1. Introduction
Petroleum coke is often used as fuel for power generation in power plants and cement production mainly in China and India [
1]. Recently, other countries are also constructing facilities using petroleum coke as a fuel to save energy cost, and its usage is gradually increasing [
2]. For example, Egypt, which is the largest cement producer in the Middle East and North Africa region, has initiated the process of changing the fuel used in the various plants from gas to petroleum coke. Japan has increased the petroleum coke imports for the gasifier project, and a few plants in South Korea are also initiating the move to change the fuel from liquefied natural gas (LNG) to petroleum coke. [
3] Furthermore, several other cases regarding the use of petroleum coke to reduce energy costs have been reported [
4,
5].
Another characteristic of petroleum coke that is drawing attention for use as a power generation fuel includes its calorific value, which is similar to those of Bunker C and LNG, which are the most commonly used fuels, and its energy price (cost per producing one ton of steam) is cheaper [
6]. Moreover, because petroleum coke is the by-product in the process of refining and commercializing crude oils [
7,
8,
9,
10,
11], refineries can provide long-term stable supplies of petroleum coke through the construction and extension of related facilities. The price stabilization based on this also contributes to the increasing use of petroleum coke [
12,
13].
Consequently, petroleum coke has become a fuel that cannot be ignored owing to the various reasons mentioned above. With an increase in the use of petroleum coke, the amount of soot and other by-products produced from the combustion of petroleum coke are also increasing, and methods to process them efficiently are being studied extensively.
To examine various research and development endeavors using the characteristics of petroleum coke soot, the Nanjing University in China reported a case where volume stability was achieved by adjusting the curing time of cement using the soot obtained from the circulating fluidized bed combustion of petroleum coke and coal. They published the result of the study and concluded that soot could be used as a good admixture, which allowed less water content [
14]. Another study reported the application of the petroleum coke-mixed fuel to the development of the gasification technology through the slagging of petroleum coke soot using a mixture of anthracite and petroleum coke. With the development of the gasification technology, the use of various types of solid carbon fuels such as lignite and petroleum cokes has increased, and the results of a comparative study on the structural characteristics of soot generated using lignite, bituminous coal, and petroleum coke as fuels have been reported [
15]. Additionally, a technology for the production of an anhydrous plaster substitute using the boiler combustion material of petroleum coke has been patented. As described above, many attempts are being made to develop technologies to recycle soot and by-products generated after using petroleum coke as fuel.
Therefore, our research team attempted to recycle petroleum coke soot as an anode active material for lithium ion batteries (LIBs), which has not been previously investigated. The materials of LIBs consist of anode materials, cathode materials, electrolytes, and separation membranes. The graphite-based carbon materials are typically used for anode materials [
16,
17,
18]. As petroleum coke soot is known to be mainly composed of carbons, its value can be further increased by recycling the soot (which is a pollutant) as an energy storage material via reprocessing it as artificial graphite using the graphitization process. Hence, the soot generated after combustion in plants using petroleum coke as fuel was collected, annealed, and examined using transmission electron microscopy (TEM), energy dispersive spectroscopy (EDS), and Brunauer–Emmett–Teller (BET) analyses. These analyses were conducted on the samples collected before and after annealing. Furthermore, elemental analysis (EA) and thermogravimetric analysis (TGA) were performed to determine the residual impurities. Finally, the applicability of the petroleum coke soot was shown by testing the electrochemical performance of the LIB fabricated using the annealed soot as an anode material.
2. Experimental Methods
2.1. Material Collection
The petroleum coke soot sample for this study was collected from a steam production facility of a paper production plant (Hanchang Paper Co., Ltd.). The plant operates approximately 330 days a year and has reduced fuel costs by converting fuel to petroleum coke since 2012.
Table 1 lists the detailed specifications of this facility. The combustion gas of the boiler passes through a dust collector before it is discharged into the atmosphere. The soot sample was collected at the dust collector by the engineers in the company last year.
