A Carbide Slag-Based, Ca12Al14O33-Stabilized Sorbent Prepared by the Hydrothermal Template Method Enabling Efficient CO2 Capture
Abstract
:1. Introduction
2. Experimental
2.1. Sorbent Preparation
2.2. Cyclic CO2 Capture Test
2.3. Characterization
3. Results and Discussion
3.1. Composition of the Synthetic Sorbent
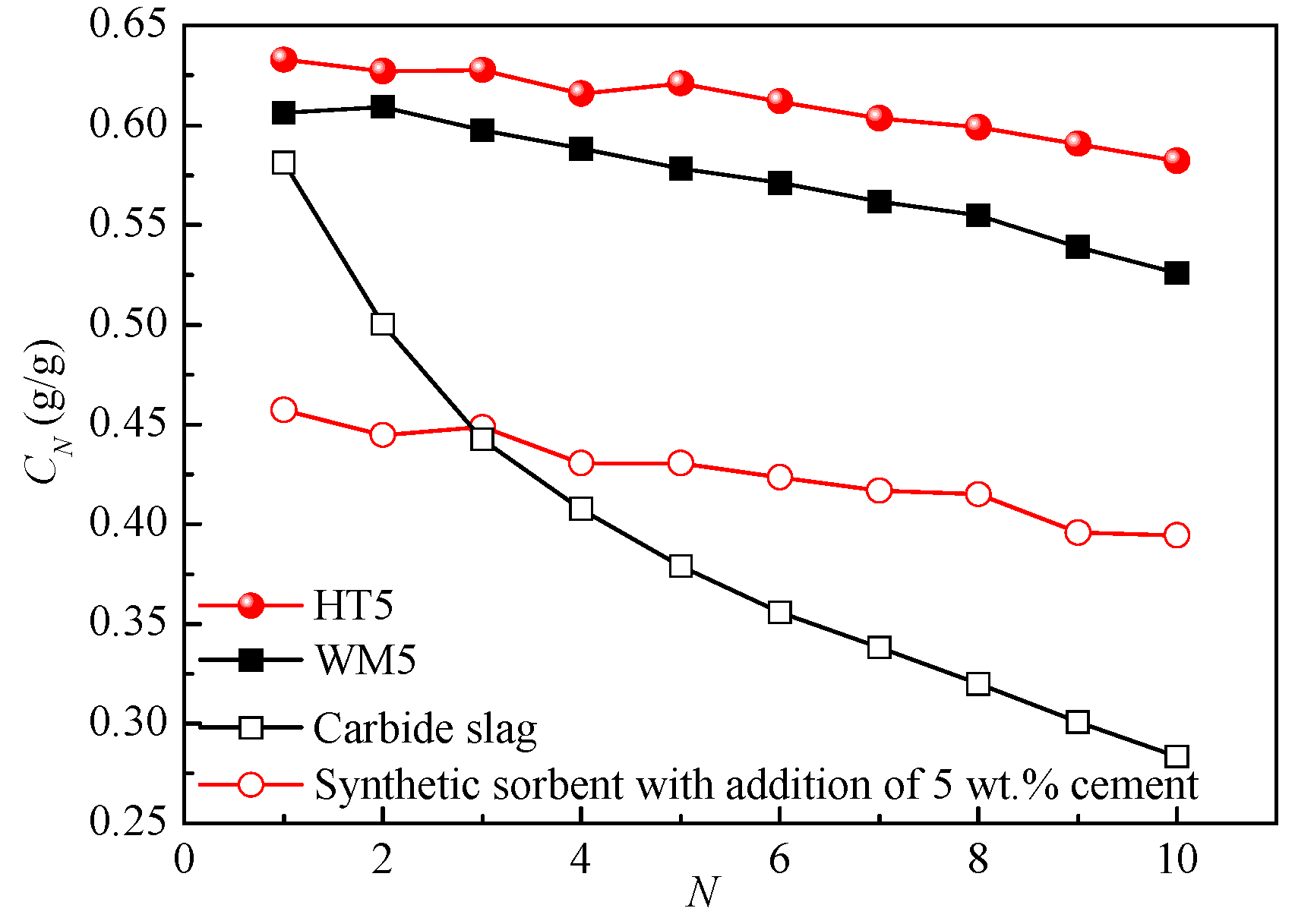
