Production of Sugar Feedstocks for Fermentation Processes from Selected Fast Growing Grasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemical Composition of Grasses
2.3. Cellulosic Pulps
2.4. Enzyme Preparation
2.5. Enzymatic Hydrolysis of Kraft Pulps
2.6. Calculation of Hydrolysis Yield
3. Results and Discussion
3.1. Characterization of Lignocellulosic Substrates and Cellulosic Pulps
3.2. Enzymatic Hydrolysis of Cellulosic Pulps
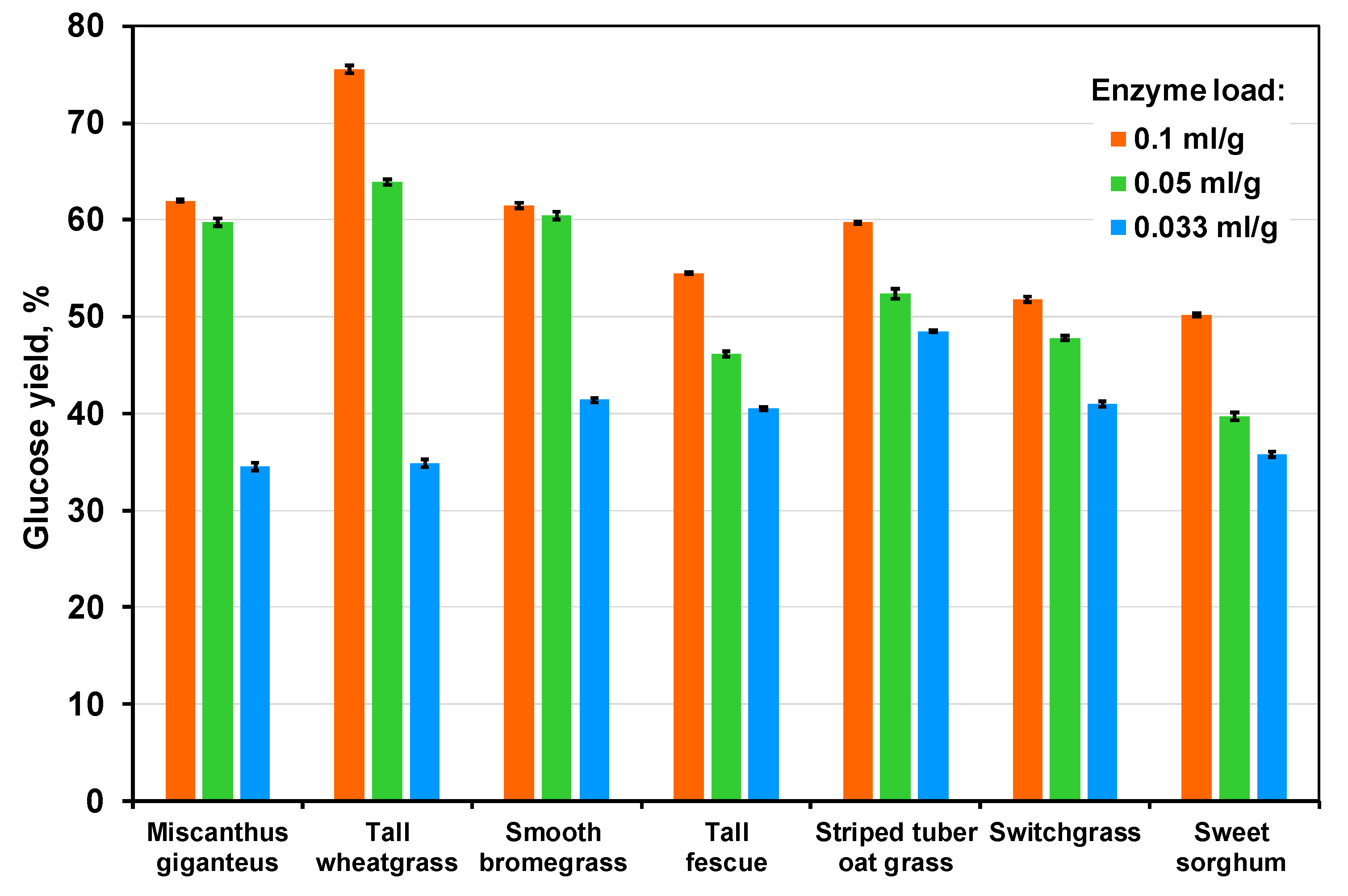
3.3. The Effect of Enzyme Concentration and Pulp Origin
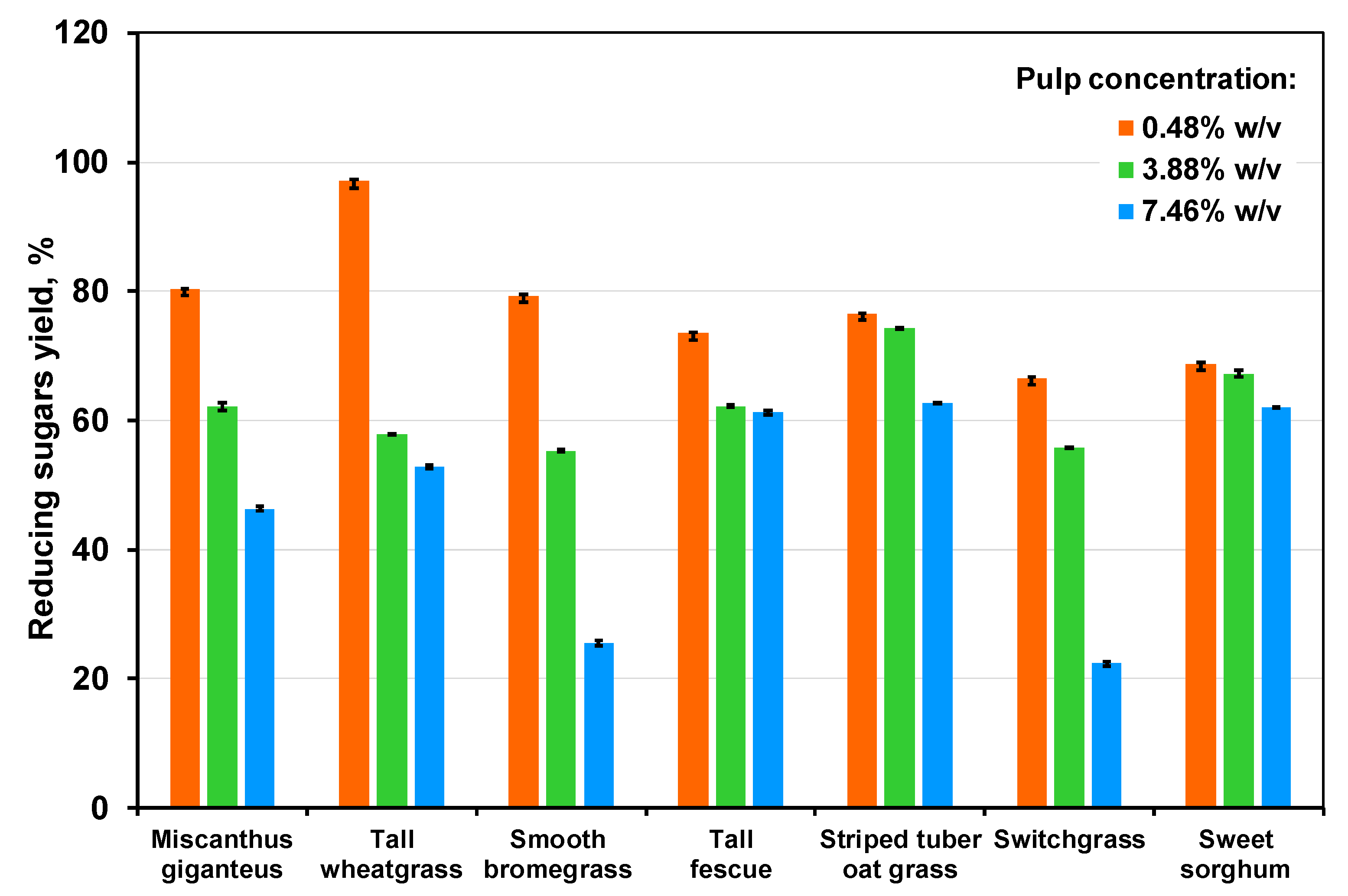
3.4. The Effect of Pulp Concentration on Hydrolysis Yield
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Pana, C.; Mihaescu, L.; Cernat, A.; Negurescu, N.; Mocanu, R.; Negreanu, G. Solutions for energy recovery of animal waste from leather industry. Energ. Convers. Manag. 2017, 149, 1085–1095. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Mihaescu, L.; Negreanu, G.; Pana, C.; Pisa, I.; Cernat, A.; Ciupageanu, D.-A. Experimental investigations of innovative biomass energy harnessing solutions. Energies 2018, 11, 3469. [Google Scholar] [CrossRef]
- Lazaroiu, G.; Pop, E.; Negreanu, G.; Pisa, I.; Mihaescu, L.; Bondrea, A.; Berbece, V. Biomass combustion with hydrogen injection for energy applications. Energy 2017, 127, 351–357. [Google Scholar] [CrossRef]
- Tilvikiene, V.; Venslauskas, K.; Navickas, K.; Župerka, V.; Dabkevičius, Z.; Kadžiulienė, Ž. The biomass and biogas productivity of perennial grasses. Zemdirbyste 2012, 99, 17–22. [Google Scholar]
- Monti, A.; Virgilio, N.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass. Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Schwarz, K.U.; Hastings, A. History of the development of Miscanthus as a bioenergy crop: From small beginnings to potential realization. Biol. Environ. Proc. R. Ir. Acad. 2015, 115, 45–57. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for lignocellulosic biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Kryževičienė, A.; Navickas, K.; Župerka, V. Perennial grasses for biogas production. Vagos 2005, 69, 76–82. [Google Scholar]