2.2. Graphitization Process
To remove the impurities from the soot collected from the petroleum coke boiler and improve crystallization, annealing was performed in an ultra-high-temperature electric furnace (Thermvac Engineering, Korea) under an argon gas flow (4 L/min) at two temperature conditions of 2000 °C and 2700 °C. The heating rate was set at 10 °C/min until 1800 °C, and 5 °C/min until 2700 °C. After maintaining the two temperature conditions (2000 °C, 2700 °C) for 2 hours, the electric furnace was naturally cooled to the ambient temperature. The graphitization process was carried out using equipment at KIST (Korea Institute of Science and Technology).
2.3. Soot Characterization
To verify the structural characteristics before and after the annealing of the petroleum coke soot, a transmission electron microscope equipped with an EDS system of 200 kV acceleration voltage (JEM-2100F; JEOL, Japan) was used. The BET surface areas were calculated from N2 adsorption isotherms obtained using a Quantachrome sorption analyzer (Autosorb-1, USA). Before the BET measurement, every sample was dehydrated by annealing at 200 °C under N2 for 2 h. To analyze the residue weight in the soot according to the annealing temperature, thermogravimetric analysis (TGA) was performed using a thermogravimetric analyzer (TGA Q500, TA Instrument, England). The heating medium was Ar gas and the heating rate was 10 °C/min. To analyze the element composition of the residue according to the annealing temperature, the composition ratios of elements were measured using a carbon, hydrogen, nitrogen, and sulfur (CHNS) analyzer (Thermo Fisher Scientific, EA1112, USA).
2.4. Electrochemical Measurement
The electrochemical test of petroleum coke was performed using 2032-coin type (Wellcos Corp.) half-cells. The slurry for electrode fabrication was produced with petroleum coke (80 wt %), carboxymethyl cellulose (CMC) / styrene-butadiene rubber (SBR) (10 wt %) as binder, Super P (10 wt %) as conductive material, and distilled water as solvent. The fabricated slurry was coated on the Cu-foil substrate using the doctor blade coater, and the solvent was removed by drying at 40 °C for 12 h under vacuum (–700 mmHg). Next, it was pressed to a thickness of 35 μm using a roll press and the typical mass loading for the electrodes was 0.0039 