3.2. Comparison of Preparation Methods
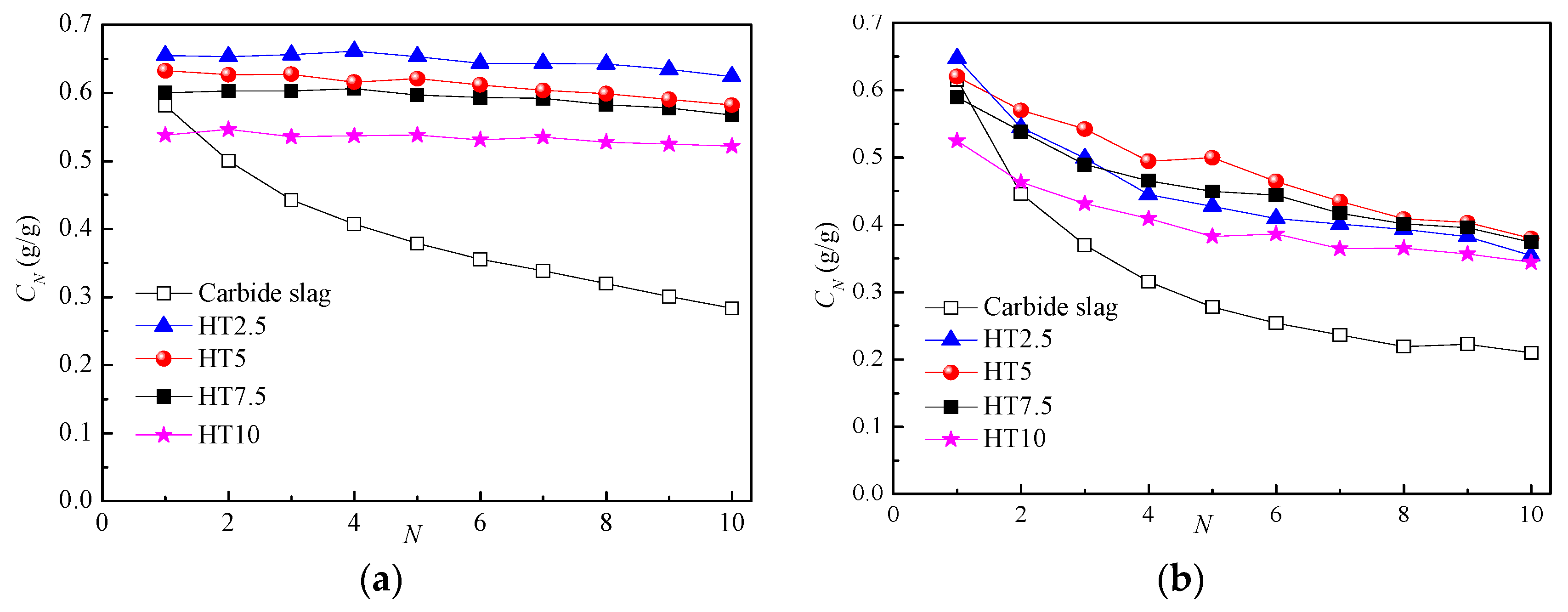
3.3. Effect of Support Ratios
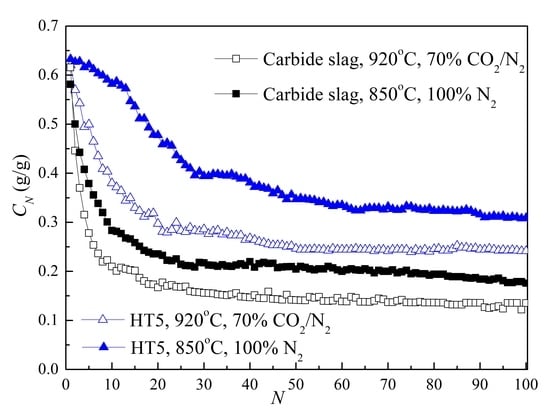
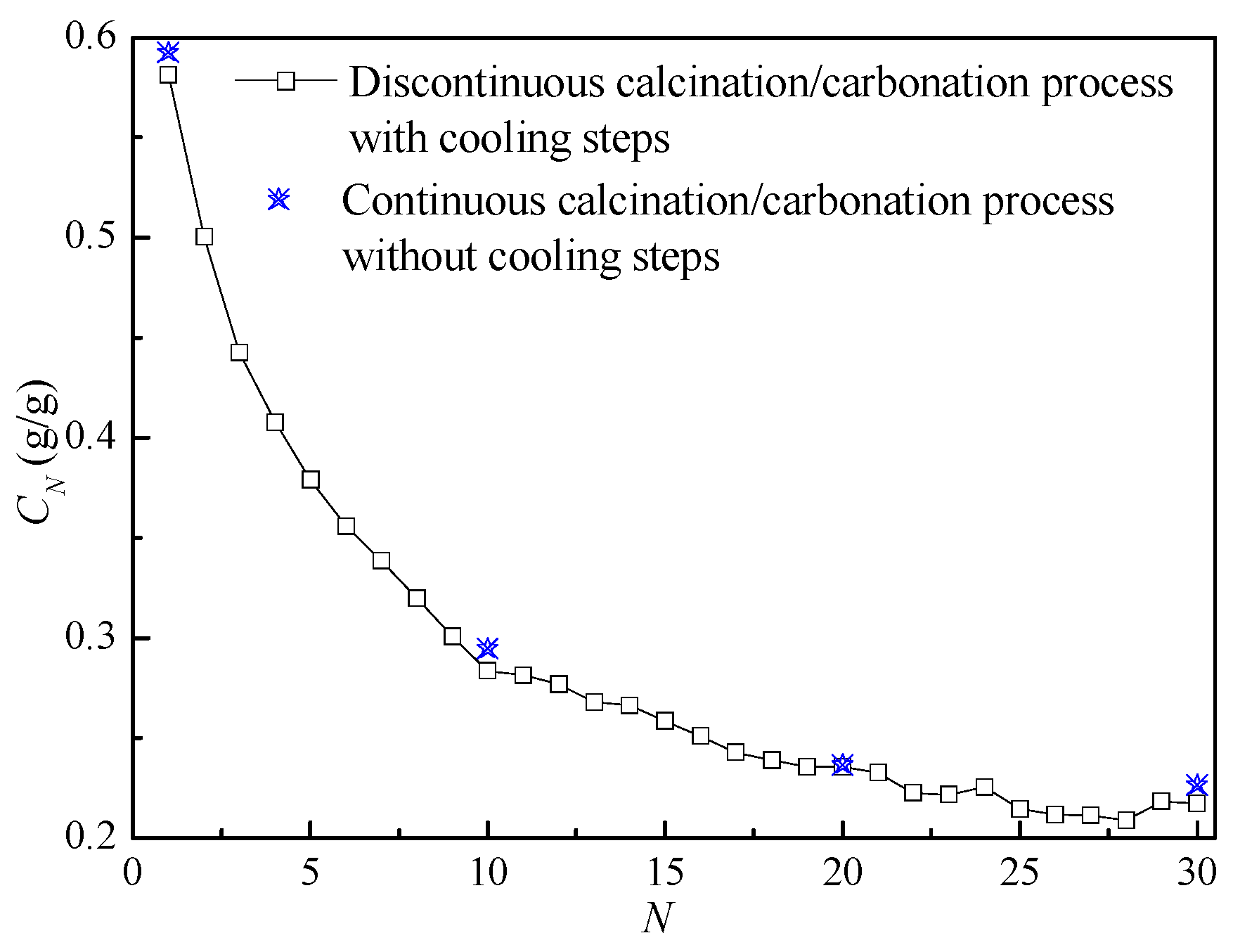
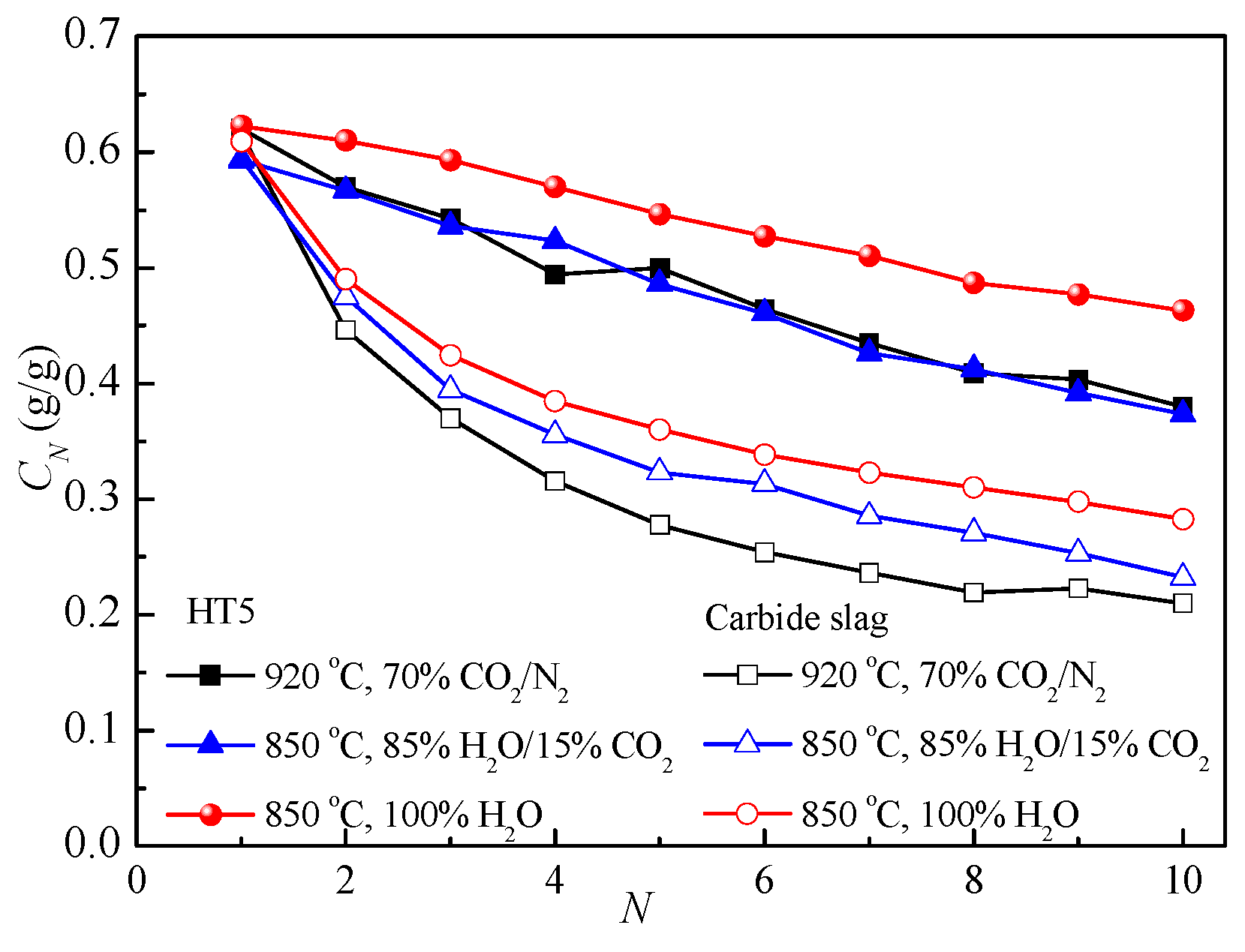
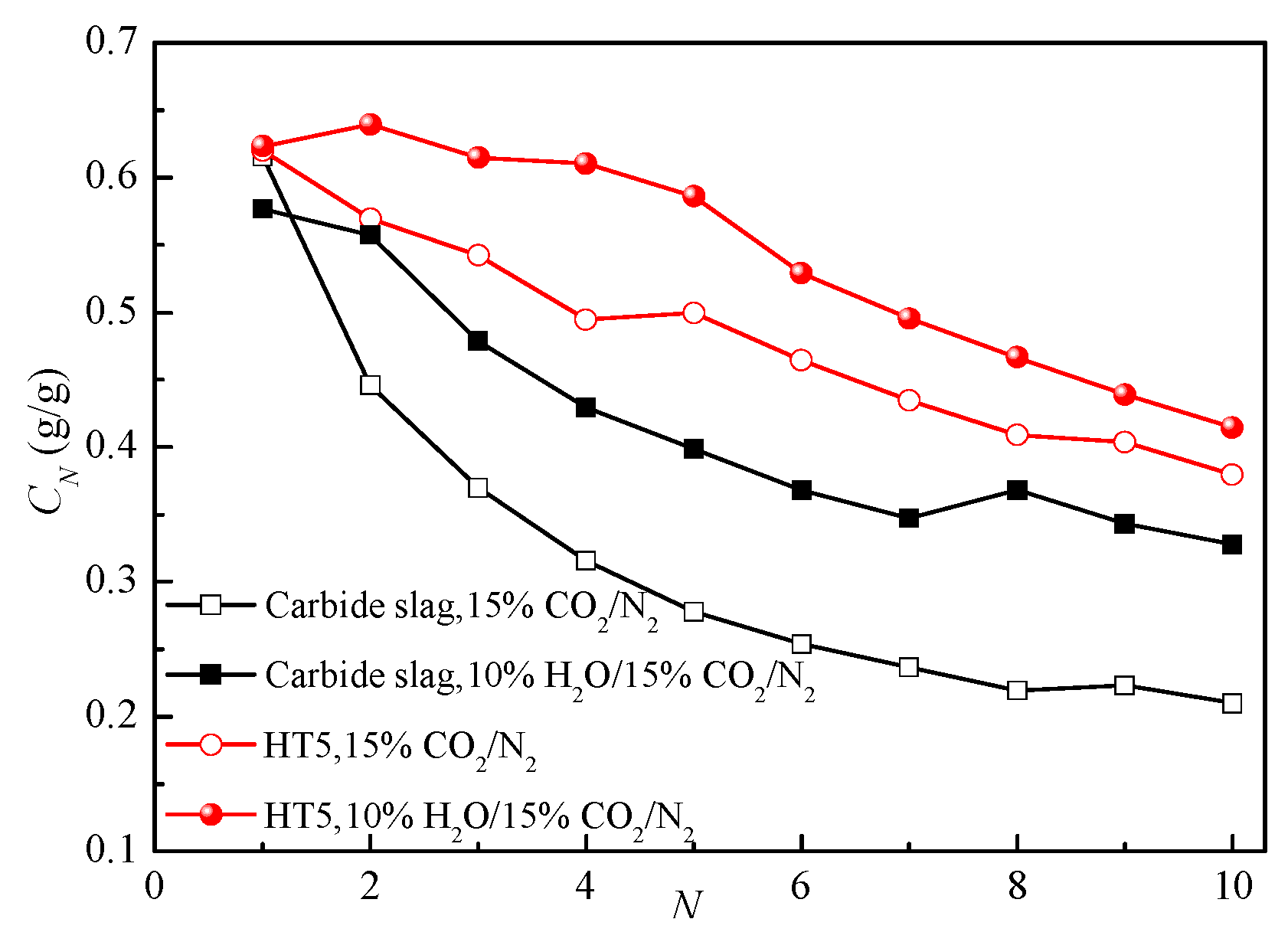
3.4. Effect of Reaction Conditions
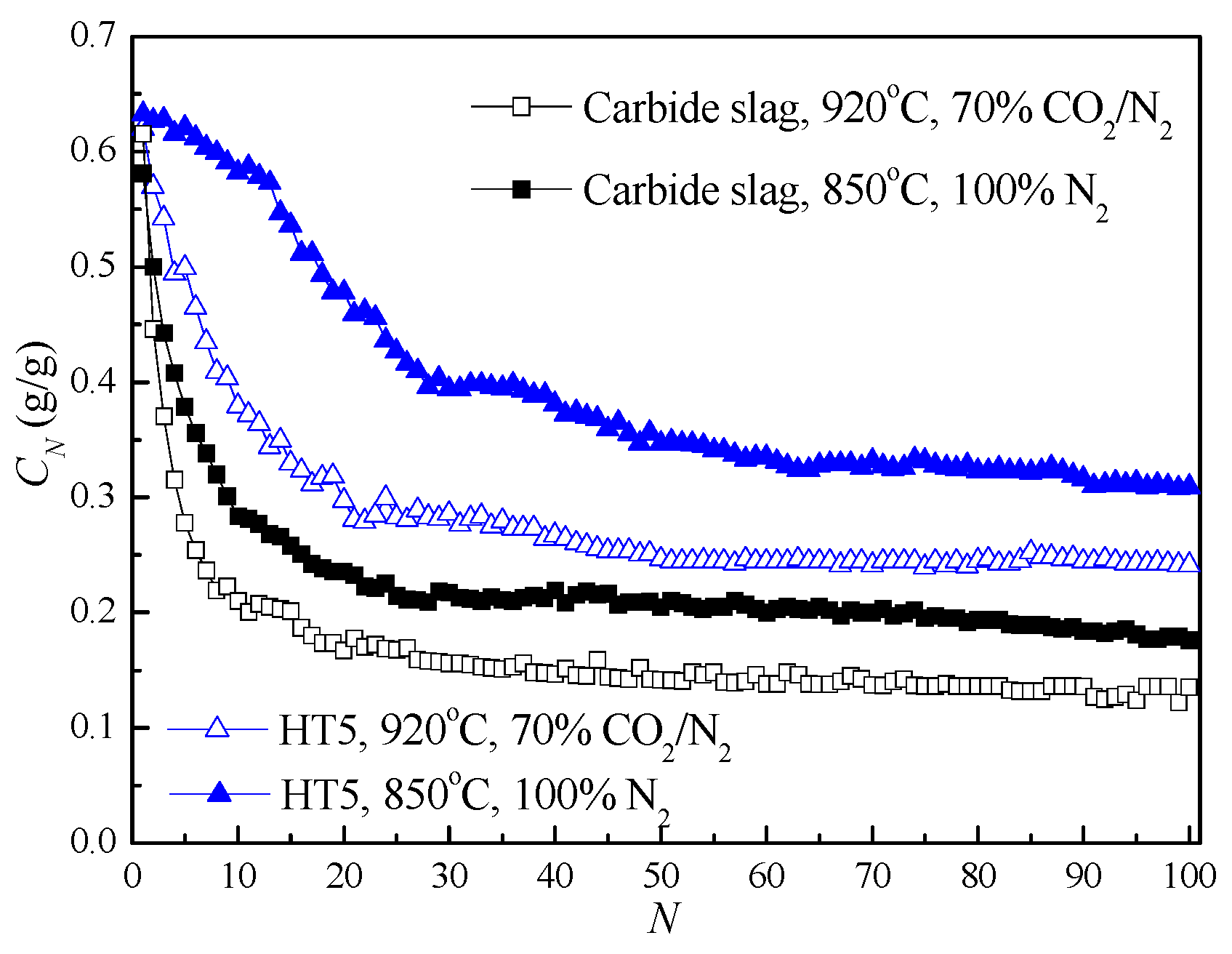
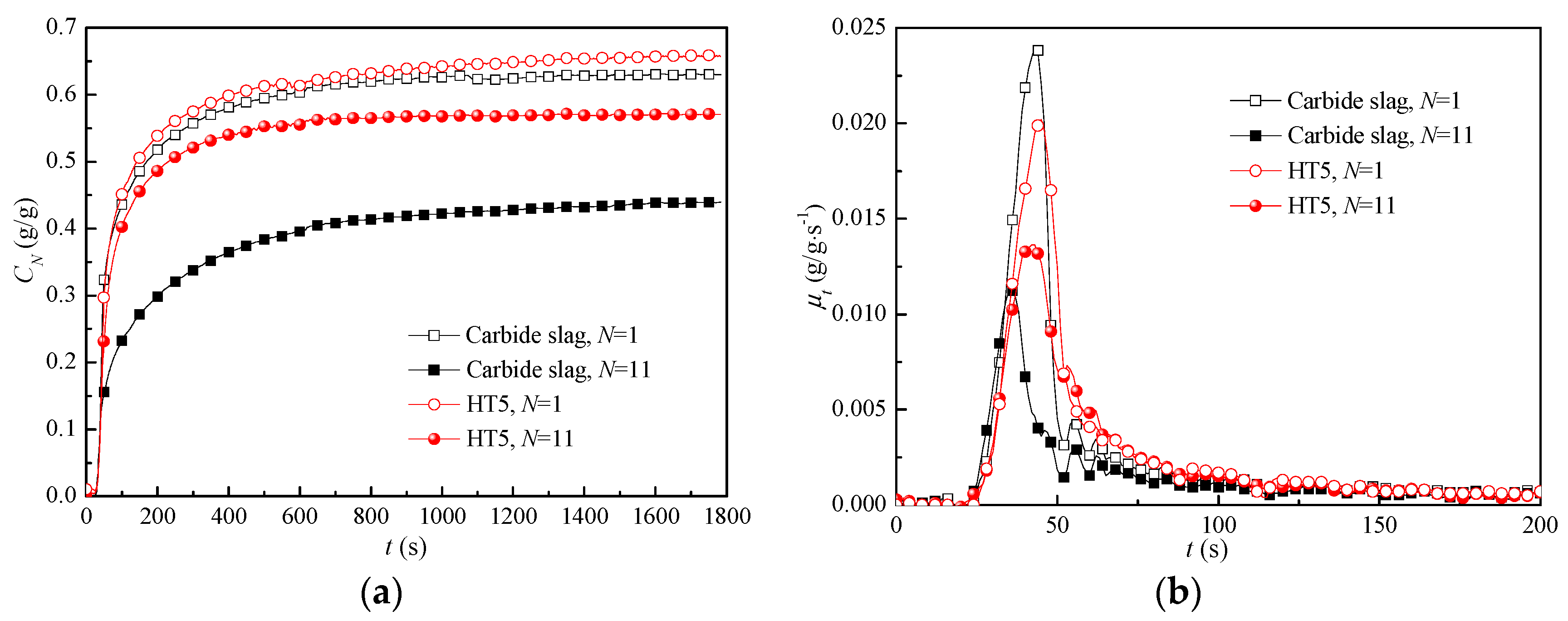
