Melting Behavior of Phase Change Material in Honeycomb Structures with Different Geometrical Cores
Abstract
1. Introduction
2. Mathematical Formulation and Numerical Validation
2.1. Problem Statement
2.2. Mathematical Formulation
2.3. Initial and Boundary Conditions
2.4. Numerical Method
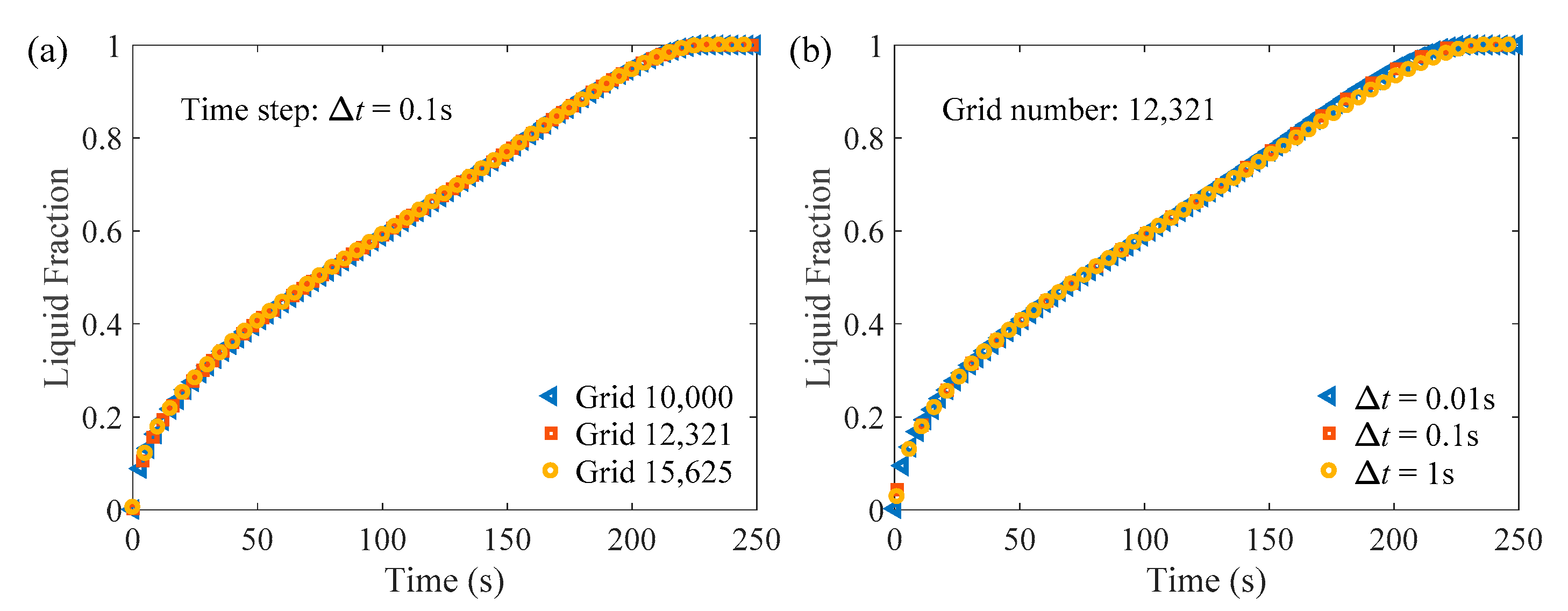
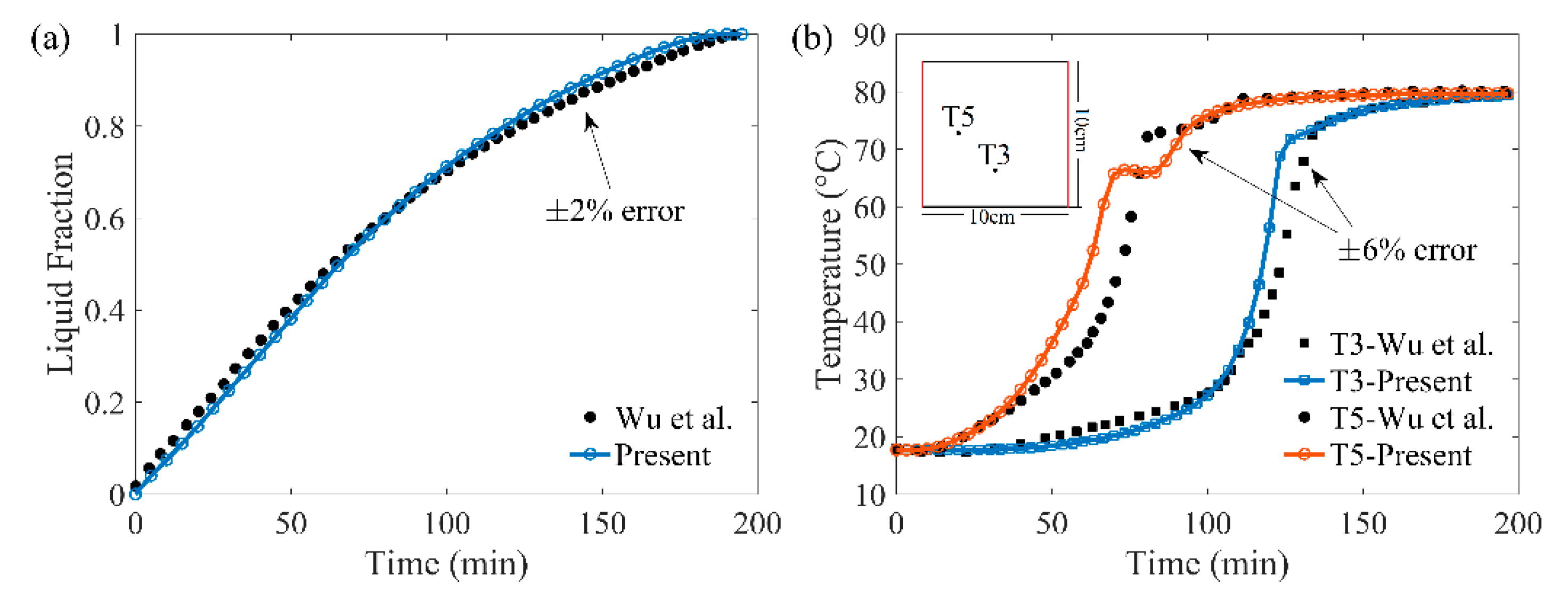
2.5. Numerical Verification and Validation
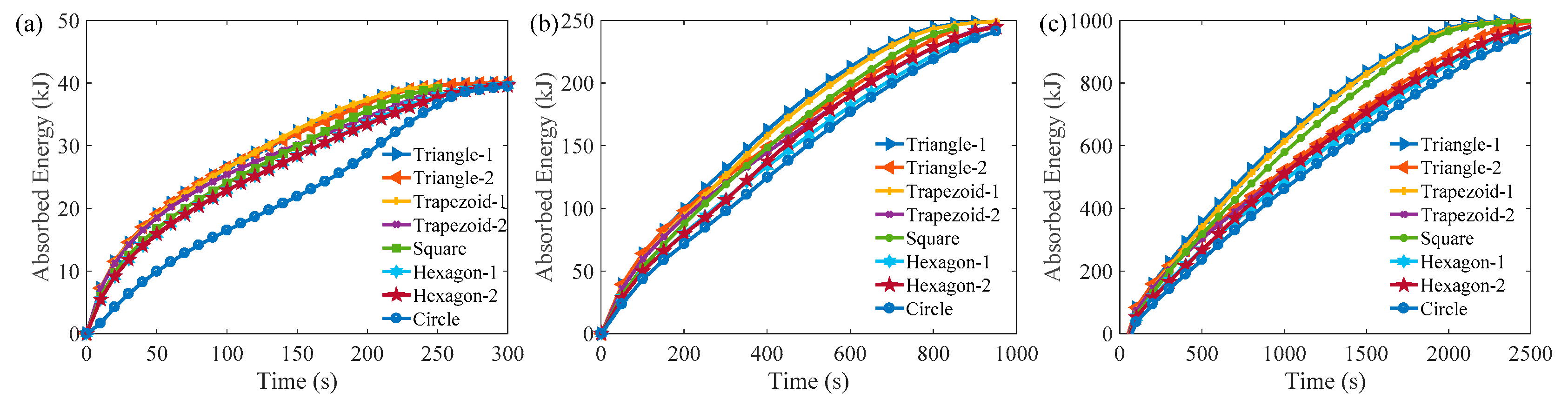
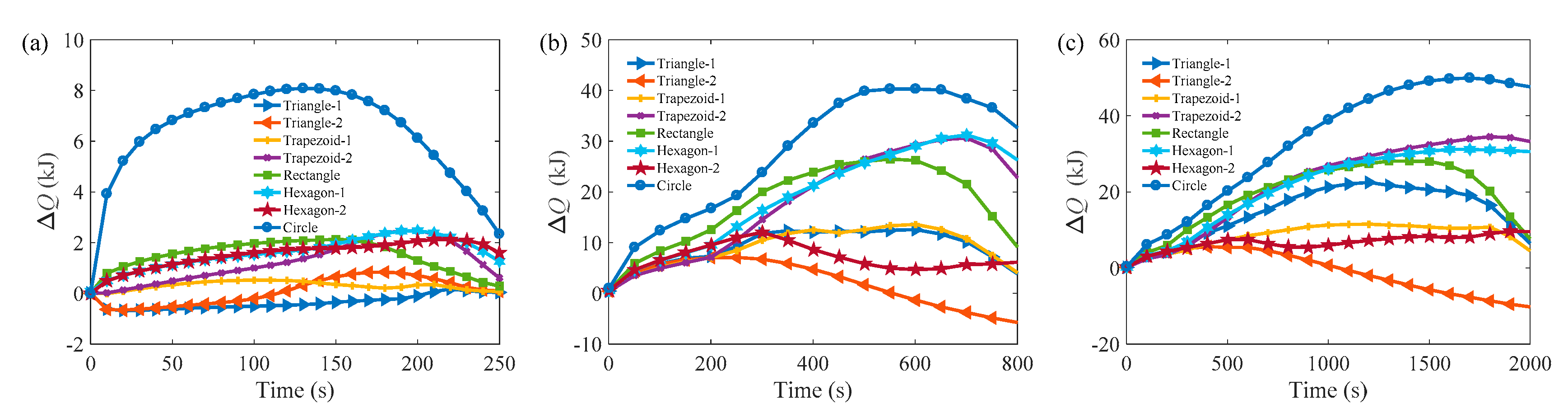
3. Results and Discussion
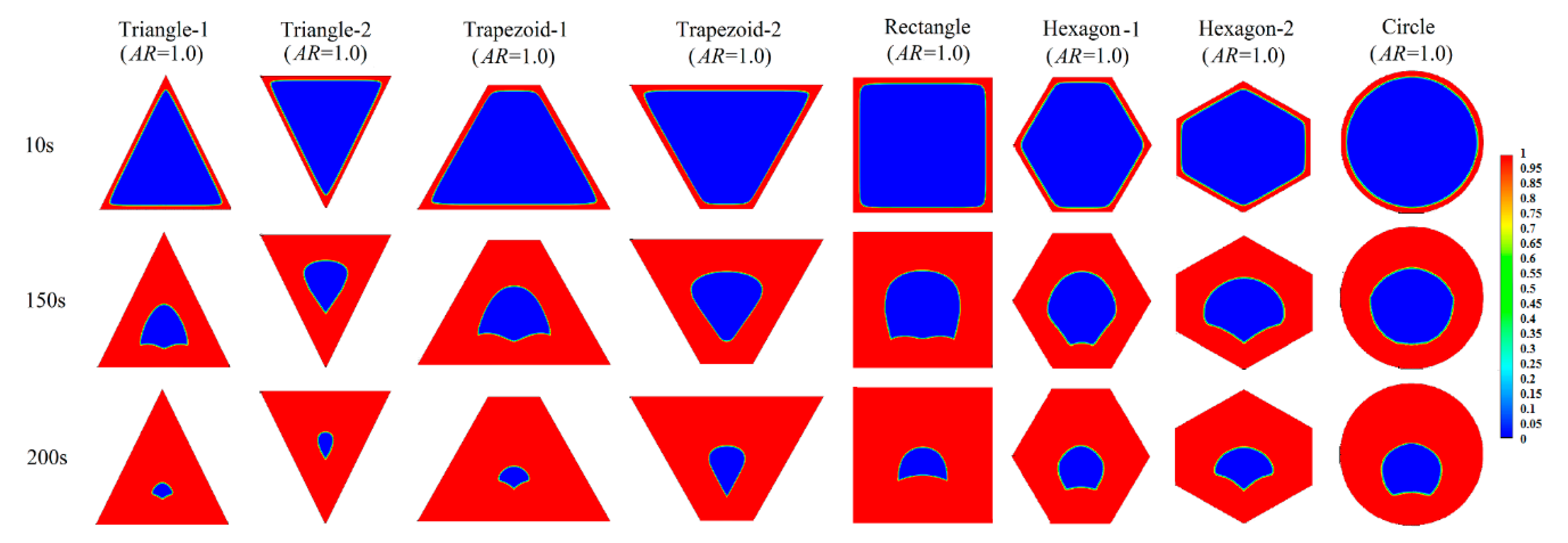
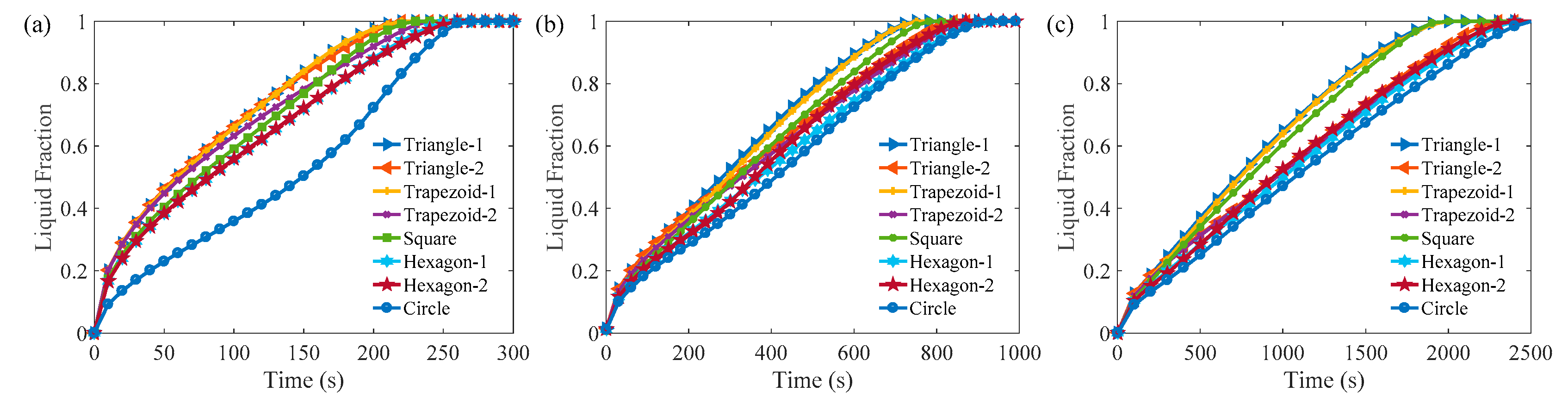
3.1. Melting Process in Cells with AR = 1.0
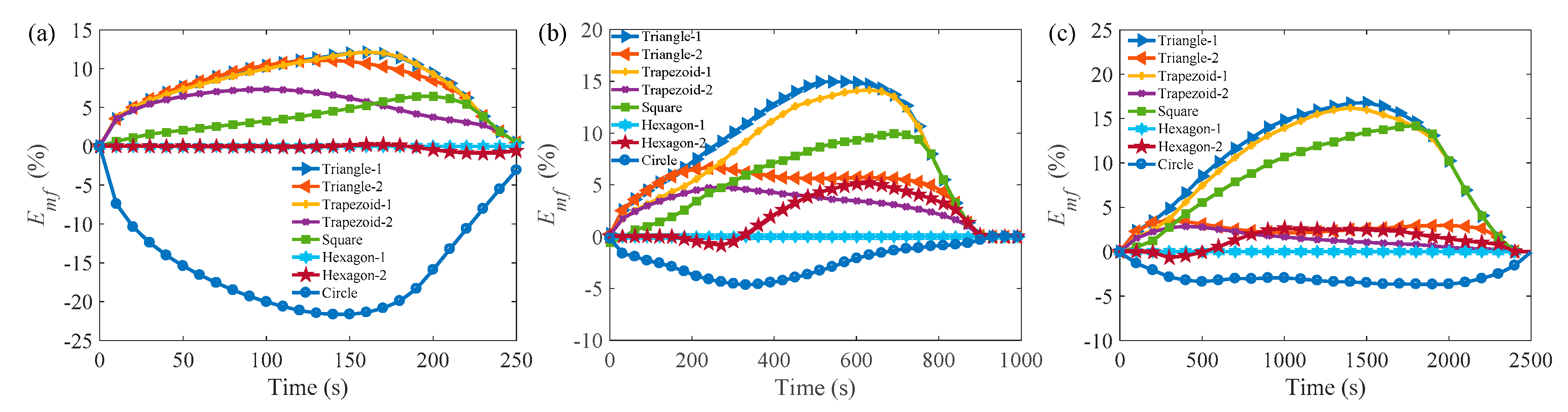
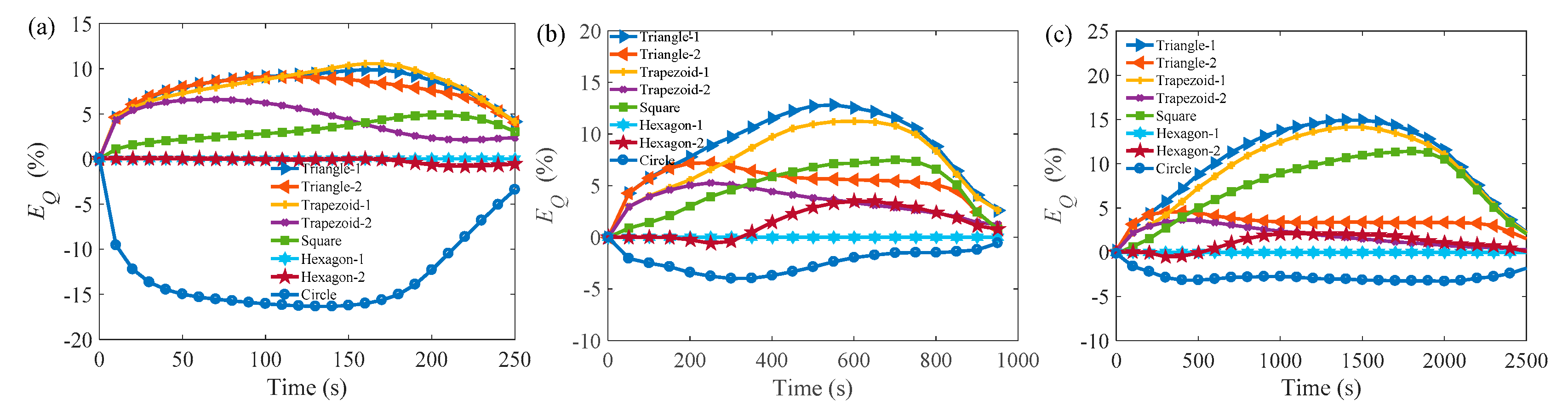
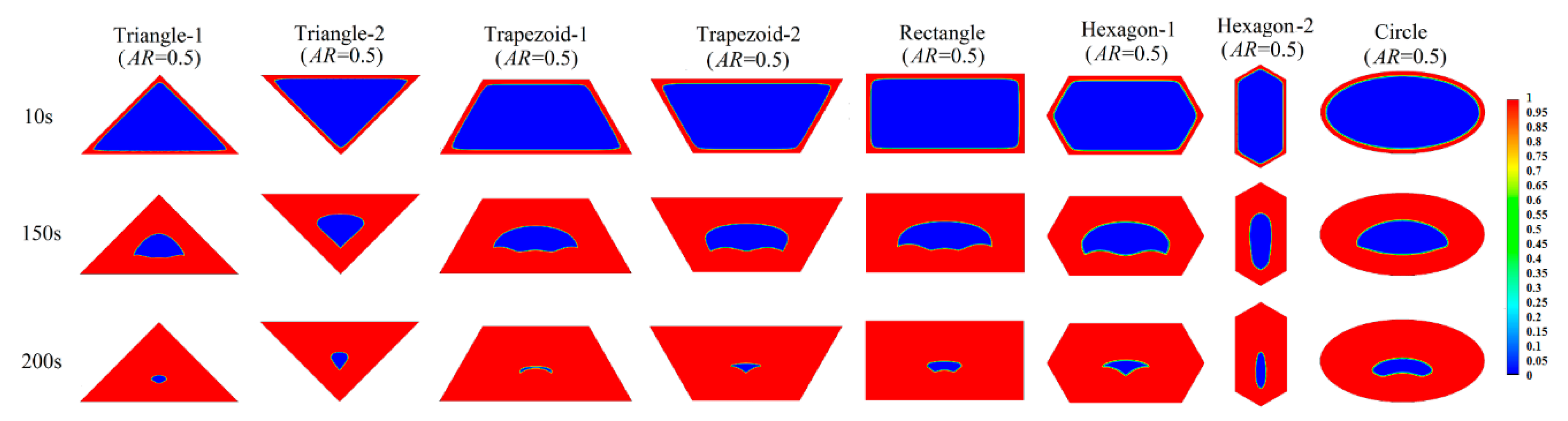
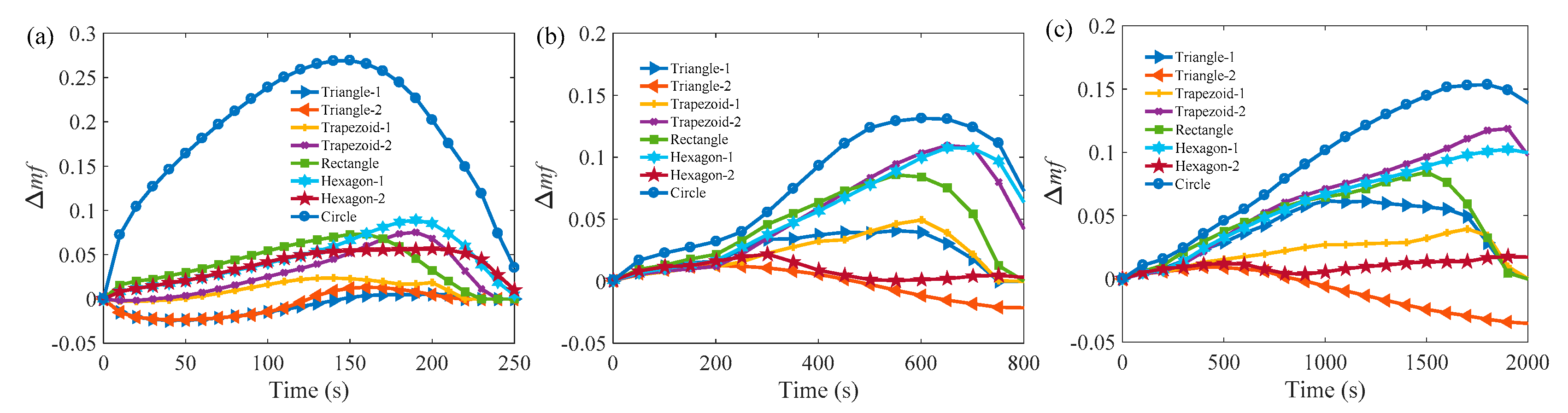
3.2. Melting Process in Cells with AR = 0.5
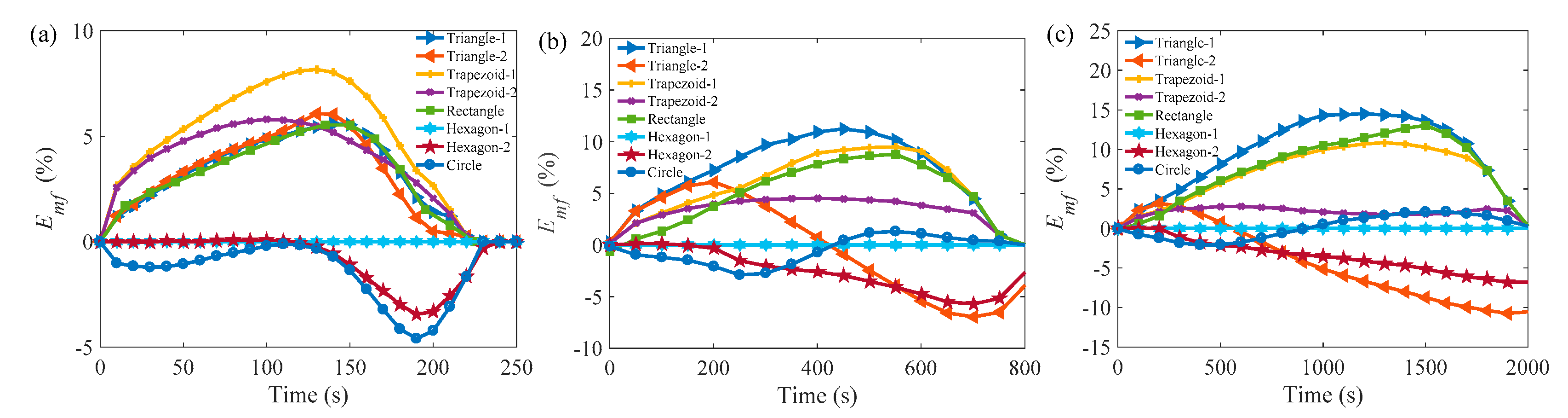
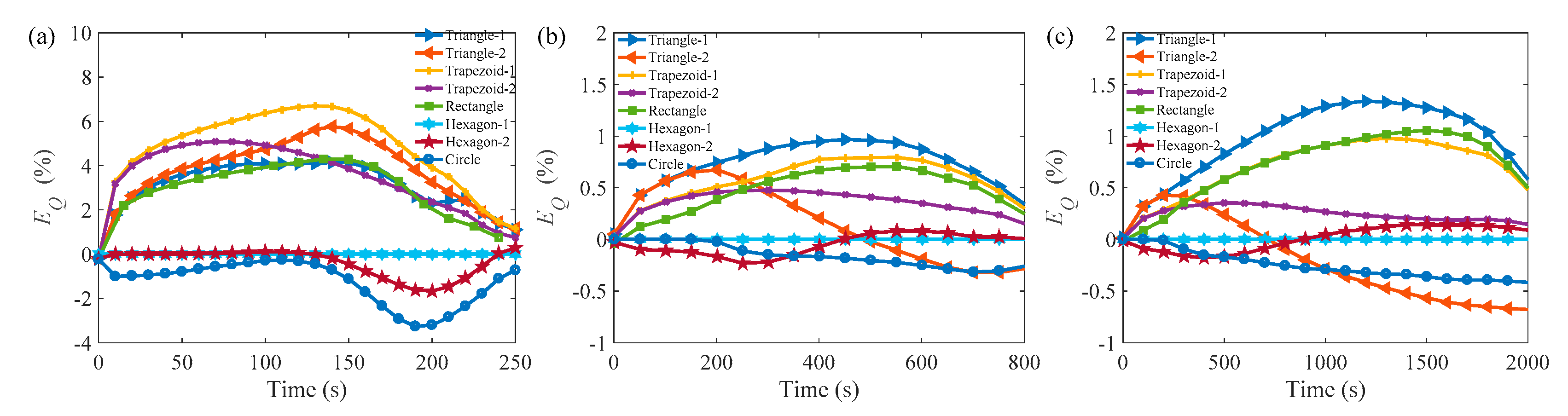
3.3. Comparison of the Melting Time in Different Rayleigh Numbers and ARs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| C | mushy zone constant [kg/m3·s] |
| Hy | height of the one honeycomb cell [m] |
| Wx | width of the one honeycomb cell [m] |
| cp | specific heat [J/kg·K] |
| p | pressure [N/m2] |
| t | time [s] |
| hsf | latent heat [J/kg] |
| T | temperature [K] |
| k | thermal conductivity [W/m·K] |
| u, v | velocity in the x- and y-direction, respectively [m/s] |
| g | gravitational acceleration [m/s2] |
| Q | instantaneous total heat transfer rate [J] |
| AR | aspect ratio |
| GF | geometrical factor |
| Greek symbols | |
| ρ | density [kg/m3] |
| μ | dynamic viscosity [kg/m·s] |
| α | thermal diffusivity [m2/s] |
| β | thermal expansion coefficient [1/K] |
| γ | liquid fraction of a calculated cell |
