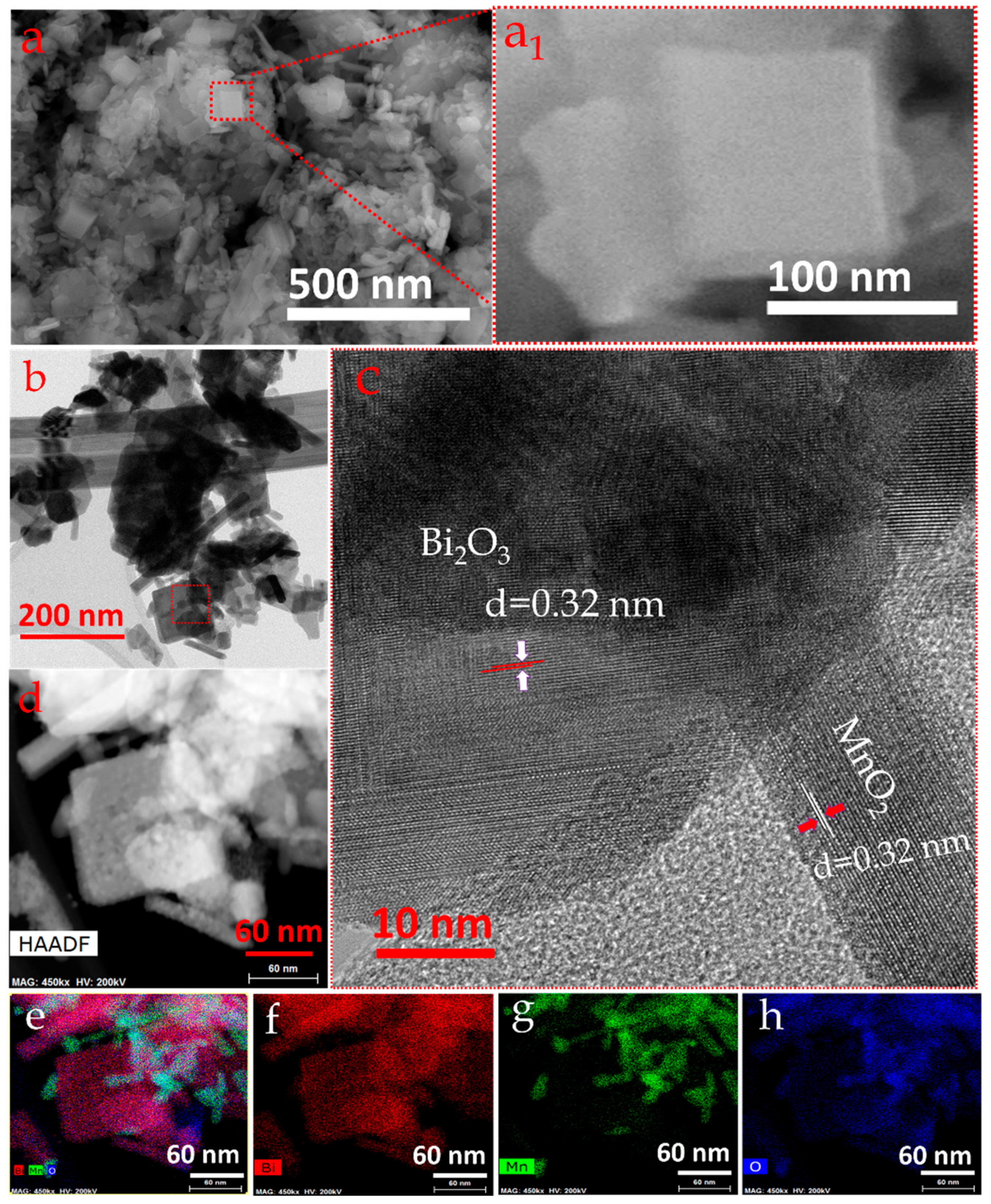
3.3. Structure–Morphology Correlation and Reaction Mechanism
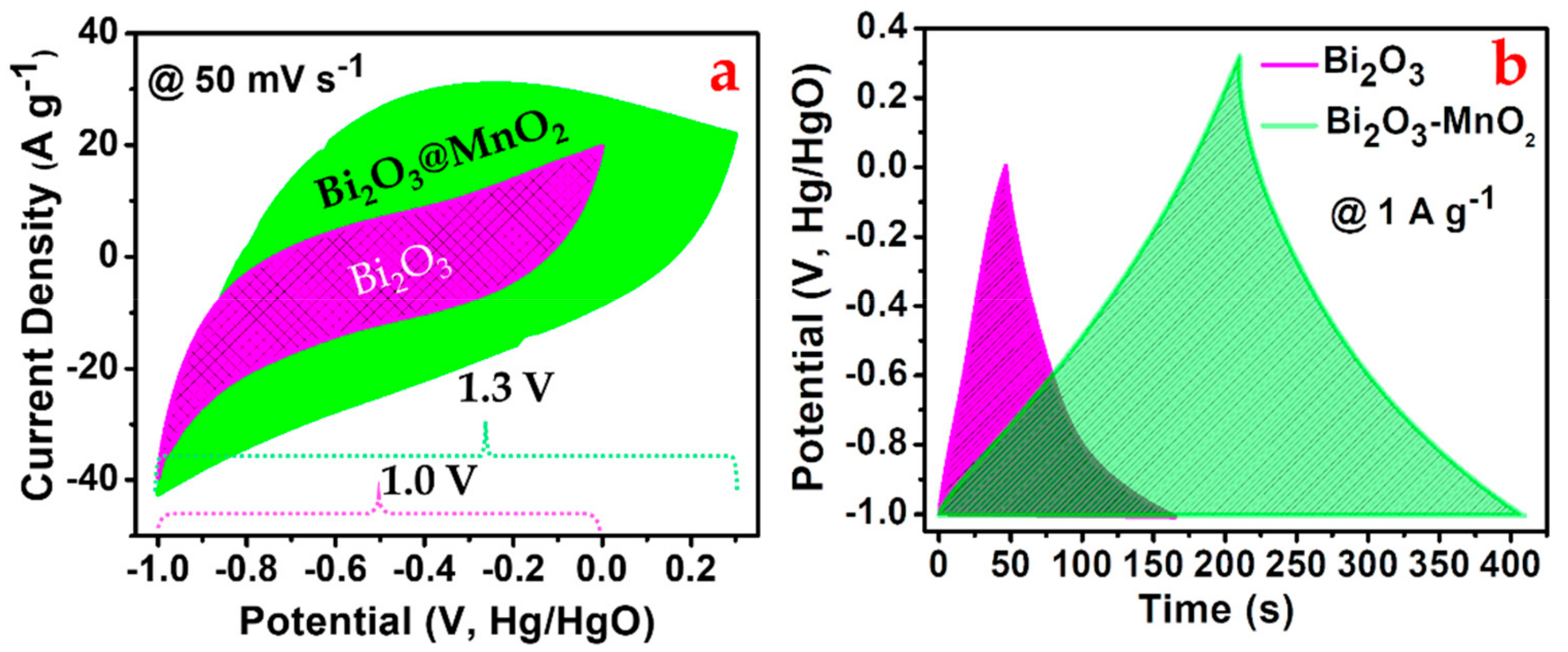
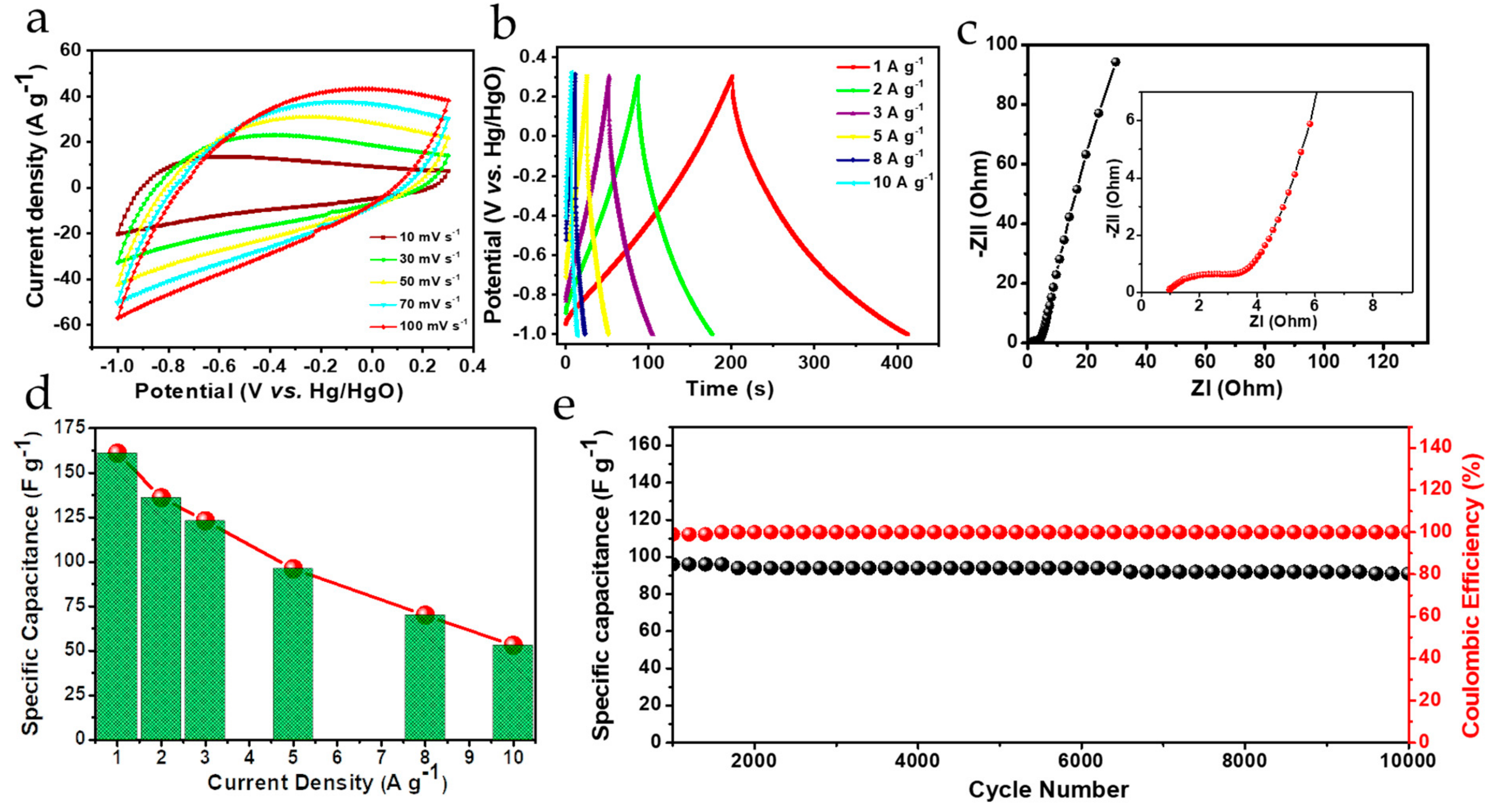
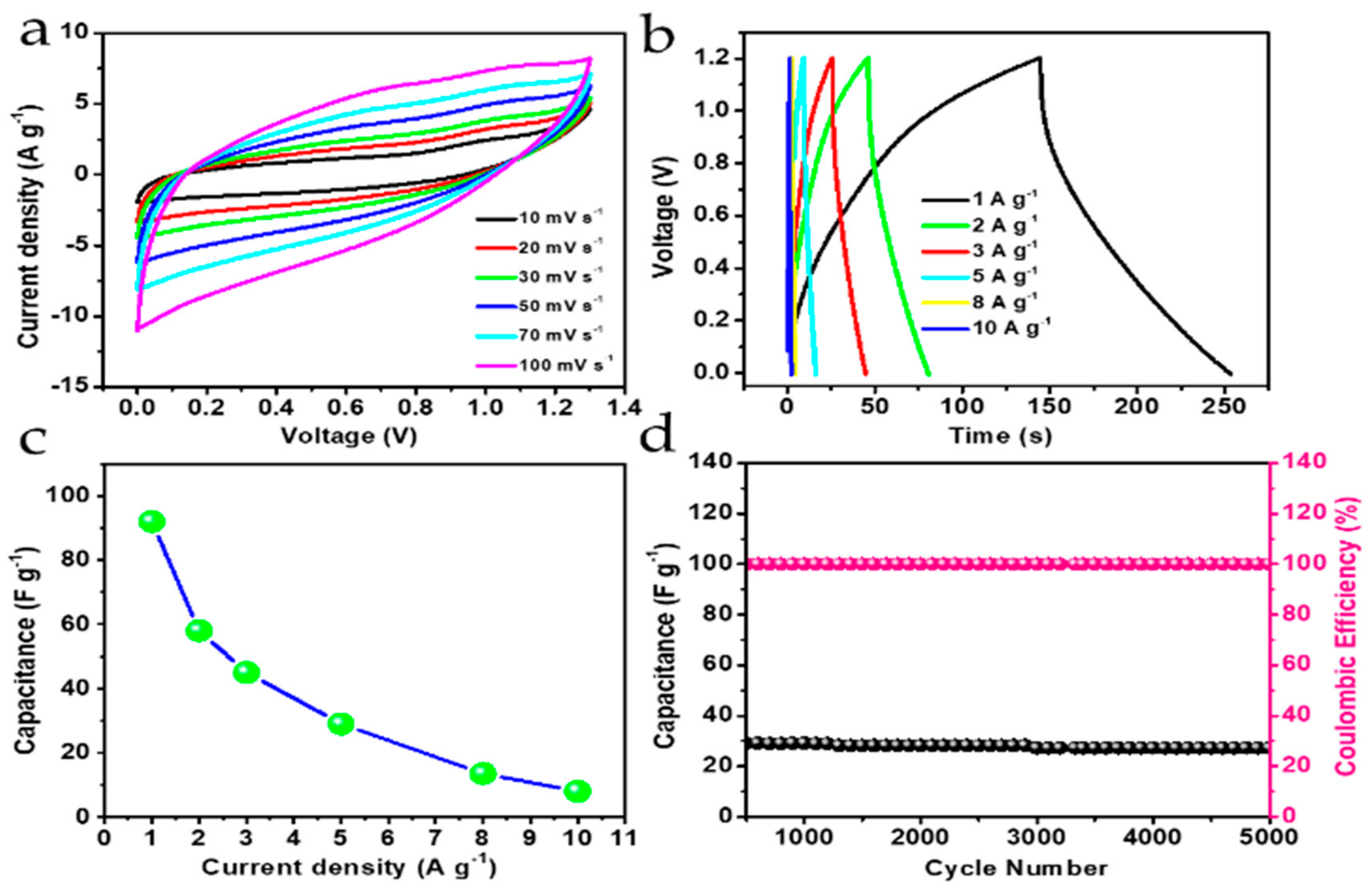
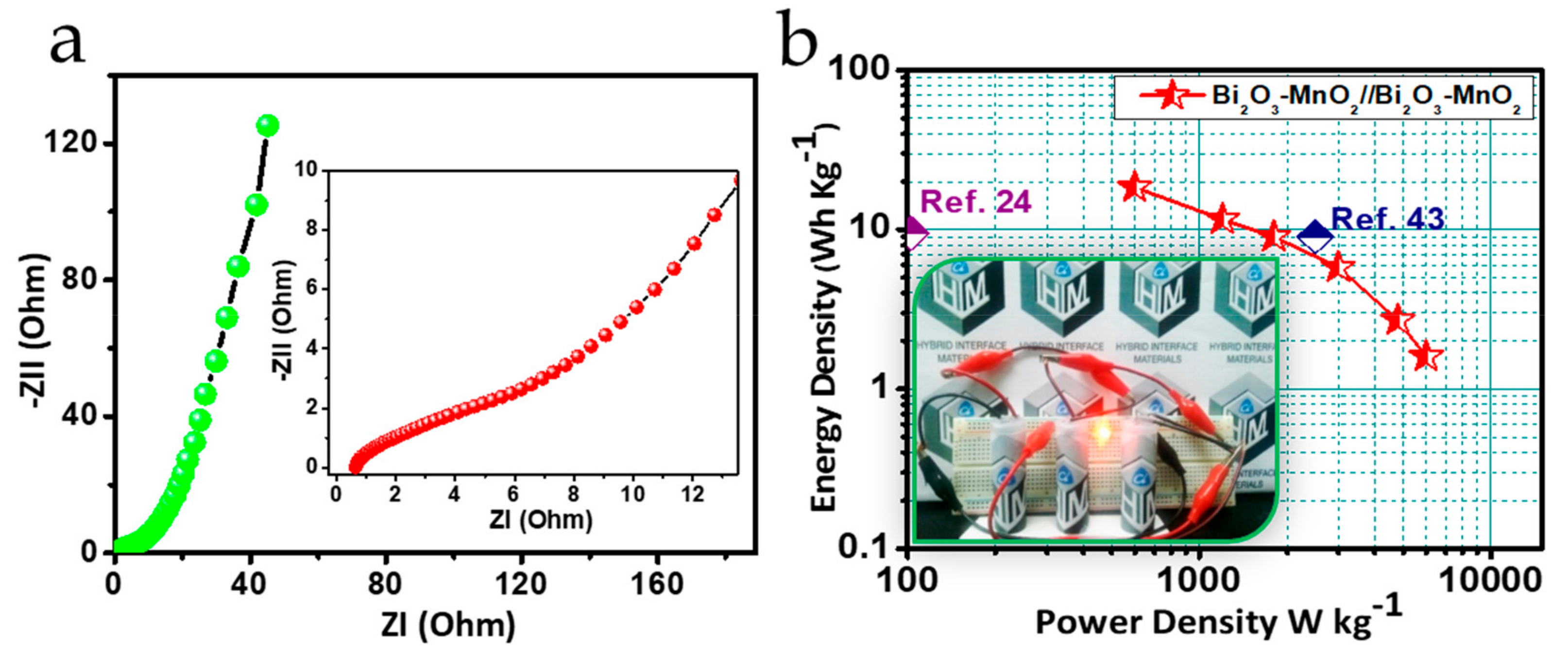
3.4. Electrochemical Performance Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hall, P.J.; Bain, E.J. Energy-storage technologies and electricity generation. Energy Policy 2008, 36, 4352–4355. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.L.; Arabatzis, G. Electrical energy storage systems in electricity generation: Energy policies, innovative technologies, and regulatory regimes. Renew. Sust. Energ. Rev. 2016, 56, 1044–1067. [Google Scholar] [CrossRef]
- Vlad, A.; Singh, N.; Rolland, J.; Melinte, S.; Ajayan, P.M.; Gohy, J.F. Hybrid supercapacitor-battery materials for fast electrochemical charge storage. Sci. Rep. 2014, 4, 4315. [Google Scholar] [CrossRef]
- Kötz, R.; Müller, S.; Bärtschi, M.; Schnyder, B.; Dietrich, P.; Büchi, F.; Tsukada, A.; Scherer, G.; Rodatz, P.; Garcia, O. Supercapacitors for peak-power demand in fuel-cell-driven cars. Electrochem. Soc. 2001, 21, 564–575. [Google Scholar]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef]
- Cherusseri, J.; Kar, K.K. Hierarchically mesoporous carbon nanopetal based electrodes for flexible supercapacitors with super-long cyclic stability. J. Mater. Chem. A 2015, 3, 21586–21598. [Google Scholar] [CrossRef]