3.5. CO2 Capture during Extended Cycles
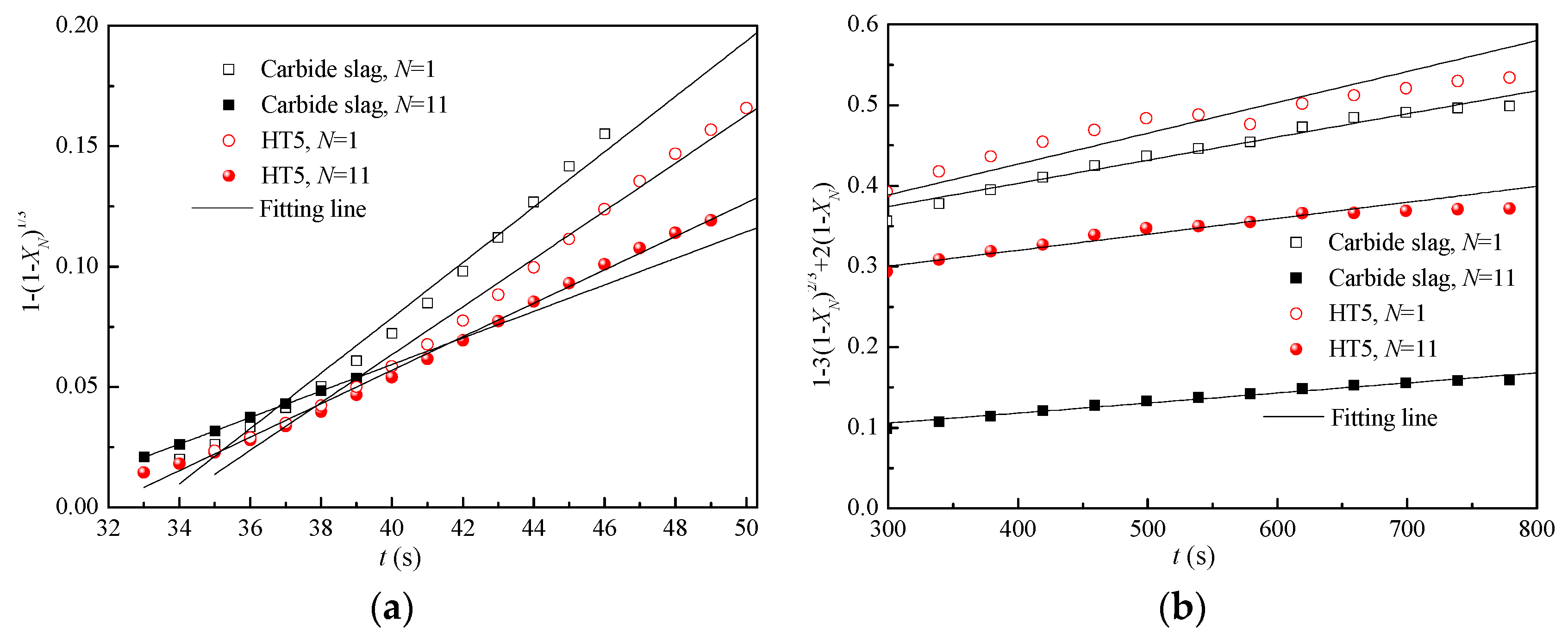
3.6. Apparent CO2 Capture Kinetics
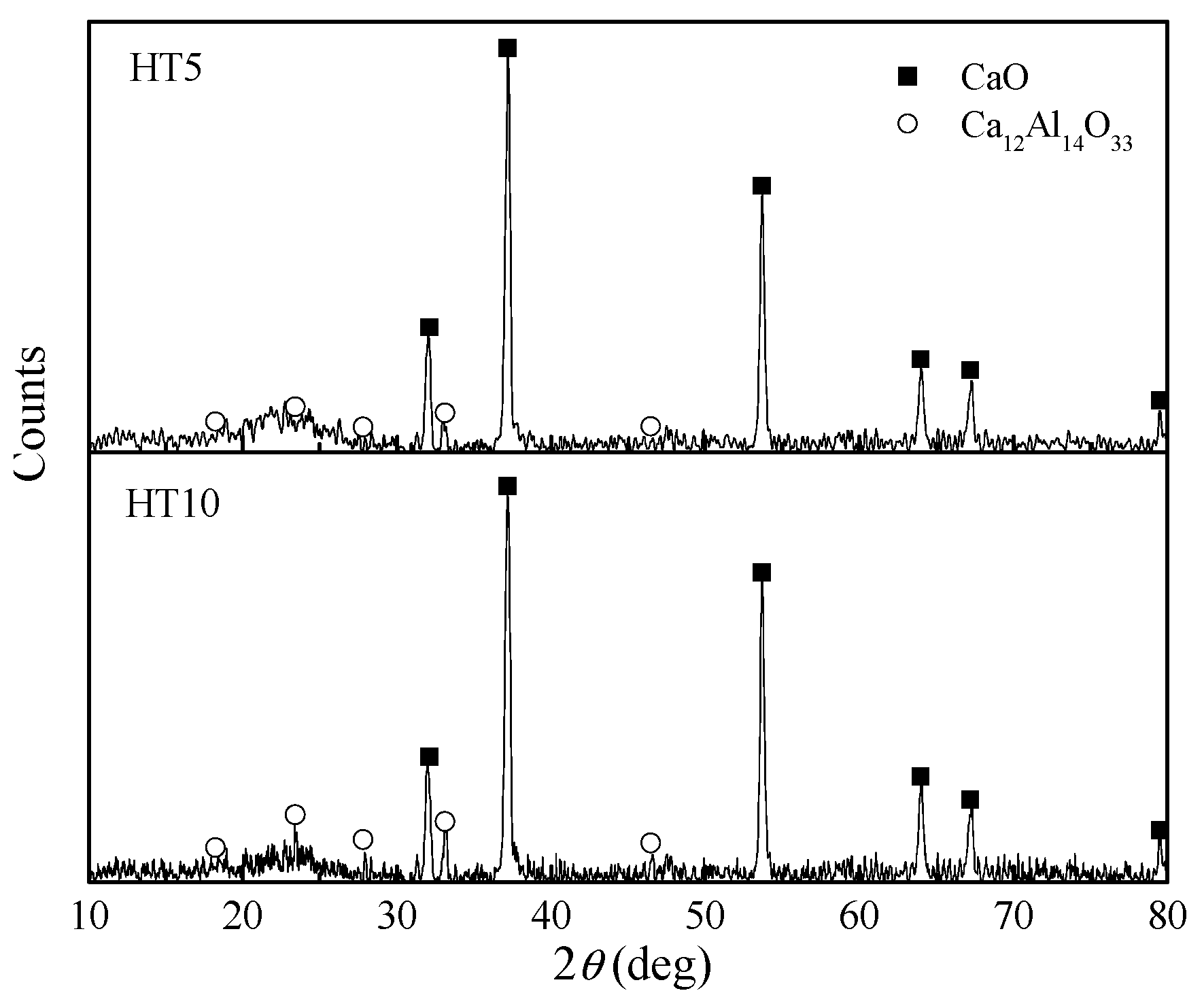
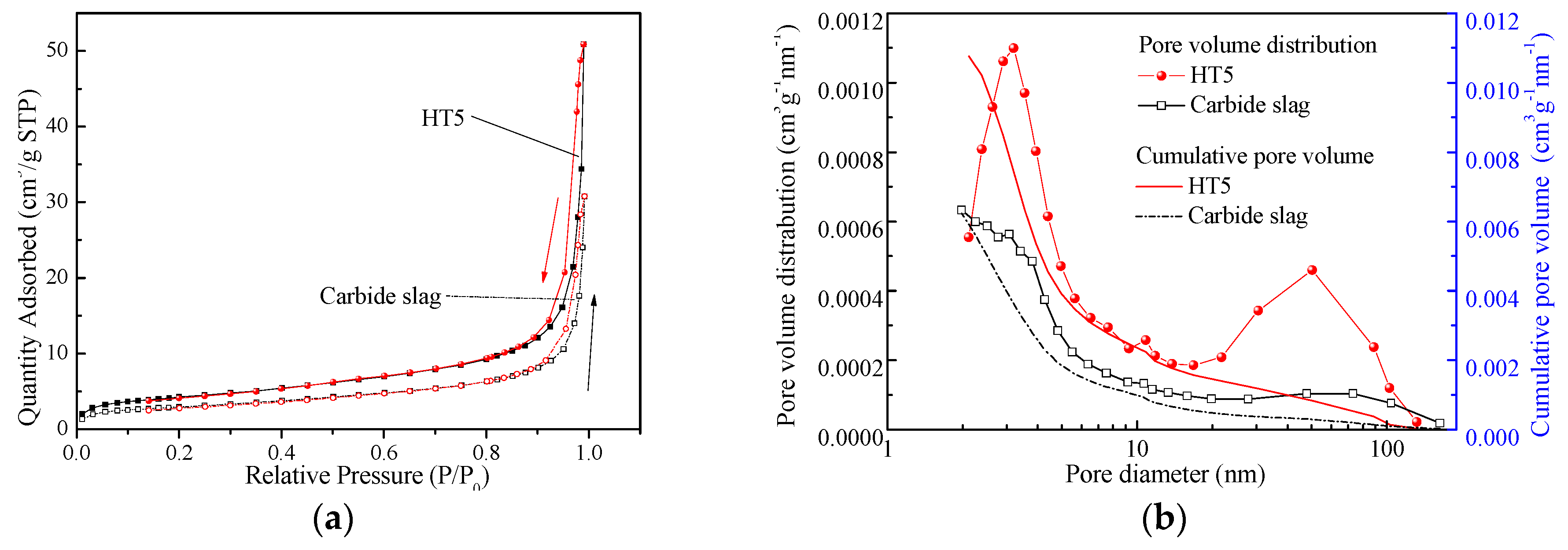
3.7. Microstructure Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nazir, S.; Bolland, O.; Amini, S. Analysis of combined cycle power plants with chemical looping reforming of natural gas and pre-combustion CO2 capture. Energies 2018, 11, 147. [Google Scholar] [CrossRef]
- Zaimes, G.G.; Beck, A.W.; Janupala, R.R.; Resasco, D.E.; Crossley, S.P.; Lobban, L.L.; Khanna, V. Multistage torrefaction and in situ catalytic upgrading to hydrocarbon biofuels: Analysis of life cycle energy use and greenhouse gas emissions. Energy Environ. Sci. 2017, 10, 1034–1050. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, F.; Liu, Z.; Hao, H. China’s electric vehicle deployment: Energy and greenhouse gas emission impacts. Energies 2018, 11, 3353. [Google Scholar] [CrossRef]
- Special Report on Global Warming of 1.5 °C. Available online: https://www.ipcc.ch/sr15/ (accessed on 23 May 2019).