| ε | a small number, typically approximately 10−3 |
| Subscripts | |
| w | hot wall |
| s | solid |
| l | liquid |
| m | melting |
Appendix A
Appendix A.1 Melting Process in Cells with AR = 1.0
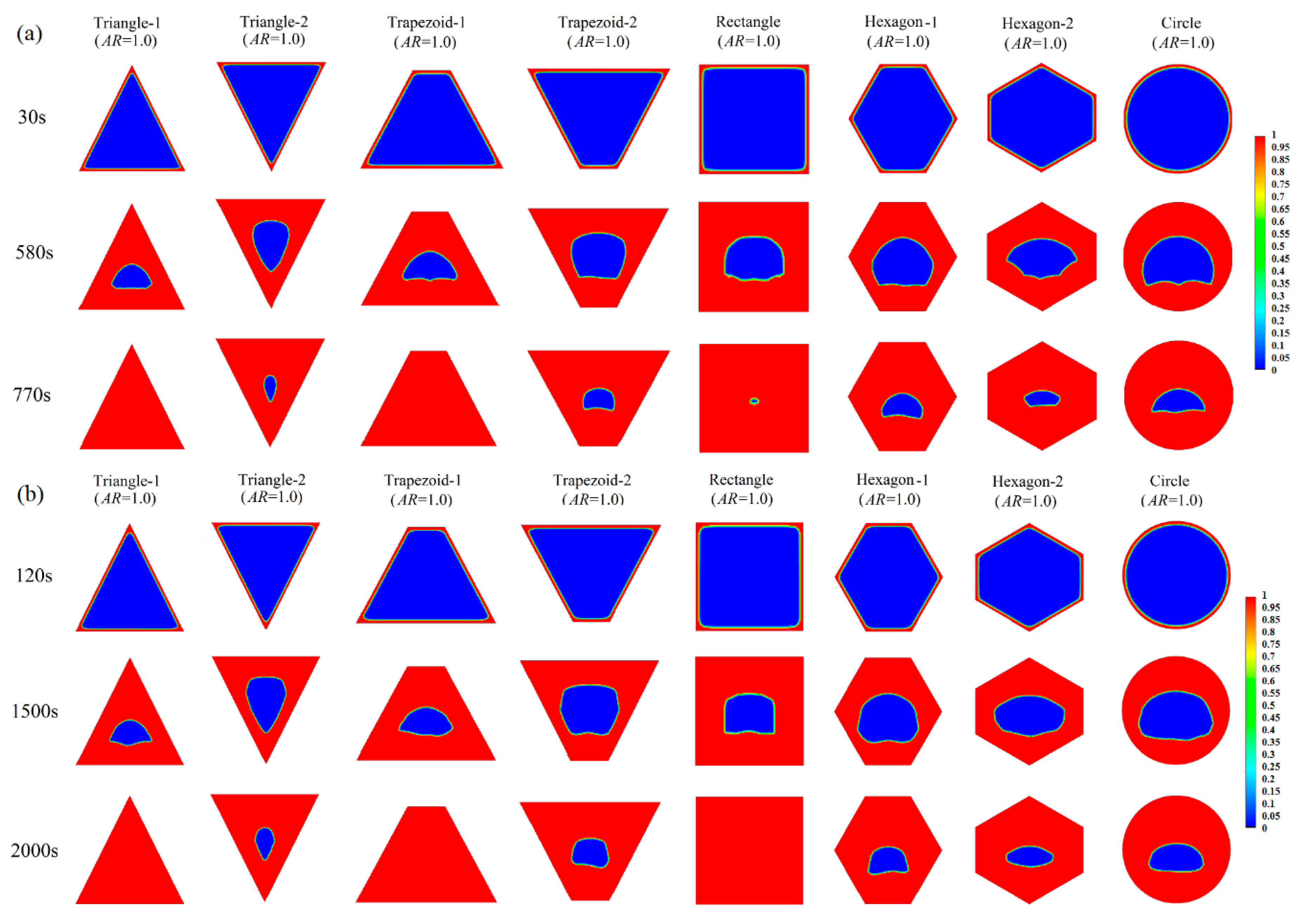
Appendix A.2 Melting Process in Cells with AR = 0.5

References
- Arnault, A.; Mathieu-Potvin, F.; Gosselin, L. Internal surfaces including phase change materials for passive optimal shift of solar heat gain. Int. J. Therm. Sci. 2010, 49, 2148–2156. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Zhang, Y.P. A new method to estimate optimal phase change material characteristics in a passive solar room. Energy Convers. Manag. 2011, 52, 2437–2441. [Google Scholar] [CrossRef]
- Sun, X.Q.; Zhang, Q.; Medina, M.A.; Lee, K.O. Energy and economic analysis of a building enclosure outfitted with a phase change material board (PCMB). Energy Convers. Manag. 2014, 83, 73–78. [Google Scholar] [CrossRef]
- Ye, H.; Long, L.S.; Zhang, H.T.; Zou, R.Q. The performance evaluation of shape-stabilized phase change materials in building applications using energy saving index. Appl. Energy 2014, 113, 1118–1126. [Google Scholar] [CrossRef]
- Mazzeo, D.; Oliveti, G.; Arcuri, N. Definition of a new set of parameters for the dynamic thermal characterization of PCM layers in the presence of one or more liquid-solid interfaces. Energy Build. 2017, 141, 379–396. [Google Scholar] [CrossRef]
- Mazzeo, D.; Oliveti, G.; Gracia, A.; Coma, J.; Sole, A.; Cabeza, L.F. Experimental validation of the exact analytical solution to the steady periodic heat transfer problem in a PCM layer. Energy 2017, 140, 1131–1147. [Google Scholar] [CrossRef]
