Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae and Growth Medium
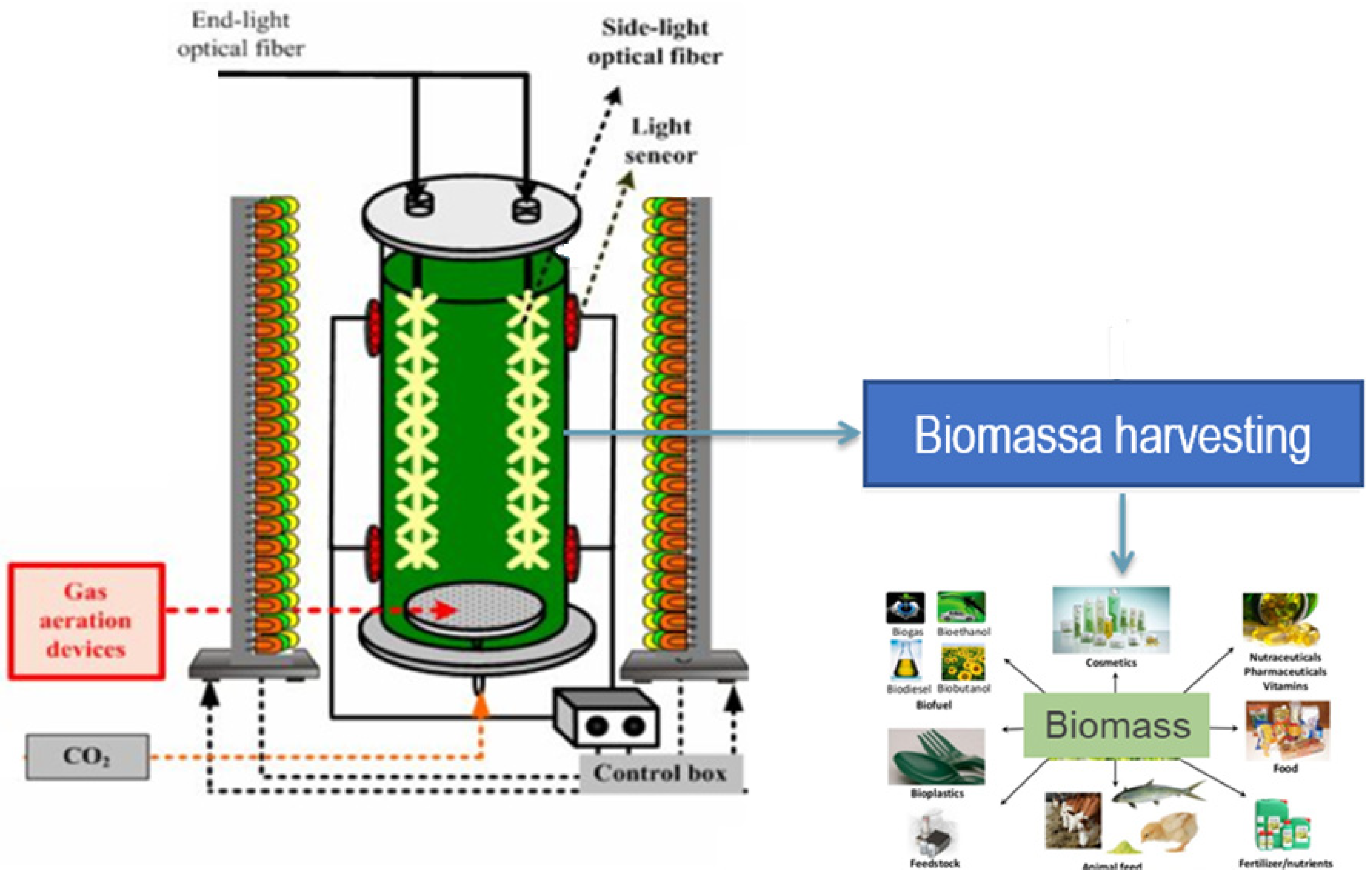
2.2. Photo-Bioreactor
2.3. Growth Conditions
2.4. Average CO2 Capture Rate and CO2 Conversion Efficiency
2.5. Analytical Methods
2.5.1. Determination of Algal Cell Concentration and Dry Cell Weight
2.5.2. Accelerated Solvent Extraction
2.5.3. Nutrient Consumption Chemical Analysis
2.5.4. Lutein Analysis
3. Results and Discussion
3.1. Effect of CO2 Content on Culture Medium pH
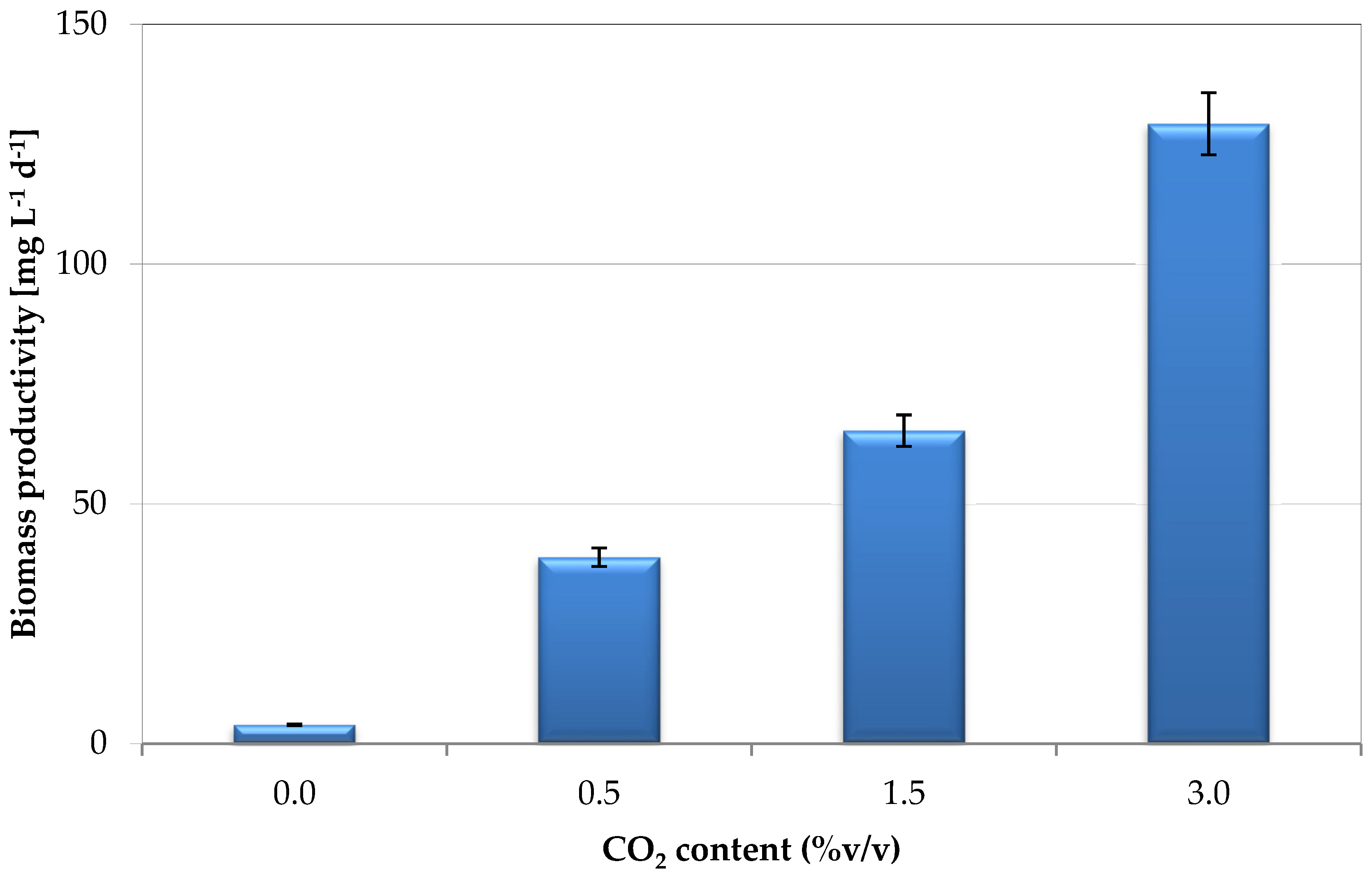
3.2. Effect of CO2 Content on Biomass Concentration
3.3. Effect of CO2 Content on Carbon Content, Average CO2 Capture Rate, and CO2 Conversion Efficiency
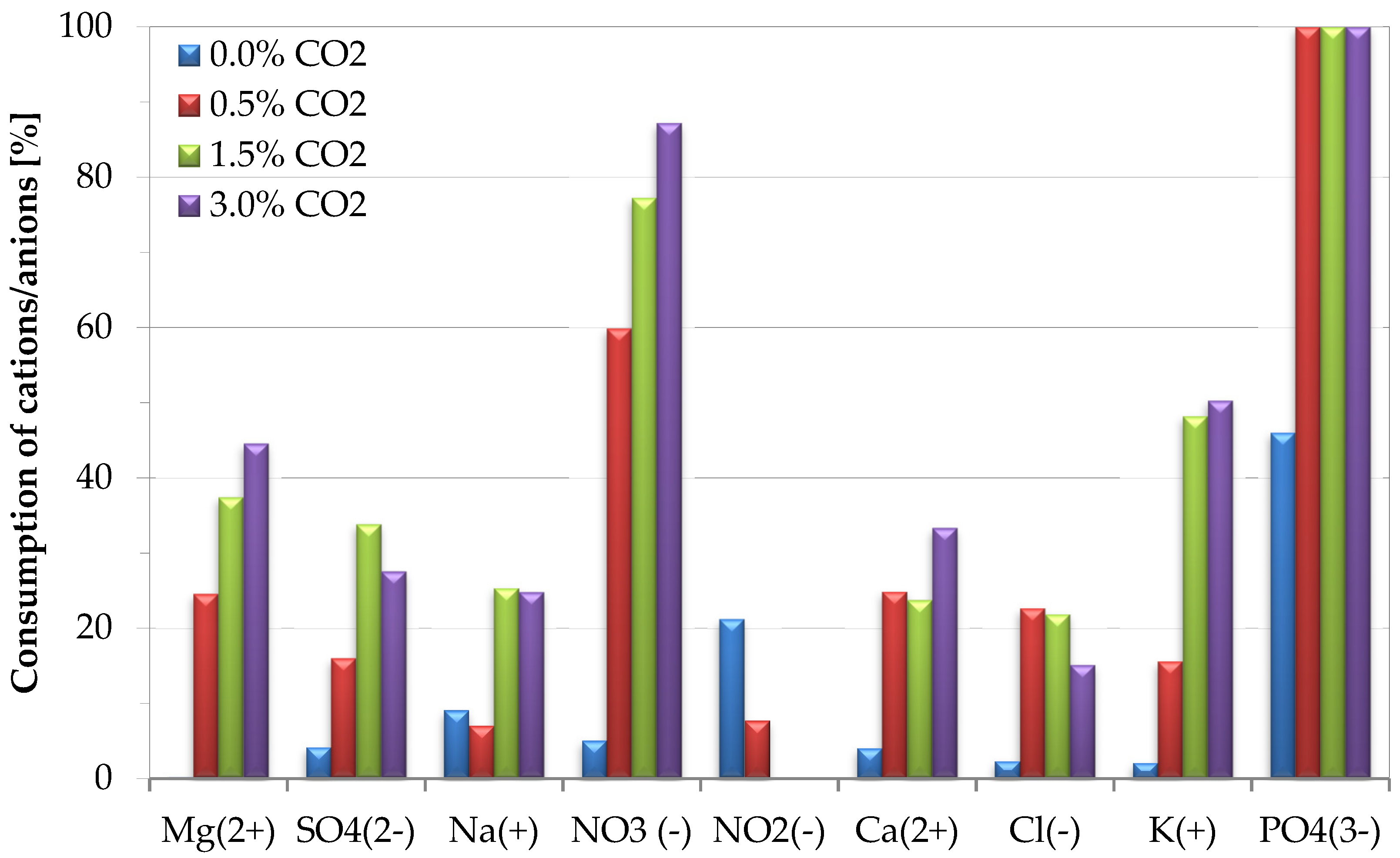
3.4. Role of CO2 Content on Nutrient Consumption
3.5. Extraction Yield and Lutein Production from S. almeriensis
3.6. Comparison of Biomass Productivity and Lutein Content
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cerón, M.C.; Campos, I.; Sánchez, J.F.; Acién, F.G.; Molina, E.; Fernández-Sevilla, J.M. Recovery of lutein from microalgae biomass: Development of a process for Scenedesmus almeriensis biomass. J. Agric. Food Chem. 2008, 56, 11761–11766. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, K.; Zhu, Y.; Yang, W.; Zhou, J.; Cen, K. Transcriptome sequencing and metabolic pathways of astaxanthin accumulated in Haematococcus pluvialis mutant under 15% CO2. Bioresour. Technol. 2017, 228, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.Y.; Kao, C.Y.; Chen, C.H.; Kuan, T.C.; Ong, S.C.; Lin, C.S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Joun, J.M.; Lee, J.; Hong, M.E.; Pham, H.M.; Chang, W.S.; Sim, S.J. Development of large-scale and economic pH control system for outdoor cultivation of microalgae Haematococcus pluvialis using industrial flue gas. Bioresour. Technol. 2017, 244, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- de Assis, T.C.; Calijuri, M.L.; Assemany, P.P.; de Paula, A.S.A.; Martins, M.A. Using atmospheric emissions as CO2 source in the cultivation of microalgae: Productivity and economic viability. J. Clean. Prod. 2019, 215, 1160–1169. