Catalytic Effect of Cobalt Additive on the Low Temperature Oxidation Characteristics of Changqing Tight Oil and Its SARA Fractions
Abstract
1. Introduction
2. Experimental
2.1. Materials
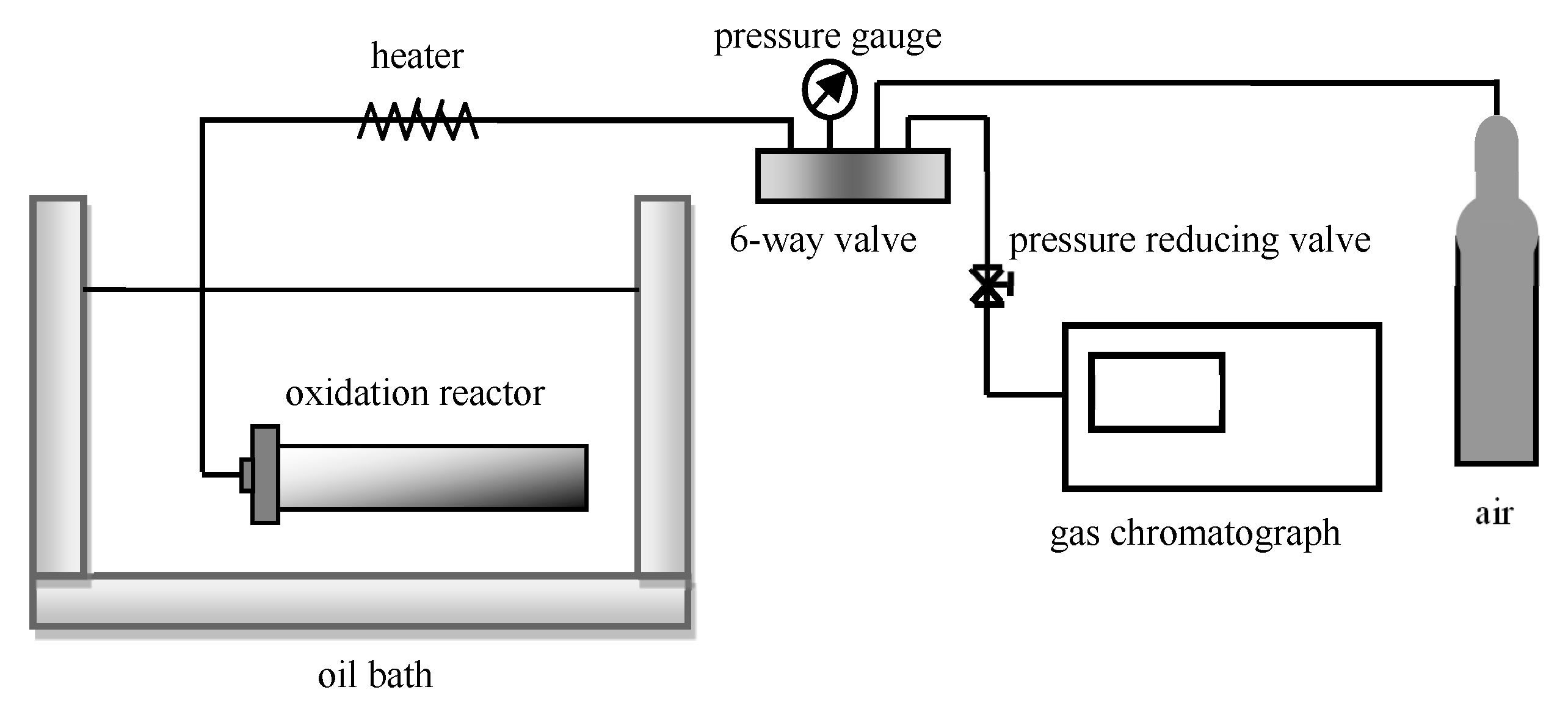
2.2. Experimental Setup
2.3. Experimental Procedures
2.3.1. Separation of SARA Fractions