- Aravinda, L.; Nagaraja, K.; Nagaraja, H.; Bhat, K.U.; Bhat, B.R. ZnO/carbon nanotube nanocomposite for high energy density supercapacitors. Electrochim. Acta 2013, 95, 119–124. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Zhi, L.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.; Qian, W.; Wei, F. A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef]
- Wu, H.; Xu, C.; Xu, J.; Lu, L.; Fan, Z.; Chen, X.; Song, Y.; Li, D. Enhanced supercapacitance in anodic TiO2 nanotube films by hydrogen plasma treatment. Nanotechnology 2013, 24, 455401. [Google Scholar] [CrossRef]
- Dou, S.; Tao, L.; Wang, R.; El Hankari, S.; Chen, R.; Wang, S. Plasma-Assisted Synthesis and Surface Modification of Electrode Materials for Renewable Energy. Adv. Mater. 2018, 30, 1705850. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A cost-effective supercapacitor material of ultrahigh specific capacitances: Spinel nickel cobaltite aerogels from an epoxide-driven sol–gel process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; Cao, P.; Cai, C.; Fu, Y.; Ma, X. Mesoporous composite nickel cobalt oxide/graphene oxide synthesized via a template-assistant co-precipitation route as electrode material for supercapacitors. J. Power Sources 2016, 306, 742–752. [Google Scholar] [CrossRef]
- Li, L.; Guo, X.; Hao, F.; Zhang, X.; Chen, J. Solid-state grinding/low-temperature calcining synthesis of carbon coated MnO2 nanorods and their electrochemical capacitive property. New J. Chem. 2015, 39, 4731–4736. [Google Scholar] [CrossRef]
- Patil, S.A.; Shinde, D.V.; Patil, D.V.; Tehare, K.K.; Jadhav, V.V.; Lee, J.K.; Mane, R.S.; Shrestha, N.K.; Han, S.-H. A simple, room temperature, solid-state synthesis route for metal oxide nanostructures. J. Mater. Chem. A 2014, 2, 13519–13526. [Google Scholar] [CrossRef]