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass. Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Kline, L.; Johnson, A.; Kim, P.; Jackson, S.; Labbe, N. Monitoring switchgrass composition to optimize harvesting periods for bioenergy and value-added products. Biomass. Bioenergy 2013, 56, 29–37. [Google Scholar] [CrossRef]
- Schmer, M.R.; Vogel, K.P.; Mitchell, R.B.; Perrin, R.K. Net energy of cellulosic ethanol from switchgrass. PNAS 2008, 105, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Bhagia, S.; Meng, X.; Evans, B.R.; Dunlap, J.R.; Bali, G.; Chen, J.; Reeves, K.S.; Chun Ho, H.; Davison, B.H.; Pu, Y.; et al. Ultrastructure and Enzymatic Hydrolysis of Deuterated Switchgrass. Sci. Rep. 2018, 8, 13226. [Google Scholar] [CrossRef] [PubMed]
- Hickman, G.C.; Vanloocke, A.; Dohleman, F.G.; Bernacchi, C.J. A comparison of canopy evapotranspiration for maize and two perennial grasses identified as potential bioenergy crops. GCB Bioenergy 2010, 2, 157–168. [Google Scholar] [CrossRef]
- Chung, J.H.; Kim, D.S. Miscanthus as a potential bioenergy crop in East Asia. J. Crop Sci. Biotech. 2012, 15, 65–77. [Google Scholar] [CrossRef]
- Wannasek, L.; Ortner, M.; Amon, B.; Amon, T. Sorghum, a sustainable feedstock for biogas production? Impact of climate, variety and harvesting time on maturity and biomass yield. Biomass. Bioenergy 2017, 106, 137–145. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Dolat, A.; Steinberger, Y.; Wang, X.; Osman, A.; Xie, G.H. Biomass yield and changes in chemical composition of sweet sorghum cultivars grown for biofuel. Field Crop Res. 2009, 111, 55–64. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Lyberatos, G. Effect of pretreatment of sweet sorghum biomass on methane generation. Waste Biomass Valori. 2013, 4, 583–591. [Google Scholar] [CrossRef]
- Mast, B.; Lemmer, A.; Oechsner, H.; Reinhardt-Hanisch, A.; Claupein, W.; Graeff, S. Methane yield potential of novel perennial biogas crops influenced by harvest date. Ind. Crops Prod. 2014, 58, 194–203. [Google Scholar] [CrossRef]
- Piłat, J.; Majtkowski, W.; Majtkowska, G.; Zurek, G.; Mikolajczak, J.; Brucknerova, M. The feeding value assessment of forage from some C-4 grass species in different phases of vegetation. Part II. Miscanthus Sacchariflorus. (Maxim.) Hack. Plant Breed. Seed Sci. 2007, 55, 55–63. [Google Scholar]