- Mazzeo, D.; Oliveti, G. Thermal field and heat storage in a cyclic phase change process caused by several moving melting and solidification interfaces in the layer. Int. J. Therm. Sci. 2018, 129, 462–488. [Google Scholar] [CrossRef]
- Erlbeck, L.; Schreiner, P.; Schlachter, K.; Dörnhofer, P.; Fasel, F.; Methner, F.J.; Rädle, M. Adjustment of thermal behavior by changing the shape of PCM inclusions in concrete blocks. Energy Convers. Manag. 2018, 158, 256–265. [Google Scholar] [CrossRef]
- Dardir, M.; Panchabikesan, K.; Haghighat, F.; Mankibi, M.E.; Yuan, Y.P. Opportunities and challenges of PCM-to-air heat exchangers (PAHXs) for building free cooling applications—A comprehensive review. J. Energy Storage 2019, 22, 157–175. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Wu, Z.G. Heat transfer enhancement of high temperature thermal energy storage using metal foams and expanded graphite. Sol. Energy Mater. Sol. Cells 2011, 95, 636–643. [Google Scholar] [CrossRef]
- Esapour, M.; Hamzehnezhad, A.; Darzi, A.A.R.; Jourabian, M. Melting and solidification of PCM embedded in porous metal foam in horizontal multi-tube heat storage system. Energy Convers. Manag. 2018, 171, 398–410. [Google Scholar] [CrossRef]
- Wang, G.; Wei, G.S.; Xu, C.; Ju, X.; Yang, Y.P.; Du, X.Z. Numerical simulation of effective thermal conductivity and pore-scale melting process of PCMs in foam metals. Appl. Therm. Eng. 2019, 147, 464–472. [Google Scholar] [CrossRef]
- Hu, X.; Patnaik, S.S. Modeling phase change material in micro-foam under constant temperature condition. Int. J. Heat Mass Transf. 2014, 68, 677–682. [Google Scholar] [CrossRef]
- Sidik, N.A.C.; Kean, T.H.; Chow, H.K.; Rajaandra, A.; Rahman, S.; Kaur, J. Performance enhancement of cold thermal energy storage system using nanofluid phase change materials: A review. Int. Commun. Heat Mass Transf. 2018, 94, 85–95. [Google Scholar] [CrossRef]