- Vanga, G.; Gattia, D.M.; Stendardo, S.; Scaccia, S. Novel synthesis of combined CaO-Ca12Al14O33-Ni sorbent-catalyst material for sorption enhanced steam reforming processes. Ceram. Int. 2019, 45, 7594–7605. [Google Scholar] [CrossRef]
- Fernández, J.; Sotenko, M.; Derevschikov, V.; Lysikov, A.; Rebrov, E.V. A radiofrequency heated reactor system for post-combustion carbon capture. Chem. Eng. Process. Process Intensif. 2016, 108, 17–26. [Google Scholar] [CrossRef]
- Sotenko, M.; Fernández, J.; Hu, G.; Derevschikov, V.; Lysikov, A.; Parkhomchuk, E.; Rebrov, E.V. Performance of novel CaO-based sorbents in high temperature CO2 capture under RF heating. Chem. Eng. Process. 2017, 122, 487–492. [Google Scholar] [CrossRef]
- Su, C.; Duan, L.; Donat, F.; Anthony, E.J. From waste to high value utilization of spent bleaching clay in synthesizing high-performance calcium-based sorbent for CO2 capture. Appl. Energy 2018, 210, 117–126. [Google Scholar] [CrossRef]
- Sisinni, M.; Di Carlo, A.; Bocci, E.; Micangeli, A.; Naso, V. Hydrogen-rich gas production by sorption enhanced steam reforming of woodgas containing tar over a commercial Ni catalyst and calcined dolomite as CO2 sorbent. Energies 2013, 6, 3167–3181. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, L.; Liu, F.; Liu, K. Analytical evaluation of CaO-CO2 loop for CO2 removal. CIESC J. 2015, 66, 3233–3241. [Google Scholar]
- Dou, B.; Wang, C.; Song, Y.; Chen, H.; Jiang, B.; Yang, M.; Xu, Y. Solid sorbents for In-Situ CO2 removal during sorption-enhanced steam reforming process: A review. Renew. Sustain. Energy Rev. 2016, 53, 536–546. [Google Scholar] [CrossRef]
- Bhatta, L.K.G.; Subramanyam, S.; Chengala, M.D.; Olivera, S.; Venkatesh, K. Progress in hydrotalcite like compounds and metal-based oxides for CO2 capture: A review. J. Clean. Prod. 2015, 103, 171–196. [Google Scholar] [CrossRef]
- Blamey, J.; Zhao, M.; Manovic, V.; Anthony, E.J.; Dugwell, D.R.; Fennell, P.S. A shrinking core model for steam hydration of CaO-based sorbents cycled for CO2 capture. Chem. Eng. J. 2016, 291, 298–305. [Google Scholar] [CrossRef]
- Salaudeen, S.A.; Acharya, B.; Dutta, A. CaO-based CO2 sorbents: A review on screening, enhancement, cyclic stability, regeneration and kinetics modelling. J. CO2 Util. 2018, 23, 179–199. [Google Scholar] [CrossRef]
- Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Role of looping-calcination conditions on self-reactivation of thermally pretreated CO2 sorbents based on CaO. Energy Fuels 2013, 27, 3373–3384. [Google Scholar] [CrossRef]
- Chen, Z.; Song, H.S.; Portillo, M.; Lim, C.J.; Grace, J.R.; Anthony, E.J. Long-term calcination/carbonation cycling and thermal pretreatment for CO2 capture by limestone and dolomite. Energy Fuels 2009, 23, 1437–1444. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, J.; Li, Y.; Wang, K.; Yin, Z.; Zhou, Z.; Su, Z. Synthesis of efficient CaO sorbents for CO2 capture using a simple organometallic calcium-based carbon template route. Energy Fuels 2016, 30, 7543–7550. [Google Scholar] [CrossRef]
- Ping, H.; Wu, S. CO2 sorption durability of Zr-modified nano-CaO sorbents with cage-like hollow sphere structure. ACS Sustain. Chem. Eng. 2016, 4, 2047–2055. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Jian, S.; Li, M.; Yang, X.; Yang, Z.; Liu, X.; Xu, M. Structurally improved CaO-based sorbent by organic acids for high temperature CO2 capture. Fuel 2016, 167, 17–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Pi, S.; He, D.; Qin, C.; Ran, J. Investigation of pore-formers to modify extrusion-spheronized CaO-based pellets for CO2 capture. Processes 2019, 7, 62. [Google Scholar] [CrossRef]
- Sun, J.; Liu, W.; Chen, H.; Zhang, Y.; Hu, Y.; Wang, W.; Li, X.; Xu, M. Stabilized CO2 capture performance of extruded-spheronized CaO-based pellets by microalgae templating. Proc. Combust. Inst. 2017, 36, 3977–3984. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, X.; Chen, X.; Yin, L.; Zhang, J.; Liu, W. Performance of synthetic CaO-based sorbent pellets for CO2 capture and kinetic analysis. Fuel 2018, 232, 205–214. [Google Scholar] [CrossRef]
- Shi, J.; Li, Y.; Zhang, Q.; Ma, X.; Duan, L.; Zhou, X. CO2 capture performance of a novel synthetic CaO/sepiolite sorbent at calcium looping conditions. Appl. Energy 2017, 203, 412–421. [Google Scholar] [CrossRef]
- Rui, H.; Gao, J.; Wei, S.; Su, Y.; Qin, Y. Development of highly effective CaO@Al2O3 with hierarchical architecture CO2 sorbents via a scalable limited-space chemical vapor deposition technique. J. Mater. Chem. A 2018, 6, 3462–3470. [Google Scholar]
- Chen, H.; Wang, F.; Zhao, C.; Khalili, N. The effect of fly ash on reactivity of calcium based sorbents for CO2 capture. Chem. Eng. J. 2017, 309, 725–737. [Google Scholar] [CrossRef]
