Abstract
In this study, Scenedesmus almeriensis as green microalga was cultivated on bench-scale for carbon dioxide (CO2) capture and lutein production. The autotrophic cultivation of S. almeriensis was carried out by using a vertical bubble column photo-bioreactor (VBC-PBR) with a continuous flow of a gaseous mixture of oxygen (O2), nitrogen (N2), and CO2, the latter in content of 0.0–3.0 %v/v. The liquid phase was batch. S. almeriensis growth was optimized. In addition, lutein extraction was carried out by using accelerated solvent extraction with ethanol as Generally Recognized as Safe (GRAS) solvent at 67 °C and 10 MPa. Upon optimization of CO2 concentration, the maximum biomass productivity, equal to 129.24 mg·L−1·d−1, was achieved during the cultivation by using a content of CO2 equal to 3.0 %v/v and it allowed to obtain a lutein content of 8.54 mg·g−1, which was 5.6-fold higher in comparison to the analogous process carried out without CO2 addition. The ion chemical analysis in the growth medium showed that by gradually increasing CO2 content, the nutrient consumption during the growth phase also increased. This study may be of potential interest for lutein extraction at industrial scale, since it is focused on pigment production from a natural source with a concomitantly CO2 capture.
1. Introduction
Microalgae cultivation is widely accepted as a valid method for carbon dioxide (CO2) capture from industrial plants, and for the extraction of high-value products, such as carotenoids and fatty acids [1,2,3,4,5,6,7,8]. Microalgae-based CO2 biofixation and biomass production are strongly dependent on the microalgae strain selection and the adopted growth conditions [5]. Scenedesmus almeriensis, being the highest lutein natural producer, has attracted growing interest and it represents the only commercialized microalgae for pigment extraction [9,10,11,12]. Lutein is classified as a primary xanthophyll because of the presence of two hydroxyl functional groups in the structure [13,14,15]. It acts as a light energy harvesting compound, which improves the photosynthesis efficiency and it prevents photodamage [16]. A daily lutein intake of 1 mg/kg body weight was suggested by the European Food Safely Authority (EFSA) [17], which could provide several health benefits. Moreover, lutein also acts as an antioxidant, anti-inflammatory, and colorant, which promotes its application for nutraceutical and pharmaceutical purposes [9]. Lutein is accumulated in the macula of the human eye retina and it is able to prevent or ameliorate cardiovascular diseases [18].
An enhanced production of lutein from microalgae can be achieved through a proper design of suitable culture strategies. The main parameters typically considered for potential microalgal producers of lutein are lutein content and biomass productivity. As reported by several authors, lutein content can be improved by optimizing the main physico-chemical (CO2 content, inoculum concentration, light intensity, temperature, and pH) and hydrodynamic (flow rate, distribution by mixing, and CO2 mass transfer) parameters [1,7,9,14,15,19,20,21,22,23,24,25,26,27,28,29]. Furthermore, under autotrophic growth conditions, microalgae cultivation is free of contamination from external sources, and the pH, if it is necessary, can also be regulated by feeding CO2 [15]. Autotrophic cultivation of microalgae could offer several environmental benefits, such as CO2 sequestration for a reduced greenhouse gas (GHG) emission [15,29,30,31]. The autotrophic growth requires inorganic carbon sources under illumination, which could potentially be derived from sun light, thus optimizing the overall production cost.
Xie et al. [14] evaluated the effect of CO2 concentration on microalgae biomass and lutein production from Desmodesmus sp. F51, and they found that by fixing CO2 concentration at 0.03, 2.5, 5.0, 7.5, 10.0, and 12.5%, biomass productivity and specific growth rate increased with increasing CO2 concentration from 0.03% to 2.5%, and they showed a decrease when CO2 concentration was further increased to 12.5% [14]. Therefore, in this study, a CO2 content of 3 %v/v as a maximum value was selected.
The extraction of intracellular compounds from microalgae biomass by using chemical solvents is the most reliable method to extract compounds by microalgae. Extraction efficiency and lutein recovery by organic solvents are higher than those associated with other physical methods. However, the effectiveness of the used solvent relies intensely on microalgae strains and on physical properties of microalgae biomass. Additionally, after the solvent extraction, lutein could be used in nutraceutical and pharmaceutical industries, while the exhausted biomass could be used in an integrated biorefinery for bioenergy production [32,33,34].
The effect of different CO2 contents, in the range of 0–3 %v/v, on biomass and lutein production, and on average CO2 capture rate and CO2 conversion efficiency, was investigated. Biomass harvesting was carried out through vacuum filtration, while the subsequent solvent extraction of lutein was performed by using the accelerated solvent extraction (ASE) technique. Ethanol, as Generally Recognized as Safe (GRAS) solvent, was used for the extraction step, operating at 67 °C, 10 MPa. Extraction was performed on mechanically pretreated S. almeriensis biomass. Lutein analysis was carried out by means of u-HPLC analysis.