- Hideno, A.; Kawashima, A.; Anzoua, K.G.; Yamada, T. Comparison of the enzymatic digestibility of physically and chemically pretreated selected line of diploid-Miscanthus sinensis Shiozuka and triploid-M.×giganteus. Bioresour. Technol. 2013, 146, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Chirino-Valle, I.; Kandula, D.; Littlejohn, C.; Hill, R.; Walker, M.; Shields, M.; Cummings, N.; Hettiarachchi, D.; Wrattenet, S. Potential of the beneficial fungus Trichoderma to enhance ecosystem-service provision in the biofuel grass Miscanthus x giganteus in agriculture. Sci. Rep. 2016, 6, 25109. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, K. Lignocellulose: A chewy problem. Nature 2011, 474, 12–14. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. Biotech 2013, 3, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci. Rep. 2016, 6, 39354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Chandra, R.; Chung, P.A.; Saddler, J. Can the same steam pretreatment conditions be used for most softwoods to achieve good, enzymatic hydrolysis and sugar yields? Bioresour. Technol. 2010, 101, 7827–7833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Enhancement of methane production from Cotton Stalk using different pretreatment techniques. Sci. Rep. 2018, 8, 3463. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Lignocellulosic ethanol production by starch-base industrial yeast under PEG detoxification. Sci. Rep. 2016, 6, 20361. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Teller, P.J.; Ahring, B.K. Pretreatment of forest residues of Douglas fir by wet explosion for enhanced enzymatic saccharification. Bioresour. Technol. 2015, 192, 46–53. [Google Scholar] [CrossRef]
- Pan, X.; Arato, C.; Gilkes, N.; Gregg, D.; Mabee, W.; Pye, K.; Xiao, Z.; Zhang, X.; Saddler, J. Biorefining of softwoods using ethanol organosolv pulping: Preliminary evaluation of process streams for manufacture of fuel-grade ethanol and co-products. Biotechnol. Bioengy 2005, 90, 473–481. [Google Scholar] [CrossRef]
