Global Warming Potential of Biomass-to-Ethanol: Review and Sensitivity Analysis through a Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioethanol from Jerusalem Artichoke
2.2. Life Cycle Assessment
2.2.1. Functional Unit
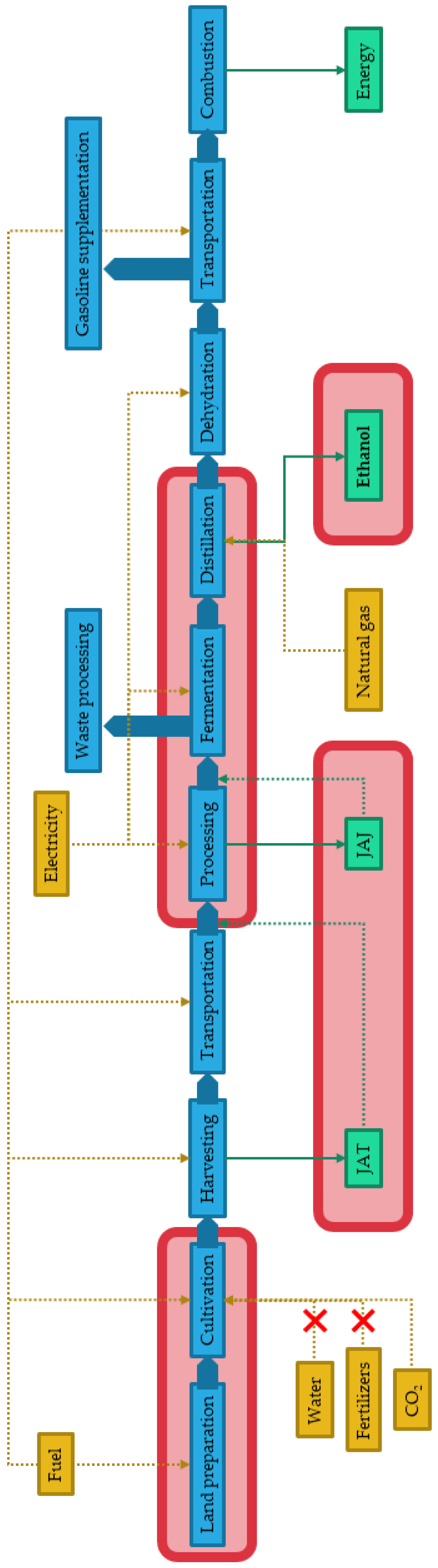
2.2.2. System Boundaries
2.2.3. Life Cycle Inventory
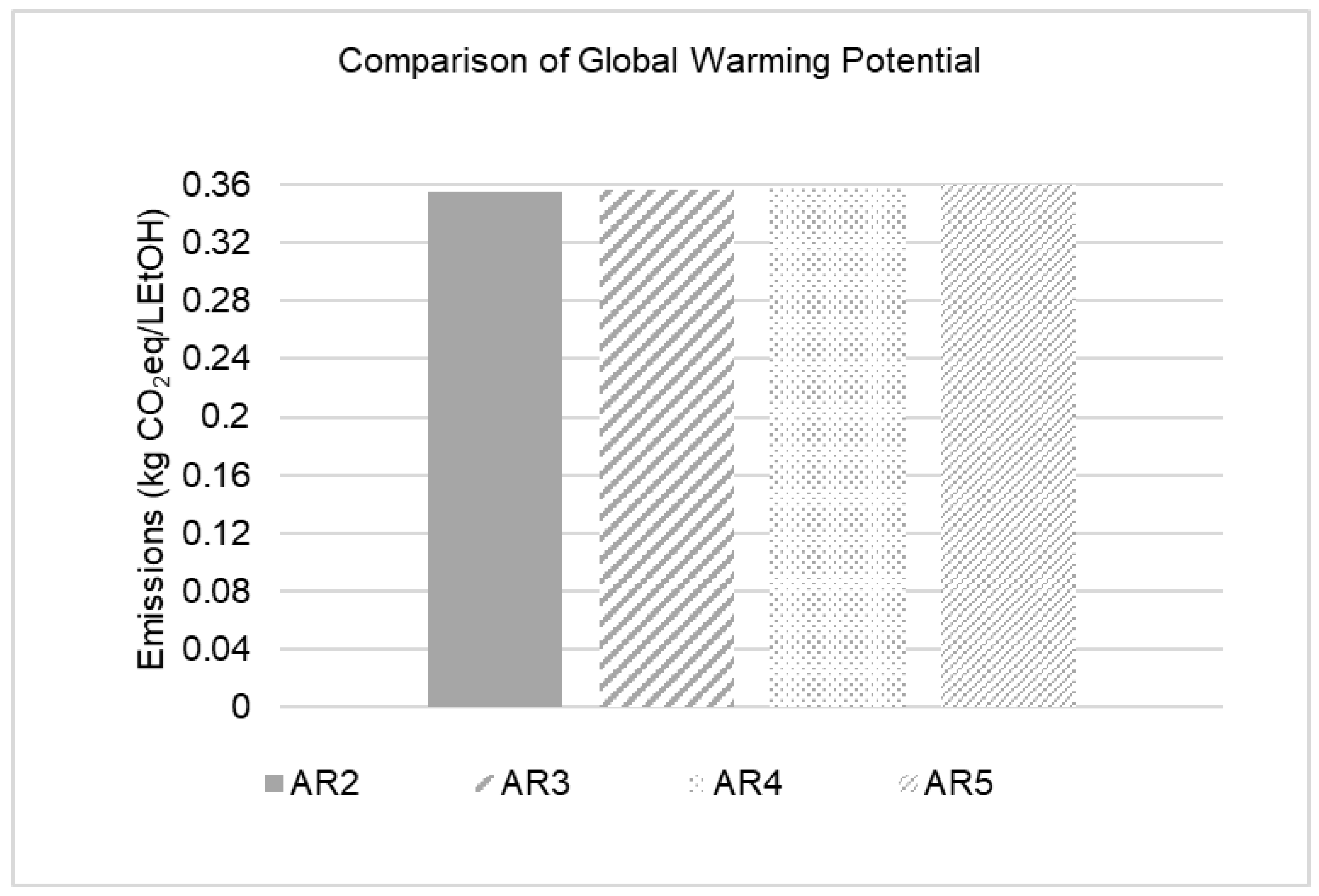
2.2.4. Impact Assessment
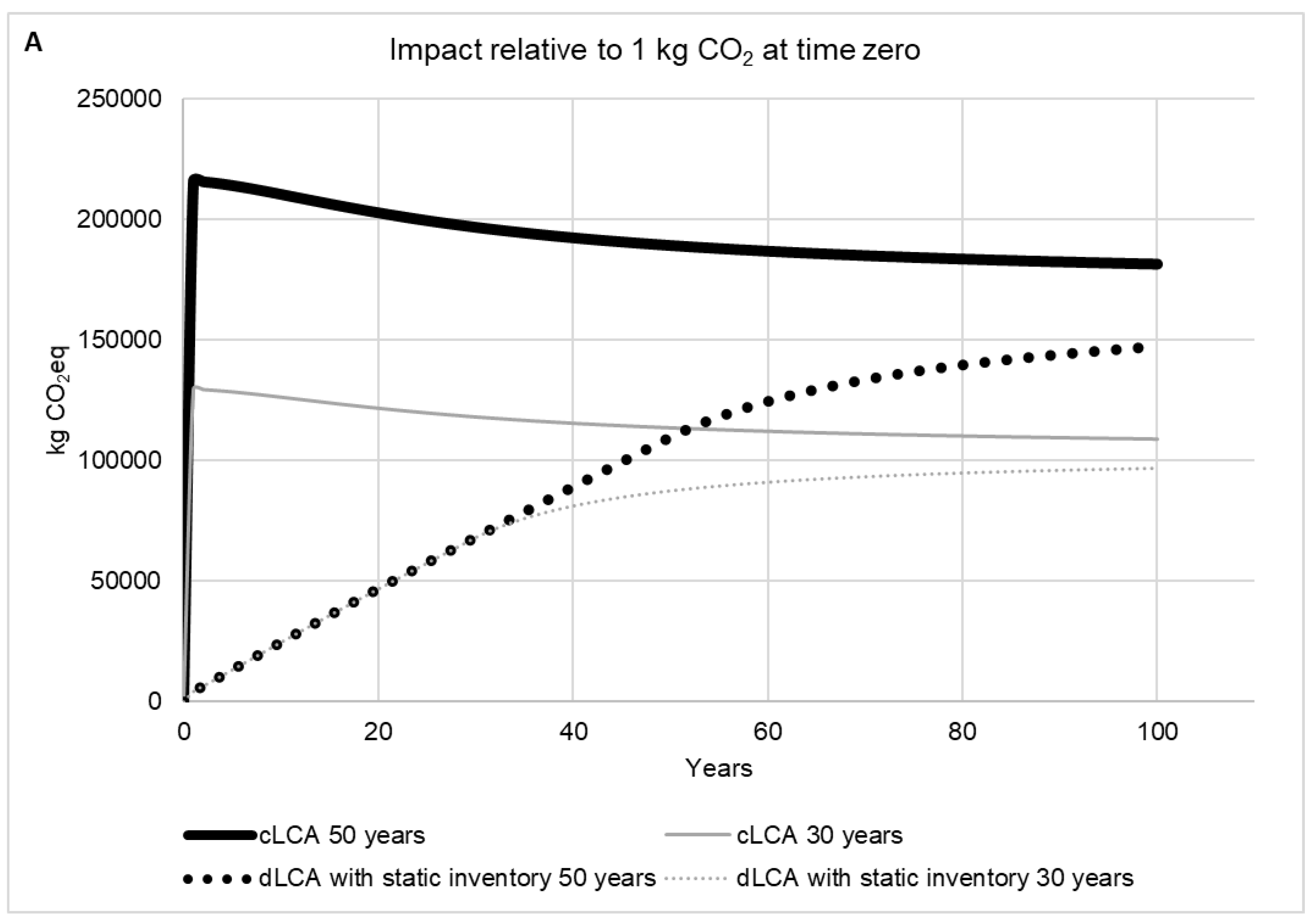
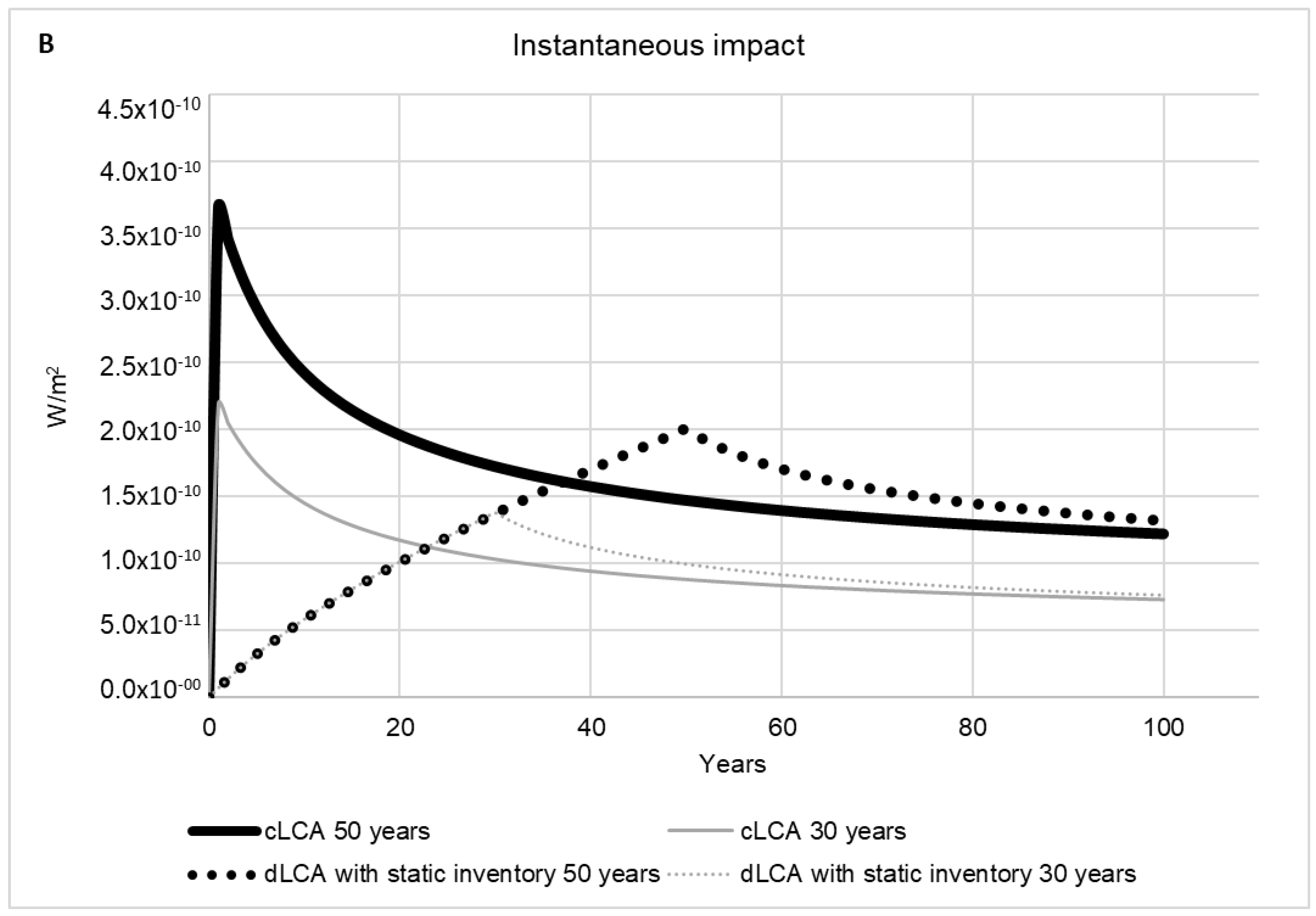