g/cm2. Lithium coin chips were used for counter and reference electrodes, and Celgard 2400 for separation membrane. The electrolytes were obtained by dissolving 1 M LiPF6 in a solvent made by adding 20 wt % of fluoroethylene carbonate (FEC) in ethylene carbonate (EC)/diethyl carbonate (DEC) (1:1 v/v). The FEC additive was used to prevent the instability of SEI film formed at the edge of the ring surface due to shrinkage and expansion during charging and discharging. The coin was produced in a glove box filled with Ar. The electrochemical test was performed in the voltage range of 0.01–3 V (vs Li / Li +) using the BCS-805 biologic battery test system (Biologic, France).
3. Results and Discussion
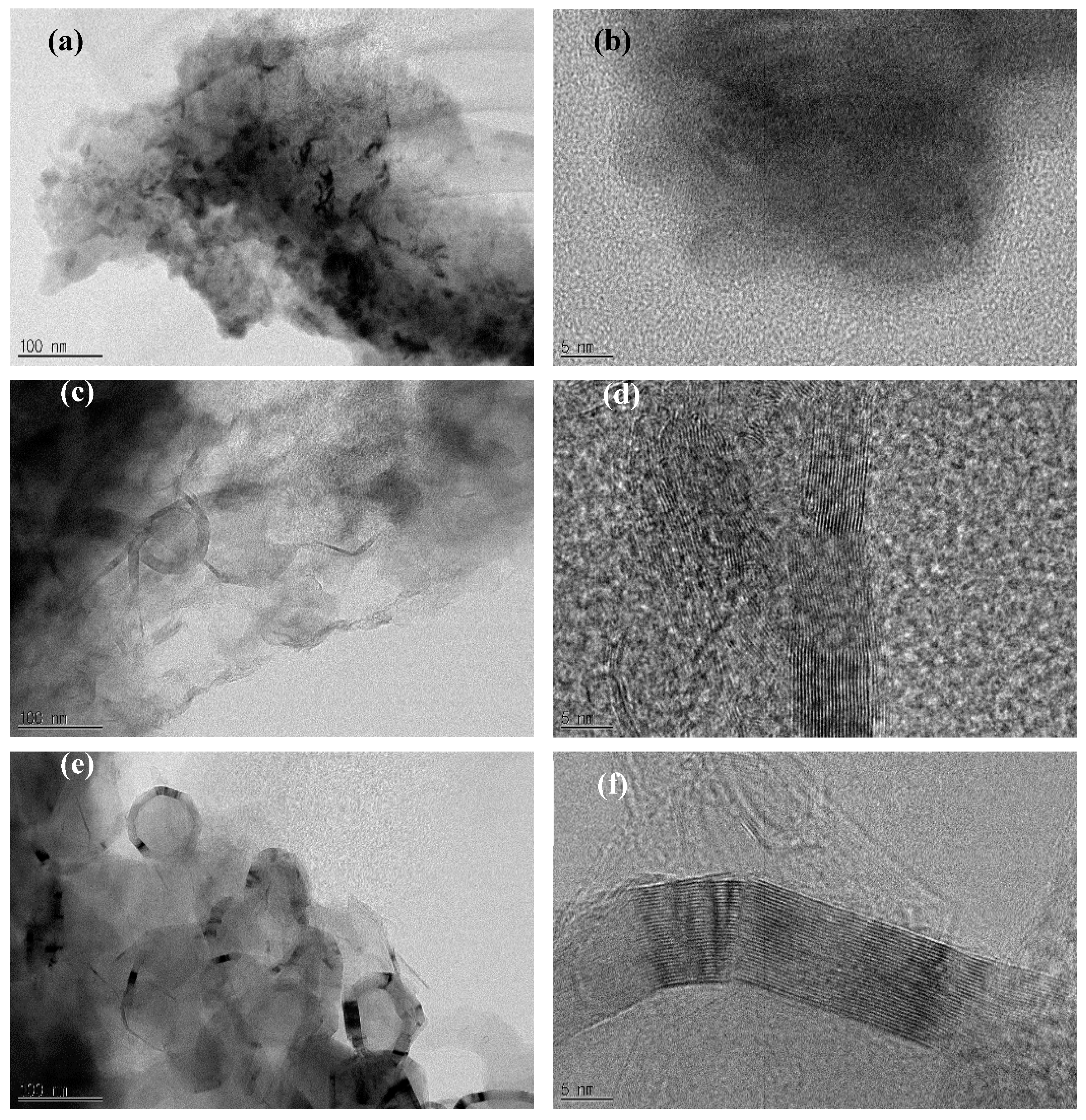
The soot morphology was observed before and after annealing using TEM.
Figure 1a,b shows the TEM and HR-TEM images, respectively, of petroleum coke soot collected from the boiler. These images confirm that the soot has an amorphous structure with no crystallization. TEM and HR-TEM images of the soot annealed at temperature up to 2000 °C (
Figure 1c,d) reveal a graphite layer that is crystallized by annealing. The TEM and HR-TEM images of the soot annealed at 2700 °C (
Figure 1e,f) exhibit a highly crystallized graphite layer. Furthermore, as the annealing temperature increased, the soot was crystallized in shape of rings approximately 100 nm in diameter, and the rings were graphitized with thicknesses ranging from 5 nm to 20 nm. The crystallization did not progress into the rings, and this was moderately different from the morphology in the crystallization of the soot generated after the combustion of general diesel fuel by annealing.
The CHNS elements were analyzed to determine the elemental composition of residual impurities in the soot, which could affect the electrochemical performance; the results are listed in
Table 2. A large quantity of sulfur was detected in the soot before annealing, whereas the quantities of hydrogen and nitrogen were less. The quantity of hydrogen was less in the petroleum coke soot than that in the soot obtained from Bunker C or LNG fuels because the composition of petroleum coke more closely resembles pure carbon lumps than hydrocarbons. The amount of impurities decreased significantly after annealing, and no nitrogen was detected.