2. Materials and Methods
2.1. Microalgae and Growth Medium
Microalgae S. almeriensis were supplied by AlgaRes Srl, Rome, Italy, and used as the inoculum for cultivation under laboratory conditions. Microalgae cells were cultivated using a modified Mann and Myers medium [19,35], which consisted of: NaNO3 (1.0 g/L), K2HPO4 (0.1 g/L), MgSO4·7H2O (1.2 g/L), and CaCl2 (0.3 g/L). Also, a micronutrient solution of 10 mL was added into 990 mL of Mann and Myers medium. The micronutrients stock solution contained Na2EDTA (0.001 mg/L), MnCl2 (1.4 mg/L), ZnSO4·7H2O (0.33 mg/L), FeSO4·2H2O (2 mg/L), CuSO4·5H2O (0.002 mg/L), and Co(NO3)2·6H2O (0.007 mg/L).
2.2. Photo-Bioreactor
S. almeriensis was cultivated in a vertical bubble column photo-bioreactor (VBC-PBR), made of Plexiglas, with a working volume of 28.5 L (effective height: 680 mm; external diameter: 250 mm; thickness: 10 mm) and with the volume to surface ratio (V/S) of 56.5 L/m2. The VBC-PBR was equipped with control and monitoring systems for monitoring and regulating gaseous mixture flow rate, temperature, pH, and light intensity. VBC-PBR was fed by a gaseous mixture (N2/ON2/CO2) from cylinders. Gaseous mixture flow rate was controlled by using Bronkhorst controllers (The Netherlands), with flow control accuracy of 0.5%. The bottom of the reactor was equipped with 6 sintered steel spargers, installed through 6 filleted holes (1/2”), to feed the gaseous mixture into the photo-bioreactor. The top of the reactor was equipped with a temperature sensor (thermocouple) and with a pH sensor. Temperature measurement was used to drive the temperature control system by modifying the flow rate of a cooling fluid flowing into an AISI 316L coaxial pipe (diameter = 60.3 mm, thickness = 1 mm). A temperature control system allowed the regulation of the temperature inside the reactor with a precision of ±1 °C in the range of 15–35 °C, thanks to a heat pump.
The lighting system consisted of a semi-cylinder, located at a distance of 100 mm from the VBC-PBR with blue, white, and red lights from a selective LED system (only blue/only white/only red, or a mix of them), with a light intensity in the range of 0–5000 lux on the surface of the VBC-PBR. The diameter of the lighting system was 350 mm, which was controlled and regulated by SCADA (Supervisory Control and Data Acquisition). The SCADA system was equipped with a touch-screen, custom software and PC to collect and record experimental data of temperature, gas flow rate, pH, and light intensity. A schematization of the experimental set-up in Figure 1 is sketched.
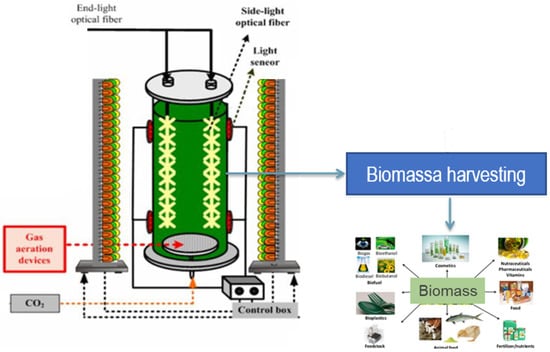
Figure 1.
Experimental set-up schematization.
2.3. Growth Conditions
Cultivation of microalgae was carried out by using a modified Mann and Myers medium with the above mentioned concentration (Section 2.1.). During investigations, a desired amount of inoculum was added to achieve an optical density of 0.6–0.7 at 420 nm. Each investigation was carried out by using a working volume of 28.5 L. The microalgae growth was performed under white light with a lux intensity on the VBC-PBR surface of 4000 lux, and by continuously feeding, for all the cultivation time, a gaseous mixture stream consisting of O2, N2, and CO2 with a flow rate of 300 mL·min−1. The CO2 content was varied in the range of 0–3.0 %v/v (O2 = 21 %v/v). Cultivation was carried out by keeping temperature constant at 28 °C. Each experimental condition was investigated three times, and for each condition, the standard deviation (SD) value was calculated.
2.4. Average CO2 Capture Rate and CO2 Conversion Efficiency
Average CO2 capture rate, RCO2 (gCO2·L−1·d−1), can be assessed by considering the elemental carbon content of the microalgae cell and the biomass productivity, according to the following equation [36]:
where Cc is the carbon content of the microalgae cell (% wt), P is the biomass productivity (gbiomass·L−1·d−1), MCO2 is the molecular weight of CO2, and MC is the molecular weight of carbon.
Average CO2 capture efficiency can be assessed according to the following equation [37]:
where RCO2, VPBR, VCO2, and ρCO2 are the average CO2 capture rate (gCO2·L−1·d−1), the volume of the VBC-PBR (L), total CO2 consumed (L) during the cultivation time t (d), and the density of CO2 (g·L−1), respectively.
2.5. Analytical Methods
2.5.1. Determination of Algal Cell Concentration and Dry Cell Weight
S. almeriensis cell growth was monitored by determining the absorbance of each sample at 420 and 690 nm for Chlorophyll-a, and 480 and 620 nm for Chlorophyll-b, by using a UV/Visible spectrophotometer (Multiskan, Thermo Fisher Scientific, USA). The biomass dry cell weight (DCW) was calculated using the absorbance values at different biomass concentrations evaluated during the growth phase, obtaining a calibration curve between absorbance and concentration as showed in Equation (3).
where DCW is concentration of biomass on dry cell weight (g/L) and A is the total absorbance obtained from the sum of the absorbance values obtained for the four chlorophyll wavelengths.