- Ebadi, S.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Melting of nano–PCM inside a cylindrical thermal energy storage system: Numerical study with experimental verification. Energy Convers. Manag. 2018, 166, 241–259. [Google Scholar] [CrossRef]
- Farsani, R.Y.; Raisi, A.; Nadooshan, A.A.; Vanapalli, S. Does nanoparticles dispersed in a phase change material improve melting characteristics? Int. Commun. Heat Mass Transf. 2017, 89, 219–229. [Google Scholar] [CrossRef]
- Kamkari, B.; Shokouhmand, H. Experimental investigation of phase change material melting in rectangular enclosures with horizontal partial fins. Int. J. Heat Mass Transf. 2014, 78, 839–851. [Google Scholar] [CrossRef]
- Abdulateef, A.M.; Abdulateef, J.; Mat, S.; Sopian, K.; Elhub, B.; Mussa, M.A. Experimental and numerical study of solidifying phase-change material in a triplex–tube heat exchanger with longitudinal/triangular fins. Int. Commun. Heat Mass Transf. 2018, 90, 73–84. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Lohrasbi, S.; Ganji, D.D.; Nsofor, E.C. Accelerated melting of PCM in energy storage systems via novel configuration of fins in the triplex-tube heat exchanger. Int. J. Heat Mass Transf. 2018, 124, 663–676. [Google Scholar] [CrossRef]
- Ji, C.Z.; Qin, Z.; Dubey, S.; Choo, F.H.; Duan, F. Simulation on PCM melting enhancement with double-fin length arrangements in a rectangular enclosure induced by natural convection. Int. J. Heat Mass Transf. 2018, 127, 255–265. [Google Scholar] [CrossRef]
- Xie, J.L.; Lee, H.M.; Xiang, J.H. Numerical study of thermally optimized metal structures in a Phase Change Material (PCM) enclosure. Appl. Therm. Eng. 2019, 148, 825–837. [Google Scholar] [CrossRef]
