A 3-In-1 Approach to Evaluate Gas Hydrate Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
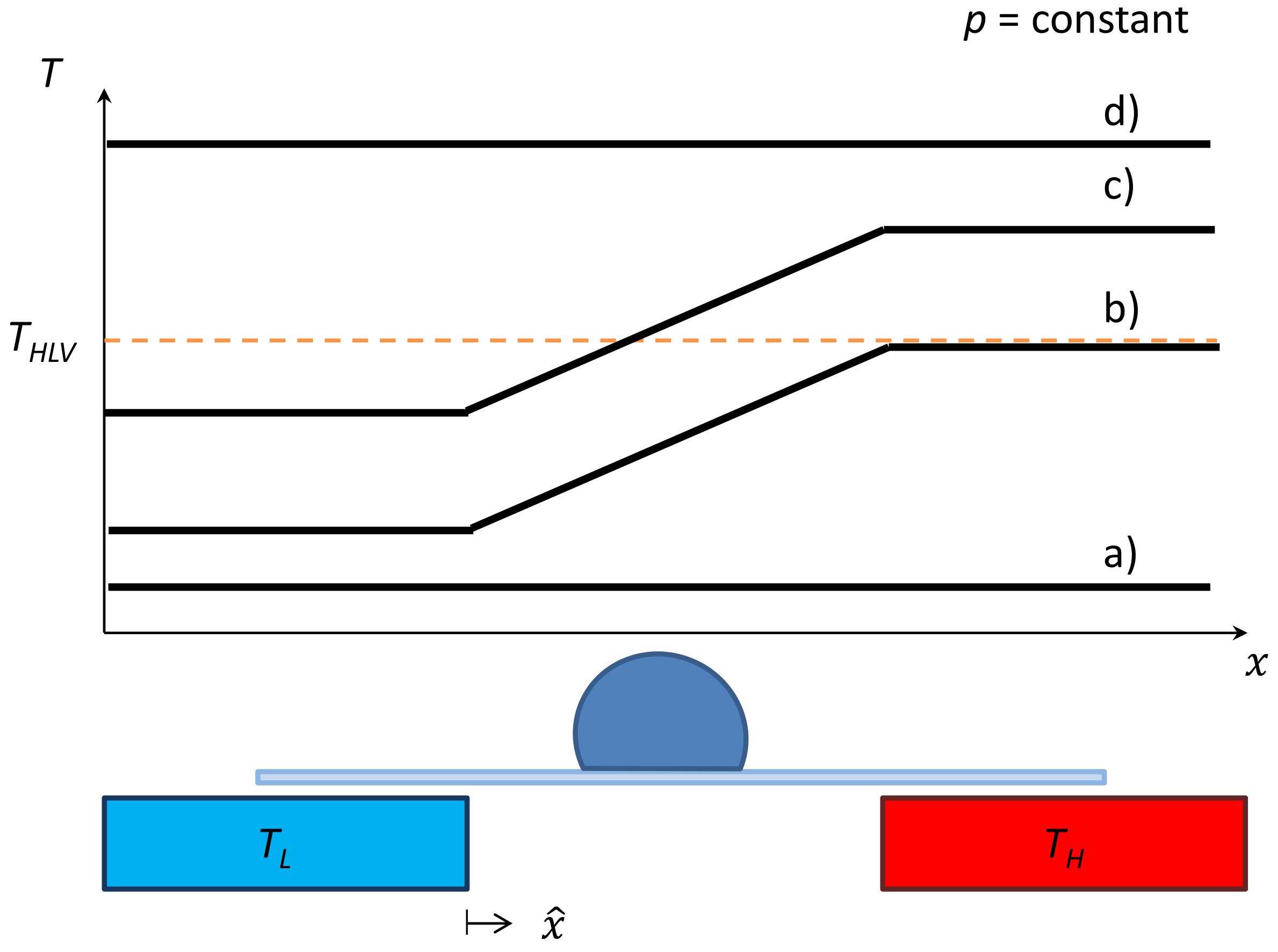
2.2. Apparatus
2.3. Methods
2.3.1. Crystal Formation History
2.3.2. Hydrate Formation
2.3.3. Hydrate Dissociation
3. Results and Discussion
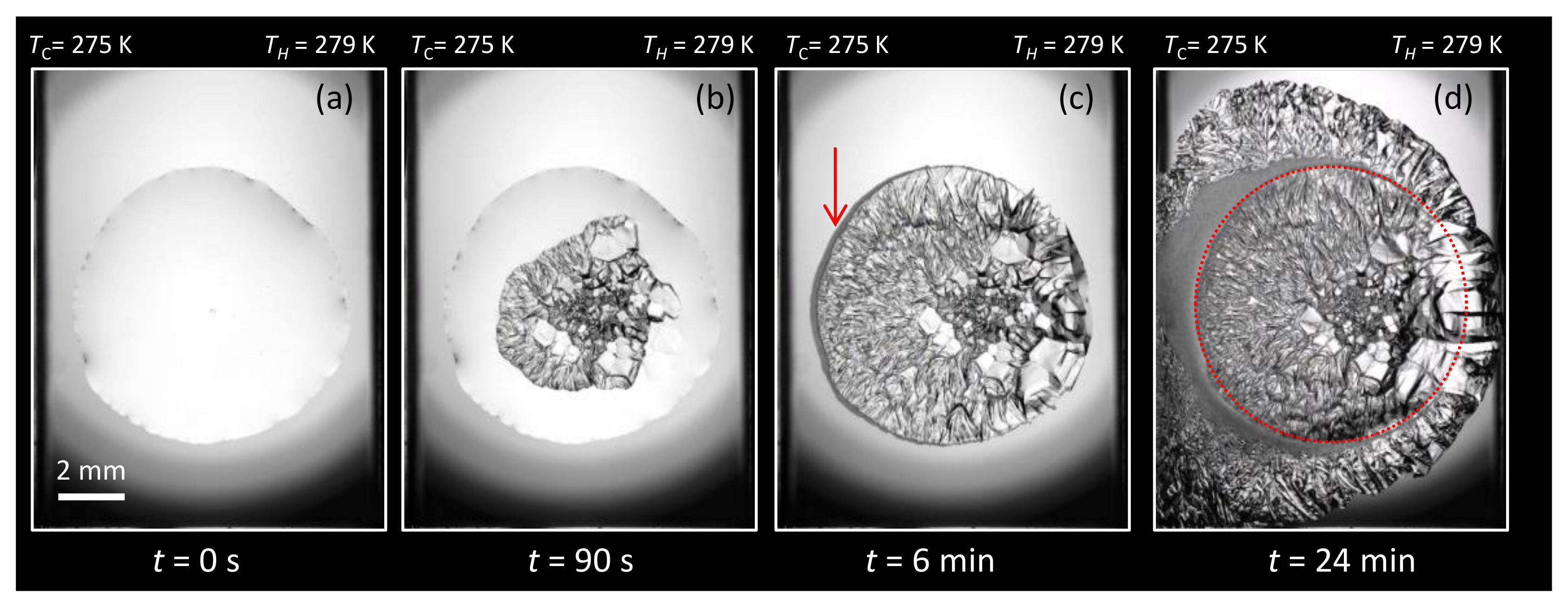
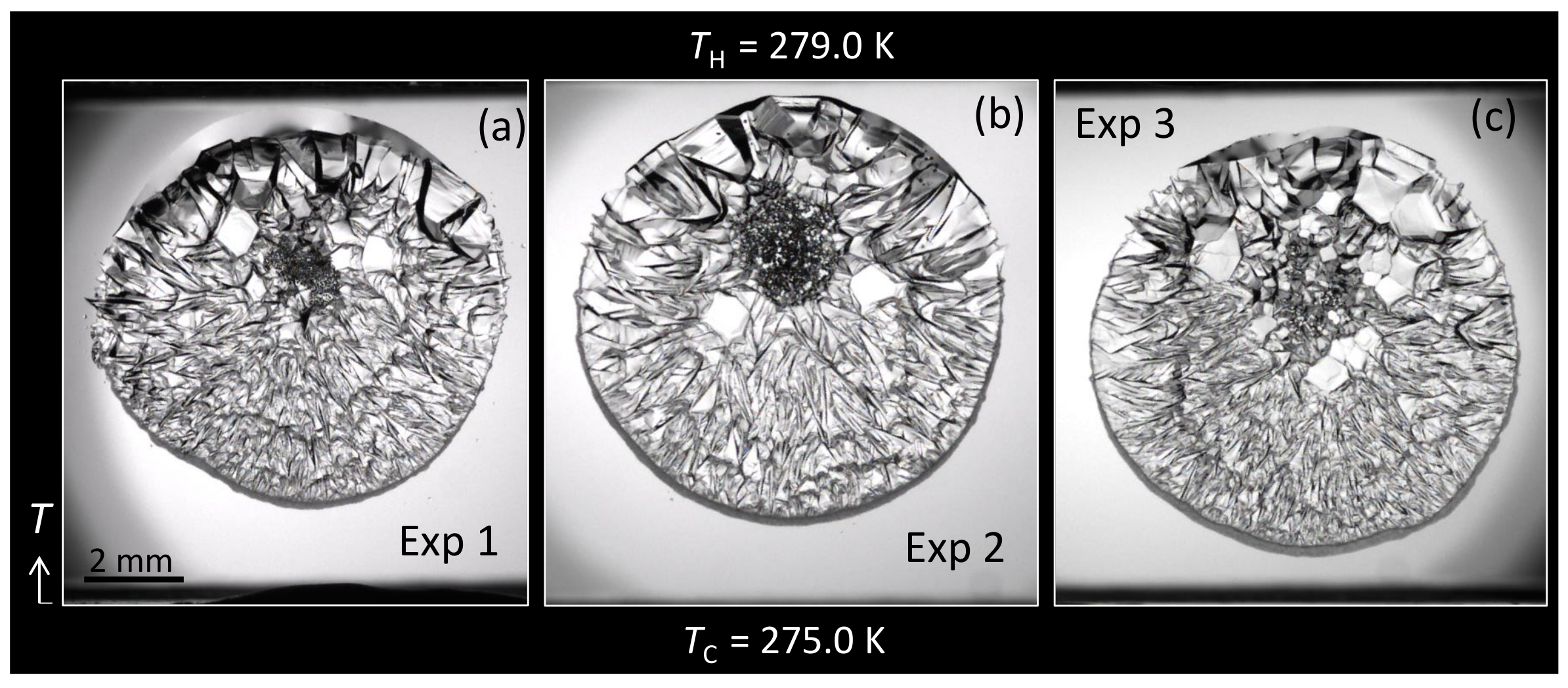
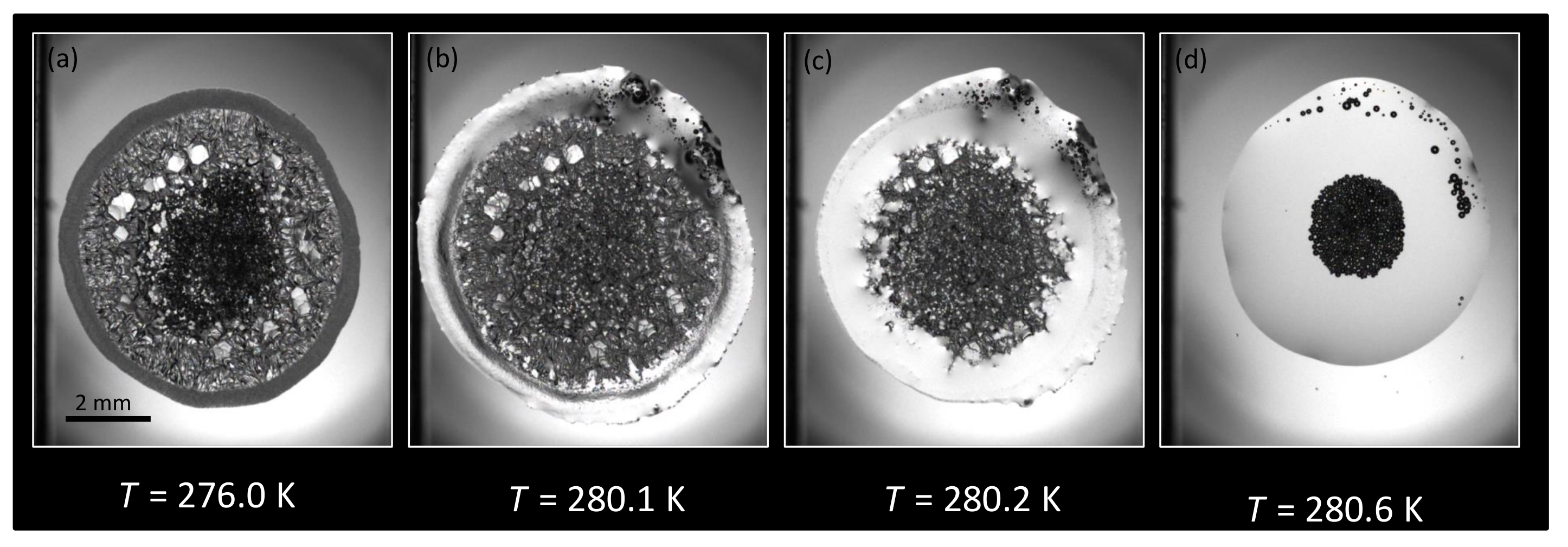
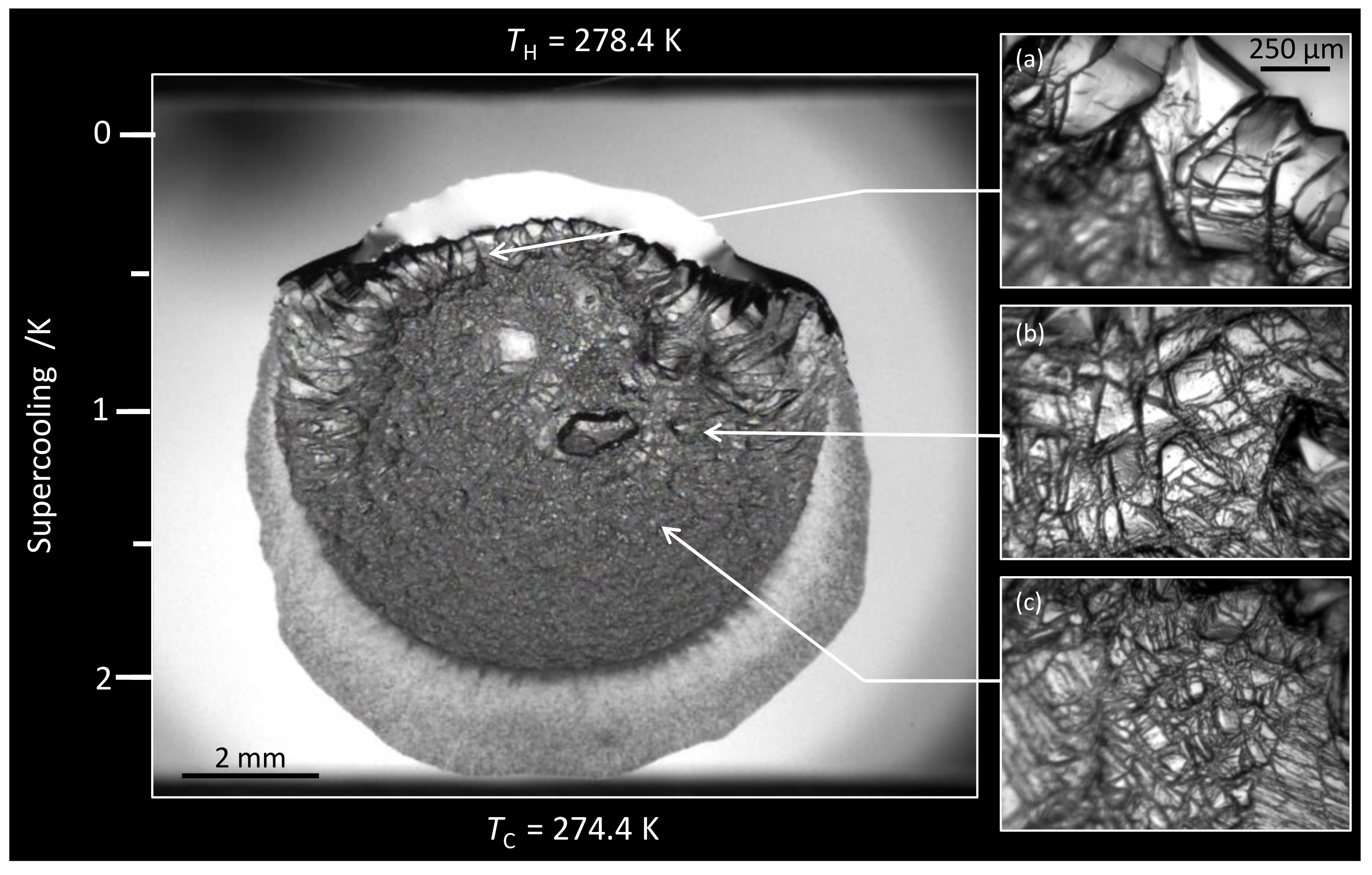
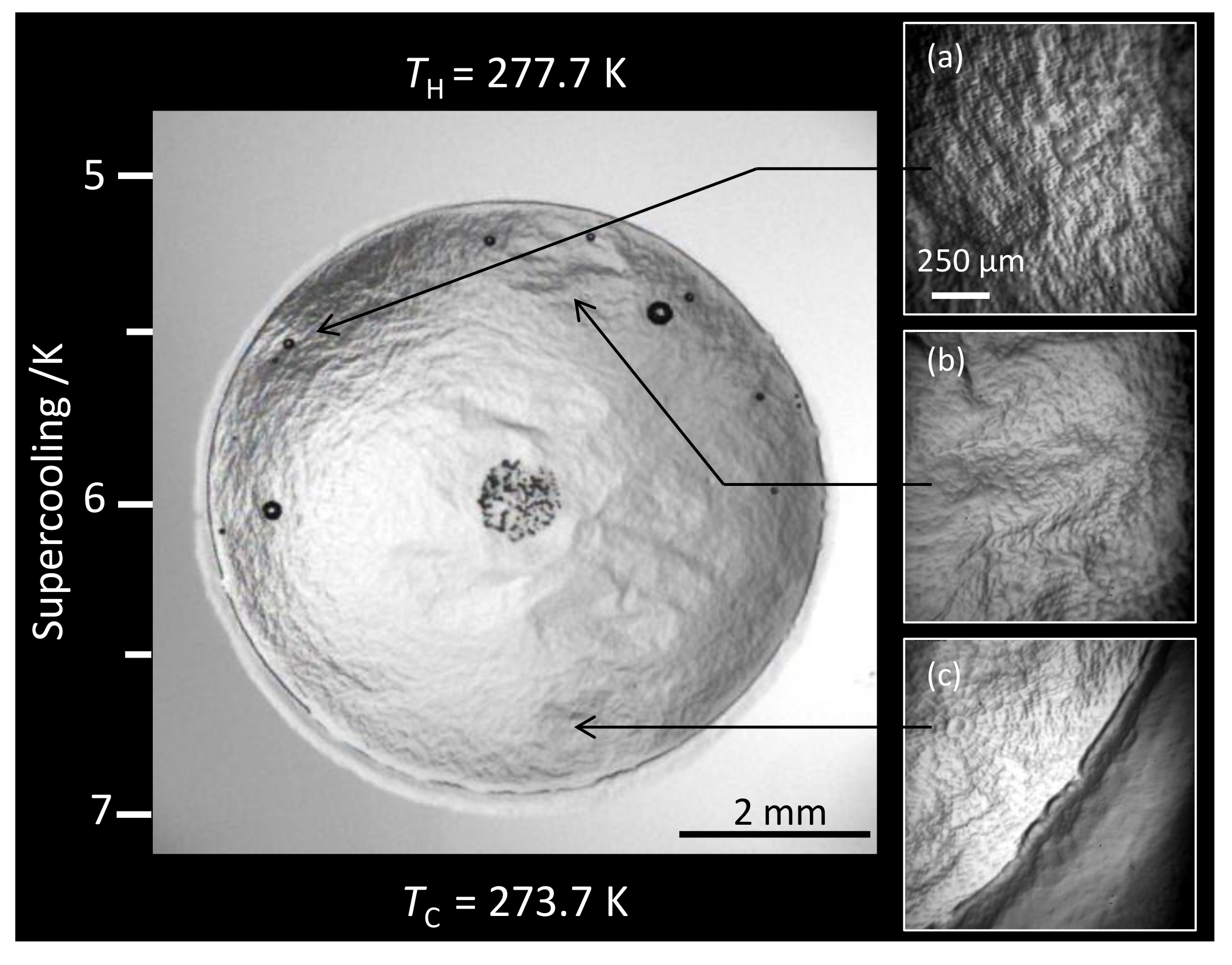
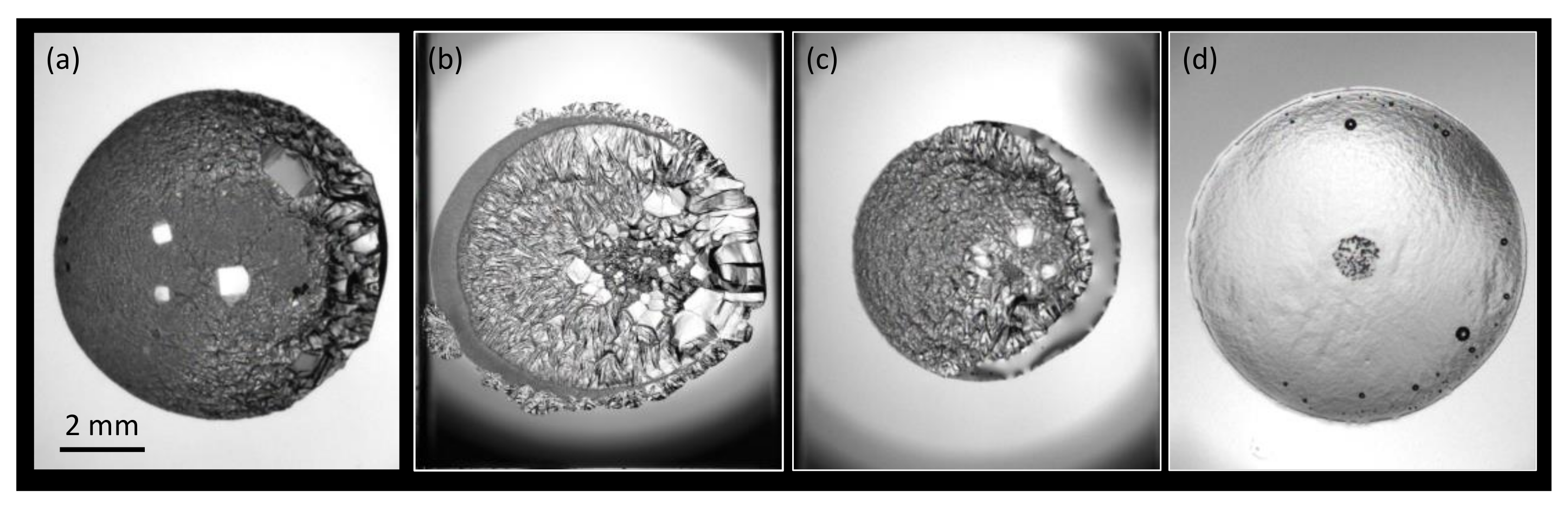
3.1. Morphology
3.1.1. Water + + MEG ()
3.1.2. Water + CH + NaCl ( = 5.44%)
3.1.3. Water + CH + PVP ()
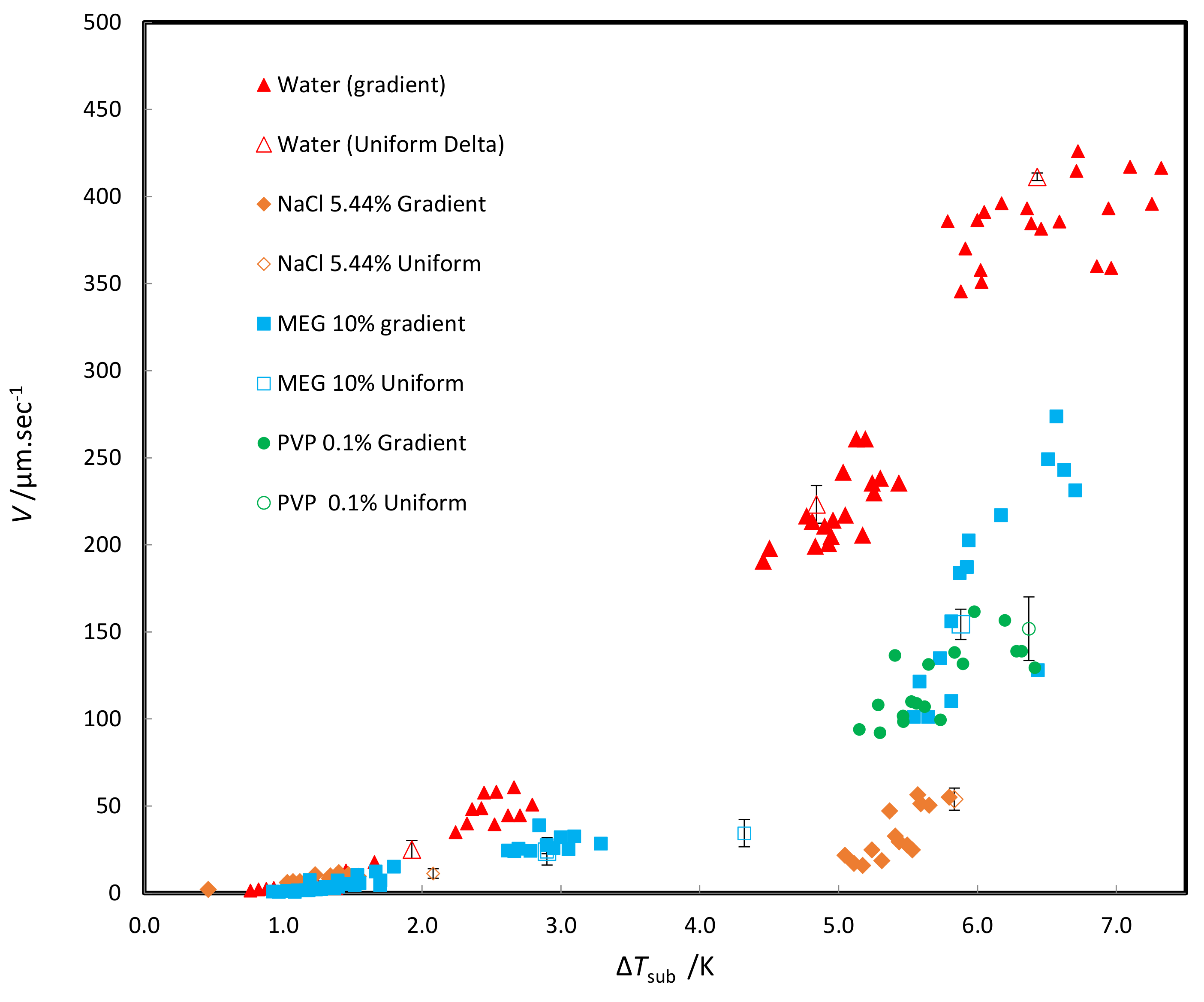
3.2. Overall Result
3.2.1. Overall Morphology
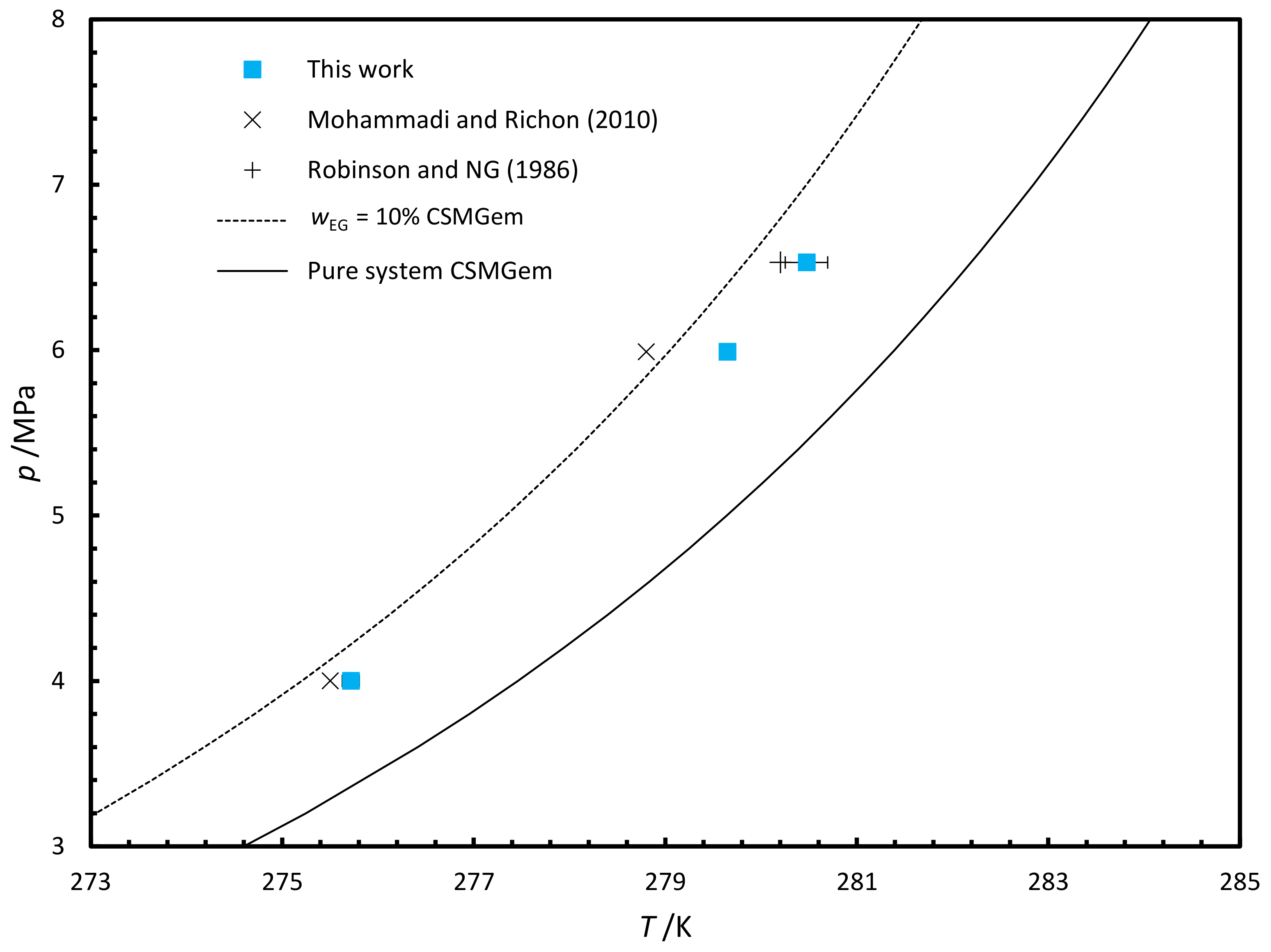
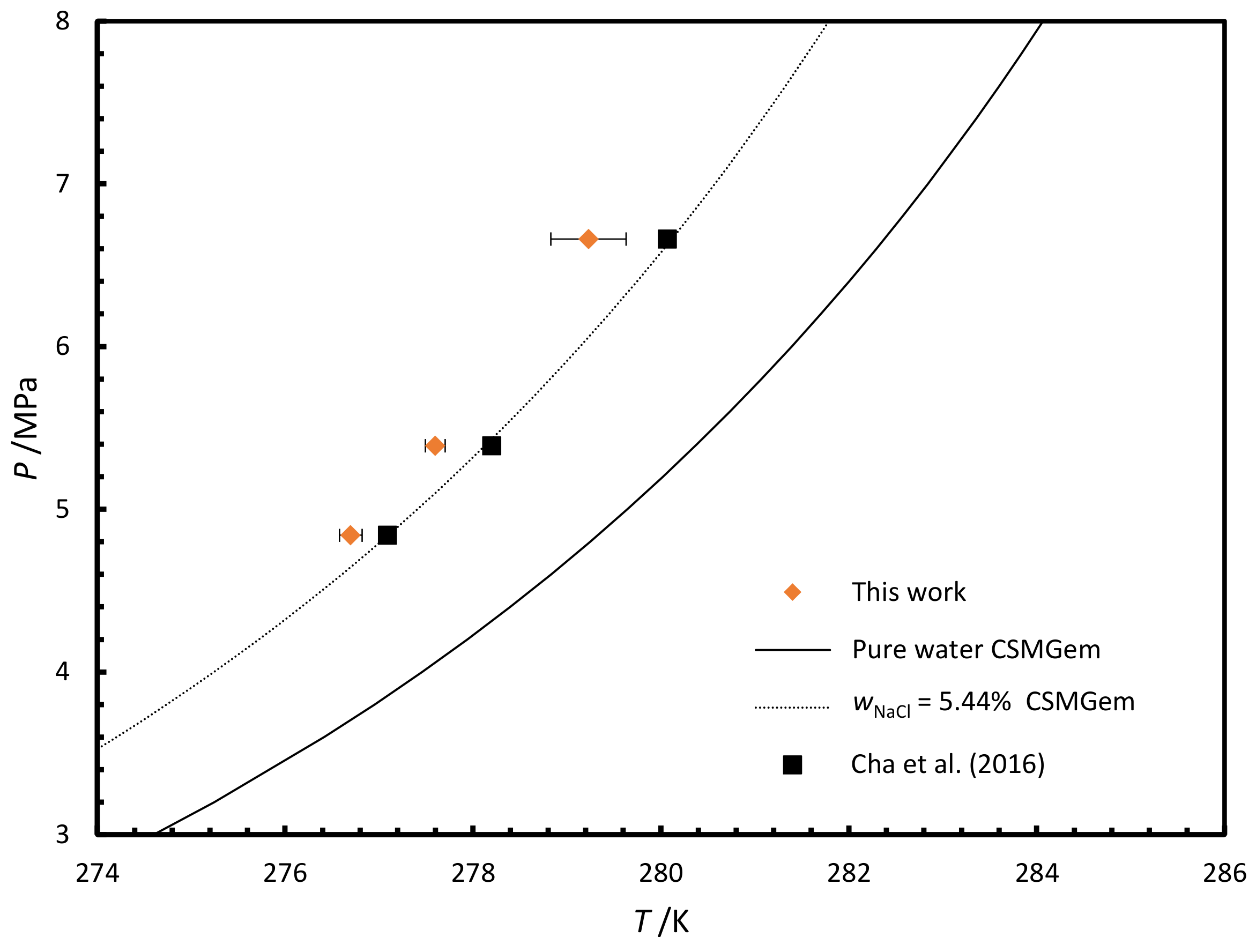
3.2.2. Phase Equilibria
3.2.3. Apparent Kinetics
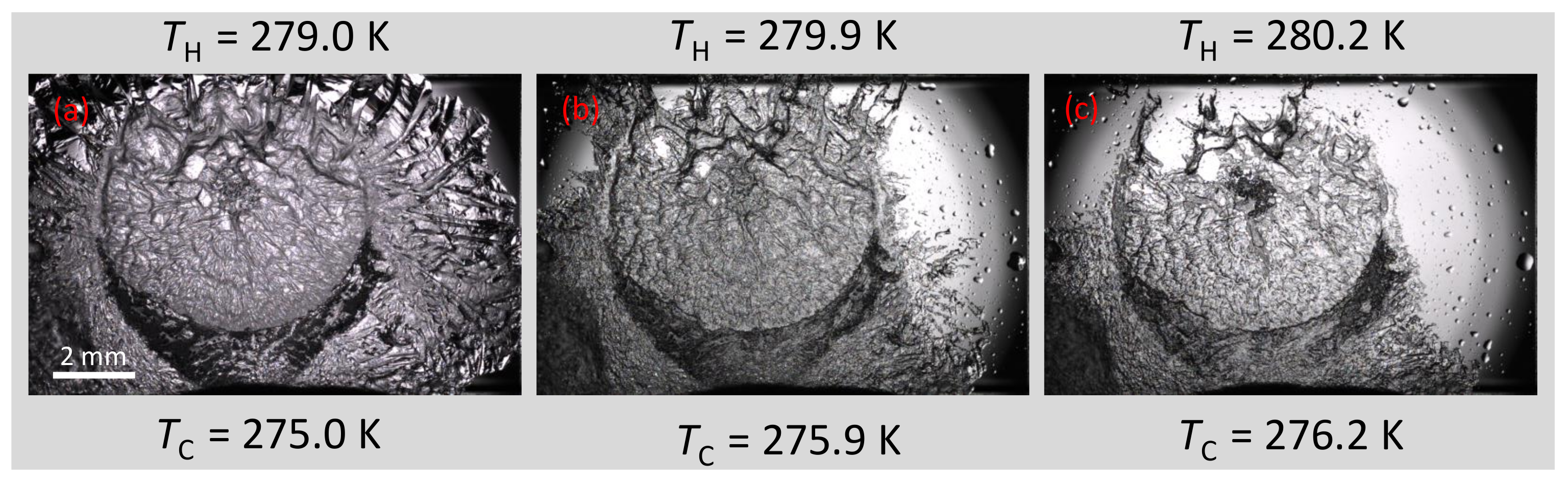
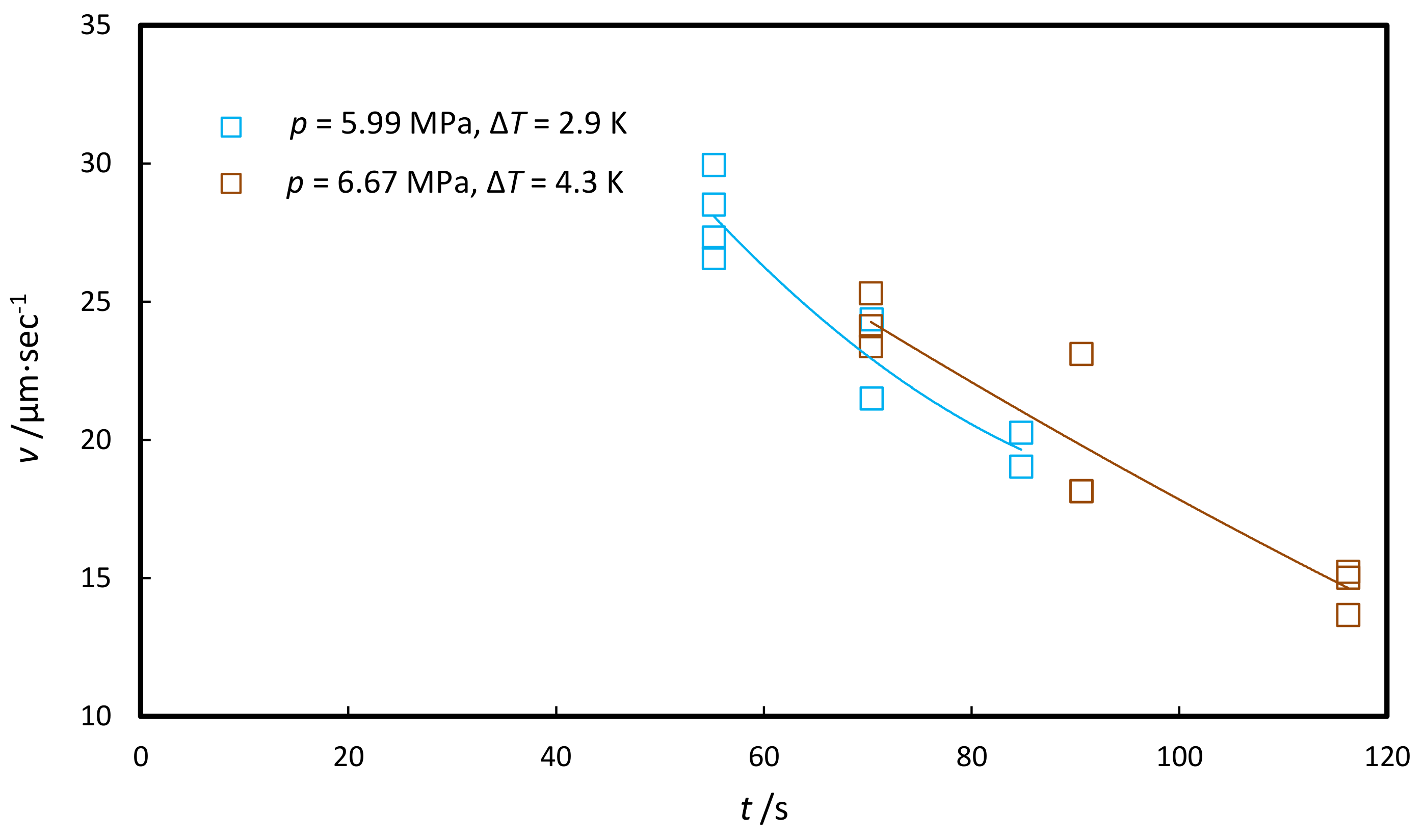
3.2.4. Hydrate Halo Growth
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van der Waals, J.H.; Platteeuw, J.C. Clathrate solutions. Adv. Chem. Phys. 1959, 2, 1–57. [Google Scholar]
- Schicks, J.M.; Reepmester, J.A. The Coexistence of Two Different Methane Hydrate Phases under Moderate Pressure and Temperature Conditions: Kinetic versus Thermodynamic Products. Chem. Int. Ed. 2004, 43, 3310–3313. [Google Scholar] [CrossRef] [PubMed]
- Urdahl, O.; Lund, A.; Mork, P.; Nilsen, T. Inhibition of gas hydrate formation by means of chemical additives—I. Development of an experimental set-up for characterization of gas hydrate inhibitor efficiency with respect to flow properties and deposition. Chem. Eng. Sci. 1995, 50, 863–870. [Google Scholar] [CrossRef]
- Lafond, P.G.; Olcott, K.A.; Sloan, E.D.; Koh, C.A.; Sum, A.K. Measurements of methane hydrate equilibrium in systems inhibited with NaCl and methanol. J. Chem. Thermodyn. 2012, 48, 1–6. [Google Scholar] [CrossRef]
- Perfeldt, C.M.; Chua, P.C.; Daraboina, N.; Friis, D.; Kristiansen, E.; Ramlov, H.; Woodley, J.M.; Kelland, M.A.; Von Solms, N. Inhibition of Gas Hydrate Nucleation and Growth: Efficacy of an Antifreeze Protein from the longhorn beetle Rhagium mordax. Energy Fuels 2014, 28, 3666–3672. [Google Scholar] [CrossRef]