DCW = (0.0867·A) − 0.1868
The final dry weight was determined through cell culture dewatering by a vacuum filtration system, using vacuum filters with a pore size of 0.45 μm (Sigma-Aldrich, USA); microalgal pellets from dewatering were lyophilized for 24 h.
2.5.2. Accelerated Solvent Extraction
Lutein extraction was carried out from mechanically pre-treated biomass of S. almeriensis cells, by using Dionex-ASE 200 extractor (Salt Lake City, UT, USA). The pretreatment was performed at optimized conditions, according to the procedure described elsewhere [38]. Four consecutive extraction cycles (single extraction cycle = 20 min) were performed by using ethanol at optimized extraction conditions, 67 °C and 10 MPa, for the complete biomass discoloring, as reported in a previous work [39]. At the end of each extraction cycle, extracts were collected into 40 mL amber glass vials by flushing the system with 6.6 mL of fresh solvent, and the system was purged for 1 minute with nitrogen (Purity ≥ 99.999%).
2.5.3. Nutrient Consumption Chemical Analysis
The nutrient (anions and cations) concentrations were analyzed using an ion Chromatograph (Dionex™ ICS-1100, Thermo Scientific, Massachusetts, USA), as reported by Pruvost et al. [40], at the beginning and at the end of the growth period. The Dionex ICS-1100 was an integrated ion chromatography system equipped with a pump, injection valve, and conductivity detector. Several nutrients such as Mg2+, SO42−, Na+, NO3−, NO2−, Ca2+, Cl−, K+, and PO43−, which are essential for growth of microalgae, were analyzed.
2.5.4. Lutein Analysis
The extract obtained after each extraction cycle was equally transferred into two different vials by adding BHT at 0.1 % wt as antioxidant for gravimetric analysis and saponification. The total lutein content was gravimetrically quantified, after the complete removal of the solvent using a Zymark TurboVap evaporator (Zymark, Hopkinton, MA, USA). Lutein analysis consisted of two steps: The first one was the saponification of samples to avoid the overlap of the spectra with the species present in the carotenoid family (lipids and chlorophylls) [41,42,43]; the second one was the measurement by using u-HPLC technology (Agilent 1290 Infinity II). The detailed procedure is reported elsewhere [41,42,43].
3. Results and Discussion
3.1. Effect of CO2 Content on Culture Medium pH
For each growth condition investigated, during the days of microalgal cultivation, a slight variation of pH was observed. In particular, pH increased from the initial value of 7.5 at the beginning of the cultivation, to the final value of 8.5 at the end of the cultivation; therefore, for the CO2 concentration range investigated (0–3.0 %v/v), the pH of the culture medium was marginally affected by the CO2 content, as reported by several authors [44,45]. The increase of the pH value during the cultivation can be attributed to the alkalinization of the medium as a result of CO2 biofixation during photosynthesis: Carbonate and bicarbonate, and hydroxide contents increased, while CO2 concentration decreased [46].
3.2. Effect of CO2 Content on Biomass Concentration
The effect of CO2 on the concentration of the S. almeriensis and on biomass productivity, i.e., the biomass produced per reactor liter and per day, are shown in Figure 2 and Figure 3, respectively.
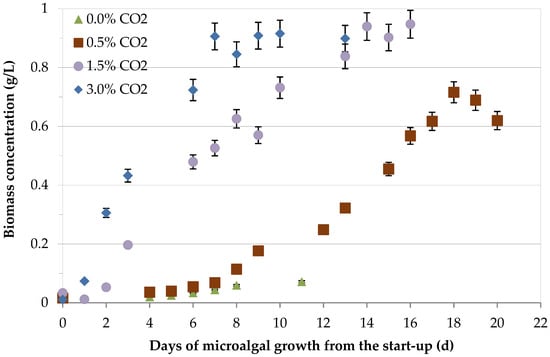
Figure 2.
Effect of CO2 content on S. almeriensis biomass concentration during cultivation.
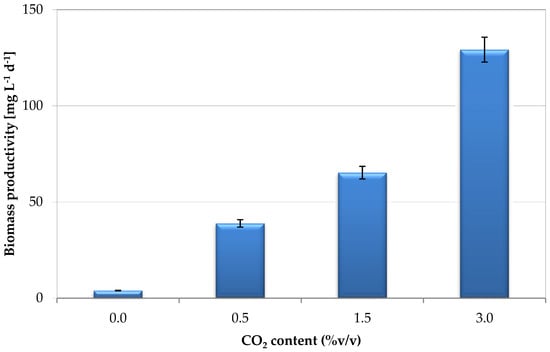
Figure 3.
Effect of CO2 content on S. almeriensis biomass productivity.