2.3.2. Static Oxidation Experiments
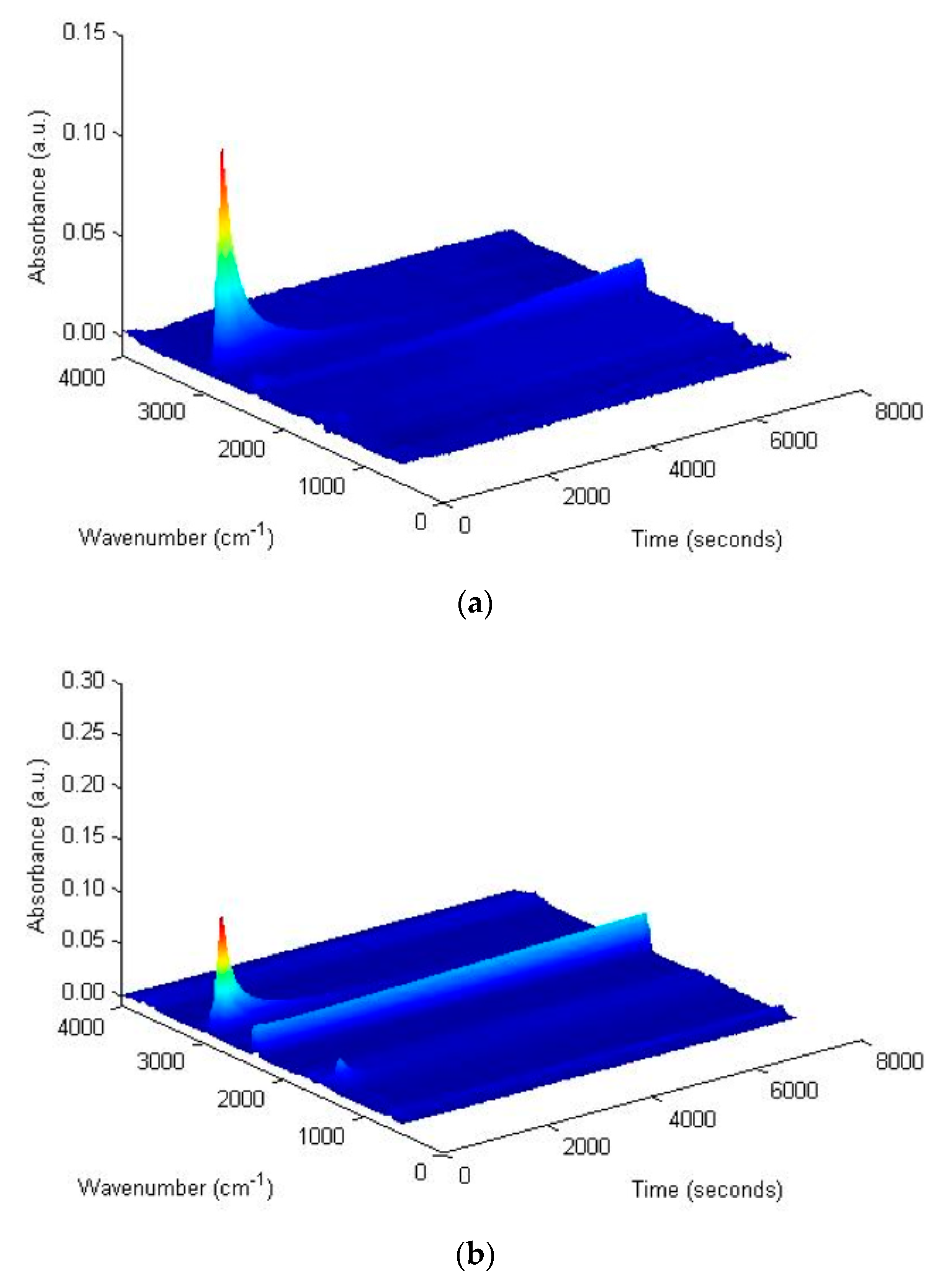
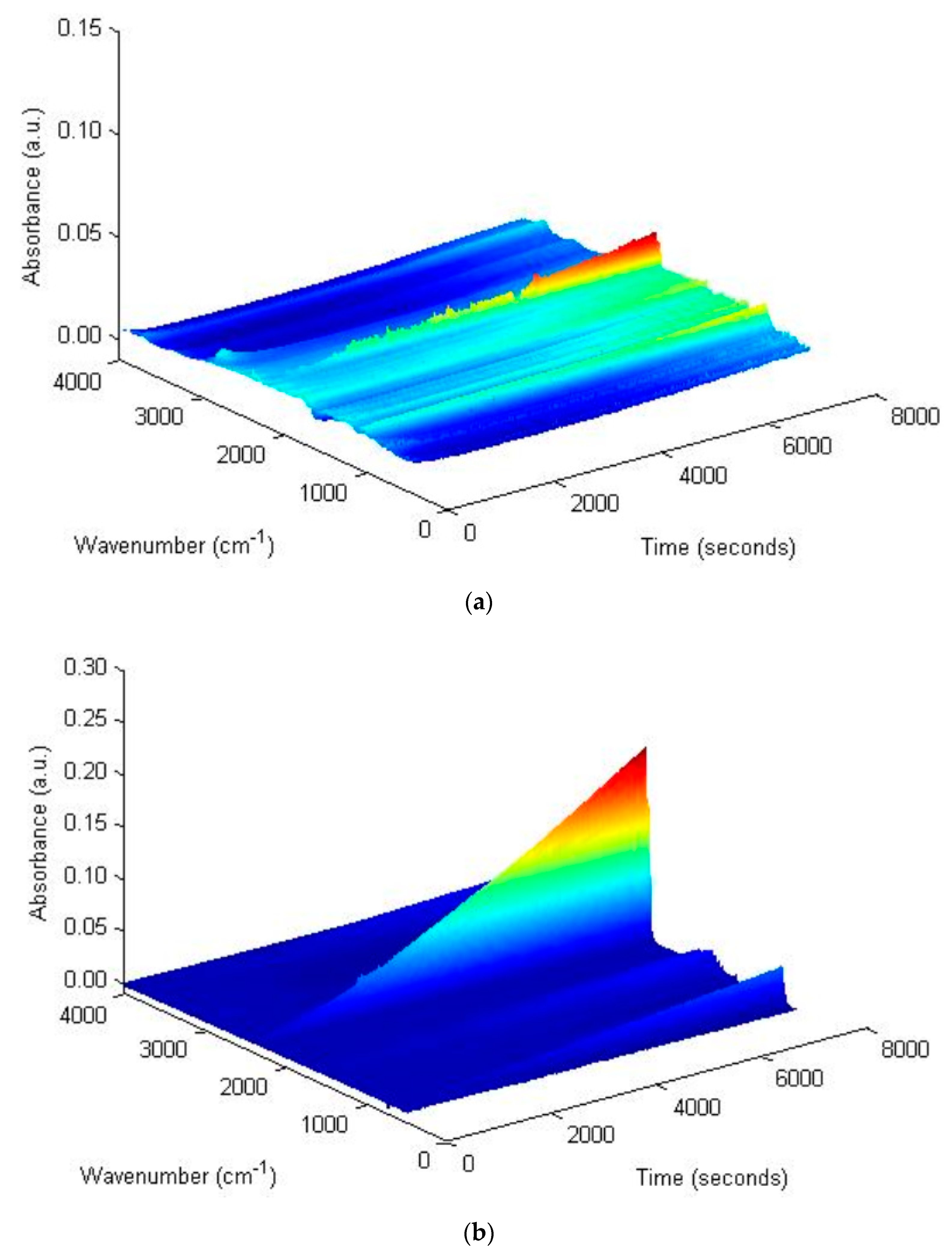
2.3.3. TG-FTIR Tests
3. Results and Discussion
3.1. Catalytic Oxidation Kinetics
3.2. CLTO Reaction Process of Changqing Tight Oil Fractions
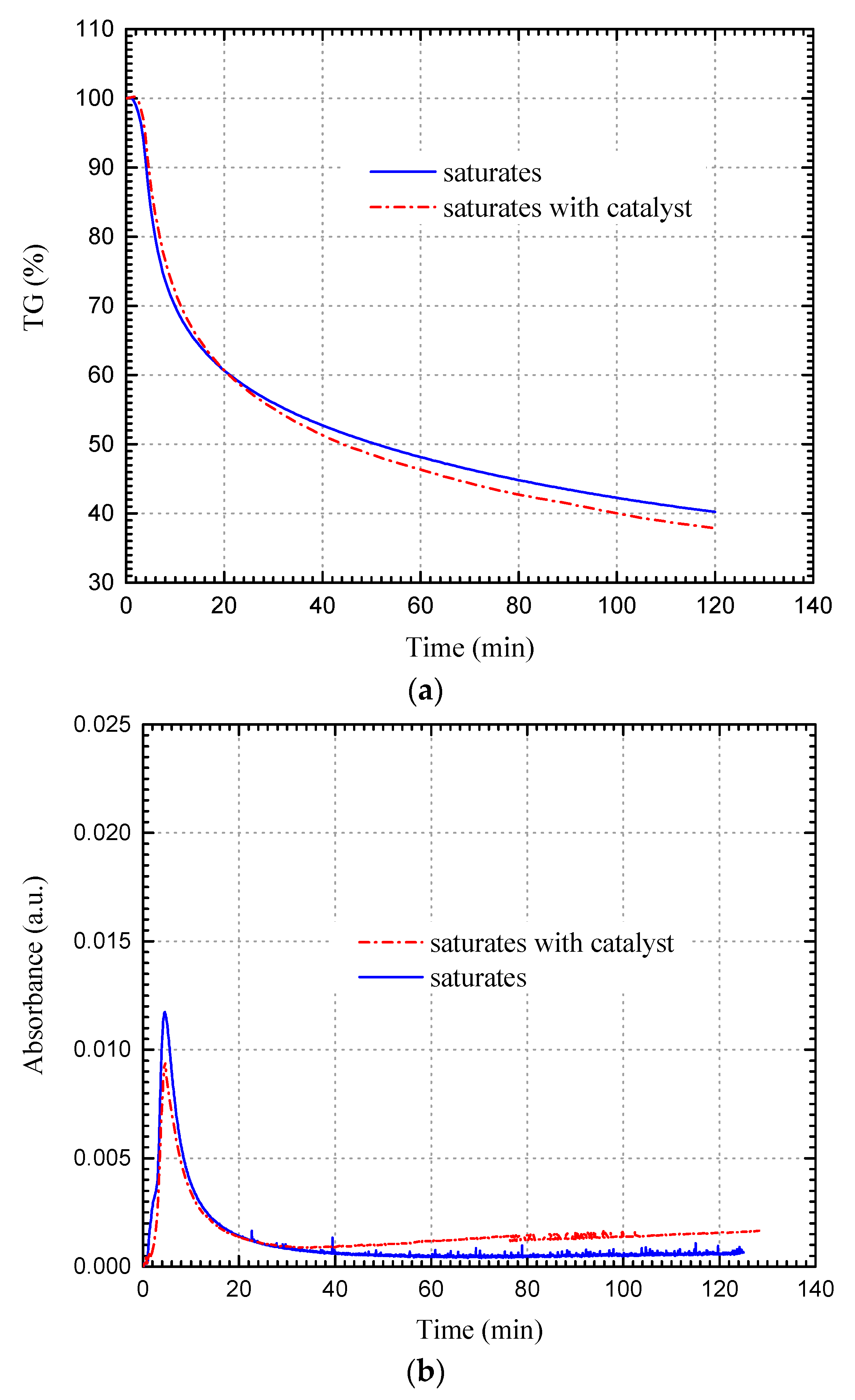