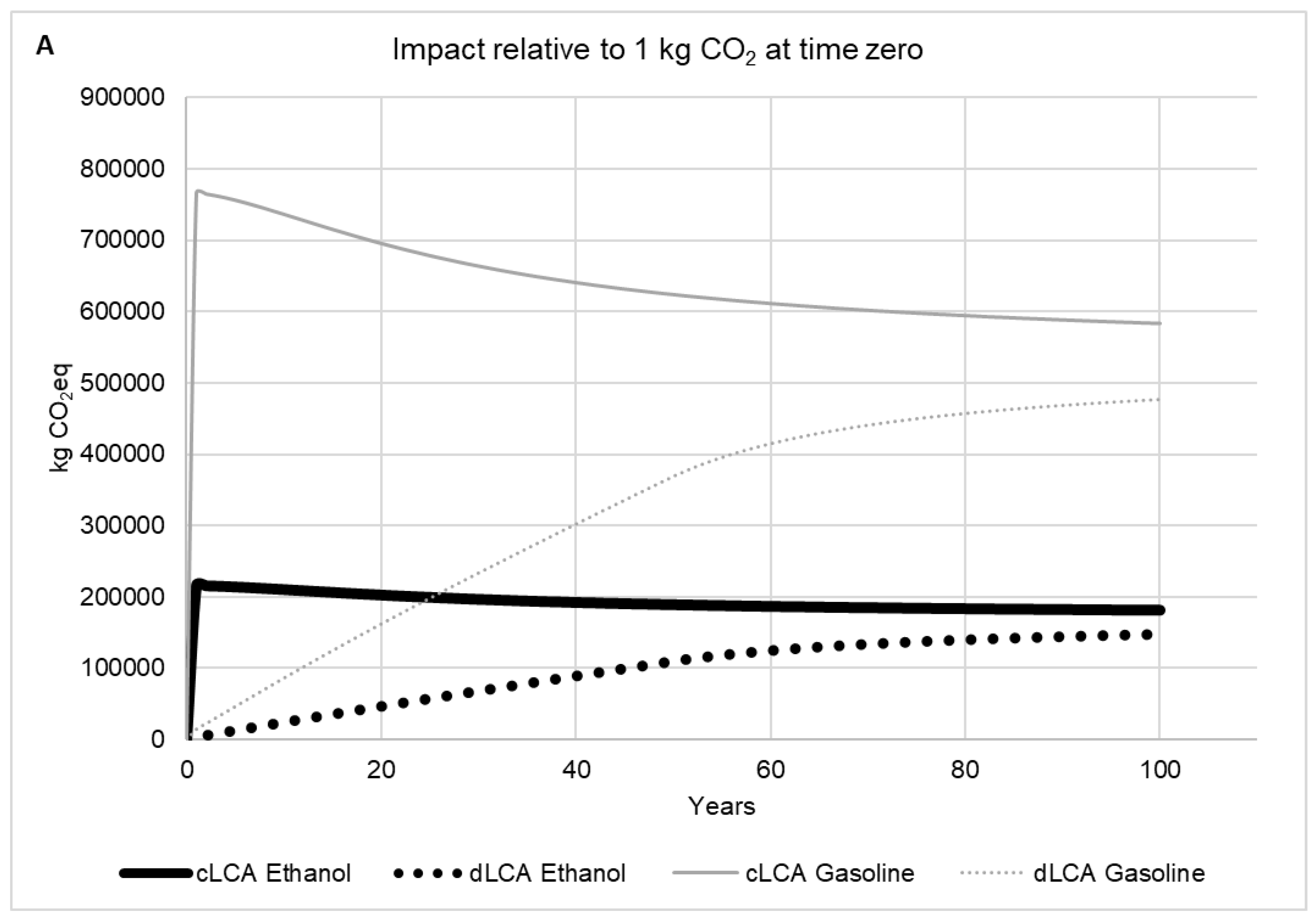
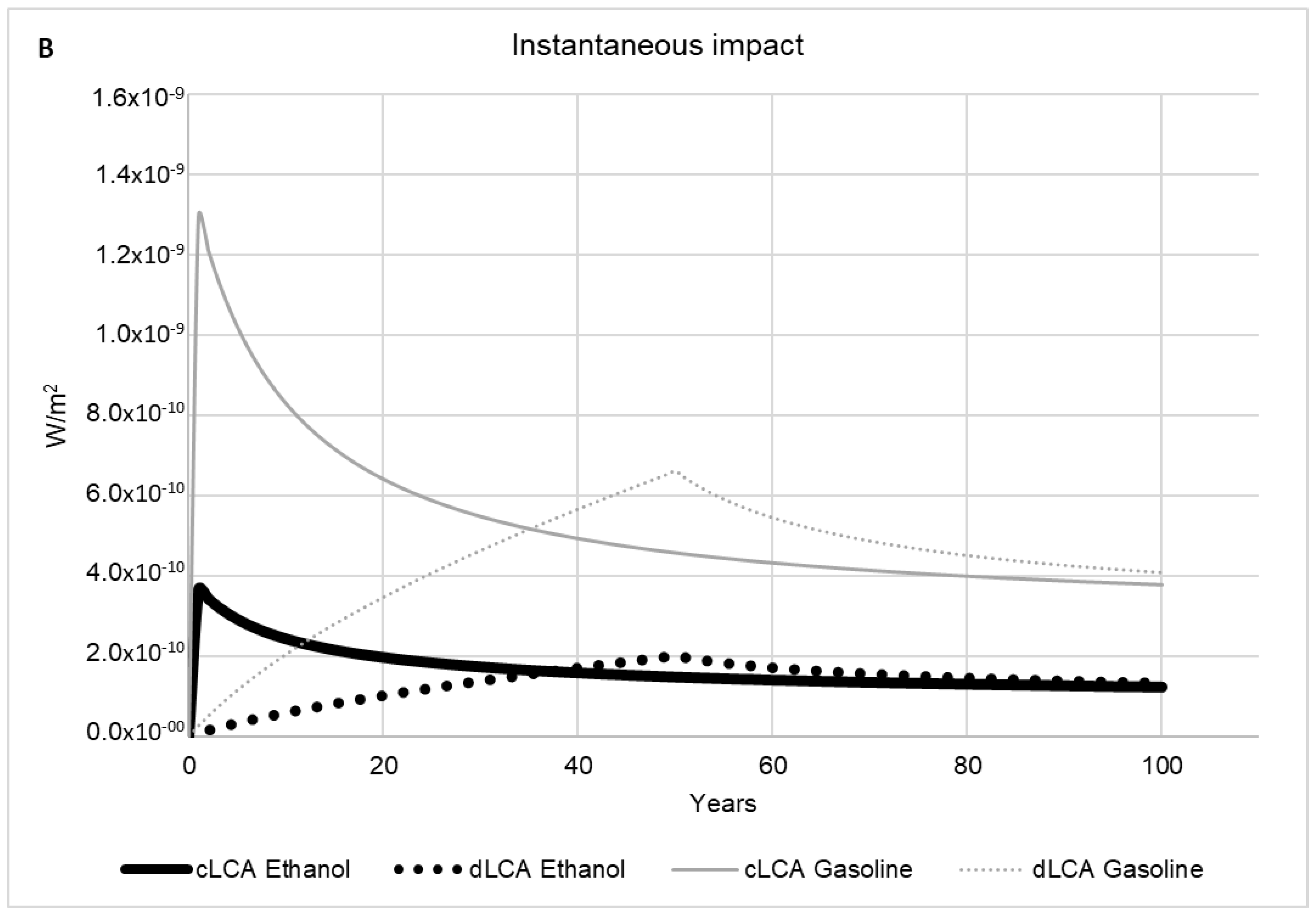
2.2.5. Time Horizon Influence
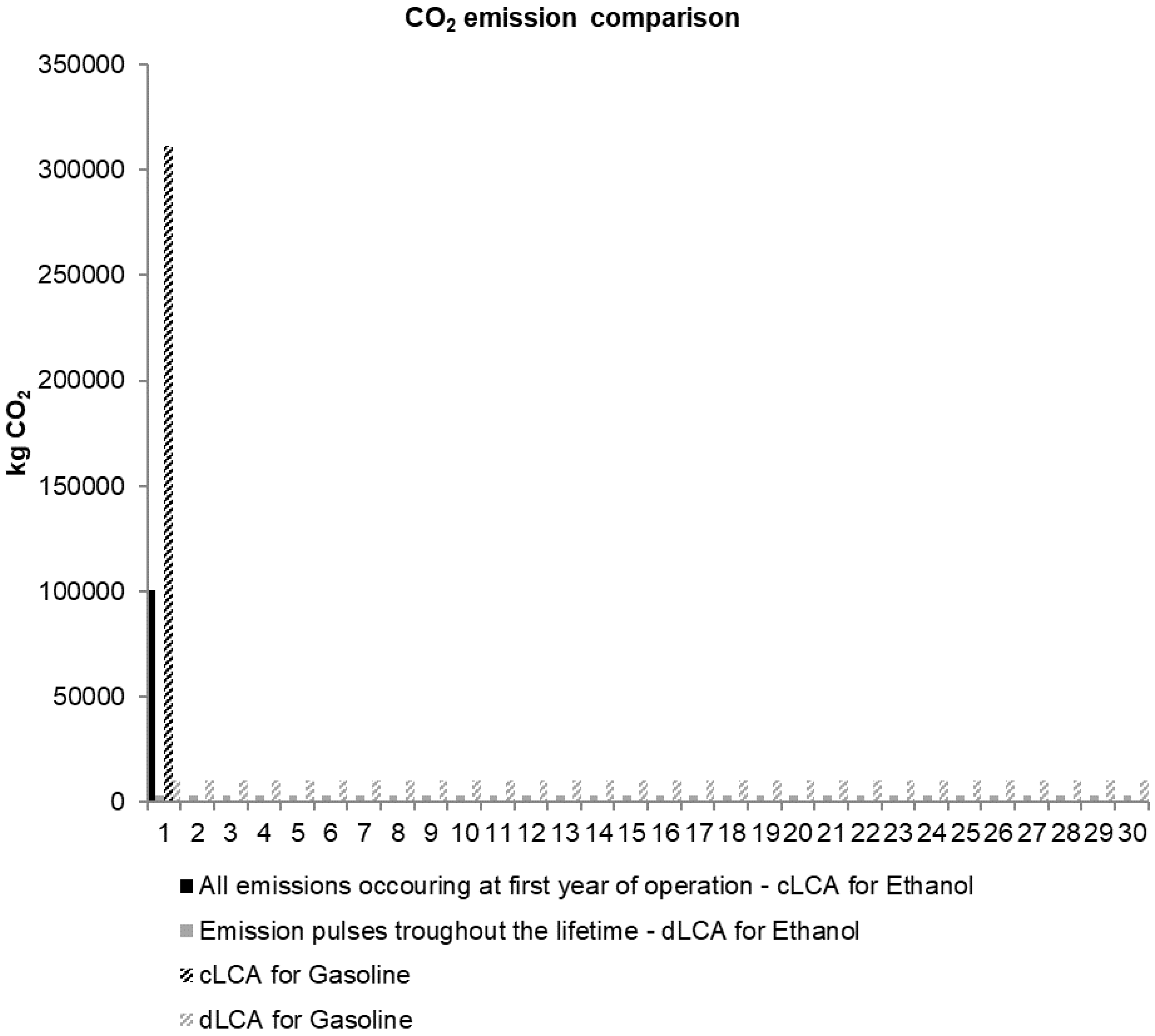
2.2.6. Dynamic LCA versus Conventional LCA
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Energy Information Administration—U.S. Department of Energy. EIA International Energy Outlook 2009; Technical Report for Energy Information Administration: Washington, DC, USA, May 2009.