By increasing the cultivation time, the biomass concentration increased until the achievement of a maximum value (Figure 2). By increasing CO2 content, an increase of both biomass productivity, with the consequence reduction of the cultivation time required for the achievement of the maximum value, and of the concentration of the biomass were observed, despite the concentration of the microalgae fed with a gaseous mixture with a CO2 content of 1.5 %v/v and the one fed with a gaseous mixture with a CO2 content of 3.0 %v/v being comparable, even if the cultivation time decreased. The observed increase in biomass concentration reflected an efficient photosynthesis rate, promoting an upregulated expression of RuBisCO in the presence of a determined inorganic carbon content [47]. However, the optimum CO2 content also contributed to maintain pH close to the neutral value. For high CO2 adaptation, cells temporarily reduced the synthesis of organic carbon and provided more ATP to assure intracellular pH stability through gene regulation and increasing the energy allocation proportion PSI/PSII [29].
With a CO2 content of 0 %v/v (O2 = 21 %v/v − N2 = 79 %v/v), a maximum concentration of biomass equal to 0.07 g·L−1 after 11 days of cultivation was observed, while it increased to 0.72 g·L−1 after 18 days with a content of CO2 of 0.5 %v/v. With CO2 contents of 1.5 %v/v and of 3 %v/v, the maximum biomass concentrations equal to 0.95 g·L−1 after a cultivation time of 16 days, and to 0.92 mg·L−1 after a cultivation time of 10 days, were measured, respectively. By increasing CO2 content from 0 to 3 %v/v, biomass productivity increased from about 4 mg·L−1·d−1 to about 130 mg·L−1·d−1, with productivities of about 39 mg·L−1·d−1 and 65 mg·L−1·d−1 at CO2 contents of 0.5 %v/v and 1.5 %v/v, respectively. Passing from 0 %v/v to 3 %v/v, biomass productivity increased of about 32-fold.
Cheng et al. [48] reported that the amount of CO2 present in the air (0.04%) is inadequate to achieve high cell density and on the other hand, an excess may inhibit the carbonic anhydrase enzyme, which in turn may reduce biomass productivity. Sepulveda et al. [49] reported that S. almeriensis was one of the most productive strains when using it in CO2 capture processes, with a biomass productivity of 1.0 g·L−1·day−1 and with 2.8 g·L−1·day−1 of CO2 consumed. Acién et al. [50] reported a consumption of CO2 of 2.31 gCO2·gb−1 for S. almeriensis.
3.3. Effect of CO2 Content on Carbon Content, Average CO2 Capture Rate, and CO2 Conversion Efficiency
Carbon content, average CO2 capture rate, and CO2 conversion efficiency, including biomass productivity, are shown in Table 1. As reported, the highest carbon element of microalgae cells (CC) and the highest average CO2 capture rate (RCO2) were found with the highest CO2 content (3 %v/v), while the highest CO2 conversion efficiency (ECO2) was found with the lowest CO2 content (0.5 %v/v).

Table 1.
Carbon elemental, average CO2 capture rate, and CO2 capture efficiency.
Average CO2 capture rates assessed in the present work are comparable with the ones available in the literature for Scenedesmus sp.; however, it should be considered that different growth conditions and fluid dynamics were used. Chaudhary et al. [37] investigated CO2 capture rate through Scenedesmus obliquus cultivation by using an airlift bubble column photo-bioreactor (PBR) (working volume = 7 L), supplied with gas (air + CO2 5 %v/v) at a flow rate of 1.4 L·min−1 and irradiated with cool white fluorescent light (3 tubes × 24 W), finding an average CO2 capture rate of 0.13 gCO2·L−1·d−1. CO2 capture rate through Scenedesmus obliquus cultivation by using tubular-type PBR (working volume = 1.8 L), supplied with gas (air + CO2 12 %v/v) at a gas volume per liquid volume per min of 0.3 and irradiated with 3200 lux brightness provided by 40 W fluorescent daylight type lamps, was also investigated by Radmann et al. [51]; they found a biomass productivity of 0.6 gbiomass·L−1·d−1 and a CO2 capture rate of 0.11 gCO2·L−1·d−1. Jin et al. [52] investigated CO2 capture rate through Scenedesmus sp. cultivation by using a bubble column PBR (working volume = 1 L), supplied with gas (air + CO2 10 %v/v) at a gas volume per liquid volume per min of 0.2 and irradiated with a light intensity of 200 μmol·m−2·s−1, finding an average CO2 capture rate of 0.46 gCO2·L−1·d−1. Ong et al. [53] found a CO2 capture rate of 0.021 gCO2·L−1·d−1 for Chlorella sp. by using a vertical bubble column (working volume = 40 L) supplied with gas (air + CO2 5 %v/v) at a gas volume per liquid volume per min of 0.25 and irradiated with a light intensity of 1500 μmol·m−2·s−1. Hsueh et al. [53] found a CO2 capture rate of 0.141 gCO2·L−1·d−1 for Thermosynechococcus sp. by using a bubble column (working volume = 40 L) supplied with gas (air + CO2 10 %v/v) at 1 L·min−1 and irradiated with a light intensity of 10000 lux. Nayak et el. [54] reported that the CO2 biofixation rate of Scenedesmus sp., when aerated with more than 1 %v/v CO2 by using a bubble column photo-bioreactor (working volume = 0.5), was in the range of 0.27–0.37 g·L−1·d−1.
In terms of CO2 capture efficiency, S. almeriensis performance are comparable with performances of several microalgal species [55,56].