- Li, Y.; Guo, B.; Huang, G.F.; Kubo, S.C.; Shu, P.C. Characterization and thermal performance of nitrate mixture/SiC ceramic honeycomb composite phase change materials for thermal energy storage. Appl. Therm. Eng. 2015, 81, 193–197. [Google Scholar] [CrossRef]
- Egolf, P.W.; Amacker, N.; Gottschalk, G.; Courret, G.; Noume, A.; Hutter, K. A translucent honeycomb solar collector and thermal storage module for building façades. Int. J. Heat Mass Transf. 2018, 127, 781–795. [Google Scholar] [CrossRef]
- Hasse, C.; Grenet, M.; Bontemps, A.; Dendievel, R.; Sallee, H. Realization, test and modeling of honeycomb wallboards containing a phase change material. Energy Build. 2011, 43, 232–238. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yu, Z.; Yang, T.T.; Qin, D.; Li, S.S.; Zhang, G.Q.; Haghighat, F.; Joybari, M.M. A review on macro-encapsulated phase change material for building envelope applications. Build. Environ. 2018, 144, 281–294. [Google Scholar] [CrossRef]
- Xie, B.; Cheng, W.L.; Xu, Z.M. Studies on the effect of shape-stabilized PCM filled aluminum honeycomb composite material on thermal control. Int. J. Heat Mass Transf. 2015, 91, 135–143. [Google Scholar] [CrossRef]
- Li, J.H.; Huang, J.H.; Cao, M. Properties enhancement of phase-change materials via silica and Al honeycomb panels for the thermal management of LiFeO4 batteries. Appl. Therm. Eng. 2018, 131, 660–668. [Google Scholar] [CrossRef]
- Lai, C.; Hokoi, S. Thermal performance of an aluminum honeycomb wallboard incorporating microencapsulated PCM. Energy Build. 2014, 73, 37–47. [Google Scholar] [CrossRef]
- Abuşka, M.; Şevik, S.; Kayapunar, A. A comparative investigation of the effect of honeycomb core on the latent heat storage with PCM in solar air heater. Appl. Therm. Eng. 2019, 148, 684–693. [Google Scholar] [CrossRef]