- Zhang, L.; Ou, M.; Yao, H.; Li, Z.; Qu, D.; Liu, F.; Wang, J.; Wang, J.; Li, Z. Enhanced supercapacitive performance of graphite-like C3N4 assembled with NiAl-layered double hydroxide. Electrochim. Acta 2015, 186, 292–301. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Yan, Y.; Che, R.; Chen, M.; Wu, L. One-Step Fabrication of Ultrathin Porous Nickel Hydroxide-Manganese Dioxide Hybrid Nanosheets for Supercapacitor Electrodes with Excellent Capacitive Performance. Adv. Energy Mater. 2013, 3, 1636–1646. [Google Scholar] [CrossRef]
- Xu, H.; Hu, X.; Yang, H.; Sun, Y.; Hu, C.; Huang, Y. Flexible Asymmetric Micro-Supercapacitors Based on Bi2O3 and MnO2 Nanoflowers: Larger Areal Mass Promises Higher Energy Density. Adv. Energy Mater. 2015, 5, 1401882. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Zan, G. A High-Performance Supercapacitor with Well-Dispersed Bi2O3 Nanospheres and Active-Carbon Electrodes. Eur. J. Inorg. Chem. 2015, 2015, 5751–5756. [Google Scholar] [CrossRef]
- Feng, X.Q.; Li, Y.H.; Chen, G.S.; Liu, Z.; Ning, X.H.; Hu, A.P.; Tang, Q.L.; Chen, X.H. Free-standing MnO2/nitrogen-doped graphene paper hybrids as binder-free electrode for supercapacitor applications. Mater. Lett. 2018, 231, 114–118. [Google Scholar] [CrossRef]
- Lin, L.Y.; Huang, M.C.; Ning, M.L.; Wu, K.W.; Li, H.W.; Hussain, S.; Zhao, S.Q. Facile ordered ZnCo2O4@MnO2 nanosheet arrays for superior-performance supercapacitor electrode. Solid State Sci. 2018, 84, 51–56. [Google Scholar] [CrossRef]