3.4. Role of CO2 Content on Nutrient Consumption
Nutrients are essential for microalgae cell growth, and a similar concentration of nutrients was kept in each growth phase. The concentration of the main ions of the growth medium at the beginning of the cultivation are reported in Table 2. The concentration of NO3− ions was the highest with respect to the rest of nutrients. It is worth highlighting that in the case of growth with a CO2 content of 0.5 %v/v, nutrient concentration was slightly lower because the growth was continued from the 0.0 %v/v CO2 content step.

Table 2.
Initial concentration of nutrients during the S. almeriensis cultivation.
Figure 4 illustrates the effect of CO2 content on nutrient consumption, measured at the end of the growth of S. almeriensis. Data highlighted a complete consumption of phosphate ions, which could limit the cell growth. The increase of CO2 content increased the nutrient consumption rate. However, the longer cultivation times (i.e., 20 days for CO2 = 0.5 %v/v; 16 days for CO2 = 1.5 %v/v; 13 days for CO2 = 3.0 %v/v) also promoted the nutrient intake. Among all the supplied nutrients, nitrate and phosphate were highly consumed during the growth phase. The possible explanation for this phenomenon is that a nitrogen source is essential for protein synthesis, and lutein exists as a nitrogenous macromolecule (i.e., light-harvesting complexes; LHCII) in microalgae [15]. NO3− and PO43− ions are the most important nutrients for cell proliferation during growth of microalgae [57], as confirmed by the present study. However, the consumption of NO3− ions was 5.0, 59.88, 77.26, and 87.22% during the growth with CO2 contents of 0.0, 0.5, 1.5, and 3.0 %v/v, respectively. This observation can be justified by considering a limited carbon source, which leads to a stress condition on microalgae growth cells, causing a lower biological consumption of nutrients. At the end of the growth, a lower consumption of both Na+ and Cl− ions was observed, in comparison to the consumption of other nutrients, with a consumption below 25%. The consumption of Cl− ions decreased by increasing CO2 content from 0.5 %v/v to 3.0 %v/v, which could be explained by considering a lower cultivation time. Similar results were observed during the cultivation of Chlorella vulgaris [58]. Chen et al. [15] evaluated the effect of nitrate and they reported that a sufficient amount of nitrogen in the medium was required to enhance lutein accumulation in the Chlorella sorokiniana Mb-1 strain. More importantly, it could be possible that the lutein content may depend on the residual nitrogen concentration [14].
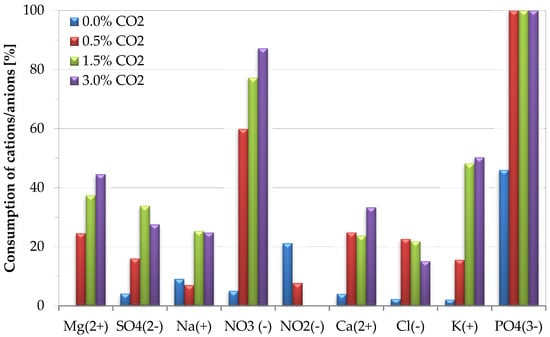
Figure 4.
Effect of CO2 content on nutrient consumption efficiency during cultivation of S. almeriensis. Standard deviation was less than 5% in all operative conditions.
3.5. Extraction Yield and Lutein Production from S. almeriensis
The increase of CO2 content of the gaseous mixture from 0.0 %v/v to 3.0 %v/v improved the extraction yield and the lutein content, as shown in Figure 5. The harvested S. almeriensis biomass during the growth with a content of CO2 of 0.0 %v/v showed extraction yield of about 172.28 mg·g−1 with a lutein content of 1.51 mg·g−1. With a content of CO2 of 0.5 %v/v, an increase of the extraction yield of 1.4-fold and a lutein content of 3.1-fold higher than that measured in absence of CO2 were found. The highest extraction yield and the highest lutein content, equal to 328.05 mg·g−1 and 8.54 mg·g−1, respectively, were obtained by feeding microalgae with a gaseous mixture containing a CO2 concentration of 3.0 %v/v. The increase of lutein content in biomass growth in stressed conditions might indicate that lutein could play a role in protecting cells from photodamage [20].
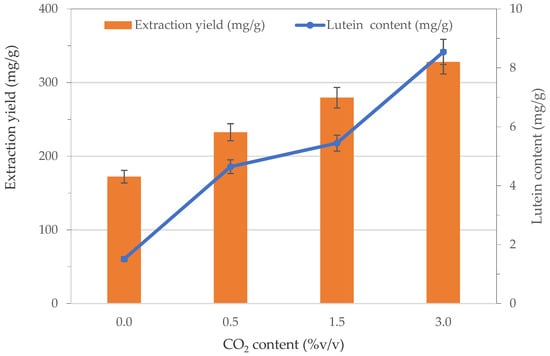
Figure 5.
Effect of CO2 content on extraction yield and lutein content from S. almeriensis.