- Raj, A.K.; Srinivas, M.; Jayaraj, S. A cost-effective method to improve the performance of solar air heaters using discrete macro-encapsulated PCM capsules for drying applications. Appl. Therm. Eng. 2019, 146, 910–920. [Google Scholar] [CrossRef]
- Dhaidan, N.S.; Khodadadi, J.M. Melting and convection of phase change materials in different shape containers: A review. Renew. Sustain. Energy Rev. 2015, 43, 449–477. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Papadokostaki, K.G.; Favvas, E.P.; Efthimiadou, E.K.; Karellas, S. Preparation and investigation of distinct and shape stable paraffin/SiO2 composite PCM nanospheres. Energy Convers. Manag. 2018, 168, 382–394. [Google Scholar] [CrossRef]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. A comprehensive study of properties of paraffin phase change materials for solar thermal energy storage and thermal management applications. Energy 2018, 162, 1169–1182. [Google Scholar] [CrossRef]
- Farzanehnia, A.; Khatibi, M.; Sardarabadi, M.; Passandideh-Fard, M. Experimental investigation of multiwall carbon nanotube/paraffin based heat sink for electronic device thermal management. Energy Convers. Manag. 2019, 179, 314–325. [Google Scholar] [CrossRef]
- Tan, F.L. Constrained and unconstrained melting inside a sphere. Int. Commun. Heat Mass Transf. 2008, 35, 466–475. [Google Scholar] [CrossRef]
- Brent, A.D.; Voller, V.R.; Reid, K.J. Enthalpy-porosity technique for modeling convection-diffusion phase change: Application to the melting of a pure metal. Numer. Heat Transf. 1988, 13, 297–318. [Google Scholar] [CrossRef]