- Cha, M.; Shin, K.; Kim, J.; Chang, D.; Seo, Y.; Lee, H.; Kang, S. Thermodynamic and kinetic hydrate inhibition performance of aqueous ethylene glycol solutions for natural gas. Chem. Eng. Sci. 2013, 99, 184–213. [Google Scholar] [CrossRef]
- Chong, Z.R.; Chana, A.H.M.; Babu, P.; Yanga, M.; Linga, P. Effect of NaCl on methane hydrate formation and dissociation in porous media. J. Nat. Gas Sci. Eng. 2015, 27, 178–189. [Google Scholar] [CrossRef]
- Nagashima, K.; Yamamoto, Y.; Komai, T.; Hoshino, H.; Ohga, K. Interferometric Observation of Salt Concentration Distribution in Liquid Phase Around THF Clathrate Hydrate During Directional Growth. J. Jpn. Inst. Energy 1999, 78, 326–331. [Google Scholar] [CrossRef]
- Davenport, J.R.; Musa, O.M.; Paterson, M.J.; Piepenbrock, M.M.; Fucke, K.; Steed, J.W. A simple chemical model for clathrate hydrate inhibition by polyvinylcaprolactam. Chem. Commun. 2011, 35, 9891–9893. [Google Scholar] [CrossRef]
- Kelland, M.A.; Svartaas, T.M.; Ovsthus, J.; Tomita, T.; Mizuta, K. Studies on some alkylamide surfactant gas hydrate antiagglomerants. Chem. Eng. Sci. 2006, 61, 4290–4298. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007; pp. 32–58. [Google Scholar]
- Posteraro, D.; Ivall, J.; Maric, M.; Servio, P. New insights into the effect of polyvinylpyrrolidone (PVP) concentration on methane hydrate growth. 2. Liquid phase methane mole fraction. Chem. Eng. Sci. 2015, 126, 91–98. [Google Scholar] [CrossRef]
- Posteraro, D.; Verrett, J.; Maric, M.; Servio, P. Hydrate anti-agglomeration and synergy effect in normal octane at varying water cuts and salt concentrations. J. Nat. Gas Sci. Eng. 2016, 34, 1–5. [Google Scholar] [CrossRef]
- Bruusgaard, H.; Lessard, L.D.; Servio, P. Morphology study of structure I methane hydrate formation and decomposition of water droplets in the presence of biological and polymeric kinetic inhibitors. Cryst. Growth Des. 2009, 9, 3014–3023. [Google Scholar] [CrossRef]