- Questell-Santiago, Y.M.; Zambrano-Valera, R.; Talebi Amiri, M.; Luterbacher, J.S. Carbohydrate stabilization extends the kinetic limits of chemical polysaccharide depolymerization. Nat. Chem. 2018, 10, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Vasco, C.; Zhang, X. Alkaline hydrogen peroxide (AHP) pretreatment of softwood: Enhanced enzymatic hydrolysability at low peroxide loadings. Biomass. Bioenergy 2017, 96, 96–102. [Google Scholar] [CrossRef]
- Sun, Z.; Bottari, G.; Afanasenko, A.; Stuart, M.C.A.; Deuss, P.J.; Fridrich, B.; Barta, K. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Sticklen, M.B. Plant genetic engineering for biofuel production: Towards affordable cellulosic ethanol. Nat. Rev. Genet. 2008, 9, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Q.; Li, H.; Qureshi, A.S.; Zhang, J.; Bao, X.; Bao, J. Dry biorefining maximizes the potentials of simultaneous saccharification and co-fermentation for cellulosic ethanol production. Biotechnol. Bioeng. 2018, 115, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crop Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Przybysz-Buzała, K.; Kalinowska, H.; Małachowska, E.; Przybysz, P. The utility of selected kraft hardwood and softwood pulps for fuel ethanol production. Ind. Crops. Prod. 2017, 108, 824–830. [Google Scholar] [CrossRef]
- Przybysz, K.; Małachowska, E.; Martyniak, D.; Boruszewski, P.; Iłowska, J.; Kalinowska, H.; Przybysz, P. Yield of pulp, dimensional properties of fibers, and properties of paper produced from fast growing trees and grasses. BioResour. 2018, 13, 1372–1387. [Google Scholar] [CrossRef]
- TAPPI T222 om-02. Acid Insoluble Lignin in Wood and Pulp; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2006.
- TAPPI T204 cm-07. Solvent Extractives of Wood and Pulp; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2007.
- TAPPI T249 cm-09. Carbohydrate Composition of Extractive-Free Wood and Wood Pulp by Gas-Liquid Chromatography; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2009.
- TAPPI T203 cm-09. Alpha, Beta and Gamma-Cellulose in Pulp; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2009.
- TAPPI T211 om-12. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 Degrees C; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2012.