3.2.1. Saturates Fraction
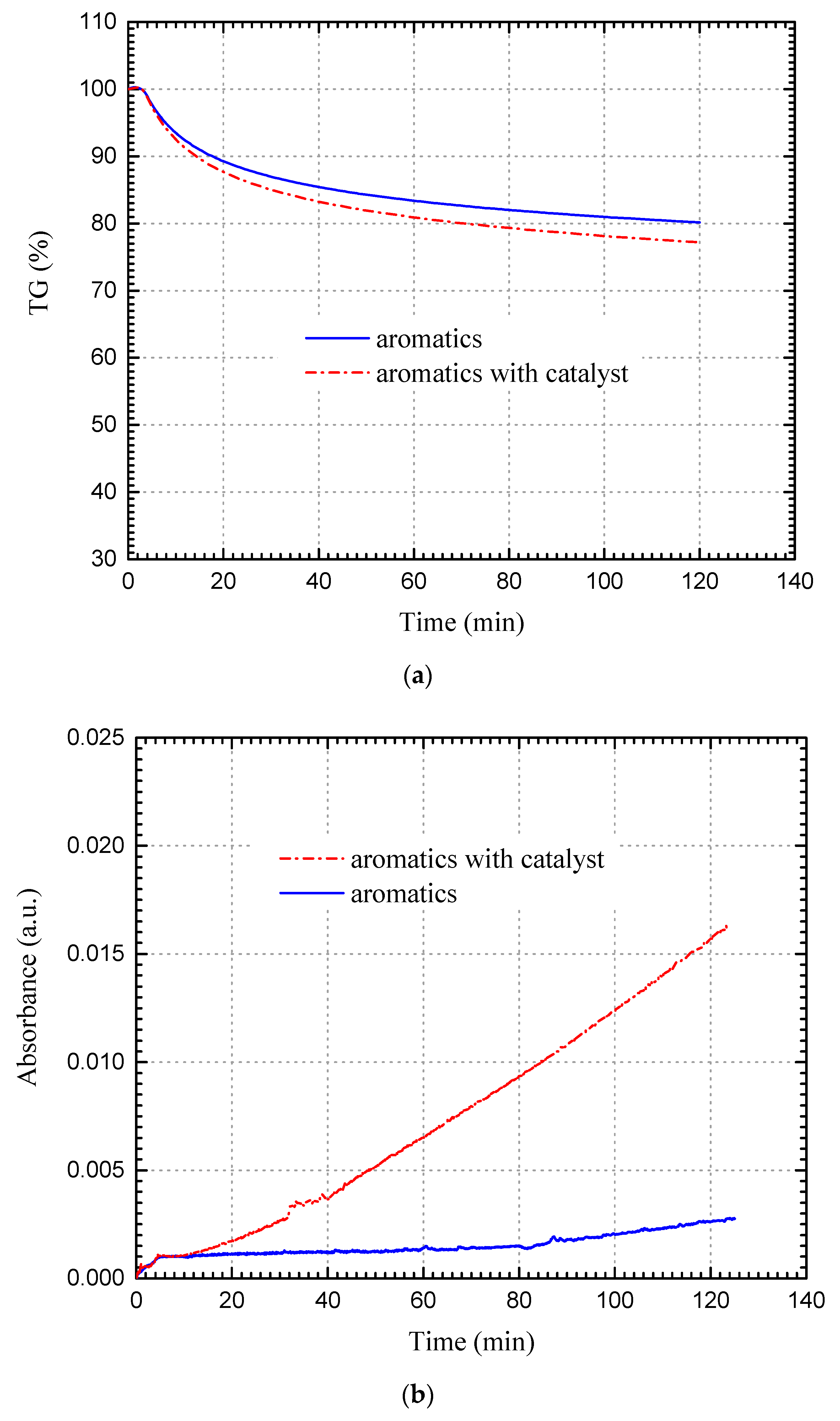
3.2.2. Aromatics Fraction
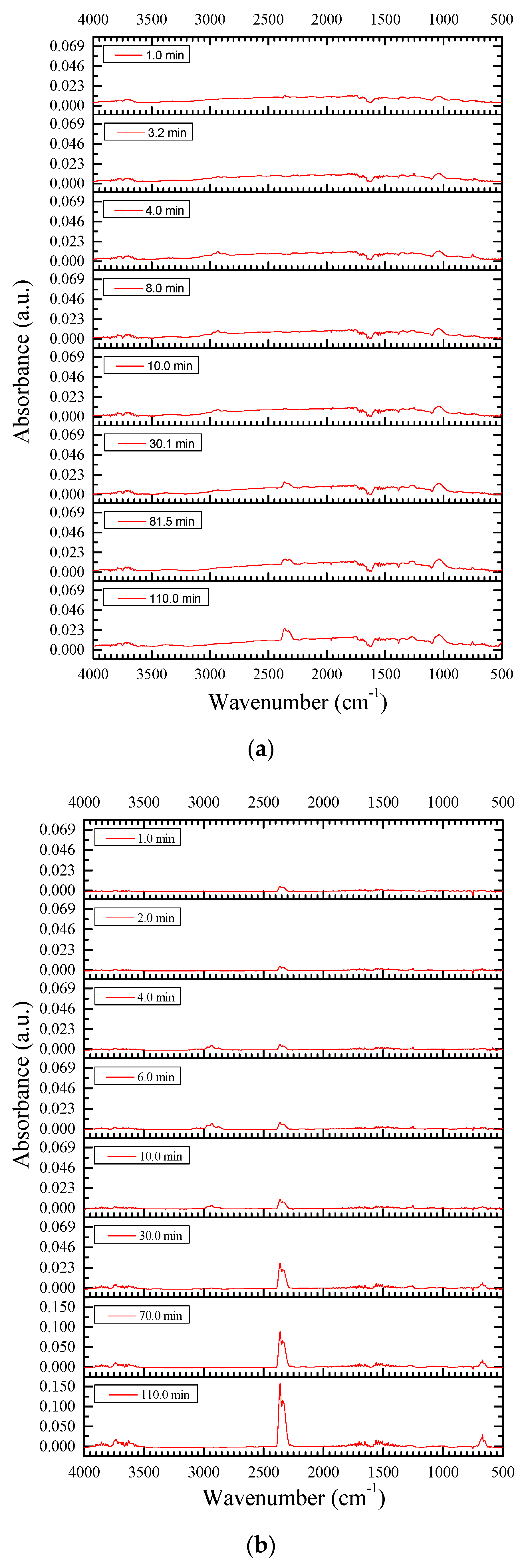
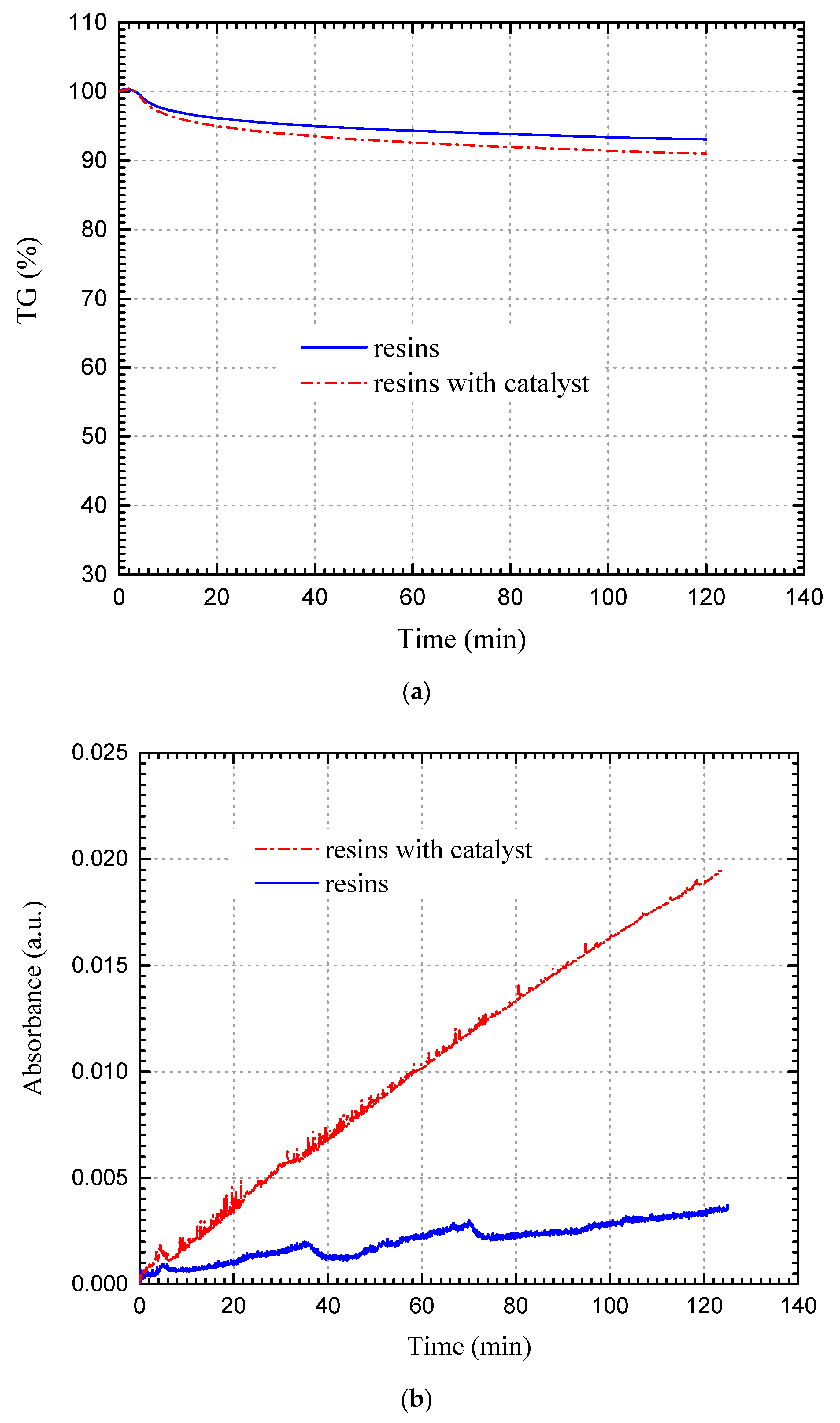
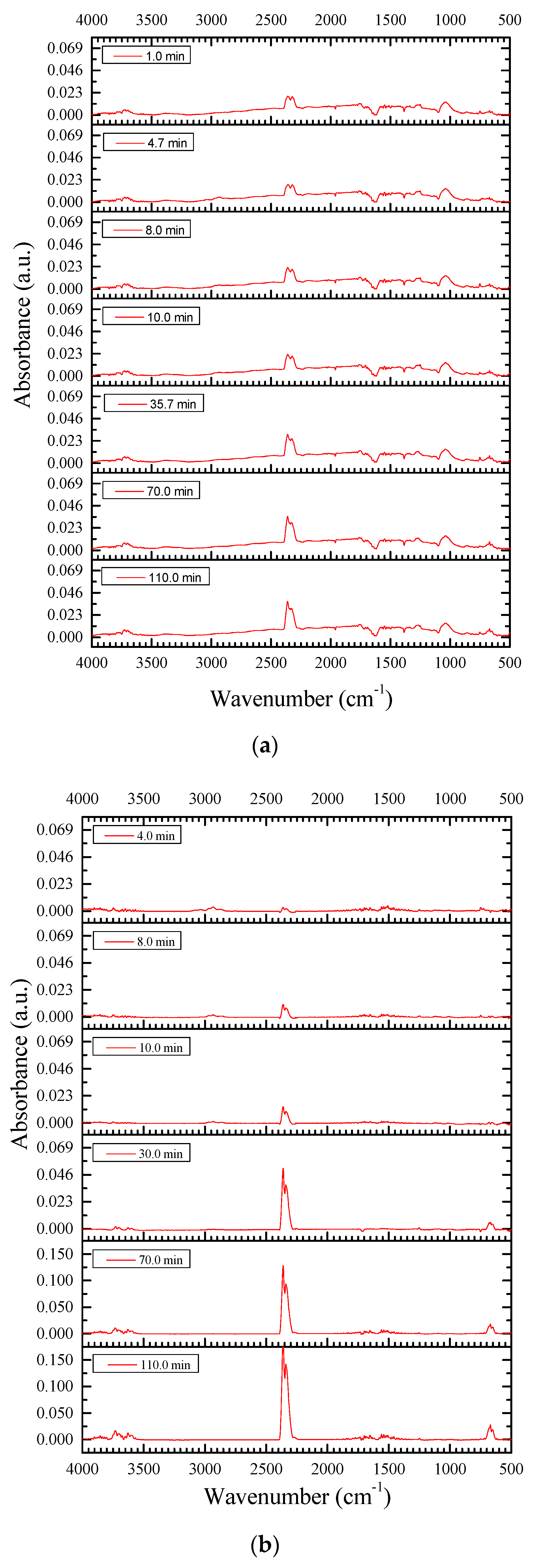
3.2.3. Resins Fraction
3.3. Catalytic Oxidation Characteristics of Changqing Tight Oil and SARA Fraction
4. Conclusions
- The cobalt-salt catalyst can reduce the LTO reaction activation energies of Changqing tight oil and its SARA fractions. The catalytic effect on LTO of SARA fractions is the intrinsic reason for the significant catalysis of Changqing tight oil oxidation.