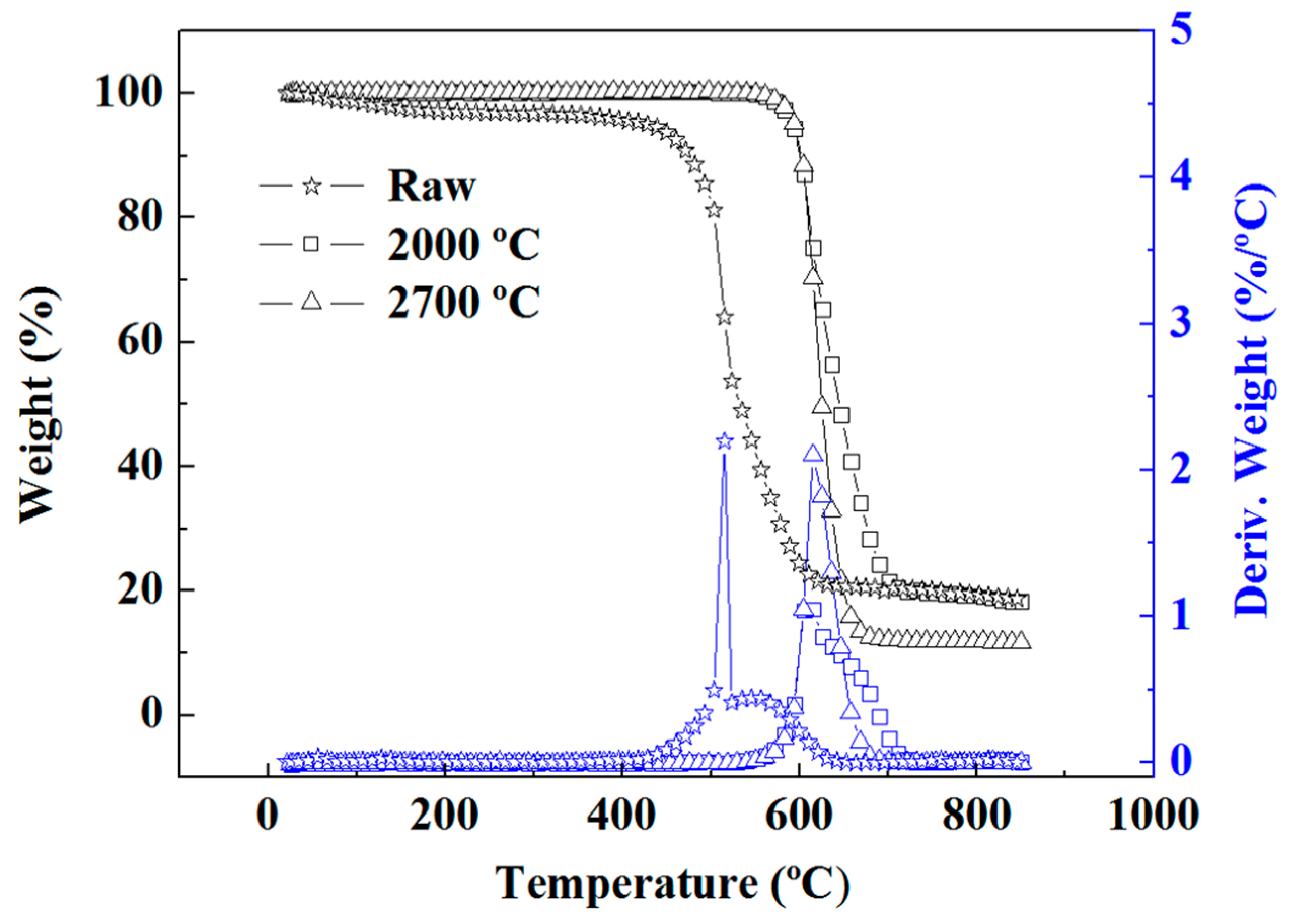
The existence of impurities other than CHNS elements was determined using TGA. The results (
Figure 2,
Table 3) confirmed that large quantities of organic matter were removed through annealing; however, 11.73% of residues remained even after annealing at 2700 °C.