- Walker, V.K.; Zeng, H.; Ohno, H.; Daraboina, N.; Sharifi, H.; Bagherzadeh, S.A.; Alavi, S.; Englezos, P. Antifreeze proteins as gas hydrate inhibitors. Can. J. Chem. 2015, 93, 839–849. [Google Scholar] [CrossRef]
- Bergeron, S.; Beltran, J.G.; Servio, P. Reaction rate constant of methane clathrate formation. Fuel 2010, 2, 294–301. [Google Scholar] [CrossRef]
- Englezos, P.; Kalogerakis, N.; Dholabhai, P.D.; Bishnoi, P.R. Kinetics of formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1987, 42, 2647–2658. [Google Scholar] [CrossRef]
- Lederhos, J.P.; Long, J.P.; Sum, A.; Christiansen, R.L.; Sloan, E.D. Effective kinetic inhibitors for natural gas hydrates. Chem. Eng. Sci. 1996, 51, 1221–1229. [Google Scholar] [CrossRef]
- Tohidi, B.; Anderson, R.; Mozaffar, H.; Tohidi, F. The return of kinetic hydrate inhibitors. Energy Fuels 2015, 29, 8254–8260. [Google Scholar] [CrossRef]
- Bruusgaard, H.; Beltran, J.G.; Servio, P. Vapor-liquid water-hydrate equilibrium data for the system N2 + CO2 + H2O. J. Chem. Eng. Data 2008, 53, 2594–2597. [Google Scholar] [CrossRef]
- Luna-Ortiz, E.; Healey, M.; Anderson, R.; Sorhaug, E. Crystal Growth Inhibition Studies for the Qualification of a Kinetic Hydrate Inhibitor under Flowing and Shut-In Conditions. Energy Fuels 2014, 28, 2902–2913. [Google Scholar] [CrossRef]
- Huo, Z.; Freer, E.; Lamar, M.; Sannigrahi, B.; Knauss, D.M.; Sloan, E.D. Hydrate plug prevention by anti-agglomeration. Chem. Eng. Sci. 2001, 56, 4979–4991. [Google Scholar] [CrossRef]
- Chua, P.C.; Kelland, M.A. Study of the Gas Hydrate Anti-agglomerant Performance of a Series of n-Alkyl-tri (n-butyl) ammonium Bromides. Energy Fuels 2013, 27, 1285–1292. [Google Scholar] [CrossRef]
- Bishnoi, P.R. Gas Hydrate—My Personal Ecperinces and Challenges. In Proceedings of the Fifth International Conference on Gas Hydrates, Trondheim, Norway, 13–16 June 2005; pp. 1–18. [Google Scholar]
- DuQuesnay, J.R.; Posada, M.C.D.; Beltran, J.G. Novel gas hydrate reactor design: 3-in-1 assessment of phase equilibria, morphology and kinetics. Fluid Phase Equilib. 2015, 413, 148–157. [Google Scholar] [CrossRef]