3.6. Comparison of Biomass Productivity and Lutein Content
Biomass productivity and lutein content under the optimal growth condition defined in this study, including the comparison with literature data, are reported in Table 3. Heterotrophic and mixotrophic cultivations support higher biomass productivity; a feasible lutein production from a mixotrophic microalgae culture, which has been rarely mentioned in the literature, is also shown—however, lutein content is lower than the one of most microalgae autotrophic cultivation (Table 3). The biomass productivity is lower during autotrophic cultivation of different microalgae strains, while a significant amount of lutein can be produced. Moreover, autotrophic cultivation brings several advantages such as capture of CO2, helping in reduction of the GHG and allowing the development of integrated biorefinery based on flue gas carbon sequestration through microalgae, and the simultaneous production of commercially valuable microalgal products, such as lutein and biodiesel [7]. Furthermore, autotrophic cultivation of microalgae might use sun as an economic source of light, which could be an effective strategy to economize the microalgal bioprocess [59]. Recently, Patel et al. [59] proposed the mixotrophic cultivation of Chlorella protothecoides, which could enhance the biomass productivity. Therefore, integration of different strategies could be a potential tool for the production of higher amounts of lutein with other bioactive compounds.

Table 3.
Biomass productivity and lutein content comparison.
Interestingly, a significant amount of lutein was attained in this study during autotrophic cultivation of S. almeriensis, which is the highest among the ones reported in Table 3.
4. Conclusions
The present study shows the role of CO2 content on cultivation of S. almeriensis for biomass and lutein production; moreover, CO2 capture rate and CO2 conversion efficiency for S. almeriensis were also assessed. The optimal amount of inorganic carbon source improved the photosynthetic efficiency. By increasing the CO2 content, the biomass productivity increased; the highest biomass concentration of about 0.94 g·L−1 was obtained with a content of CO2 of 1.5 %v/v, with a direct correlation with the extraction yield and the lutein content. Moreover, a similar trend for nutrient consumption was also found. The nutrient chemical analysis shows that a content of CO2 of 3.0 %v/v better supports nutrient intake during the growth phase, while consumptions of nitrate and phosphate have been found as the highest ones for each test. The increase of CO2 content increased the extraction yield, as well as the lutein content. With a CO2 content of 3 %v/v, the extraction yield and lutein content were about 328 mg·g−1 and 8 mg·g−1 of dry biomass, respectively. It is worth pointing out that due to the impressive lutein content, S. almeriensis could be a suitable candidate for potential commercial production of microalgae-derived natural lutein.
This study could positively contribute to the present scientific literature showing the sustainable integration of CO2 sequestration with the concomitant microalgae cultivation for the production of lutein and of other high value-added chemicals.
Author Contributions
Conceptualization, D.M. and A.M.; Data Curation, S.M. and A.I.; Formal Analysis, A.I.; Investigation, S.M.; Methodology, S.C., D.K., A.I., P.C., and A.M.; Writing—Original Draft, S.M. and T.M.; Writing—Review and Editing, S.C. and D.M.; Project Administration, A.M.; Resources, A.M.; Supervision, D.M. and A.M.
Funding
This research was funded by a Bio Based Industries Joint Undertaking under the European Union’s Horizon 2020 research and innovation program, grant agreement No. 745695 (VALUEMAG).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cerón, M.C.; Campos, I.; Sánchez, J.F.; Acién, F.G.; Molina, E.; Fernández-Sevilla, J.M. Recovery of lutein from microalgae biomass: Development of a process for Scenedesmus almeriensis biomass. J. Agric. Food Chem. 2008, 56, 11761–11766. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, K.; Zhu, Y.; Yang, W.; Zhou, J.; Cen, K. Transcriptome sequencing and metabolic pathways of astaxanthin accumulated in Haematococcus pluvialis mutant under 15% CO2. Bioresour. Technol. 2017, 228, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.Y.; Kao, C.Y.; Chen, C.H.; Kuan, T.C.; Ong, S.C.; Lin, C.S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.-Y.; Kao, C.-Y.; Tsai, M.-T.; Ong, S.-C.; Chen, C.-H.; Lin, C.-S. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Joun, J.M.; Lee, J.; Hong, M.E.; Pham, H.M.; Chang, W.S.; Sim, S.J. Development of large-scale and economic pH control system for outdoor cultivation of microalgae Haematococcus pluvialis using industrial flue gas. Bioresour. Technol. 2017, 244, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- de Assis, T.C.; Calijuri, M.L.; Assemany, P.P.; de Paula, A.S.A.; Martins, M.A. Using atmospheric emissions as CO2 source in the cultivation of microalgae: Productivity and economic viability. J. Clean. Prod. 2019, 215, 1160–1169. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Dash, S.K.; Sen, R. Process integration for microalgal lutein and biodiesel production with concomitant flue gas CO2 sequestration: a biorefinery model for healthcare, energy and environment. RSC Adv. 2015, 5, 73381–73394. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas Control 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef]