EDS mapping (
Figure 3) was performed to determine the composition of the residue Among the metal components, a large quantity of vanadium was observed in the petroleum coke soot [
19,
20,
21]. Many prior studies have suggested various physical and chemical methods to remove this metal. Therefore, this metal can be effectively removed from the petroleum coke soot for application as the electrode material in LIB in future studies. In this study, petroleum coke soot was used as the electrode material for LIB without removing the metal components.
Figure 4 shows the nitrogen adsorption/desorption isotherms of the annealed soot, and
Table 4 outlines the specific surface area calculated using this curve. To calculate the specific surface area, a linear BET range in the relative pressure range of 0.05–0.35 was used. The graphite used in LIBs is generally micron-scale, and thus, has a very low BET specific surface area (less than 2 m
2/g). The petroleum coke shows a specific surface area of approximately 3 m
2/g regardless of annealing. Thus, the specific surface area is not affected by annealing.
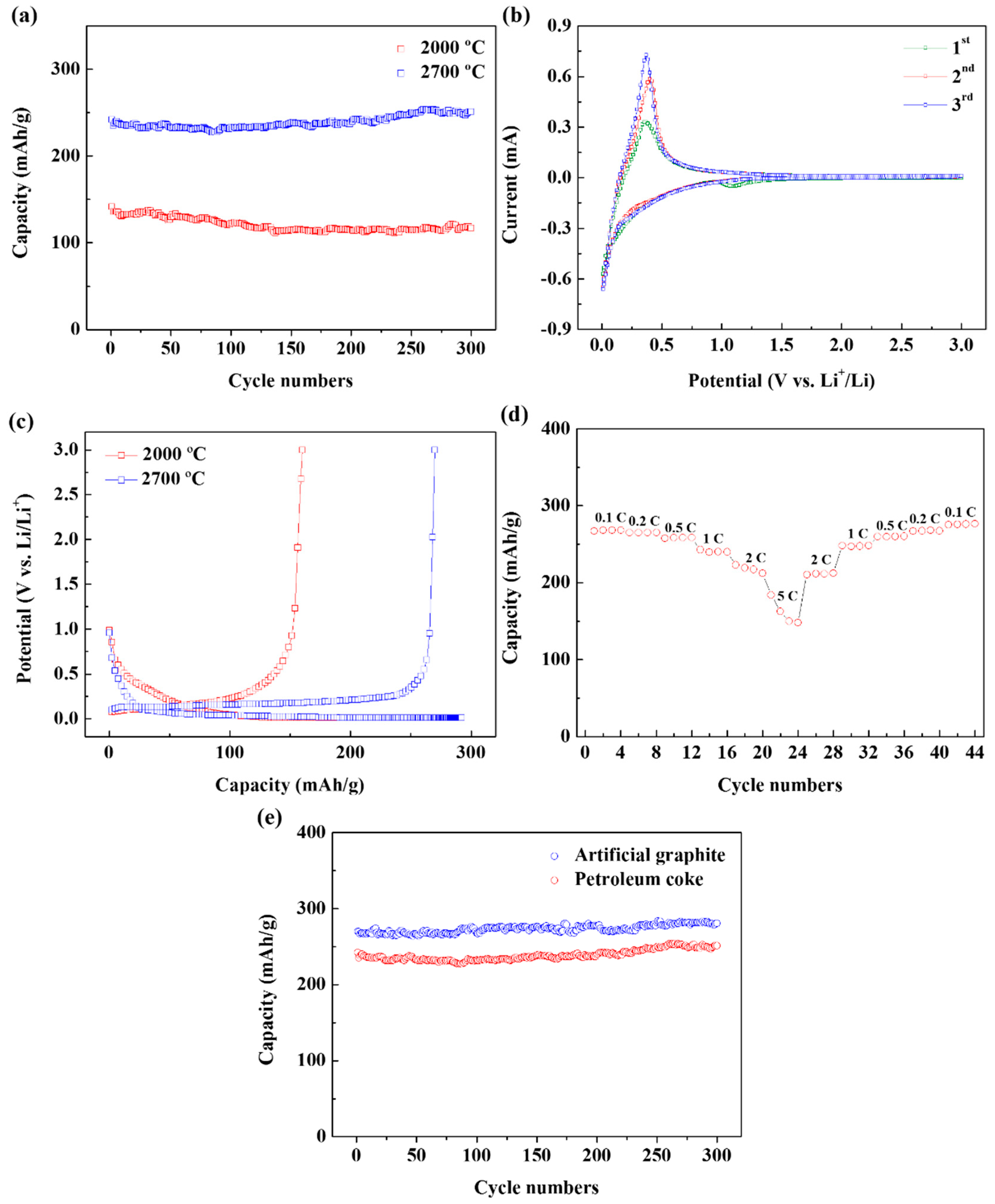
To evaluate the electrochemical performance of the petroleum coke soot as an anode active material, the galvanostatic charge/discharge experiment was performed.