- Udegbunam, L.U.; DuQuesnay James, R.; Osorio, L.; Walker, V.K.; Beltran, J.G. Phase equilibria, kinetics and morphology of methane hydrate inhibited by antifreeze proteins: Application of a novel 3-in-1 method. J. Chem. Thermodyn. 2018, 117, 155–163. [Google Scholar] [CrossRef]
- Sowa, B.; Maeda, N. Statistical Study of the Memory Effect in Model Natural Gas Hydrate Systems. J. Phys. Chem. A 2015, 119, 10784–10790. [Google Scholar] [CrossRef]
- Takeya, S.; Hori, A.; Hondoh, T.; Uchida, T. Freezing-Memory Effect of Water on Nucleation of CO2 Hydrate Crystals. J. Phys. Chem. B 2000, 104, 4164–4168. [Google Scholar] [CrossRef]
- Buchanan, P.; Soper, A.K.; Westacott, R.E.; Creek, J.L.; Hobson, G.; Koh, C.A. Search for memory effects in methane hydrate: Structure of water before hydrate formation and after hydrate decomposition. J. Chem. Phys. 2005, 123, 164507. [Google Scholar] [CrossRef]
- Thompson, H.; Soper, A.K.; Buchanan, P.; Aldiwan, N.; Creek, J.L.; Koh, C.A. Methane hydrate formation and decomposition: Structural studies via neutron diffraction and empirical potential structure refinement. J. Chem. Phys. 2006, 124, 164508–464508. [Google Scholar] [CrossRef]
- Esmail, S.; Beltran, J.G. Methane hydrate propagation on surfaces of varying wettability. J. Nat. Gas Sci. Eng. 2016, 35, 1535–1543. [Google Scholar] [CrossRef]
- Beltran, J.G.; Servio, P. Morphological Investigations of Methane- Hydrate Films Formed on a Glass Surface. Cryst. Growth Des. 2010, 10, 4339–4347. [Google Scholar] [CrossRef]
- Sakemoto, R.; Sakamoto, H.; Shiraiwa, K.; Ohmura, R.; Uchida, T. Clathrate hydrate crystal growth at the seawater/hydrophobic- guest- liquid interface. Cryst. Growth Des. 2009, 10, 1296–1300. [Google Scholar] [CrossRef]
- Folas, G.K.; Berg, O.J.; Solbraa, E.; Fredheim, A.O.; Kontogeorgis, A.M.; Michelsen, M.L.; Stenby, E.H. High-pressure vapor–liquid equilibria of systems containing ethylene glycol, water and methane: Experimental measurements and modeling. Fluid Phase Equilib. 2007, 251, 52–58. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.; Han, G.; Guo, X.; Guo, T. Experimental study on the solubility of natural gas components in water with or without hydrate inhibitor. Fluid Phase Equilib. 