- Derevschikov, V.; Semeykina, V.; Bitar, J.; Parkhomchuk, E.; Okunev, A. Template technique for synthesis of CaO-based sorbents with designed macroporous structure. Micropor. Mesopor. Mater. 2017, 238, 56–61. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, Y.; Sun, Y.; Li, P.; Yu, J. Preparation of CaO-Al2O3 sorbent and CO2 capture performance at high temperature. Fuel 2013, 111, 636–642. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, C.; Li, S.; Huang, Z.; Yan, S.; Wang, S.; Ma, X.; Gong, J. Sorption enhanced steam reforming of ethanol on Ni-CaO-Al2O3 multifunctional catalysts derived from hydrotalcite-like compounds. Energy Environ. Sci. 2012, 5, 8942–8949. [Google Scholar] [CrossRef]
- Luo, C.; Zheng, Y.; Ding, N.; Wu, Q.L.; Zheng, C.G. SGCS-made ultrafine CaO/Al2O3 sorbent for cyclic CO2 capture. Chin. Chem. Lett. 2011, 22, 615–618. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, P.; Xie, M.; Cheng, Z.; Yuan, W. Modeling of the carbonation kinetics of a synthetic CaO-based sorbent. Chem. Eng. Sci. 2013, 95, 283–290. [Google Scholar] [CrossRef]
- Li, Y.; Su, M.; Xie, X.; Wu, S.; Liu, C. CO2 capture performance of synthetic sorbent prepared from carbide slag and aluminum nitrate hydrate by combustion synthesis. Appl. Energy 2015, 145, 60–68. [Google Scholar] [CrossRef]
- Liu, W.; Feng, B.; Wu, Y.; Wang, G.; Barry, J.; Diniz da Costa, J.O.C. Synthesis of sintering-resistant sorbents for CO2 capture. Environ. Sci. Technol. 2010, 44, 3093–3097. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, C.; Yu, W. Calcium-based sorbent doped with attapulgite for CO2 capture. Appl. Energy 2013, 112, 67–74. [Google Scholar] [CrossRef]
- Ridha, F.N.; Manovic, V.; Macchi, A.; Anthony, E.J. High-temperature CO2 capture cycles for CaO-based pellets with kaolin-based binders. Int. J. Greenh. Gas Control 2012, 6, 164–170. [Google Scholar] [CrossRef]
- Ridha, F.N.; Manovic, V.; Macchi, A.; Anthony, E.J. The effect of SO2 on CO2 capture by CaO-based pellets prepared with a kaolin derived Al(OH)3 binder. Appl. Energy 2012, 92, 415–420. [Google Scholar] [CrossRef]
- Luo, C.; Zheng, Y.; Xu, Y.; Ding, H.; Zheng, C.; Qin, C.; Feng, B. Cyclic CO2 capture characteristics of a pellet derived from sol-gel CaO powder with Ca12Al14O33 support. Korean J. Chem. Eng. 2015, 32, 934–938. [Google Scholar] [CrossRef]
- Sun, J.; Liu, W.; Hu, Y.; Li, M.; Yang, X.; Zhang, Y.; Xu, M. Structurally improved, core-in-shell, CaO-based sorbent pellets for CO2 capture. Energy Fuels 2015, 29, 6636–6644. [Google Scholar] [CrossRef]
- Wu, Y.; Manovic, V.; He, I.; Anthony, E.J. Modified lime-based pellet sorbents for high-temperature CO2 capture: Reactivity and attrition behavior. Fuel 2012, 96, 454–461. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Liu, C.; Liu, H.; Lu, C. CO2 capture by carbide slag from chlor-alkali plant in calcination/carbonation cycles. Int. J. Greenh. Gas Control 2012, 9, 117–123. [Google Scholar] [CrossRef]
- Sun, J.; Liu, W.; Hu, Y.; Wu, J.; Li, M.; Yang, X. Enhanced performance of extruded-spheronized carbide slag pellets for high temperature CO2 capture. Chem. Eng. J. 2016, 285, 293–303. [Google Scholar] [CrossRef]
- Cheng, J.; Zhou, J.H.; Liu, J.Z.; Cao, X.Y.; Cen, K.F. Physicochemical characterizations and desulfurization properties in coal combustion of three calcium and sodium industrial wastes. Energy Fuels 2009, 23, 2506–2516. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Chen, H.; Liang, C.; Duan, L.; Zhou, W. Modified CaO-based sorbent looping cycle for CO2 mitigation. Fuel 2009, 88, 697–704. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Duan, L.; Anthony, E.; Liu, H. CO2 capture performance of calcium-based synthetic sorbent with hollow core-shell structure under calcium looping conditions. Appl. Energy 2018, 225, 402–412. [Google Scholar] [CrossRef]
- Martavaltzi, C.S.; Lemonidou, A.A. Parametric study of the CaO-Ca12Al14O33 synthesis with respect to high CO2 sorption capacity and stability on multicycle operation. Ind. Eng. Chem. Res. 2008, 47, 9537–9543. [Google Scholar] [CrossRef]
- Martavaltzi, C.S.; Pampaka, E.P.; Korkakaki, E.S.; Lemonidou, A.A. Hydrogen production via steam reforming of methane with simultaneous CO2 capture over CaO-Ca12Al14O33. Energy Fuels 2010, 24, 2589–2595. [Google Scholar] [CrossRef]
- Duan, L.; Yu, Z.; Erans, M.; Li, Y.; Manovic, V.; Anthony, E.J. Attrition study of cement-supported biomass-activated calcium sorbents for CO2 capture. Ind. Eng. Chem. Res. 2016, 55, 9476–9484. [Google Scholar] [CrossRef]
- Luo, C.; Zheng, Y.; Yin, J.J.; Qin, C.L.; Ding, N.; Zheng, C.G.; Feng, B. Effect of support material on carbonation and sulfation of synthetic CaO-based sorbents in calcium looping cycle. Energy Fuels 2013, 27, 4824–4831. [Google Scholar] [CrossRef]
- Broda, M.; Muller, C.R. Synthesis of highly efficient, Ca-based, Al2O3-stabilized, carbon gel-templated CO2 sorbents. Adv. Mater. 2012, 24, 3059–3064. [Google Scholar] [CrossRef]
- Zhou, Z.; Qi, Y.; Xie, M.; Cheng, Z.; Yuan, W. Synthesis of CaO-based sorbents through incorporation of alumina/aluminate and their CO2 capture performance. Chem. Eng. Sci. 2012, 74, 172–180. [Google Scholar] [CrossRef]
- Valverde, J.M.; Medina, S. Crystallographic transformation of limestone during calcination under CO2. Phys. Chem. Chem. Phys. 2015, 17, 21912–21926. [Google Scholar] [CrossRef]