Figure 5a shows the data of the reversible capacity measurements using the soot annealed at 2000 °C and 2700 °C as anodes. When annealed at 2000 °C, it shows a cycle and capacity performance lower than those of the commercial graphite anodes, but when annealed at 2700 °C, an excellent performance is observed with a capacity of 250 mAh/g even for more than 300 cycles at a rate of 1 C. Consistent with previous results, as the annealing temperature increases, better performance is obtained because the soot structure is highly crystallized. The experiment results confirm that when annealed at a high temperature of 2700 °C, the soot shows a performance that is comparable to that of the commercial graphite anodes.
Figure 5b shows the first three continuous voltage–current (CV) curves of the soot anode annealed at 2700 °C, consisting of three separate reduction peaks. The first peak at 1.1 V corresponds to the irreversible reduction of fluoroethylene carbonate (FEC). The second broad anode peak of 0.25–1.1 V corresponds to the ethylene carbonate/dimethyl carbonate (EC/DEC) decomposition and the solid electrolyte interface (SEI) layer. Finally, a sharp peak below 0.25 V indicates the insertion of Li ions. After the first cycle, the anode reduction peak disappears, and the CV curves are almost overlapped with no clear changes in the magnitudes of peak current or potential. This shows the excellent reversibility and cycling stability of the Li insertion and extraction reaction. In the anodic reaction, most lithium ions are delithiated at a voltage below 1.5 V.
Figure 5c shows the initial Coulomb efficiency values of the annealed soot, which are 82.2% and 92.3%. The improvement in Coulomb efficiency with an increase in temperature increased cannot be explained by the change in the specific surface area because the specific surface area did not change significantly in the BET results. It is speculated that the improvement in Coulomb efficiency is observed because the basal plane area increases as crystallized rings are generated during the graphitization process.
The rate-capability of the annealed soot is shown in
Figure 5d. The initial high capacity of 270 mAh/g is observed at a current density of 0.1 C after five discharge/charge cycles. The capacity of the annealed soot, is measured as 255, 240, 205, and 150 mAh/g current density when the current speed is set continuously at 0.5, 1, 2, and 5 C. When the current speed returns to a value of 0.1 C, the capacity is fully restored. This result shows that the structure of the annealed soot is stable at various current densities.
A comparison of the performance of the cell with that of the graphite fabricated under identical conditions is shown in
Figure 5e. The annealed soot shows 92% discharge capacity in comparison with commercial graphite, and therefore, confirms that the performance of annealed soot is equivalent to that of the commercial graphite.
These results show the potential of the annealed soot as a promising candidate for a low-cost anode material for LIBs.
4. Conclusions
Soot, a combustion product of petroleum coke, was recycled and used as an active material for lithium ion batteries for the first time in this study. With the increasing use of petroleum coke as an emerging fuel, the amount of soot generated has increased, and many studies have been conducted to efficiently process this byproduct. The collected soot was graphitized and used as an anode active material after annealing at 2700 °C. The morphology and structure of the annealed soot were examined using high-resolution TEM. The graphitized soot was found to crystallize into ring-shaped structures of approximately 100 nm in diameter and transform into highly crystallized graphite.
The results of the electrochemical experiments demonstrated that the annealed soot with an intrinsic graphite multilayer structure was a promising a candidate for anode material with high reversible capacity, excellent cycling, and high first cycle Coulomb efficiency for rechargeable LIBs.