- Liu, P.B.; Zhu, Y.D.; Gao, X.G.; Huang, Y.; Wang, Y.; Qin, S.Y.; Zhang, Y.Q. Rational construction of bowl-like MnO2 nanosheets with excellent electrochemical performance for supercapacitor electrodes. Chem. Eng. J. 2018, 350, 79–88. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Song, Y.; Feng, D.-Y.; Sun, Z.; Sun, X.; Liu, X.-X. High mass loading MnO2 with hierarchical nanostructures for supercapacitors. ACS Nano 2018, 12, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhu, S.; Shan, Q.; Liu, S.; Zhang, Y.; Dong, F.; Liu, H. Facile synthesis of flower-like (BiO)2CO3@ MnO2 and Bi2O3@ MnO2 nanocomposites for supercapacitors. Electrochim. Acta 2015, 168, 97–103. [Google Scholar] [CrossRef]
- Ng, C.H.; Lim, H.N.; Hayase, S.; Zainal, Z.; Shafie, S.; Huang, N.M. Effects of temperature on electrochemical properties of bismuth oxide/manganese oxide pseudocapacitor. Ind. Eng. Chem. Res. 2018, 57, 2146–2154. [Google Scholar] [CrossRef]
- Singh, S.; Shinde, N.M.; Xia, Q.X.; Gopi, C.; Yun, J.M.; Mane, R.S.; Kim, K.H. Tailoring the morphology followed by the electrochemical performance of NiMn-LDH nanosheet arrays through controlled Co-doping for high-energy and power asymmetric supercapacitors. Dalton Trans. 2017, 46, 12876–12883. [Google Scholar] [CrossRef]
- Shinde, N.M.; Xia, Q.X.; Yun, J.M.; Singh, S.; Mane, R.S.; Kim, K.H. A binder-free wet chemical synthesis approach to decorate nanoflowers of bismuth oxide on Ni-foam for fabricating laboratory scale potential pencil-type asymmetric supercapacitor device. Dalton Trans. 2017, 46, 6601–6611. [Google Scholar] [CrossRef]