2003, 1, 143–154. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lim, B.D.; Lee, J.E.; Lee, C.S. Solubilities of Carbon Dioxide, Methane, and Ethane in Sodium Chloride Solution Containing Gas Hydrate. J. Chem. Eng. Data 2008, 53, 1351–1354. [Google Scholar] [CrossRef]
- Bzdek, B.R.; Power, R.M.; Simpson, S.H.; Reid, J.P.; Royall, C.P. Precise, contactless measurements of the surface tension of picolitre aerosol droplets. Chem. Sci. 2016, 1, 274–285. [Google Scholar] [CrossRef]
- Tsierkezos, N.G.; Molinou, I.E. Thermodynamic properties of water+ ethylene glycol at 283.15, 293.15, 303.15, and 313.15 K. J. Chem. Eng. Data 1998, 6, 989–993. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.C.; Lee, J.D. Morphology study of methane–propane clathrate hydrates on the bubble surface in the presence of SDS or PVCap. J. Cryst. Growth 2014, 402, 249–259. [Google Scholar] [CrossRef]
- Wu, R.; Kozielski, K.A.; Hartley, P.G.; May, E.F.; Boxall, J.; Maeda, N. Methane–propane mixed gas hydrate film growth on the surface of water and Luvicap EG solutions. Energy Fuels 2013, 27, 2548–2554. [Google Scholar] [CrossRef]
- Cha, M.; Shin, K.; Seo, Y.; Shin, J.; Kang, S. Catastrophic growth of gas hydrates in the presence of kinetic hydrate inhibitors. J. Phys. Chem. A 2013, 117, 13988–13995. [Google Scholar] [CrossRef]
- Kumar, R.; Lee, J.D.; Song, M.; Englezos, P. Kinetic inhibitor effects on methane/propane clathrate hydrate-crystal growth at the gas/water and water/n-heptane interfaces. J. Cryst. Growth 2008, 310, 1154–1166. [Google Scholar] [CrossRef]
- Lee, J.D.; Englezos, P. Unusual kinetic inhibitor effects on gas hydrate formation. Chem. Eng. Sci. 2006, 61, 1368–1376. [Google Scholar] [CrossRef]
- Sharifi, H.; Englezos, P. Accelerated Hydrate Crystal Growth in the Presence of Low Dosage Additives Known as Kinetic Hydrate Inhibitors. J. Chem. Eng. Data 2014, 60, 336–342. [Google Scholar] [CrossRef]