- Hong, Y.X.; Ye, W.B.; Du, J.; Huang, S.M. Solid-liquid phase-change thermal storage and release behaviors in a rectangular cavity under the impacts of mushy region and low gravity. Int. J. Heat Mass Transf. 2019, 130, 1120–1132. [Google Scholar] [CrossRef]
- Wu, Z.G.; Sheng, W.C.; Tao, W.Q.; Li, Z. A novel experimental–numerical method for studying the thermal behaviors of phase change material in a porous cavity. Sol. Energy 2018, 169, 325–334. [Google Scholar] [CrossRef]
- Kakac, S.; Aung, W.; Viskanta, R. (Eds.) Natural Convection: Fundamentals and Applications; Hemisphere: Washington, DC, USA, 1985. [Google Scholar]
- Wesseling, P. Principles of Computational Fluid Dynamics; Springer: Berlin, Germany, 2001. [Google Scholar]
- Kamkaria, B.; Amlashi, H.J. Numerical simulation and experimental verification of constrained melting of phase change material in inclined rectangular enclosures. Int. Commun. Heat Mass Transf. 2017, 88, 211–219. [Google Scholar] [CrossRef]
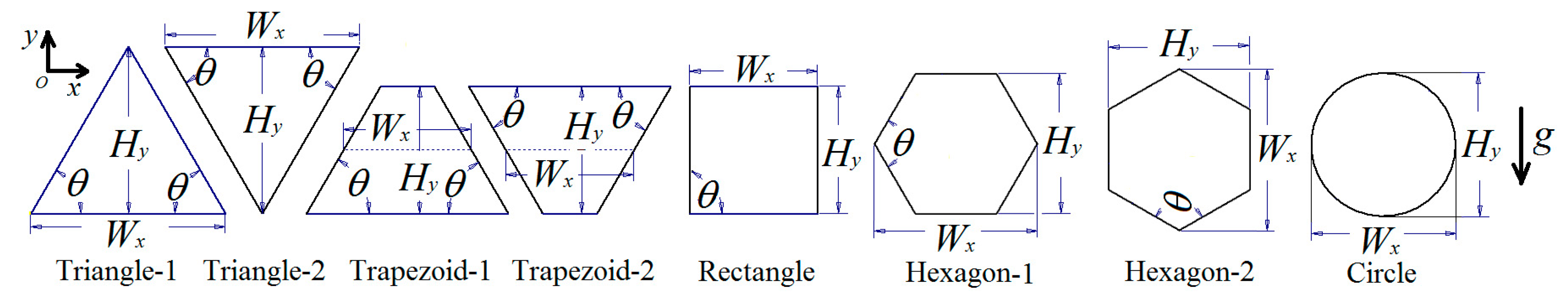


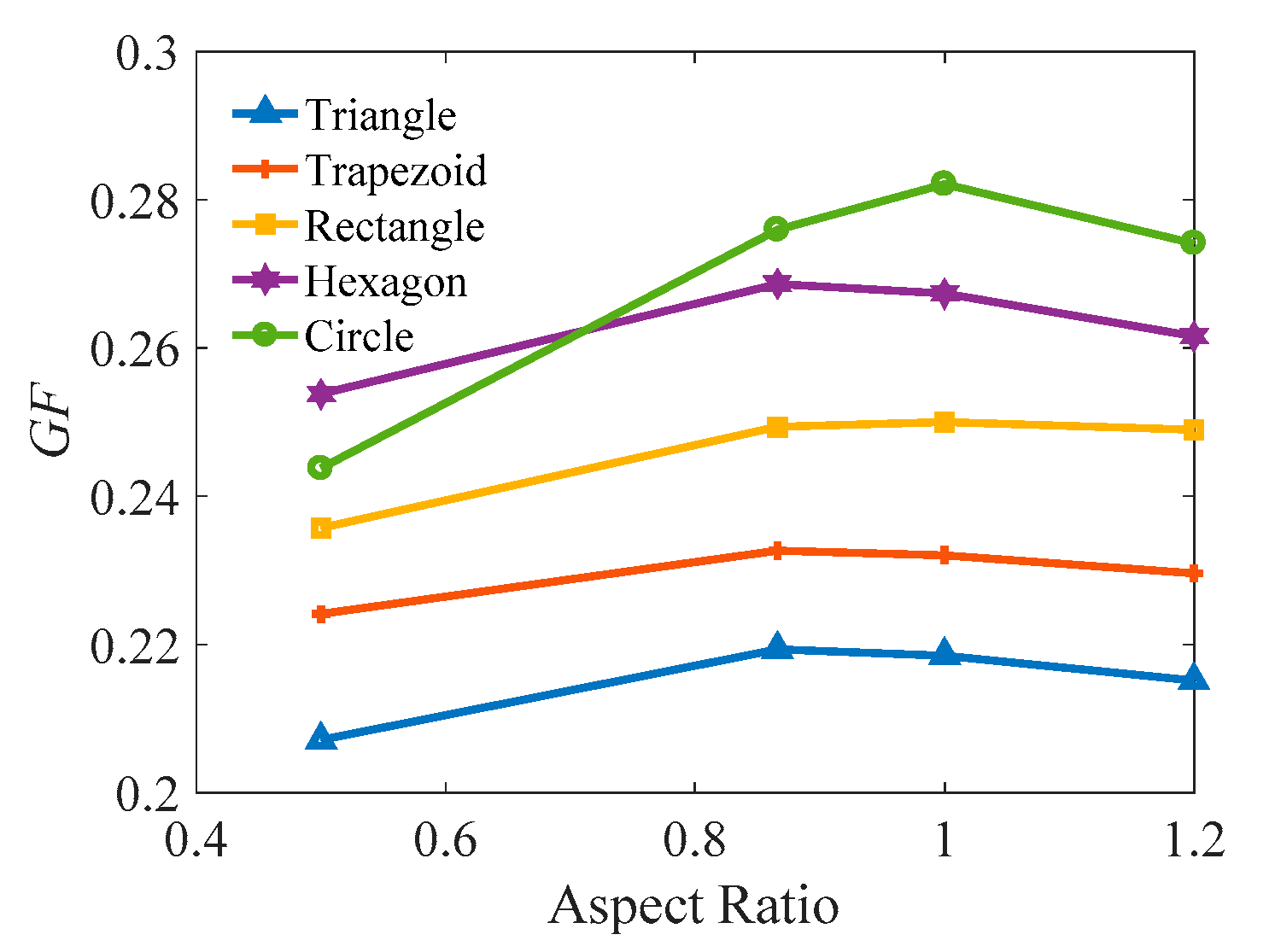
| Cells | AR = 0.5 | AR = 0.866 | AR = 1.0 | AR = 1.2 |
|---|---|---|---|---|
| Triangle | 45° | 60° | 63.43° | 67.38° |
| Trapezoid | 60° | 60° | 60° | 60° |
| Rectangle | 90° | 90° | 90° | 90° |
| Hexagon | 120° | 120° | 120° | 120° |
| Circle | - | - | - | - |
| Parameters | Symbol | Paraffin | Aluminum |
|---|---|---|---|
| Solidus/liquidus density [kg/m3] | ρs/ρl | 980/980 | 2719 |
| Specific heat capacity [J/kg·K] | cp | 3400 | 871 |
| Thermal conductivity [W/m·K] | k | 0.2 | 202.4 |
| Dynamic viscosity [kg/m·s] | μ | 0.006 | - |
| Thermal expansion coefficient [1/K] | β | 3.85 × 10−4 | - |
| Latent heat [J/kg] | hsf | 224,000 | - |
| Solidus/liquidus temperature [K] | Ts/Tl | 323/323 | - |