- Manovic, V.; Charland, J.P.; Blamey, J.; Fennell, P.S.; Lu, D.; Anthony, E.J. Influence of calcination conditions on carrying capacity of CaO-based sorbent in CO2 looping cycles. Fuel 2009, 88, 1893–1900. [Google Scholar] [CrossRef]
- Adánez, J.; de Diego, L.F.; García-Labiano, F. Calcination of calcium acetate and calcium magnesium acetate: Effect of the reacting atmosphere. Fuel 1999, 78, 583–592. [Google Scholar] [CrossRef]
- Mai, M.C.; Edgar, T.F. Surface area evolution of calcium hydroxide during calcination and sintering. AIChE J. 2010, 35, 30–36. [Google Scholar] [CrossRef]
- Labotka, T.C.; Cole, D.R.; Fayek, M.J.; Chacko, T. An experimental study of the diffusion of C and O in calcite in mixed CO2-H2O fluid. Am. Miner. 2011, 96, 1262–1269. [Google Scholar] [CrossRef]
- Kronenberg, A.K.; Yund, R.A.; Giletti, B.J. Carbon and oxygen diffusion in calcite—Effects of Mn content and Ph2O. Phys. Chem. Miner. 1984, 11, 101–112. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Chen, H.; Ren, Q.; Duan, L. CO2 capture efficiency and energy requirement analysis of power plant using modified calcium-based sorbent looping cycle. Energy 2011, 36, 1590–1598. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; He, Z.; Ma, X.; Song, H. CO2 capture by carbide slag calcined under high-concentration steam and energy requirement in calcium looping conditions. Appl. Energy 2017, 206, 869–878. [Google Scholar] [CrossRef]
- Wu, Z.H.; Kou, P.; Yu, Z.W. The modulation of desulphurization properties of calcium oxide by alkali carbonates. J. Therm. Anal. Calorim. 2002, 67, 745–750. [Google Scholar] [CrossRef]
- Wieczorek-Ciurowa, K. Peculiarities of interactions in the CaCO3/CaO-SO2/SO3-air system a review. J. Therm. Anal. Calorim. 1998, 53, 649–658. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.; Zheng, C.; Zhao, H. Research on the carbonation reaction of CaO. J. Huazhong Univ. Sci. Techno. Nat. Sci. Ed. 2003, 31, 54–55. [Google Scholar]
- Szekely, J.; Evans, J.W.; Sohn, H.Y. Gas-Solid Reactions; Academic Press Inc.: London, UK, 1976. [Google Scholar]
- Hughes, R.W.; Lu, D.; Anthony, E.J.; Wu, Y. Improved long-term conversion of limestone-derived sorbents for in situ capture of CO2 in a fluidized bed combustor. Ind. Eng. Chem. Res. 2004, 43, 5529–5539. [Google Scholar] [CrossRef]


| CaO | MgO | SiO2 | Al2O3 | Fe2O3 | SrO | Ti2O | Others | Loss on Ignition |
|---|---|---|---|---|---|---|---|---|
| 69.52 | 0.02 | 2.34 | 1.52 | 0.17 | 0.03 | 0.03 | 0.57 | 25.80 |
| Calcium Aluminates | CaO Ratios (wt.%) | Precursors | Methods | Reaction Conditions | C10 |
|---|---|---|---|---|---|
| Ca3Al2O6 [41] | 90 | Carbide slag; Al(NO3)3 | Combustion | Carbonation: T1, A1, t3; Calcination: T3, A3, t1 | 0.45 |
| Ca9Al6O18 [49] | 90 | Ca(C6H5O7)2; Al(NO3)3 | Mixing | Carbonation: T1, A1, t3; Calcination: 800 °C, A3, t1 | 0.55 |
| Ca12Al14O33 [47] | 80 | Ca(NO3)2; Al(NO3)3 | Sol-gel | Carbonation: T2, A1, 2.5 min; Calcination: 950 °C, CO2, 2.5 min | 0.35 |
| Ca12Al14O33 [36] | 75 | Sol-gel CaO; Cement | Dry mixing | Carbonation: T2, A1, 15 min; Calcination: T3, A3, t1 | 0.48 |
| Ca12Al14O33 [37] | 90 | Limestone; Cement | Wet mixing | Carbonation: T2, A1, t3; Calcination: 900 °C, A3, t1 | 0.28 |
| Ca12Al14O33 [48] | 90 | Ca(NO3)2; Al(NO3)3 | Carbon gel Templating | Carbonation:750 °C, 60%CO2, t2; Calcination: 750 °C, A3, t2 | 0.56 |
| Ca12Al14O33 (this work) | 90 | Carbide slag; Al(NO3) 3 | Hydrothermal Templating | Carbonation: T1, A1, t2; Calcination: T3, A3, t1 | 0.58 |
| Samples. | N | kC | R2 | kD × 103 | R2 |
|---|---|---|---|---|---|
| Carbide slag | 1 | 0.119 | 0.983 | 2.978 | 0.968 |
| HT5 | 1 | 0.103 | 0.990 | 3.964 | 0.942 |
| Carbide slag | 11 | 0.057 | 1 | 1.292 | 0.974 |
| HT5 | 11 | 0.072 | 0.994 | 2.054 | 0.959 |
| Samples | N | BET Surface Area m2/g | BJH Pore Volume cm3/g |
|---|---|---|---|
| Carbide slag | 0 | 10 | 0.048 |
| 10 | 3 | 0.017 | |
| HT5 | 0 | 12 | 0.053 |
| 10 | 7 | 0.038 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Li, Y.; Qian, Y.; Wang, Z. A Carbide Slag-Based, Ca12Al14O33-Stabilized Sorbent Prepared by the Hydrothermal Template Method Enabling Efficient CO2 Capture. Energies 2019, 12, 2617. https://doi.org/10.3390/en12132617
Ma X, Li Y, Qian Y, Wang Z. A Carbide Slag-Based, Ca12Al14O33-Stabilized Sorbent Prepared by the Hydrothermal Template Method Enabling Efficient CO2 Capture. Energies. 2019; 12(13):2617. https://doi.org/10.3390/en12132617
Chicago/Turabian StyleMa, Xiaotong, Yingjie Li, Yi Qian, and Zeyan Wang. 2019. "A Carbide Slag-Based, Ca12Al14O33-Stabilized Sorbent Prepared by the Hydrothermal Template Method Enabling Efficient CO2 Capture" Energies 12, no. 13: 2617. https://doi.org/10.3390/en12132617
APA StyleMa, X., Li, Y., Qian, Y., & Wang, Z. (2019). A Carbide Slag-Based, Ca12Al14O33-Stabilized Sorbent Prepared by the Hydrothermal Template Method Enabling Efficient CO2 Capture. Energies, 12(13), 2617. https://doi.org/10.3390/en12132617