- Sharifi, H.; Ripmeester, J.; Walker, V.K.; Englezos, P. Kinetic inhibition of natural gas hydrates in saline solutions and heptane. Fuel 2014, 117, 109–117. [Google Scholar] [CrossRef]
- Sharifi, H.; Walker, V.K.; Ripmeester, J.; Englezos, P. Insights into the behavior of biological clathrate hydrate inhibitors in aqueous saline solutions. Cryst. Growth Des. 2014, 14, 2923–2930. [Google Scholar] [CrossRef]
- Sharifi, H.; Ripmeester, J.; Englezos, P. Recalcitrance of gas hydrate crystals formed in the presence of kinetic hydrate inhibitors. J. Nat. Gas Sci. Eng. 2016, 35, 1573–1578. [Google Scholar] [CrossRef] [Green Version]
- Daraboina, N.; Ripmeester, J.A.; Walker, V.K.; Englezos, P. Natural gas hydrate formation and decomposition in the presence of kinetic inhibitors. 1. High pressure calorimetry. Energy Fuels 2011, 10, 4392–4397. [Google Scholar] [CrossRef]
- Daraboina, N.; Ripmeester, J.A.; Walker, V.K.; Englezos, P. Natural gas hydrate formation and decomposition in the presence of kinetic inhibitors. 3. Structural and compositional changes. Energy Fuels 2011, 10, 4398–4404. [Google Scholar] [CrossRef]
- Makogon, Y.F.; Makogon, T.Y.; Holditch, S.A. Kinetics and mechanisms of gas hydrate formation and dissociation with inhibitors. Ann. N. Y. Acad. Sci. 2000, 912, 777–796. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–363. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Gas hydrate phase equilibrium in the presence of ethylene glycol or methanol aqueous solution. Ind. Eng. Chem. Res. 2010, 49, 8865–8869. [Google Scholar] [CrossRef]
- Robinson, D.B. Hydrate Formation and Inhibition in Gas or Gas Condensate Streams. Can. Pet. Technol. 1986, 25. [Google Scholar] [CrossRef]
- Cha, M.; Hu, Y.; Sum, A.K. Methane hydrate phase equilibria for systems containing NaCl, KCl, and NH4Cl. Fluid Phase Equilib. 2016, 413, 2–9. [Google Scholar] [CrossRef]
- Anderson, R.; Mozaffar, H.; Tohidi, B.; Glenat, P. Applicatio of a crystal growth inhibition based KHI evaluation method to commercial formulation assessment. In Proceedings of the 7th International Conference on Gas Hydrates, Edinburgh, Scotland, 17–21 July 2011. [Google Scholar]