- Zhang, L.; Ghimire, P.; Phuriragpitikhon, J.; Jiang, B.; Gonçalves, A.A.; Jaroniec, M. Facile formation of metallic bismuth/bismuth oxide heterojunction on porous carbon with enhanced photocatalytic activity. J. Colloid Interface Sci. 2018, 513, 82–91. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Banerjee, D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am. Miner. 1998, 83, 305–315. [Google Scholar] [CrossRef]
- Zampieri, G.; Abbate, M.; Prado, F.; Caneiro, A. Mn-2p XPS spectra of differently hole-doped Mn perovskites. Solid State Commun. 2002, 123, 81–85. [Google Scholar] [CrossRef]
- John, R.E.; Chandran, A.; Thomas, M.; Jose, J.; George, K. Surface-defect induced modifications in the optical properties of α-MnO2 nanorods. Appl. Surf. Sci. 2016, 367, 43–51. [Google Scholar] [CrossRef]
- Andreeva, A.Y.; Sukhikh, T.S.; Kozlova, S.G.; Konchenko, S.N. Exchange interactions and XPS O1s spectra in polynuclear lanthanide complexes with dibenzoylmethanide and 4-hydroxy-2,1,3-benzothiadiazole. J. Mol. Struct. 2018, 1166, 190–194. [Google Scholar] [CrossRef]
- Kotsis, K.; Staemmler, V. Ab initio calculations of the O1s XPS spectra of ZnO and Zn oxo compounds. Phys. Chem. Chem. Phys. 2006, 8, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Nanba, T.; Miura, Y.; Sakida, S. Consideration on the correlation between basicity of oxide glasses and O1s chemical shift in XPS. J. Ceram. Soc. Jpn. 2005, 113, 44–50. [Google Scholar] [CrossRef]