- The catalyst greatly promotes the oxygen addition reaction and bond scission reaction of saturates, aromatics and resins, and the promotion effect on the bond scission reaction is stronger. The promotion effect on the bond scission reaction is the most pronounced for the resins, followed by the aromatics, and the weakest for the saturates.
- The catalyst significantly changes the reaction pathways of the LTO of SARA fractions. The formation of hydroxyl-containing oxides and CO2 from the oxidation of saturates, aromatics and resins is promoted, while the formation of ethers from the oxidation of aromatics and resins is inhibited.
Author Contributions
Funding
Conflicts of Interest
References
- Jia, H.; Sheng, J.J. Numerical modeling on air injection in a light oil reservoir: Recovery mechanism and scheme optimization. Fuel 2016, 172, 70–80. [Google Scholar] [CrossRef]
- Huang, S.; Sheng, J.J. Discussion of thermal experiments’ capability to screen the feasibility of air injection. Fuel 2017, 195, 151–164. [Google Scholar] [CrossRef]
- Ren, S.R.; Greaves, M.; Rathbone, R.R. Air injection LTO process: An IOR technique for light-oil reservoirs. SPE J. 2002, 7, 90–99. [Google Scholar] [CrossRef]
- Gutierrez, D.; Taylor, A.R.; Kumar, V.; Ursenbach, M.G.; Moore, R.G.; Mehta, S.A. Recovery factors in high-pressure air injection projects revisited. SPE Reserv. Eval. Eng. 2008, 11, 1097–1106. [Google Scholar] [CrossRef]
- Fraim, M.L.; Moffitt, P.D.; Yannimaras, D.V. Laboratory testing and simulation results for high pressure air injection in a waterflooded North Sea oil reservoir. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 5–8 October 1997. [Google Scholar]
- Adetunji, L.A.; Teigland, R. Light-oil air-injection performance: Sensitivity to critical parameters. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 9–12 October 2005. [Google Scholar]
- Clara, C.; Durandeau, M.; Quenault, G.; Nguyen, T.H. Laboratory studies for light-oil air injection projects: Potential application in Handil field. SPE Reserv. Eval. Eng. 2000, 3, 239–248. [Google Scholar] [CrossRef]
- Duiveman, M.W.; Herwin, H.; Grivot, P.G. Integrated management of water, lean gas and air injection: The successful ingredients to IOR projects on the mature Handil field. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 5–7 April 2005. [Google Scholar]
- Pascual, M.; Crosta, D.; Lacentre, P.; Coombe, D. Air injection into a mature waterflooded light oil reservoir-laboratory and simulation results for Barrancas field, Argentina. In Proceedings of the 67th EAGE Conference Exhibition, Madrid, Spain, 13–16 June 2005. [Google Scholar]
- Fassihi, M.R.; Yannimaras, D.V.; Kumar, V.K. Estimation of recovery factor in light-oil air-injection projects. SPE Reserv. Eng. 1997, 12, 173–178. [Google Scholar] [CrossRef]
- Fassihi, M.R.; Gillham, T.H. The use of air injection to improve the double displacement processes. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 3–6 October 1993. [Google Scholar]
- Gillham, T.H.; Cerveny, B.W.; Turek, E.A.; Yannimaras, D.V. Keys to increasing production via air injection in Gulf Coast light oil reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 5–8 October 1997. [Google Scholar]
- Glandt, C.A.; Pieterson, R.; Dombrowski, A.; Balzarini, M.A. Coral creek field study: A comprehensive assessment of the potential of high-pressure air injection in a mature waterflood project. In Proceedings of the SPE Mid-continent operations symposium, Oklahoma, OK, USA, 28–31 March 1999. [Google Scholar]
- Stokka, S.; Oesthus, A.; Frangeul, J. Evaluation of air injection as an IOR method for the Giant Ekofisk Chalk Field. In Proceedings of the SPE International Improved Oil Recovery Conference in Asia Pacific, Kuala Lumpur, Malaysia, 5–6 December 2005. [Google Scholar]
- Gutierrez, D.; Kumar, V.; Moore, R.G.; Mehta, S.A. Air injection and waterflood performance comparison of two adjacent units in the Buffalo Field. SPE Reserv. Eval. Eng. 2008, 11, 848–857. [Google Scholar] [CrossRef]
- Denney, D. 30 years of successful high-pressure air injection: Performance evaluation of Buffalo field, South Dakota. J. Pet. Technol. 2011, 63, 50–53. [Google Scholar] [CrossRef]
- Germain, P.; Geyelin, J.L. Air injection into a light oil reservoir: The Horse Creek project. In Proceedings of the SPE Middle East Oil Show and Conference, Awali, Bahrain, 15–18 March 1997. [Google Scholar]
- Bagci, S.; Celebioglu, D. Light oil combustion with metallic additives in limestone medium. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 8–10 June 2004. [Google Scholar]
- Shallcross, D.C.; De los Rios, C.F.; Castanier, L.M.; Brigham, W.E. Modifying in-situ combustion performance by the use of water-soluble additives. SPE Reserv. Eng. 1991, 6, 287–294. [Google Scholar] [CrossRef]