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Dash, S.K.; Sen, R. Process integration for microalgal lutein and biodiesel production with concomitant flue gas CO2 sequestration: a biorefinery model for healthcare, energy and environment. RSC Adv. 2015, 5, 73381–73394. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas Control 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Acién-Fernández, G.; Molina-Grima, E.; Fernández-Sevilla, J.M.; Pérez-Sacristán, B.; Blanco-Navarro, I. In vitro bioaccesibility of lutein and zeaxanthin from the microalgae Scenedesmus almeriensis. Food Chem. 2009, 114, 747–752. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein in specific marigold flowers and microalgae. J. Taiwan Inst. Chem. Eng. 2015, 49, 90–94. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.E.; Herbst-Espinosa, S.M.; Hussain, E.A.; Stacewicz-Sapuntzakis, M. Esterification Does Not Impair Lutein Bioavailability in Humans. J. Nutr. 2018, 132, 3668–3673. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.H.; Wang, B.; Chang, J.S.; Shen, Y. Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour. Technol. 2017, 244, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Ho, S.H.; Liu, C.C.; Chang, J.S. Enhancing lutein production with Chlorella sorokiniana Mb-1 by optimizing acetate and nitrate concentrations under mixotrophic growth. J. Taiwan Inst. Chem. Eng. 2017, 79, 88–96. [Google Scholar] [CrossRef]
- Ruban, A.V.; Berera, R.; Ilioaia, C.; Van Stokkum, I.H.M.; Kennis, J.T.M.; Pascal, A.A.; Van Amerongen, H.; Robert, B.; Horton, P.; Van Grondelle, R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 2007, 450, 575–578. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the re-evaluation of lutein (E 161b) as a food additive. EFSA J. 2010, 8, 1678. [CrossRef]