- Sopha, H.; Spotz, Z.; Michalicka, J.; Hromadko, L.; Bulanek, R.; Wagner, T.; Macak, J.M. Bismuth Oxychloride Nanoplatelets by Breakdown Anodization. ChemElectroChem 2019, 6, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Kitchaev, D.A.; Peng, H.W.; Liu, Y.; Sun, J.W.; Perdew, J.P.; Ceder, G. Energetics of MnO2 polymorphs in density functional theory. Phys. Rev. B 2016, 93, 5. [Google Scholar] [CrossRef]
- Manthiram, A.; Murugan, A.V.; Sarkar, A.; Muraliganth, T. Nanostructured electrode materials for electrochemical energy storage and conversion. Energy Environ. Sci. 2008, 1, 621–638. [Google Scholar] [CrossRef]
- Gujar, T.P.; Shinde, V.R.; Lokhande, C.D.; Mane, R.S.; Han, S.H. Bismuth oxide thin films prepared by chemical bath deposition (CBD) method: Annealing effect. Appl. Surf. Sci. 2005, 250, 161–167. [Google Scholar] [CrossRef]
- Yu, X.; Yun, S.; Yeon, J.S.; Bhattacharya, P.; Wang, L.; Lee, S.W.; Hu, X.; Park, H.S. Emergent Pseudocapacitance of 2D Nanomaterials. Adv. Energy Mater. 2018, 8, 1702930. [Google Scholar] [CrossRef]
- Ghodbane, O.; Ataherian, F.; Wu, N.-L.; Favier, F. In situ crystallographic investigations of charge storage mechanisms in MnO2-based electrochemical capacitors. J. Power Sources 2012, 206, 454–462. [Google Scholar] [CrossRef]
- Yuan, D.; Zeng, J.; Kristian, N.; Wang, Y.; Wang, X. Bi2O3 deposited on highly ordered mesoporous carbon for supercapacitors. Electrochem. Commun. 2009, 11, 313–317. [Google Scholar] [CrossRef]
- Wang, S.; Jin, C.; Qian, W. Bi2O3 with activated carbon composite as a supercapacitor electrode. J. Alloy. Compd. 2014, 615, 12–17. [Google Scholar] [CrossRef]