- Gerritsen, M.; Kovscek, A.; Castanier, L.; Nilsson, J.; Younis, R.; He, B. Experimental investigation and high resolution simulator of in-situ combustion processes; 1. Simulator design and improved combustion with metallic additives. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium and Western Regional Meeting, Bakersfield, CA, USA, 16–18 March 2004. [Google Scholar]
- Ramirez-Garnica, M.A.; Mamora, D.D.; Nares, R.; Schacht-Hernandez, P.; Mohammad, A.A.; Cabrera, M. Increase heavy-oil production in combustion tube experiments through the use of catalyst. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 15–18 April 2007. [Google Scholar]
- Ramirez-Garnica, M.A.; Hernandez Perez, J.R.; Cabrera-Reyes, M.D.C.; Schacht-Hernandez, P.; Mamora, D.D. Increase oil recovery of heavy oil in combustion tube using a new catalyst based on nickel ionic solution. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium, Calgary, AB, Canada, 20–23 October 2008. [Google Scholar]
- Fassihi, M.R.; Brigham, W.E.; Ramey, H.J., Jr. Reaction kinetics of in-situ combustion: Part 1—Observations. SPE J. 1984, 24, 399–407. [Google Scholar] [CrossRef]
- Fassihi, M.R.; Brigham, W.E.; Ramey, H.J., Jr. Reaction kinetics of in-Situ combustion: Part 2—Modeling. SPE J. 1984, 24, 408–416. [Google Scholar] [CrossRef]
- Castanier, L.M.; Baena, C.J.; Holt, R.J.; Brigham, W.E.; Tavares, C. In situ combustion with metallic additives. In Proceedings of the SPE Latin America Petroleum Engineering Conference, Caracas, Venezuela, 8–11 March 1992. [Google Scholar]
- Drici, O.; Vossoughi, S. Catalytic effect of heavy metal oxides on crude oil combustion. SPE Reserv. Eng. 1987, 2, 591–595. [Google Scholar] [CrossRef]
- He, B.; Chen, Q.; Castanier, L.M.; Kovscek, A.R. Improved in-situ combustion performance with metallic salt additives. In Proceedings of the SPE Western Regional Meeting, Irvine, CA, USA, 30 March–1 April 2005. [Google Scholar]
- Wang, H.; Tang, X.; Meng, K.; Cui, Y.; Wang, Z.; Wang, F. Preparation and evaluation of catalyst for heavy oil injected air catalytic oxidation production. Fine Chem. 2009, 33, 179–188. [Google Scholar]
- Tang, X.; Cui, Y.; He, B.; Liu, Q.; Meng, K. Experimental study on the viscosity reduction of Liaohe heavy oil by catalytic air oxidation. Oilfield Chem. 2008, 25, 316–319. [Google Scholar]
- Pu, W.; Liu, P.; Li, Y.; Jin, F.; Liu, Z. Thermal characteristics and combustion kinetics analysis of heavy crude oil catalyzed by metallic additives. Ind. Eng. Chem. Res. 2015, 54, 11525–11533. [Google Scholar] [CrossRef]
- Amanam, U.U.; Kovscek, A.R. Analysis of the effects of copper nanoparticles on in-situ combustion of extra heavy-crude oil. J. Pet. Sci. Eng. 2017, 152, 406–415. [Google Scholar] [CrossRef]
- Zhao, Y. Effect of Clay Minerals on Oxidation Characteristics of Air Flooding. Ph.D. Dissertation, Southwest Petroleum University, Chengdu, China, 2014. [Google Scholar]
- Jia, H.; Zhao, J.Z.; Pu, W.F.; Zhao, J.; Kuang, X.Y. Thermal study on light crude oil for application of high-pressure air injection (HPAI) process by TG/DTG and DTA tests. Energy Fuels 2012, 26, 1575–1584. [Google Scholar] [CrossRef]
- Shi, G. Experimental study on low-temperature oxidation in air injection with supported-catalyst. Spec. Oil Gas. Reserv. 2012, 19, 126–129. [Google Scholar]
- Wang, J.; Wang, T.; Feng, C.; Chen, Z.; Kong, D.; Niu, Z. The catalytic effect of organometallic additives on low-temperature oxidation of light crude oil during air flooding. Pet. Sci. Technol. 2015, 33, 1118–1125. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Yang, W.; Kalitaani, S.; Deng, Z. A novel air flooding technology for light crude oil reservoirs applied under reservoir conditions. Energy Fuels 2018, 32, 4942–4950. [Google Scholar] [CrossRef]
- Wang, T.; Yang, W.; Wang, J.; Kalitaani, S.; Deng, Z. Low temperature oxidation of crude oil: Reaction progress and catalytic mechanism of metallic salts. Fuel 2018, 225, 336–342. [Google Scholar] [CrossRef]
- Groenzin, H.; Mullins, O.C. Asphaltene molecular size and structure. J. Phys. Chem. A 1999, 103, 11237–11245. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Feng, C.; Yang, C.; Chen, Z.; Lu, G. Catalytic effect of transition metallic additives on the light oil low-temperature oxidation reaction. Energy Fuels 2015, 29, 3545–3555. [Google Scholar] [CrossRef]
- Freitag, N.P.; Exelby, D.R. A SARA-based model for simulating the pyrolysis reactions that occur in high-temperature EOR processes. J. Can. Pet. Technol. 2006, 45, 38–44. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Meng, X.; Chu, G.; Liu, C. The low temperature oxidation characteristics of SARA fractions in air flooding process. Pet. Sci. Technol. 2018, 36, 1125–1130. [Google Scholar] [CrossRef]