- Utomo, R.P.; Chang, Y.R.; Lee, D.J.; Chang, J.S. Lutein recovery from Chlorella sp. ESP-6 with coagulants. Bioresour. Technol. 2013, 139, 176–180. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Serrano, C.G.; Zurano, A.S.; García, L.A.; Maldonado, S.E.; Peña, J.; Fernández, F.G.A. Variations of culture parameters in a pilot-scale thin-layer reactor and their influence on the performance of Scenedesmus almeriensis culture. Bioresour. Technol. Reports 2019, 6, 190–197. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J. Biotechnol. 2001, 85, 289–295. [Google Scholar] [CrossRef]
- Casal, C.; Cuaresma, M.; Vega, J.M.; Vilchez, C. Enhanced productivity of a lutein-enriched novel acidophile microalga grown on urea. Mar. Drugs 2011, 9, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, C.C. Optimization of lutein production with a two-stage mixotrophic cultivation system with Chlorella sorokiniana MB-1. Bioresour. Technol. 2018, 262, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of lutein production in Chlorella sorokiniana (chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, R.; Dhanarajan, G.; Dash, S.K.; Sen, R. An advanced hybrid medium optimization strategy for the enhanced productivity of lutein in Chlorella minutissima. Algal Res. 2015, 7, 24–32. [Google Scholar] [CrossRef]
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández-Sevilla, J.M.; Acién, F.G.; Cerón, M.C.; Pérez-Parra, J.; Molina-Grima, E. Biomass and lutein productivity of Scenedesmus almeriensis: Influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008, 79, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Chen, F.; Chen, G.; Zhang, X.W.; Liu, L.J.; Zhang, H. Enhanced production of lutein in heterotrophic Chlorella protothecoides by oxidative stress. Sci. China Ser. C Life Sci. 2008, 51, 1088–1093. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Yen, H.W.; Hu, I.C.; Chen, C.Y.; Ho, S.H.; Lee, D.J.; Chang, J.S. Microalgae-based biorefinery - From biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef]
- Salih, F.M. Microalgae Tolerance to High Concentrations of Carbon Dioxide: A Review. J. Environ. Prot. (Irvine. Calif). 2011, 02, 648–654. [Google Scholar] [CrossRef]