- Axelsson, L.; Franzén, M.; Ostwald, M.; Berndes, G.; Lakshmi, G.; Ravindranath, N.H. Perspective: Jatropha cultivation in southern India: Assessing farmers’ experiences. Biofuels Bioprod. Biorefin. 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Morales, M.; Quintero, J.; Conejeros, R.; Aroca, G. Life cycle assessment of lignocellulosic bioethanol: Environmental impacts and energy balance. Renew. Sustain. Energy Rev. 2015, 42, 1349–1361. [Google Scholar] [CrossRef]
- Giuntoli, J. Final Recast Renewable Energy Directive for 2021–2030 in the European Union; Report for International Council on Clean Transportation: Washington, DC, USA, July 2018. [Google Scholar]
- International Energy Agency. IEA Energy Technology Essentials Biofuel Production; Technical Report for International Energy Agency: Paris, France, 24 April 2007; pp. 1–4. [Google Scholar]
- Heywood, J. Internal Combustion Engine Fundamentals; McGraw-Hill Education: New York, NY, USA, 16 May 1988. [Google Scholar]
- Combustion of Fuels—Carbon Dioxide Emission. Available online: https://www.engineeringtoolbox.com/co2-emission-fuels-d_1085.html (accessed on 13 June 2019).
- Sendelius, J. Steam Pretreatment Optimisation for Sugarcane Bagasse in Bioethanol Production. Master’s Thesis, Lund University, Scania, Sweden, January 2005. [Google Scholar]
- Quintero, J.A.; Montoya, M.I.; Sánchez, O.J.; Giraldo, O.H.; Cardona, C.A. Fuel ethanol production from sugarcane and corn: Comparative analysis for a Colombian case. Energy 2008, 33, 385–399. [Google Scholar] [CrossRef]
- Cardona, C.A.; Sánchez, Ó.J. Fuel ethanol production: Process design trends and integration opportunities. Bioresour. Technol. 2007, 98, 2415–2457. [Google Scholar] [CrossRef]
- Cheng, K.K.; Cai, B.Y.; Zhang, J.A.; Ling, H.Z.; Zhou, Y.J.; Ge, J.P.; Xu, J.M. Sugarcane bagasse hemicellulose hydrolysate for ethanol production by acid recovery process. Biochem. Eng. J. 2008, 38, 105–109. [Google Scholar] [CrossRef]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef]
- McMillan, J.D. Bioethanol production: Status and prospects. Renew. Energy 1997, 10, 295–302. [Google Scholar] [CrossRef]
- Slininger, P.J.; Shea-Andersh, M.A.; Thompson, S.R.; Dien, B.S.; Kurtzman, C.P.; Balan, V.; Balan, V.; Sousa, L.D.C.; Uppuqundla, N.; Dale, B.E.; et al. Evolved strains of Scheffersomyces stipitis achieving high ethanol productivity on acid- and base-pretreated biomass hydrolyzate at high solids loading. Biotechnol. Biofuels 2015, 8, 1–27. [Google Scholar] [CrossRef]
- Demeke, M.M.; Dumortier, F.; Li, Y.; Broeckx, T.; Foulquié-Moreno, M.R.; Thevelein, J.M. Combining inhibitor tolerance and D-xylose fermentation in industrial Saccharomyces cerevisiae for efficient lignocellulose-based bioethanol production. Biotechnol. Biofuels 2013, 6, 1–17. [Google Scholar] [CrossRef]
- Duda, M.; Shaw, J.S. Life Cycle Assessment. Soc. Sci. Public Policy 1997, 35, 38–43. [Google Scholar] [CrossRef]
- Ekvall, T.; Finnveden, G. Allocation in ISO 14041—A critical review. J. Clean. Prod. 2001, 9, 197–208. [Google Scholar] [CrossRef]
- Finnveden, G.; Hauschild, M.Z.; Ekvall, T.; Guinée, J.; Heijungs, R.; Hellweg, S.; Koehler, A.; Pennington, D.; Suh, S. Recent developments in Life Cycle Assessment. J. Environ. Manag. 2009, 91, 1–21. [Google Scholar] [CrossRef]
- Ometto, A.R.; Hauschild, M.Z.; Roma, W.N.L. Lifecycle assessment of fuel ethanol from sugarcane in Brazil. Int. J. Life Cycle Assess. 2009, 14, 236–247. [Google Scholar] [CrossRef]
- Luo, L.; Voet, E.V.D.; Huppes, G. An energy analysis of ethanol from cellulosic feedstock-Corn stover. Renew. Sustain. Energy Rev. 2009, 13, 2003–2011. [Google Scholar] [CrossRef]
- Kadam, K.L. Environmental Life Cycle Implications of Using Bagasse-Derived Ethanol as a Gasoline Oxygenate in Mumbai (Bombay); NREL/TP-580-28705; Technical Report for National Renewable Energy Laboratory: Lakewood, CO, USA, November 2000.