- Yang, S.; Jin, L.; Kong, Q.; Li, L. Advanced Engineering Mechanics; Higher Education Press: Beijing, China, 1988; pp. 156–157, 222–223. [Google Scholar]
- Fraust, B. Infrared spectroscopy. In Modern Chemical Techniques; The Royal Society of Chemistry: London, UK, 1997; pp. 62–91. [Google Scholar]
- Niu, B.; Ren, S.; Liu, Y.; Wang, D.; Tang, L.; Chen, B. Low-temperature oxidation of oil components in an air injection process for improved oil recovery. Energy Fuels 2011, 25, 4299–4304. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Maham, Y.; Tan, X.; Babadagli, T.; Gray, M. Enhancement of the efficiency of in situ combustion technique for heavy-oil recovery by application of nickel ions. Fuel 2013, 105, 397–407. [Google Scholar] [CrossRef]



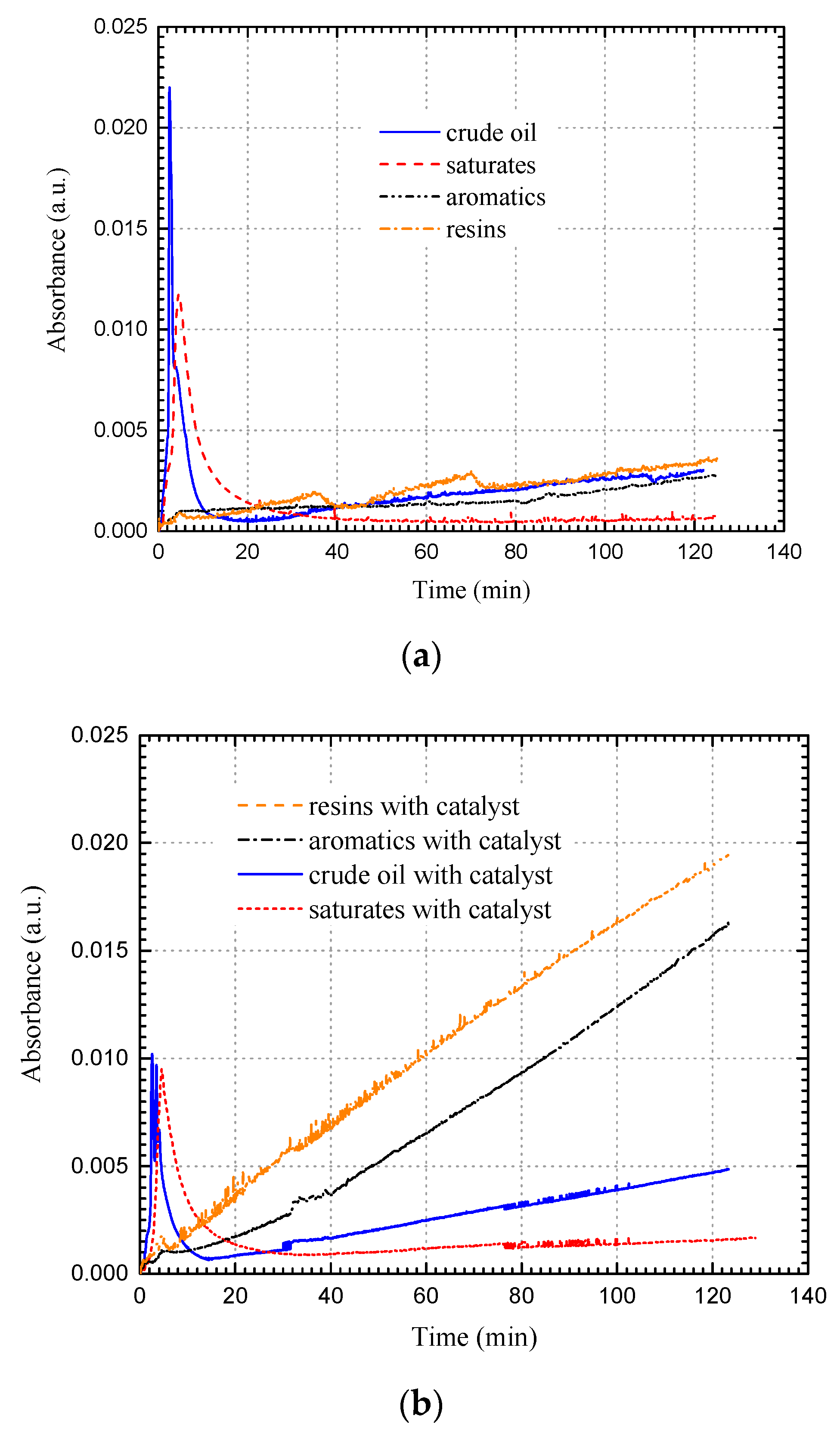
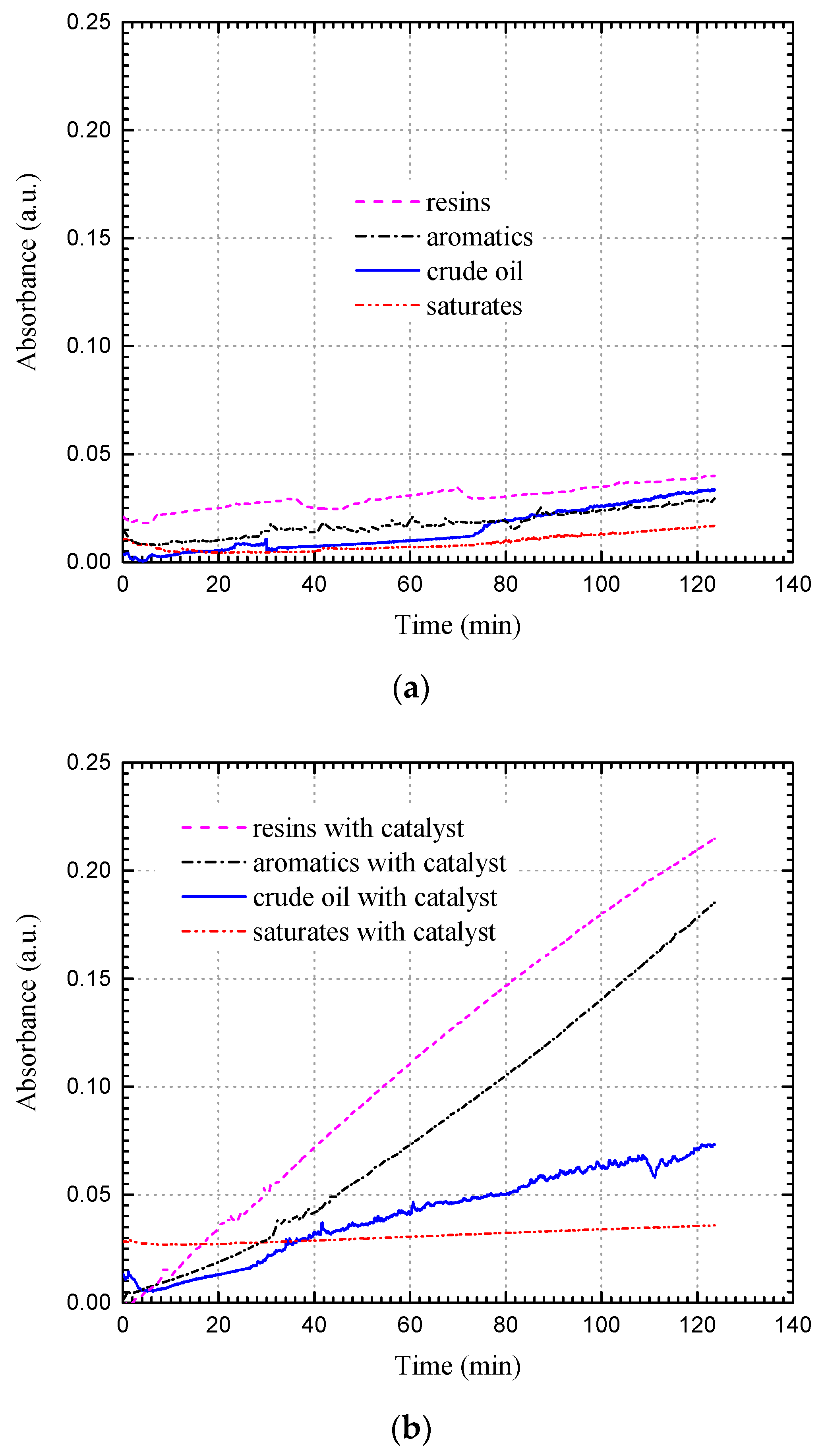
| Source | Experimental Type | Crude Oil (API Gravity) | Experimental Conditions (Temperature/Pressure) | Additives | Findings |
|---|---|---|---|---|---|
| Bagci et al. [18] | Combustion reaction kinetics experiments | Karakus (29° API) and Beykan (32° API) crude oils from Turkish oilfields | The cell was continuously heated to 500–600 °C with a 1 °C/min heating rate at pressures of 172 or 345 kPa | CuCl2, FeCl3, and MgCl2 | The catalyst type and concentration influenced the kinetic parameters (i.e., reaction order and activation energy) of high-temperature oxidation. |
| Shallcross et al. [19] | Combustion cell experiments | Californian (18.5° API) and Venezuelan (10.5° API) oils | Temperature was increased to 450 °C at a rate of 50 °C/h at pressures of 280 or 550 kPa | FeCl2, SnCl2, CuSO4, ZnCl2, MgCl2, K2Cr2O7, Al2Cl3, MnCl2, Ni(NO3)2, and CdSO4 | Iron and tin salts could enhance fuel formation, whereas copper, nickel, and cadmium salts have minor effects. |
| Gerritsen et al. [20] | Combustion tube experiments | Cymric light oil (34° API) | Temperature: 400 °C; Pressure: 68.9 kPa | iron nitrate | Metallic additive improves combustion of light oil. The Cymric light oil cannot sustain combustion without additive. |
| Ramirez et al. [21,22] | Combustion tube experiments | Heavy oil from Gulf of Mexico (12.5° API) | Ignition temperature: 300 °C; Pressures: 2070 kPa | Additives included Mo, Co, Ni, and Fe, and the anionic part of the salt was acetylacetonate or alkylhexanoate. | The addition of metallic additives could accelerate the propagation velocity of the combustion front, improve combustion efficiency, and increase oil production. |
| Fassihi et al. [23,24] | Combustion cell experiments | San Ardo oil (11.2° API), Venezuela oil (9.5° API), Huntington Beach oil (18.5° API), and Lynch Canyon oil (10° API) | Temperature was increased to 450 °C at a rate of 55 °C/h at pressures of 690, 550 or 138 kPa | Additives included Cu, Ni, Va, and Fe, but the anionic part of the salt was not reported. | The additives can lower both the activation energy and the occurrence temperature of the combustion reaction under the identical reservoir conditions. |
| Castanier et al. [25] | Combustion tube experiments | Huntington Beach oil (22° API), Hamaca oil (10° API), Cymric heavy oil (12° API), and Cymric light oil (34° API) | Ignition temperature: 315 °C; Pressure: 690 kPa | Additives included Fe, Sn, and Zn, but the anionic part of the salt was not reported. | The combustion efficiency and front velocities can be improved by the metallic additives. The H/C ratio of the fuel, heat of combustion, air requirements, and density of the produced crude are changed after the addition of metallic salts. In addition, the combustion of light oil can be improved by metallic additives. |
| Drici et al. [26] | Differential scanning calorimetry (DSC) and therrnogravimetric analysis (TGA) | Crude oil from the Iola field (19.8° API) | Temperature was increased to 600 °C at a rate of 10 °C/h at atmospheric pressure | Heavy metal oxides such as titanium, ferric, nickel, cupric, vanadium, and chromium oxides | The effect of titanium oxide is similar to that of silica and alumina. Vanadium, nickel, and ferric oxides behaves similarly in enhancing the endothermic reactions. The effect of small amount of metal oxide is weak in the presence of a large surface area such as silica. |
| He et al. [27] | Combustion tube experiments and ramped temperature oxidation (RTO) tests | Heavy (12° API) and light (34° API) oil from Cymric | Tube experiments: Ignition temperature 400 °C; Pressure 690 kPa; RTO tests: Temperature was increased to 470 °C at a rate of 60 °C/h at pressures of 690, 550 or 310 kPa | Fe(NO3)3 | The combustion reaction catalytic mechanism is cation exchange of metallic salts with clay to create activated sites that enhance the combustion reactions between oil and oxygen. The combustion of light oil can be improved by metallic additives. |