- Modrzejewski, K.; Olszewski, J.; Rutkowski, J. Analysis in Papermaking Industry, 4th ed.; Editorial Office of the Lodz University of Technology: Lodz, Poland, 1969; pp. 60–89. [Google Scholar]
- ISO 5351. Pulps—Determination of Limiting Viscosity Number in Cupriethylenediamine (CED) Solution; International Organization for Standardization: Geneva, Switzerland, 2010.
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Barham, D.; Trinder, P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes–Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Taherzadeh, M.J. A critical review on analysis in pretreatment of lignocelluloses: Degree of polymerization, adsorption/desorption, and accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Andrić, P.; Meyer, A.S.; Jensen, P.A.; Dam Johansen, K. Reactor design for minimizing product inhibition during enzymatic lignocellulose hydrolysis: I. Significance and mechanism of cellobiose and glucose inhibition on cellulolytic enzymes. Biotechnol. Adv. 2010, 28, 308–324. [Google Scholar] [CrossRef] [PubMed]

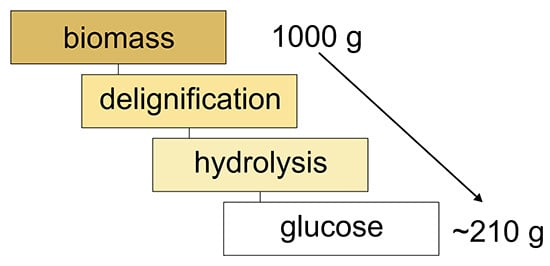
| Material | Cellulose | Hemicelluloses | Lignin | Extractives | Ash |
|---|---|---|---|---|---|
| % DW | |||||
| Miscanthus giganteus | 47.2 ± 0.1 | 25.9 ± 0.8 | 17.8 ± 0.8 | 2.6 ± 0.6 | 6.5 ± 0.2 |
| Tall wheatgrass cultivar A2 | 47.6 ± 0.4 | 29.4 ± 0.7 | 14.1 ± 0.6 | 3.0 ± 0.2 | 5.9 ± 0.1 |
| Smooth bromegrass (Budzynska cultivar) | 35.4 ± 0.1 | 40.2 ± 0.4 | 13.7 ± 0.5 | 4.2 ± 0.5 | 6.5 ± 0.2 |
| Tall fescue | 34.4 ± 0.3 | 40.9 ± 0.3 | 14.0 ± 0.8 | 3.8 ± 0.6 | 6.9 ± 0.3 |
| Striped tuber oat grass | 39.7 ± 0.4 | 39.4 ± 0.7 | 12.2 ± 0.2 | 2.8 ± 0.4 | 5.9 ± 0.3 |
| Switchgrass | 40.7 ± 0.7 | 30.4 ± 0.6 | 17.4 ± 0.9 | 4.1 ± 0.4 | 7.4 ± 0.3 |
| Sweet sorghum cultivar S-N | 32.9 ± 0.3 | 47.4 ± 0.5 | 12.8 ± 0.6 | 3.1 ± 0.2 | 3.8 ± 0.4 |
| Material | Consumption of Bases | The Yield of Pulp from Digester | The Yield of Pulp after Screening | Shives | Kappa Number | DP |
|---|---|---|---|---|---|---|
| % | % | % | % | - | - | |
| Miscanthus giganteus | 98.80 ± 0.03 | 47.82 ± 0.46 | 47.12 ± 0.31 | 1.47 ± 0.18 | 14.31 ± 0.16 | 1649 ± 26 |
| Tall wheatgrass, cultivar A2 | 98.64 ± 0.05 | 44.95 ± 0.28 | 44.22 ± 0.39 | 1.62 ± 0.12 | 13.82 ± 0.24 | 1822 ± 41 |
| Smooth bromegrass (Budzynska cultivar) | 98.84 ± 0.03 | 34.67 ± 0.28 | 34.58 ± 0.16 | 0.27 ± 0.02 | 14.42 ± 0.16 | 1623 ± 57 |
| Tall fescue | 99.35 ± 0.04 | 34.08 ± 0.29 | 34.02 ± 0.23 | 0.18 ± 0.02 | 12.71 ± 0.24 | 1663 ± 38 |