- Mehariya, S.; Patel, A.K.; Obulisamy, P.K.; Punniyakotti, E.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective. Bioresour. Technol. 2018, 265, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Limonti, C.; Mehariya, S.; Molino, A.; Calabrò, V. Biofuel production and phosphorus recovery through an integrated treatment of agro-industrial waste. Sustain. 2018, 11, 52. [Google Scholar] [CrossRef]
- Nobre, B.P.; Villalobos, F.; Barragán, B.E.; Oliveira, A.C.; Batista, A.P.; Marques, P.A.S.S.; Mendes, R.L.; Sovová, H.; Palavra, A.F.; Gouveia, L. A biorefinery from Nannochloropsis sp. microalga - Extraction of oils and pigments. Production of biohydrogen from the leftover biomass. Bioresour. Technol. 2013, 135, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.E.; Myers, J. on Pigments, Growth, and Photosynthesis of Phaeodactylum Tricornutum. J. Phycol. 1968, 4, 349–355. [Google Scholar] [CrossRef] [PubMed]
- de Morais, M.G.; Costa, J.A.V. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef]
- Chaudhary, R.; Dikshit, A.K.; Tong, Y.W. Carbon-dioxide biofixation and phycoremediation of municipal wastewater using Chlorella vulgaris and Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2018, 25, 20399–20406. [Google Scholar] [CrossRef] [PubMed]
- Mehariya, S.; Iovine, A.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.; Casella, P.; Karatza, D.; Marino, T.; Musmarra, D.; et al. Supercritical Fluid Extraction of Lutein from Scenedesmus almeriensis. Molecules 2019, 24, 1324. [Google Scholar] [CrossRef]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- Pruvost, J.; Van Vooren, G.; Le Gouic, B.; Couzinet-Mossion, A.; Legrand, J. Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour. Technol. 2011, 102, 150–158. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef] [PubMed]
- Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical Carbon Dioxide Extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Cabello, J.; Morales, M.; Revah, S. Carbon dioxide consumption of the microalga Scenedesmus obtusiusculus under transient inlet CO2 concentration variations. Sci. Total Environ. 