| Wang et al. [28] | Catalytic oxidation experiment; Viscosity reduction experiment | SZ36-1 crude oil (30° API) | Temperature: 100 °C; Pressure: 2000 kPa | Iron naphthenate, copper naphthenate, zinc naphthenate, manganese naphthenate, cobalt naphthenate | The preferred catalyst is copper naphthenate in an amount of 0.2% by mass of the crude oil. After the catalytic oxidation, the acid value and viscosity of the heavy oil increases. After adding the auxiliary solution to the oxidized oil system, the resulting surfactant can greatly reduce the viscosity of the aqueous heavy oil system by the emulsified viscosity reduction mechanism. |
| Tang et al. [29] | Catalytic oxidation experiment; Viscosity reduction experiment | Crude oil from Liaohe oilfield in China, API gravity is not given, oil viscosity is 382.6 Pa·s. | Temperature: 200 °C; Pressure: 500 kPa | Water soluble catalyst SP-1, light blue powder, MeL type compound | The catalyst promotes oxidation and cracking of heavy oil and increases the acid value of heavy oil. The surface-active materials formed in the reaction can greatly reduce the viscosity of the aqueous heavy oil system by the emulsified viscosity reduction mechanism. |
| Pu et al. [30] | Thermogravimetric testing | Tahe heavy crude oil (18.9° API) | Temperature was increased to 800 °C at a rate of 10 °C/min at atmospheric pressure | ZnSO4, CuCl2, FeCl2, and AlCl3·6H2O | Metallic additives exhibit varied catalytic effects on heavy oil oxidation. CuCl2 is found to be an excellent catalyst for improving oil production through positively influencing the oxidation reactions of Tahe heavy crude oil. |
| Amanam et al. [31] | Ramped temperature oxidation experiments; Isoconversional analysis; Thermogravimetric analysis | Zuata crude oil (9.8° API) | Temperature was increased to 600 °C at a rate of 1–3 °C/min at atmospheric pressure | Copper nanoparticles | The apparent activation energy of the high-temperature oxidation region is decreased. The presence of Cu-NP helps maintain a greater front temperature. Cu-NP changes the type of oil produced and decreases the amount of water generated during the reactions. |
| Properties | Value | Appearance | |
|---|---|---|---|
| Density (g/cm3) | 0.850 | N/A | |
| Viscosity at 70 °C (mPa·s) | 2.14 | N/A | |
| SARA composition (wt %) | Saturates | 70.91 | Colourless liquid |
| Aromatics | 16.07 | Yellow or red sticky liquid | |
| Resins | 9.78 | Brown viscous liquid | |
| Asphaltenes | 3.24 | Black fragile powder solid | |
| Test Sample | Temperature, °C | Oxidation Rate, (mol O2/d·m3 [Sample]) | Test Sample | Temperature, °C | Oxidation Rate, (mol O2/d·m3 [Sample]) |
|---|---|---|---|---|---|
| Crude oil | 50 | 26.14 | Crude oil + catalyst | 50 | 241.16 |
| 70 | 53.37 | 70 | 271.69 | ||
| 90 | 86.62 | 90 | 390.46 | ||
| 110 | 295.37 | 110 | 907.23 | ||
| Saturates | 50 | 16.97 | Saturates + catalyst | 50 | 101.78 |
| 70 | 35.02 | 70 | 185.08 | ||
| 90 | 54.64 | 90 | 297.07 | ||
| 110 | 168.81 | 110 | 704.01 | ||
| Aromatics | 50 | 26.43 | Aromatics + catalyst | 50 | 202.17 |
| 70 | 47.25 | 70 | 311.08 | ||
| 90 | 65.11 | 90 | 377.06 | ||
| 110 | 245.96 | 110 | 691.65 | ||
| Resins | 50 | 75.34 | Resins + catalyst | 50 | 139.75 |
| 70 | 137.75 | 70 | 313.27 | ||
| 90 | 210.43 | 90 | 405.37 | ||
| 110 | 492.36 | 110 | 768.33 |
| Crude oil and Fractions | Activation Energy, (J/mol) | Activation Energy with Catalyst Added, (J/mol) | |
|---|---|---|---|
| Crude oil | 39,571 | 21,864 | |
| SARA fraction | Saturates | 37,469 | 32,088 |
| Aromatics | 35,606 | 19,849 | |
| Resins | 30,970 | 27,700 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Wang, J. Catalytic Effect of Cobalt Additive on the Low Temperature Oxidation Characteristics of Changqing Tight Oil and Its SARA Fractions. Energies 2019, 12, 2848. https://doi.org/10.3390/en12152848
Wang T, Wang J. Catalytic Effect of Cobalt Additive on the Low Temperature Oxidation Characteristics of Changqing Tight Oil and Its SARA Fractions. Energies. 2019; 12(15):2848. https://doi.org/10.3390/en12152848
Chicago/Turabian StyleWang, Tengfei, and Jiexiang Wang. 2019. "Catalytic Effect of Cobalt Additive on the Low Temperature Oxidation Characteristics of Changqing Tight Oil and Its SARA Fractions" Energies 12, no. 15: 2848. https://doi.org/10.3390/en12152848
APA StyleWang, T., & Wang, J. (2019). Catalytic Effect of Cobalt Additive on the Low Temperature Oxidation Characteristics of Changqing Tight Oil and Its SARA Fractions. Energies, 12(15), 2848. https://doi.org/10.3390/en12152848