- Granado-Lorencio, F.; Herrero-Barbudo, C.; Acién-Fernández, G.; Molina-Grima, E.; Fernández-Sevilla, J.M.; Pérez-Sacristán, B.; Blanco-Navarro, I. In vitro bioaccesibility of lutein and zeaxanthin from the microalgae Scenedesmus almeriensis. Food Chem. 2009, 114, 747–752. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein in specific marigold flowers and microalgae. J. Taiwan Inst. Chem. Eng. 2015, 49, 90–94. [Google Scholar] [CrossRef]
- Lin, J.H.; Lee, D.J.; Chang, J.S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.E.; Herbst-Espinosa, S.M.; Hussain, E.A.; Stacewicz-Sapuntzakis, M. Esterification Does Not Impair Lutein Bioavailability in Humans. J. Nutr. 2018, 132, 3668–3673. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhao, X.; Chen, J.; Yang, X.; Ho, S.H.; Wang, B.; Chang, J.S.; Shen, Y. Enhancing cell growth and lutein productivity of Desmodesmus sp. F51 by optimal utilization of inorganic carbon sources and ammonium salt. Bioresour. Technol. 2017, 244, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Ho, S.H.; Liu, C.C.; Chang, J.S. Enhancing lutein production with Chlorella sorokiniana Mb-1 by optimizing acetate and nitrate concentrations under mixotrophic growth. J. Taiwan Inst. Chem. Eng. 2017, 79, 88–96. [Google Scholar] [CrossRef]
- Ruban, A.V.; Berera, R.; Ilioaia, C.; Van Stokkum, I.H.M.; Kennis, J.T.M.; Pascal, A.A.; Van Amerongen, H.; Robert, B.; Horton, P.; Van Grondelle, R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 2007, 450, 575–578. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the re-evaluation of lutein (E 161b) as a food additive. EFSA J. 2010, 8, 1678. [CrossRef]
- Utomo, R.P.; Chang, Y.R.; Lee, D.J.; Chang, J.S. Lutein recovery from Chlorella sp. ESP-6 with coagulants. Bioresour. Technol. 2013, 139, 176–180. [Google Scholar] [CrossRef]
- Barceló-Villalobos, M.; Serrano, C.G.; Zurano, A.S.; García, L.A.; Maldonado, S.E.; Peña, J.; Fernández, F.G.A. Variations of culture parameters in a pilot-scale thin-layer reactor and their influence on the performance of Scenedesmus almeriensis culture. Bioresour. Technol. Reports 2019, 6, 190–197. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J. Biotechnol. 2001, 85, 289–295. [Google Scholar] [CrossRef]
- Casal, C.; Cuaresma, M.; Vega, J.M.; Vilchez, C. Enhanced productivity of a lutein-enriched novel acidophile microalga grown on urea. Mar. Drugs 2011, 9, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Liu, C.C. Optimization of lutein production with a two-stage mixotrophic cultivation system with Chlorella sorokiniana MB-1. Bioresour. Technol. 2018, 262, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of lutein production in Chlorella sorokiniana (chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, R.; Dhanarajan, G.; Dash, S.K.; Sen, R. An advanced hybrid medium optimization strategy for the enhanced productivity of lutein in Chlorella minutissima. Algal Res. 2015, 7, 24–32. [Google Scholar] [CrossRef]
- Ho, S.H.; Chan, M.C.; Liu, C.C.; Chen, C.Y.; Lee, W.L.; Lee, D.J.; Chang, J.S. Enhancing lutein productivity of an indigenous microalga Scenedesmus obliquus FSP-3 using light-related strategies. Bioresour. Technol. 2014, 152, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.F.; Fernández, J.M.; Acién, F.G.; Rueda, A.; Pérez-Parra, J.; Molina, E. Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem. 2008, 43, 398–405. [Google Scholar] [CrossRef]
- Sánchez, J.F.; Fernández-Sevilla, J.M.; Acién, F.G.; Cerón, M.C.; Pérez-Parra, J.; Molina-Grima, E. Biomass and lutein productivity of Scenedesmus almeriensis: Influence of irradiance, dilution rate and temperature. Appl. Microbiol. Biotechnol. 2008, 79, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Chen, F.; Chen, G.; Zhang, X.W.; Liu, L.J.; Zhang, H. Enhanced production of lutein in heterotrophic Chlorella protothecoides by oxidative stress. Sci. China Ser. C Life Sci. 2008, 51, 1088–1093. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y. Process effect of microalgal-carbon dioxide fixation and biomass production: A review. Renew. Sustain. Energy Rev. 2014, 31, 121–132. [Google Scholar] [CrossRef]
- Yen, H.W.; Hu, I.C.; Chen, C.Y.; Ho, S.H.; Lee, D.J.; Chang, J.S. Microalgae-based biorefinery - From biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef]
- Salih, F.M. Microalgae Tolerance to High Concentrations of Carbon Dioxide: A Review. J. Environ. Prot. (Irvine. Calif). 2011, 02, 648–654. [Google Scholar] [CrossRef]
- Mehariya, S.; Patel, A.K.; Obulisamy, P.K.; Punniyakotti, E.; Wong, J.W.C. Co-digestion of food waste and sewage sludge for methane production: Current status and perspective. Bioresour. Technol. 2018, 265, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Limonti, C.; Mehariya, S.; Molino, A.; Calabrò, V. Biofuel production and phosphorus recovery through an integrated treatment of agro-industrial waste. Sustain. 2018, 11, 52. [Google Scholar] [CrossRef]