2017, 584–585, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wang, J.; Li, R.; Liu, T. Modeling of carbon dioxide mass transfer behavior in attached cultivation photobioreactor using the analysis of the pH profiles. Bioprocess Biosyst. Eng. 2017, 40, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, Y.; Goyal, A.; Tolbert, N.E. Alkalization of the medium by unicellular green algae during uptake dissolved inorganic carbon. Plant Cell Physiol. 1993, 34, 649–657. [Google Scholar] [CrossRef]
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour. Technol. 2016, 204, 49–54. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Chen, H.; Gao, C. Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep. Purif. Technol. 2006, 50, 324–329. [Google Scholar] [CrossRef]
- Sepulveda, C.; Gómez, C.; El Bahraoui, N.; Acién, G. Comparative evaluation of microalgae strains for CO2 capture purposes. J. CO2 Util. 2019, 30, 158–167. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Radmann, E.M.; Camerini, F.V.; Santos, T.D.; Costa, J.A.V. Isolation and application of SOX and NOX resistant microalgae in biofixation of CO2 from thermoelectricity plants. Energy Convers. Manag. 2011, 52, 3132–3136. [Google Scholar] [CrossRef]
- Jin, H.F.; Lim, B.R.; Lee, K. Influence of nitrate feeding on carbon dioxide fixation by microalgae. J. Environ. Sci. Heal. Part A Toxic/Hazardous Subst. Environ. Eng. 2006, 41, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.C.; Kao, C.Y.; Chiu, S.Y.; Tsai, M.T.; Lin, C.S. Characterization of the thermal-tolerant mutants of Chlorella sp. with high growth rate and application in outdoor photobioreactor cultivation. Bioresour. Technol. 2010, 101, 2880–2883. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Karemore, A.; Sen, R. Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Tsai, M.T.; Kao, C.Y.; Ong, S.C.; Lin, C.S. The air-lift photobioreactors with flow patterning for high-density cultures of microalgae and carbon dioxide removal. Eng. Life Sci. 2009, 9, 254–260. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Potential of using organic fertilizer to cultivate Chlorella vulgaris for biodiesel production. Appl. Energy 2012, 94, 303–308. [Google Scholar] [CrossRef]