- Spatari, S.; Zhang, Y.; Maclean, H.L. Life Cycle Assessment of Switchgrass- and Corn Automobiles. Environ. Sci. Technol. 2005, 39, 9750–9758. [Google Scholar] [CrossRef]
- Muñoz, I.; Flury, K.; Jungbluth, N.; Rigarlsford, G.; I Canals, L.M.; King, H. Life cycle assessment of bio-based ethanol produced from different agricultural feedstocks. Int. J. Life Cycle Assess. 2014, 19, 109–119. [Google Scholar] [CrossRef]
- Paixão, S.M.; Alves, L.; Pacheco, R.; Silva, C.M. Evaluation of Jerusalem artichoke as a sustainable energy crop to bioethanol: energy and CO2eq emissions modeling for an industrial scenario. Energy 2018, 150, 468–481. [Google Scholar] [CrossRef]
- Negro, M.J.; Ballesteros, I.; Manzanares, P.; Oliva, J.M.; Saez, F.; Ballesteros, M. Inulin-containing biomass for ethanol production. Appl. Biochem. Biotechnol. 2006, 132, 922–932. [Google Scholar] [CrossRef]
- Gengmao, Z.; Mehta, S.K.; Zhaopu, L. Use of saline aquaculture wastewater to irrigate salt-tolerant Jerusalem artichoke and sunflower in semiarid coastal zones of China. Agric. Water Manag. 2010, 97, 1987–1993. [Google Scholar] [CrossRef]
- Zhang, T.; Chi, Z.; Zhao, C.H.; Chi, Z.M.; Gong, F. Bioethanol production from hydrolysates of inulin and the tuber meal of Jerusalem artichoke by Saccharomyces sp. W0. Bioresour. Technol. 2010, 101, 8166–8170. [Google Scholar] [CrossRef]
- Hu, N.; Yuan, B.; Sun, J.; Wang, S.A.; Li, F.L. Thermotolerant Kluyveromyces marxianus and Saccharomyces cerevisiae strains representing potentials for bioethanol production from Jerusalem artichoke by consolidated bioprocessing. Appl. Microbiol. Biotechnol. 2012, 95, 1359–1368. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.M.; Kim, C.H. Ethanol production using whole plant biomass of jerusalem artichoke by Kluyveromyces marxianus CBS1555. Appl. Biochem. Biotechnol. 2013, 169, 1531–1545. [Google Scholar] [CrossRef]
- Macedo, I.C.; Seabra, J.E.A.; Silva, J.E.A.R. Greenhouse gases emissions in the production and use of ethanol from sugarcane in Brazil: The 2005/2006 averages and a prediction for 2020. Biomass Bioenergy 2008, 32, 582–595. [Google Scholar] [CrossRef]
- Seabra, J.E.A.; Macedo, I.C.; Chum, H.L.; Faroni, C.E.; Sarto, C.A. Life cycle assessment of Brazilian sugarcane products: GHG emissions and energy use. Biofuels Bioprod. Biorefin. 2011. [Google Scholar] [CrossRef]
- Tsiropoulos, I.; Faaij, A.P.C.; Seabra, J.E.A.; Lundquist, L.; Schenker, U.; Briois, J.F.; Patel, M.K. Life cycle assessment of sugarcane ethanol production in India in comparison to Brazil. Int. J. Life Cycle Assess. 2014, 19, 1049–1067. [Google Scholar] [CrossRef]
- Edwards, R.; Lariv_e, J.-F.; Rickeard, D.; Weindorf, W. Report Well-to-Tank Report Appendix 4-Version4a: Description, Results and Input Data per Pathway, April 2014, JRC Technical Reports—Report EUR 26237 EN, JEC-Joint Research Centre-EUCAR-CONCAWE Collaboration, 2014, 1–12. Available online: http://iet.jrc.ec.europa.eu/about-jec/downloads (accessed on 25 April 2019).
- Neeft, J.; Buck, S.; Gerlagh, T.; Gapnepain, B.; Bacovsky, D.; Ludwiczek, N.; Lavelle, P.; Thonier, G.; Lechón, Y.; Lago, C. Biograce—Harmonised Calculations of Biofuel Greenhouse Gas Emissions in Europe; Technical Report for Intelligent Energy Europe: Brussels, Belgium, June 2019. [Google Scholar]
- Greenhouse Gases, Regulated Emissions and Energy Use in Transportation (GREET). Available online: https://greet.es.anl.gov/ (accessed on 2 March 2019).
- The Ecoinvent Database Version 3 (Part I): Overview and Methodology. Available online: http://link.springer.com/10.1007/s11367-016-1087-8 (accessed on 20 November 2018).