| Striped tuber oat grass | 99.51 ± 0.04 | 33.23 ± 0.39 | 33.19 ± 0.29 | 0.12 ± 0.02 | 8.86 ± 0.25 | 1563 ± 44 |
| Switchgrass | 99.32 ± 0.06 | 34.47 ± 0.31 | 34.09 ± 0.22 | 1.11 ± 0.04 | 13.71 ± 0.12 | 1640 ± 52 |
| Sweet sorghum cultivar S-N | 95.87 ± 0.06 | 15.49 ± 0.23 | 15.46 ± 0.31 | 0.22 ± 0.09 | 7.54 ± 0.14 | 1310 ± 29 |
| Material | Cellulose | Hemicelluloses | Lignin | Extractives | Ash |
|---|---|---|---|---|---|
| % | |||||
| Miscanthus giganteus | 91.5 ± 0.4 | 3.6 ± 0.2 | 2.2 ± 0.2 | 0.3 ± <0.1 | 2.4 ± 0.2 |
| Tall wheatgrass, cultivar A2 | 91.7 ± 0.6 | 4.1 ± 0.3 | 2.0 ± 0.2 | 0.2 ± <0.1 | 2.0 ± 0.1 |
| Smooth bromegrass (Budzynska cultivar) | 90.0 ± 0.5 | 5.4 ± 0.4 | 2.1 ± 0.3 | 0.3 ± <0.1 | 2.2 ± 0.2 |
| Tall fescue | 90.1 ± 0.7 | 5.3 ± 0.4 | 1.7 ± 0.1 | 0.3 ± <0.1 | 2.6 ± 0.1 |
| Striped tuber oat grass | 92.2 ± 0.6 | 4.6 ± 0.2 | 1.1 ± 0.1 | 0.2 ± <0.1 | 1.9 ± 0.1 |
| Switchgrass | 91.4 ± 0.6 | 3.5 ± 0.1 | 2.0 ± 0.1 | 0.2 ± <0.1 | 2.9 ± 0.2 |
| Sweet sorghum cultivar S-N | 92.7 ± 1.2 | 5.1 ± 0.2 | 1.1 ± 0.3 | 0.1 ± <0.1 | 1.0 ± 0.1 |
| Material | Cellulose | Hemicelluloses | Lignin | Extractives | Ash |
|---|---|---|---|---|---|
| % | |||||
| Miscanthus giganteus | 92.7 ± 0.4 | 6.6 ± 0.6 | 5.9 ± 0.4 | 5.5 ± 0.2 | 17.7 ± 0.9 |
| Tall wheatgrass, cultivar A2 | 86.6 ± 0.2 | 6.3 ± 0.5 | 6.4 ± 0.6 | 3.0 ± 0.4 | 15.2 ± 0.6 |
| Smooth bromegrass (Budzynska cultivar) | 88.1 ± 0.1 | 4.7 ± 0.5 | 5.3 ± 0.3 | 2.5 ± 0.4 | 11.7 ± 0.6 |
| Tall fescue | 89.3 ± 0.4 | 4.4 ± 0.4 | 4.1 ± 0.5 | 2.7 ± 0.5 | 12.8 ± 0.4 |
| Striped tuber oat grass | 77.2 ± 0.5 | 3.9 ± 0.2 | 3.0 ± 0.2 | 2.4 ± 0.6 | 10.7 ± 0.5 |
| Switchgrass | 77.4 ± 0.3 | 4.0 ± 0.4 | 4.0 ± 0.2 | 1.7 ± 0.2 | 13.5 ± 0.7 |
| Sweet sorghum cultivar S-N | 43.6 ± 0.2 | 1.7 ± 0.1 | 1.3 ± 0.1 | 0.5 ± 0.1 | 4.1 ± 0.3 |
| Pulp Origin | Insoluble Residues | ||
|---|---|---|---|
| % DW Pulp | |||
| NS-81235 Load | |||
| 0.1 mL/g | 0.05 mL/g | 0.03 mL/g | |
| Miscanthus giganteus | 7.2 ± 0.1 | 8.4 ± 0.3 | 35.3 ± 0.1 |
| Tall wheatgrass, cultivar A2 | 8.2 ± 0.3 | 8.7 ± 0.3 | 38.5 ± 0.4 |
| Smooth bromegrass (Budzynska cultivar) | 11.0 ± 0.1 | 11.7 ± 0.5 | 20.0 ± 0.3 |
| Tall fescue | 8.9 ± 0.2 | 11.2 ± 0.2 | 18.8 ± 0.4 |
| Striped tuber oat grass | 7.2 ± 0.1 | 8.4 ± 0.1 | 10.5 ± 0.3 |
| Switchgrass | 10.0 ± 0.5 | 11.4 ± 0.1 | 21.6 ± 0.3 |
| Sweet sorghum cultivar S-N | 8.4 ± 0.1 | 11.4 ± 0.1 | 32.1 ± 0.1 |