- Nobre, B.P.; Villalobos, F.; Barragán, B.E.; Oliveira, A.C.; Batista, A.P.; Marques, P.A.S.S.; Mendes, R.L.; Sovová, H.; Palavra, A.F.; Gouveia, L. A biorefinery from Nannochloropsis sp. microalga - Extraction of oils and pigments. Production of biohydrogen from the leftover biomass. Bioresour. Technol. 2013, 135, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.E.; Myers, J. on Pigments, Growth, and Photosynthesis of Phaeodactylum Tricornutum. J. Phycol. 1968, 4, 349–355. [Google Scholar] [CrossRef] [PubMed]
- de Morais, M.G.; Costa, J.A.V. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [Google Scholar] [CrossRef]
- Chaudhary, R.; Dikshit, A.K.; Tong, Y.W. Carbon-dioxide biofixation and phycoremediation of municipal wastewater using Chlorella vulgaris and Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2018, 25, 20399–20406. [Google Scholar] [CrossRef] [PubMed]
- Mehariya, S.; Iovine, A.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.; Casella, P.; Karatza, D.; Marino, T.; Musmarra, D.; et al. Supercritical Fluid Extraction of Lutein from Scenedesmus almeriensis. Molecules 2019, 24, 1324. [Google Scholar] [CrossRef]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- Pruvost, J.; Van Vooren, G.; Le Gouic, B.; Couzinet-Mossion, A.; Legrand, J. Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour. Technol. 2011, 102, 150–158. [Google Scholar] [CrossRef]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef] [PubMed]
- Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical Carbon Dioxide Extraction of Astaxanthin, Lutein, and Fatty Acids from Haematococcus pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Cabello, J.; Morales, M.; Revah, S. Carbon dioxide consumption of the microalga Scenedesmus obtusiusculus under transient inlet CO2 concentration variations. Sci. Total Environ. 2017, 584–585, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wang, J.; Li, R.; Liu, T. Modeling of carbon dioxide mass transfer behavior in attached cultivation photobioreactor using the analysis of the pH profiles. Bioprocess Biosyst. Eng. 2017, 40, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Shiraiwa, Y.; Goyal, A.; Tolbert, N.E. Alkalization of the medium by unicellular green algae during uptake dissolved inorganic carbon. Plant Cell Physiol. 1993, 34, 649–657. [Google Scholar] [CrossRef]
- Cheng, J.; Li, K.; Yang, Z.; Zhou, J.; Cen, K. Enhancing the growth rate and astaxanthin yield of Haematococcus pluvialis by nuclear irradiation and high concentration of carbon dioxide stress. Bioresour. Technol. 2016, 204, 49–54. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, L.; Chen, H.; Gao, C. Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep. Purif. Technol. 2006, 50, 324–329. [Google Scholar] [CrossRef]
- Sepulveda, C.; Gómez, C.; El Bahraoui, N.; Acién, G. Comparative evaluation of microalgae strains for CO2 capture purposes. J. CO2 Util. 2019, 30, 158–167. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Radmann, E.M.; Camerini, F.V.; Santos, T.D.; Costa, J.A.V. Isolation and application of SOX and NOX resistant microalgae in biofixation of CO2 from thermoelectricity plants. Energy Convers. Manag. 2011, 52, 3132–3136. [Google Scholar] [CrossRef]
- Jin, H.F.; Lim, B.R.; Lee, K. Influence of nitrate feeding on carbon dioxide fixation by microalgae. J. Environ. Sci. Heal. Part A Toxic/Hazardous Subst. Environ. Eng. 2006, 41, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.C.; Kao, C.Y.; Chiu, S.Y.; Tsai, M.T.; Lin, C.S. Characterization of the thermal-tolerant mutants of Chlorella sp. with high growth rate and application in outdoor photobioreactor cultivation. Bioresour. Technol. 2010, 101, 2880–2883. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Karemore, A.; Sen, R. Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar] [CrossRef]
- Rodolfi, L.; Zittelli, G.C.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Tsai, M.T.; Kao, C.Y.; Ong, S.C.; Lin, C.S. The air-lift photobioreactors with flow patterning for high-density cultures of microalgae and carbon dioxide removal. Eng. Life Sci. 2009, 9, 254–260. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Potential of using organic fertilizer to cultivate Chlorella vulgaris for biodiesel production. Appl. Energy 2012, 94, 303–308. [Google Scholar] [CrossRef]
- Hadj-Romdhane, F.; Zheng, X.; Jaouen, P.; Pruvost, J.; Grizeau, D.; Croué, J.P.; Bourseau, P. The culture of Chlorella vulgaris in a recycled supernatant: Effects on biomass production and medium quality. Bioresour. Technol. 2013, 132, 285–292. [Google Scholar] [CrossRef]
- Patel, A.K.; Joun, J.M.; Hong, M.E.; Sim, S.J. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour. Technol. 2019, 245–253. [Google Scholar] [CrossRef]
- Yen, H.W.; Sun, C.H.; Ma, T.W. The comparison of lutein production by scenesdesmus sp. in the autotrophic and the mixotrophic cultivation. Appl. Biochem. Biotechnol. 2011, 164, 353–361. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ho, S.H.; Chen, C.N.N.; Chen, C.Y.; Ng, I.S.; Jing, K.J.; Chang, J.S.; Lu, Y. Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: Effects of nitrate concentration, light intensity and fed-batch operation. Bioresour. Technol. 2013, 144, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, I.; Ruiz-Domínguez, M.C.; Márquez, M.; Vílchez, C. Cu-mediated biomass productivity enhancement and lutein enrichment of the novel microalga Coccomyxa onubensis. Process Biochem. 2012, 47, 694–700. [Google Scholar] [CrossRef]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).