- Hadj-Romdhane, F.; Zheng, X.; Jaouen, P.; Pruvost, J.; Grizeau, D.; Croué, J.P.; Bourseau, P. The culture of Chlorella vulgaris in a recycled supernatant: Effects on biomass production and medium quality. Bioresour. Technol. 2013, 132, 285–292. [Google Scholar] [CrossRef]
- Patel, A.K.; Joun, J.M.; Hong, M.E.; Sim, S.J. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour. Technol. 2019, 245–253. [Google Scholar] [CrossRef]
- Yen, H.W.; Sun, C.H.; Ma, T.W. The comparison of lutein production by scenesdesmus sp. in the autotrophic and the mixotrophic cultivation. Appl. Biochem. Biotechnol. 2011, 164, 353–361. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ho, S.H.; Chen, C.N.N.; Chen, C.Y.; Ng, I.S.; Jing, K.J.; Chang, J.S.; Lu, Y. Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: Effects of nitrate concentration, light intensity and fed-batch operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, I.; Ruiz-Domínguez, M.C.; Márquez, M.; Vílchez, C. Cu-mediated biomass productivity enhancement and lutein enrichment of the novel microalga Coccomyxa onubensis. Process Biochem. 2012, 47, 694–700. [Google Scholar] [CrossRef]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| CO2 Content (%v/v) | CC (% w/w) | P (gbiomass L−1 d−1) | RCO2 (gCO2 L−1 d−1) | ECO2 (%) |
|---|---|---|---|---|
| 0.5 | 43.4 | 0.039 | 0.06 | 44.5 |
| 1.5 | 42.4 | 0.065 | 0.10 | 24.3 |
| 3.0 | 49.9 | 0.129 | 0.24 | 28.4 |
| Nutrients (mg/L) | CO2 Content (%v/v) | |||
|---|---|---|---|---|
| 0.0 | 0.5 | 1.5 | 3.0 | |
| Mg2+ | 100.44 | 100.25 | 98.805 | 109.13 |
| SO42− | 451.28 | 432.27 | 459.06 | 442.47 |
| Na+ | 259.45 | 235.82 | 265.57 | 268.94 |
| NO3− | 620.58 | 589.12 | 680.73 | 622.24 |
| Ca2+ | 99.35 | 95.32 | 98.48 | 96.19 |
| Cl− | 165.78 | 161.87 | 185.80 | 205.22 |
| K+ | 38.8 | 37.98 | 47.78 | 48.56 |
| PO43− | 47.58 | 25.68 | 47.88 | 47.21 |
| Microalgal Strain | Operation Mode | Cultivating Conditions | Biomass Productivity (g/L/day) | Lutein Content (mg/g) | References |
|---|---|---|---|---|---|
| Chlorella protothecoides | Batch | Heterotrophic | 1.99 | 1.98 | [28] |