| Grass | Glucose Yield | |||||
|---|---|---|---|---|---|---|
| % DW Pulp | % DW Biomass | |||||
| 0.476% w/v | 3.88% w/v | 7.46% w/v | 0.476% w/v | 3.88% w/v | 7.46% w/v | |
| Miscanthus giganteus | 62.0 ± 0.1 | 47.9 ± 0.6 | 35.7 ± 0.4 | 29.2 ± 0.1 | 22.6 ± 0.6 | 16.8 ± 0.4 |
| Tall wheatgrass, cultivar A2 | 75.5 ± 0.4 | 45.0 ± 0.1 | 41.1 ± 0.2 | 33.4 ± 0.4 | 19.9 ± 0.1 | 18.2 ± 0.2 |
| Smooth bromegrass Budzynska cultivar | 61.5 ± 0.3 | 42.9 ± 0.2 | 19.8 ± 0.3 | 21.3 ± 0.3 | 14.8 ± 0.2 | 6.8 ± 0.3 |
| Tall fescue | 54.5 ± 0.1 | 46.2 ± 0.2 | 45.4 ± 0.2 | 18.5 ± 0.1 | 15.7 ± 0.2 | 15.4 ± 0.2 |
| Striped tuber oat grass | 59.7 ± 0.1 | 58.0 ± 0.1 | 49.0 ± 0.4 | 19.8 ± 0.1 | 19.3 ± 0.1 | 16.3 ± 0.4 |
| Switchgrass | 51.8 ± 0.3 | 43.4 ± 0.1 | 17.4 ± 0.5 | 17.7 ± 0.3 | 14.8 ± 0.2 | 6.8 ± 0.5 |
| Sweet sorghum cultivar S-N | 50.2 ± 0.2 | 49.0 ± 0.5 | 45.2 ± 0.1 | 7.8 ± 0.2 | 7.6 ± 0.5 | 7.0 ± 0.1 |
| Grass | Glucose Yield | ||
|---|---|---|---|
| % DW Cellulose | |||
| 0.476% w/v | 3.88% w/v | 7.46% w/v | |
| Miscanthus giganteus | 61.86 | 47.88 | 35.59 |
| Tall wheatgrass cultivar A2 | 70.17 | 41.81 | 38.24 |
| Smooth bromegrass (Budzynska cultivar) | 60.17 | 41.81 | 19.21 |
| Tall fescue | 53.78 | 45.64 | 44.77 |
| Striped tuber oat grass | 49.87 | 48.61 | 41.06 |
| Switchgrass | 43.49 | 36.36 | 14.50 |
| Sweet sorghum cultivar S-N | 23.71 | 23.10 | 21.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybysz, K.; Małachowska, E.; Martyniak, D.; Boruszewski, P.; Kalinowska, H.; Przybysz, P. Production of Sugar Feedstocks for Fermentation Processes from Selected Fast Growing Grasses. Energies 2019, 12, 3129. https://doi.org/10.3390/en12163129
Przybysz K, Małachowska E, Martyniak D, Boruszewski P, Kalinowska H, Przybysz P. Production of Sugar Feedstocks for Fermentation Processes from Selected Fast Growing Grasses. Energies. 2019; 12(16):3129. https://doi.org/10.3390/en12163129
Chicago/Turabian StylePrzybysz, Kamila, Edyta Małachowska, Danuta Martyniak, Piotr Boruszewski, Halina Kalinowska, and Piotr Przybysz. 2019. "Production of Sugar Feedstocks for Fermentation Processes from Selected Fast Growing Grasses" Energies 12, no. 16: 3129. https://doi.org/10.3390/en12163129
APA StylePrzybysz, K., Małachowska, E., Martyniak, D., Boruszewski, P., Kalinowska, H., & Przybysz, P. (2019). Production of Sugar Feedstocks for Fermentation Processes from Selected Fast Growing Grasses. Energies, 12(16), 3129. https://doi.org/10.3390/en12163129