- Bare, J.C. The tool for the reduction and assessment of chemical and other environmental impacts 2.0. J. Ind. Ecol. 2011, 13, 687–696. [Google Scholar] [CrossRef]
- Shimako, A. Contribution to the Development of a Dynamic Life Cycle Assessment Method. Ph.D. Thesis, Institut National des Sciences Appliquées de Toulouse, Toulouse, France, 2017. [Google Scholar]
- Joos, F.; Roth, R.; Fuglestvedt, J.S.; Peters, G.P.; Enting, I.G.; Bloh, W.V.; Brovkin, V.; Burke, E.J.; Eby, M.; Edwards, N.R.; et al. Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis. Atmos. Chem. Phys. 2013, 13, 2793–2825. [Google Scholar] [CrossRef]
- Levasseur, A.; Lesage, P.; Margni, M.; Deschênes, L.; Samson, R. Considering time in LCA: dynamic LCA and its application to global warming impact assessments. Environ. Sci. Technol. 2010, 44, 3169–3174. [Google Scholar] [CrossRef]
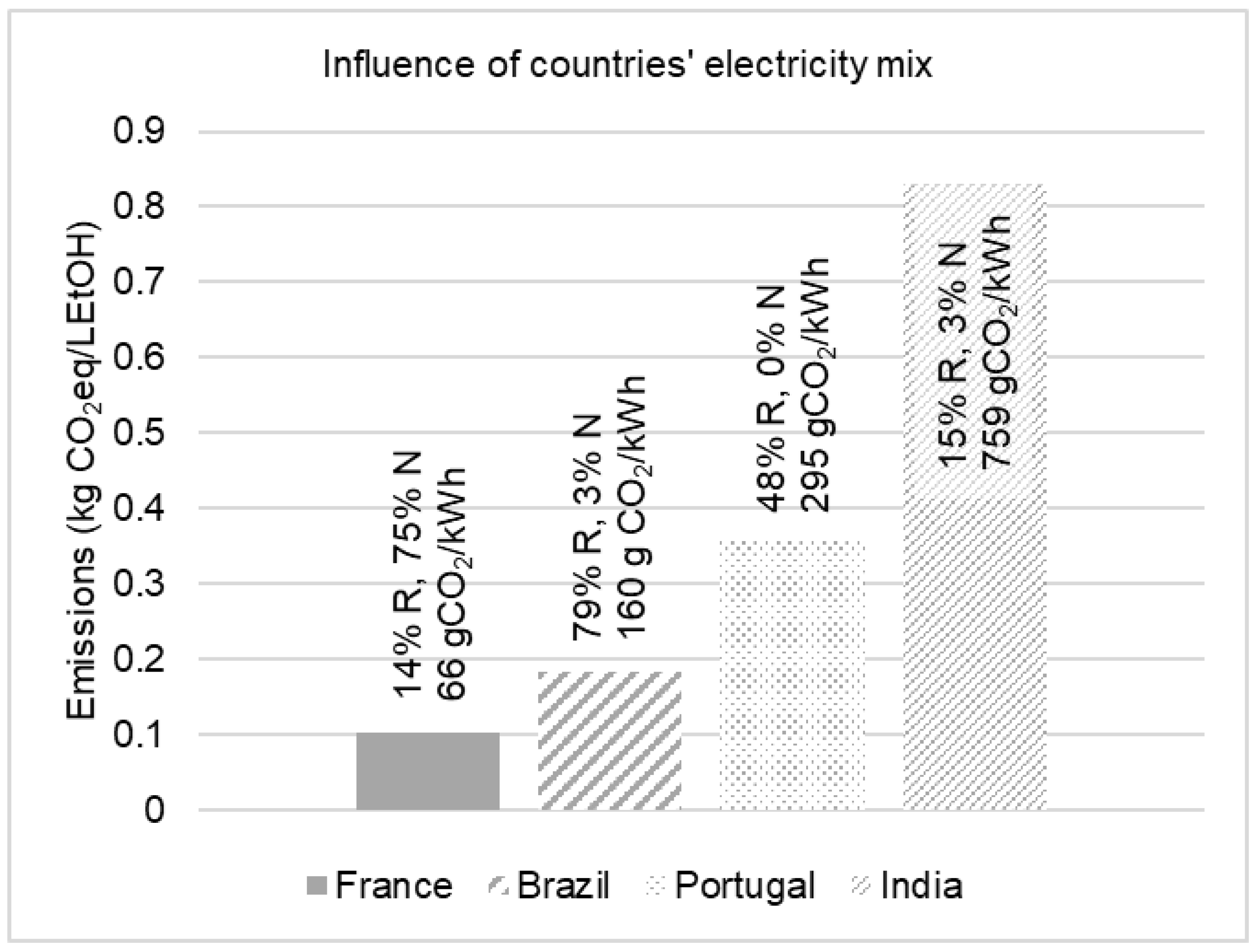
- Moro, A.; Lonza, L. Electricity carbon intensity in European Member States: Impacts on GHG emissions of electric vehicles. Transp. Res. Part D: Transp. Environ. 2018, 64, 5–14. [Google Scholar] [CrossRef]
- Climate Transparency Organization. G20 Brown to Green Report 2018; Climate Transparency, c/o Humboldt-Viadrina Governance Platform: Berlin, Germany, 2018. [Google Scholar]
- Silva, C.; Pacheco, R.; Arcentales, D.; Santos, F. Sustainability of sugarcane for energy purposes—Chapter 3. In Sugarcane Biorefinery, Technology and Perspectives; Academic Press: Cambridge, MA, USA, 2019; ISBN 978-0-12-814236-3. (in press) [Google Scholar]
- Borrion, A.L.; McManus, M.C.; Hammond, G.P. Environmental life cycle assessment of lignocellulosic conversion to ethanol: A review. Renew. Sustain. Energy Rev. 2012, 16, 4638–4650. [Google Scholar] [CrossRef]
- Kosugi, A.; Kondo, A.; Ueda, M.; Murata, Y.; Vaithanomsat, P.; Thanapase, W.; Arai, T.; Mori, Y. Production of ethanol from cassava pulp via fermentation with a surface-engineered yeast strain displaying glucoamylase. Renew. Energy 2009, 34, 1354–1358. [Google Scholar] [CrossRef]
- Yamada, R.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. Novel strategy for yeast construction using δ-integration and cell fusion to efficiently produce ethanol from raw starch. Appl. Microbiol. Biotechnol. 2010, 85, 1491–1498. [Google Scholar] [CrossRef]
- Aydemir, E. Genetic Modifications of Saccharomyces cerevisiae for Ethanol Production from Starch Fermentation: A Review. J. Bioprocess. Biotech. 2014. [Google Scholar] [CrossRef]
- Shigechi, H.; Uyama, K.; Fujita, Y.; Matsumoto, T.; Ueda, M.; Tanaka, A.; Fukuda, H.; Kondo, A. Efficient ethanol production from starch through development of novel flocculent yeast strains displaying glucoamylase and co-displaying or secreting α-amylase. J. Mol. Catal. B Enzym. 2002, 17, 179–187. [Google Scholar] [CrossRef]
- Ramachandran, N.; Joubert, L.; Gundlapalli, S.B.; Otero, R.R.C.; Pretorius, I.S. The effect of flocculation on the efficiency of raw-starch fermentation by Saccharomyces cerevisiae producing the Lipomyces kononenkoae LKA1-encoded α-amylase. Ann. Microbiol. 2008, 58, 99–108. [Google Scholar] [CrossRef]

| Gasoline | Ethanol | |
|---|---|---|
| Feedstock | crude oil | corn, sugar cane, vegetable waste |
| Gasoline equivalent | 100% | 73 to 83% |
| Density | 0.725 kg/L | 0.785 kg/L |
| Energy content (LHV) | ≈31.2 to 32.3 MJ/L | ≈21.2 MJ/L |
| Energy content (HHV) | ≈33.4 to 34.6 MJ/L | ≈23.4 MJ/L |
| Emissions | 2.49 kgCO2/L | 1.51 kgCO2/L |
| 77.82 gCO2/MJ | 71.68 gCO2/MJ |
| Study | Country | Feedstock | Generation | CO2eq Emissions (kg/LEtOH) | Ethanol Yield (LEtOH/kgfeedstock) | Included Processes |
|---|---|---|---|---|---|---|
| [21] | India (only CO2) | sugarcane bagasse (e) | 2G | 3.88 | 0.30 | bagasse transportation; ethanol production; reformulated gasoline use (includes biogenic CO2) |
| sugarcane bagasse (da) | 2G | 5.55 | 0.24 | |||
| [22] | Canada | switchgrass | 2G | 0.49 | 0.33 | biomass production; ethanol production; ethanol transportation and distribution; use |
| corn stover | 2G | 0.33 | 0.34 | |||