- Kishimoto, M.; Iijima, S.; Ohmura, R. Crystal growth of clathrate hydrate at the interface between seawater and hydrophobic-guest liquid: Effect of elevated salt concentration. Ind. Eng. Chem. Res. 2012, 51, 5224–5229. [Google Scholar] [CrossRef]
- Peng, B.; Sun, C.; Chen, G.; Yang, L.; Zhou, W.; Pang, W. Hydrate film growth at the interface between gaseous CO2 and sodium chloride solution. Sci. China Chem. 2009, 52, 676–682. [Google Scholar] [CrossRef]
- Uchida, T.; Ikeda, I.Y.; Takeya, S.; Ebinuma, T.; Nagao, J.; Narita, H. CO2 hydrate film formation at the boundary between CO2 and water: Effects of temperature, pressure and additives on the formation rate. J. Cryst. Growth 2002, 237, 383–387. [Google Scholar] [CrossRef]
- Gordienko, R.; Ohno, H.; Singh, V.K.; Jia, Z.; Ripmeester, J.A.; Walker, V.K. Towards a green hydrate inhibitor: Imaging antifreeze proteins on clathrates. PLoS ONE 2010, 5, e8953. [Google Scholar] [CrossRef]
- Jensen, L.; Ramlov, H.; Thomsen, K.; Von Solms, N. Inhibition of methane hydrate formation by ice-structuring proteins. Ind. Eng. Chem. Res. 2010, 49, 1486–1492. [Google Scholar] [CrossRef]
- Kitamura, M.; Mori, Y.H. Clathrate-hydrate film growth along water/methane phase boundaries-an observational study. Cryst. Res. Technol. 2013, 48, 511–519. [Google Scholar] [CrossRef]
- Daraboina, N.; Moudrakovski, I.L.; Ripmeester, J.A.; Walker, V.K.; Englezos, P. Assessing the performance of commercial and biological gas hydrate inhibitors using nuclear magnetic resonance microscopy and a stirred autoclave. Fuel 2013, 105, 630–635. [Google Scholar] [CrossRef]
- Ivall, J.; Pasieka, J.; Posteraro, D.; Servio, P. Profiling the Concentration of the Kinetic Inhibitor Polyvinylpyrrolidone throughout the Methane Hydrate Formation Process. Energy Fuels 2015, 29, 2329–2335. [Google Scholar] [CrossRef]
- Mori, Y.; Mochizuki, T. Modeling of Mass Transport across a Hydrate Layer Intervening between Liquid Water and ‘Guest’ Fluid Phases. In Proceedings of the 2nd International Symposium on Gas Hydrates, Daejeon, Korea, 1–2 November 1996; pp. 267–274. [Google Scholar]