- Liu, Y.; Zhitomirsky, I. Electrochemical supercapacitor based on multiferroic BiMn2O5. J. Power Sources 2015, 284, 377–382. [Google Scholar] [CrossRef]
- Yang, W.-D.; Lin, Y.-J. Preparation of rGO/Bi2O3 composites by hydrothermal synthesis for supercapacitor electrode. J. Electr. Eng. 2019, 70, 101–106. [Google Scholar]
- Ciszewski, M.; Mianowski, A.; Szatkowski, P.; Nawrat, G.; Adamek, J. Reduced graphene oxide–bismuth oxide composite as electrode material for supercapacitors. Ionics 2015, 21, 557–563. [Google Scholar] [CrossRef]
- Sundriyal, P.; Bhattacharya, S. Scalable Micro-fabrication of Flexible, Solid-State, Inexpensive, and High-Performance Planar Micro-supercapacitors through Inkjet Printing. ACS Appl. Energy Mater. 2019, 2, 1876–1890. [Google Scholar] [CrossRef]
- Yu, T.; Li, Z.; Chen, S.; Ding, Y.; Chen, W.; Liu, X.; Huang, Y.; Kong, F. Facile synthesis of flowerlike Bi2MoO6 hollow microspheres for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 7355–7361. [Google Scholar] [CrossRef]
| Sl. No. | Materials | Specific Capacitance | Electrolyte | Voltage Difference (in V) | Cycling Stability (Specific Capacitance Retention) | Ref. |
|---|---|---|---|---|---|---|
| 1 | Bi2O3/HOMC | 232 F g−1 @ 5 mV s−1 | 6 M KOH | 1.0 V | 70% after 1000 cycles | [40] |
| 2 | AC–Bi2O3 composite | 332.6 F g−1 @1 A g−1 | 6 M KOH | 1.0 V | 60% after 1000 cycles | [41] |
| 3 | BiMn2O5- MWCNT | 6.0 F cm−2 @ 2 mV s−1 | 0.5 M Na2SO4 | 1.0 V | 90% after 1000 cycles | [42] |
| 4 | rGO-Bi2O3 composite | 216 F g−1 at 1 A g−1 | 1 M KOH | 1.0 V | Not mentioned | [43] |