| Cell | Ra = 3.095 × 105 | Ra = 4.83 × 106 | Ra = 3.87 × 107 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 0.866 | 1.0 | 1.2 | 0.5 | 0.866 | 1.0 | 1.2 | 0.5 | 0.866 | 1.0 | 1.2 | |
| Tri-1 | 4.5% | ★10.8% | ★15.2% | ★13.0% | 7.7% | 15.2% | 17.8% | ★17.5% | 13.9% | 18.4% | 19.0% | 18.3% |
| Tri-2 | 1.3% | ★10.4% | ★14.4% | ★13.4% | −14.7% | 2.7% | 7.3% | ★12.5% | −20.3% | 0.3% | 4.9% | 9.4% |
| T-1 | ★8.5% | ★10.4% | 14.4% | 10.5% | 8.5% | 14.1% | 16.8% | 18.5% | 11.3% | 16.9% | 18.3% | 20.0% |
| T-2 | ★4.5% | ★10.4% | 7.0% | 10.5% | 4.3% | 2.7% | 2.4% | 3.0% | 2.4% | 0.4% | 0.5% | 0.1% |
| Rec | 2.2% | 8.0% | 11.3% | 8.5% | ★9.6% | ★10.0% | ★14.0% | ★12.5% | ★13.2% | ★14.1% | ★20.0% | ★15.5% |
| Hex-1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Hex-2 | −4.5% | −5.6% | −1.2% | −2.8% | −12.2% | −0.2% | 4.9% | 9.9% | −13.7% | −2.4% | 1.9% | 5.5% |
| Cir | −2.7% | −12.0% | −2.7% | −10.1% | 0.5% | −2.2% | −1.1% | −1.5% | 1.3% | −2.1% | −3.6% | −4.1% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, J.; Xiong, Y.; Yang, D. Melting Behavior of Phase Change Material in Honeycomb Structures with Different Geometrical Cores. Energies 2019, 12, 2920. https://doi.org/10.3390/en12152920
Duan J, Xiong Y, Yang D. Melting Behavior of Phase Change Material in Honeycomb Structures with Different Geometrical Cores. Energies. 2019; 12(15):2920. https://doi.org/10.3390/en12152920
Chicago/Turabian StyleDuan, Juan, Yongliang Xiong, and Dan Yang. 2019. "Melting Behavior of Phase Change Material in Honeycomb Structures with Different Geometrical Cores" Energies 12, no. 15: 2920. https://doi.org/10.3390/en12152920
APA StyleDuan, J., Xiong, Y., & Yang, D. (2019). Melting Behavior of Phase Change Material in Honeycomb Structures with Different Geometrical Cores. Energies, 12(15), 2920. https://doi.org/10.3390/en12152920