| Coccomyxa acidophila | Batch | Mixotrophic | 0.26 | 3.50 | [21] |
| Chlorella sorokiniana | Batch | Autotrophic | 0.84 | 3.0 | [23] |
| Scenedesmus sp. | Batch | Mixotrophic | 0.38 | 1.05 | [60] |
| Chlorella sorokiniana Mb-1 | Batch | Mixotrophic | 1.03 | 3.86 | [15] |
| Scenedesmus obliquus FSP-3 | Batch | Autotrophic | 0.92 | 4.52 | [25] |
| Chlorella zofingiensis | Batch | Autotrophic | 0.45 | 7.2 | [61] |
| Scenedesmus almeriensis | Continuous | Autotrophic | 0.87 | 5.5 | [27] |
| Scenedesmus almeriensis | Continuous | Autotrophic | 0.72 | 5.3 | [26] |
| Desmodesmus sp. F51 | Fed-batch | Autotrophic | 0.65 | 5.5 | [62] |
| Coccomyxa onubensis | Semi-continuous | Autotrophic | 0.55 | 6.2 | [63] |
| Muriellopsis sp. | Batch | Autotrophic | 0.04 | 4.3 | [64] |
| Chlorella zofingiensis | Batch | Autotrophic | 0.88 | 3.4 | [64] |
| Chlorella minutissima MCC-27 | Batch | Autotrophic | 0.57 | 6.05 | [24] |
| Chlorella minutissima | Batch | Autotrophic | 0.67 | 6.37 | [7] |
| Scenedesmus almeriensis | Batch | Autotrophic | 0.13 | 8.54 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molino, A.; Mehariya, S.; Karatza, D.; Chianese, S.; Iovine, A.; Casella, P.; Marino, T.; Musmarra, D. Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production. Energies 2019, 12, 2806. https://doi.org/10.3390/en12142806
Molino A, Mehariya S, Karatza D, Chianese S, Iovine A, Casella P, Marino T, Musmarra D. Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production. Energies. 2019; 12(14):2806. https://doi.org/10.3390/en12142806
Chicago/Turabian StyleMolino, Antonio, Sanjeet Mehariya, Despina Karatza, Simeone Chianese, Angela Iovine, Patrizia Casella, Tiziana Marino, and Dino Musmarra. 2019. "Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production" Energies 12, no. 14: 2806. https://doi.org/10.3390/en12142806
APA StyleMolino, A., Mehariya, S., Karatza, D., Chianese, S., Iovine, A., Casella, P., Marino, T., & Musmarra, D. (2019). Bench-Scale Cultivation of Microalgae Scenedesmus almeriensis for CO2 Capture and Lutein Production. Energies, 12(14), 2806. https://doi.org/10.3390/en12142806