| [9] | Colombia | corn | 1G | n/a | 0.45 | pre-treatment; hydrolysis; fermentation; separation; dehydration; wastewater treatment |
| sugarcane | 1G | n/a | 0.08 | |||
| [30] | Brazil | sugarcane (2002) | 1G | 0.39 (h) | 0.09 | sugarcane production; processing; ethanol production |
| 0.40 (a) | ||||||
| sugarcane (2005/06) | 1G | 0.42 (h) | 0.09 | |||
| 0.44 (a) | ||||||
| sugarcane (2020 scenario) | 1G | 0.33 (h) | 0.09 | |||
| 0.35 (a) | ||||||
| [31] | Brazil | sugarcane | 1G | 0.45 | 0.07 | sugarcane production; harvesting; transportation; processing; ethanol production; distribution |
| [23] | France | sugarbeet | 1G | 0.87 | 0.075 | sugarbeet production; transportation; ethanol production; distribution; ethanol disposal |
| [32] | India | sugarcane | 1G | 2.45 | 0.25 | sugarcane production; sugarcane processing to sugar; sugarcane processing to ethanol |
| [33] | Brazil | sugarcane | 1G | 0.35 | n/a | sugarcane production + local transport; ethanol production (without surplus energy credits) |
| [34] | Europe | sugarbeet | 1G | 0.8 | 0.11 | cultivation plus ethanol production, energy allocation |
| wheat | 0.52–1.45 | 0.37 | ||||
| corn | 0.88 | 0.38 | ||||
| sugarcane | 0.32 | 0.09 | ||||
| sugarbeet | 1.12 | 0.11 | cultivation plus ethanol production, no allocation | |||
| wheat | 0.85–2.42 | 0.37 | ||||
| corn | 1.61 | 0.38 | ||||
| [35] | USA | corn year 2000 | 1G | 1.29 | N.A. | cultivation plus ethanol production |
| corn year 2015 | 1.04 | |||||
| [24] | Portugal | Jerusalem artichoke | 1G | 0.42 | 0.057 | cultivation; ethanol production (juice extraction; processing; fermentation; distillation) |
| JA with biogenic CO2 | 1.43 | |||||
| JA with direct land use change | 1.56 |
| Input | Value (kWh) |
|---|---|
| Geothermal | 0.008 |
| Hard coal | 0.445 |
| Hydro | 0.306 |
| Wind | 0.241 |
| Compensation for grid losses | 0.308 |
| Emissions (gCO2/kWh) | 295 |
| Process | Item | Value |
|---|---|---|
| Land preparation | Area | 1.000 ha |
| Diesel | 0.023 L | |
| Cultivation | Water | 0.000 L |
| JA tubers yield | 39,069.700 kg | |
| Processing | Electricity | 1207.000 kWh |
| JA juice yield | 27,906.930 kg | |
| Fermentation | Electricity | 7035.118 kWh |
| EtOH yield | 2299.050 L | |
| Distillation | Natural gas | 0.179 L |
| Value (kg CO2eq/LETOH) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Method | T | IP | IL | IM | E | C20 | C100 | C500 | |
| Time horizon | 100 | 20 | 100 | 100 | 100 | 20 | 100 | 500 | |
| Country | USA | n/a | n/a | Switzerland | Denmark | Netherlands | |||
| Processes | Ethanol from JA (this study) | ||||||||
| Land preparation and cultivation | 0.0456 | 0.0469 | 0.0456 | 0.1390 | 0.1430 | 0.0466 | 0.0458 | 0.0452 | |
| Processing | 0.1130 | 0.1300 | 0.1130 | 0.1120 | 0.1160 | 0.1230 | 0.1130 | 0.1080 | |
| Fermentation | 0.2010 | 0.2310 | 0.2010 | 0.1190 | 0.2070 | 0.2190 | 0.2000 | 0.1920 | |
| Distillation | 2.56 × 10−8 | 4.90 × 10−8 | 2.56 × 10−8 | 1.88 × 10−6 | 2.56 × 10−8 | 4.01 × 10−8 | 2.48 × 10−8 | 1.85 × 10−8 | |
| Total | 0.3600 | 0.4800 | 0.3600 | 0.4490 | 0.4660 | 0.3890 | 0.3590 | 0.3450 | |
| Gasoline [32,35] | 1.154 | ||||||||
| Ethanol from sugarcane [32,35] | 0.75 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, R.; Silva, C. Global Warming Potential of Biomass-to-Ethanol: Review and Sensitivity Analysis through a Case Study. Energies 2019, 12, 2535. https://doi.org/10.3390/en12132535
Pacheco R, Silva C. Global Warming Potential of Biomass-to-Ethanol: Review and Sensitivity Analysis through a Case Study. Energies. 2019; 12(13):2535. https://doi.org/10.3390/en12132535
Chicago/Turabian StylePacheco, Rui, and Carla Silva. 2019. "Global Warming Potential of Biomass-to-Ethanol: Review and Sensitivity Analysis through a Case Study" Energies 12, no. 13: 2535. https://doi.org/10.3390/en12132535
APA StylePacheco, R., & Silva, C. (2019). Global Warming Potential of Biomass-to-Ethanol: Review and Sensitivity Analysis through a Case Study. Energies, 12(13), 2535. https://doi.org/10.3390/en12132535