| 5 | RGO-Bi2O3 composite | 94 F g−1 @ 0.2 A g−1 | 6 M KOH | 1.0 V | 90% after 3000 cycles | [44] |
| 6 | AC-Bi2O3 | 0.5127 F cm−2 | 6 M KOH | 0.9 V | 92.2% after 20,000 cycles | [45] |
| 7 | Bi2MoO6 | 182 F g−1 @ 1 A g−1 | 3 M KOH | 0.5 V | 80% after 3000 cycles | [46] |
| 8 | Bi2O3@MnO2 | 93.1 F g−1 @ 1 A g−1 | 1 M Na2SO4 | 1 V | 112% after 1000 cycles | [23] |
| 9 | Bi2O3/MnO2 | 79.4 F g−1 @ 1 A g−1 | 1 M Na2SO4 | 1.0 V | 95% after 1000 cycles | [24] |
| 10 | Bi2O3-MnO2 | 161 F g−1 @ 1 A g−1 | 1 M NaOH | 1.3 (GCD) | 95% after 10,000 cycles | This Work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Sahoo, R.K.; Shinde, N.M.; Yun, J.M.; Mane, R.S.; Kim, K.H. Synthesis of Bi2O3-MnO2 Nanocomposite Electrode for Wide-Potential Window High Performance Supercapacitor. Energies 2019, 12, 3320. https://doi.org/10.3390/en12173320
Singh S, Sahoo RK, Shinde NM, Yun JM, Mane RS, Kim KH. Synthesis of Bi2O3-MnO2 Nanocomposite Electrode for Wide-Potential Window High Performance Supercapacitor. Energies. 2019; 12(17):3320. https://doi.org/10.3390/en12173320
Chicago/Turabian StyleSingh, Saurabh, Rakesh K. Sahoo, Nanasaheb M. Shinde, Je Moon Yun, Rajaram S. Mane, and Kwang Ho Kim. 2019. "Synthesis of Bi2O3-MnO2 Nanocomposite Electrode for Wide-Potential Window High Performance Supercapacitor" Energies 12, no. 17: 3320. https://doi.org/10.3390/en12173320
APA StyleSingh, S., Sahoo, R. K., Shinde, N. M., Yun, J. M., Mane, R. S., & Kim, K. H. (2019). Synthesis of Bi2O3-MnO2 Nanocomposite Electrode for Wide-Potential Window High Performance Supercapacitor. Energies, 12(17), 3320. https://doi.org/10.3390/en12173320