- Mori, Y.H.; Mochizuki, T. Mass transport across clathrate hydrate films a capillary permeation model. Chem. Eng. Sci. 1997, 52, 3613–3616. [Google Scholar] [CrossRef]
- Davies, S.R.; Sloan, E.D.; Sum, A.K.; Koh, C.A. In situ studies of the mass transfer mechanism across a methane hydrate film using high-resolution confocal Raman spectroscopy. J. Phys. Chem. C 2009, 114, 1173–1180. [Google Scholar] [CrossRef]
- Austvik, T.; Li, X.; Gjertsen, L.H. Hydrate plug properties: Formation and removal of plugs. Ann. N. Y. Acad. Sci. 2000, 912, 294–303. [Google Scholar] [CrossRef]





| Chemical Name | Source | Purity | Purity Units |
|---|---|---|---|
| MEG | Fisher Scientific, Canada | ≥99.80% | Mass Fraction |
| NaCl | Caledon Laboratory Ltd. | ≥99.00% | Mass Fraction |
| PVP | Sigma-Aldrich Canada Co. | N/A | N/A |
| CH4 | Air Liquide, QC Canada | 99.99% | Mass Fraction |
| N2 | Air Liquide, QC Canada | 99.99% | Mass fraction |
| Distilled Water | In-house | See caption | See caption |
| System | T/K | /K | p/ MPa |
|---|---|---|---|
| Water | 277.48 | 0.14 | 4.00 |
| Water | 281.31 | 0.08 | 6.01 |
| PVP | 281.38 | 0.08 | 6.15 |
| PVP | 281.83 | 0.06 | 6.28 |
| PVP | 281.82 | 0.10 | 6.25 |
| NaCl | 276.72 | 0.12 | 4.84 |
| NaCl | 277.61 | 0.11 | 5.39 |
| NaCl | 279.23 | 0.40 | 6.66 |
| MEG | 279.65 | 0.08 | 5.99 |
| MEG | 280.48 | 0.22 | 6.53 |
| MEG | 275.72 | 0.06 | 4.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Chowdhury, N.B.; Beltran, J.G. A 3-In-1 Approach to Evaluate Gas Hydrate Inhibitors. Energies 2019, 12, 2921. https://doi.org/10.3390/en12152921
Kumar N, Chowdhury NB, Beltran JG. A 3-In-1 Approach to Evaluate Gas Hydrate Inhibitors. Energies. 2019; 12(15):2921. https://doi.org/10.3390/en12152921
Chicago/Turabian StyleKumar, Narendra, Niaz Bahar Chowdhury, and Juan G. Beltran. 2019. "A 3-In-1 Approach to Evaluate Gas Hydrate Inhibitors" Energies 12, no. 15: 2921. https://doi.org/10.3390/en12152921
APA StyleKumar, N., Chowdhury, N. B., & Beltran, J. G. (2019). A 3-In-1 Approach to Evaluate Gas Hydrate Inhibitors. Energies, 12(15), 2921. https://doi.org/10.3390/en12152921




