Optimization of Oleuropein and Luteolin-7-O-Glucoside Extraction from Olive Leaves by Ultrasound-Assisted Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Extraction Methods
2.3. Design of Experiments and Statistical Analysis
2.4. Extraction Yield
2.5. TPC and TFC of Olive Leaves Extracts
2.6. Antioxidant Capacities of Olive Leaves Extracts
2.7. Analysis of Phenolic Compounds by HPLC-DAD
3. Results and Discussion
3.1. Influence of UAE Factors on the Extraction of Bioactive Compounds from Olive Leaves
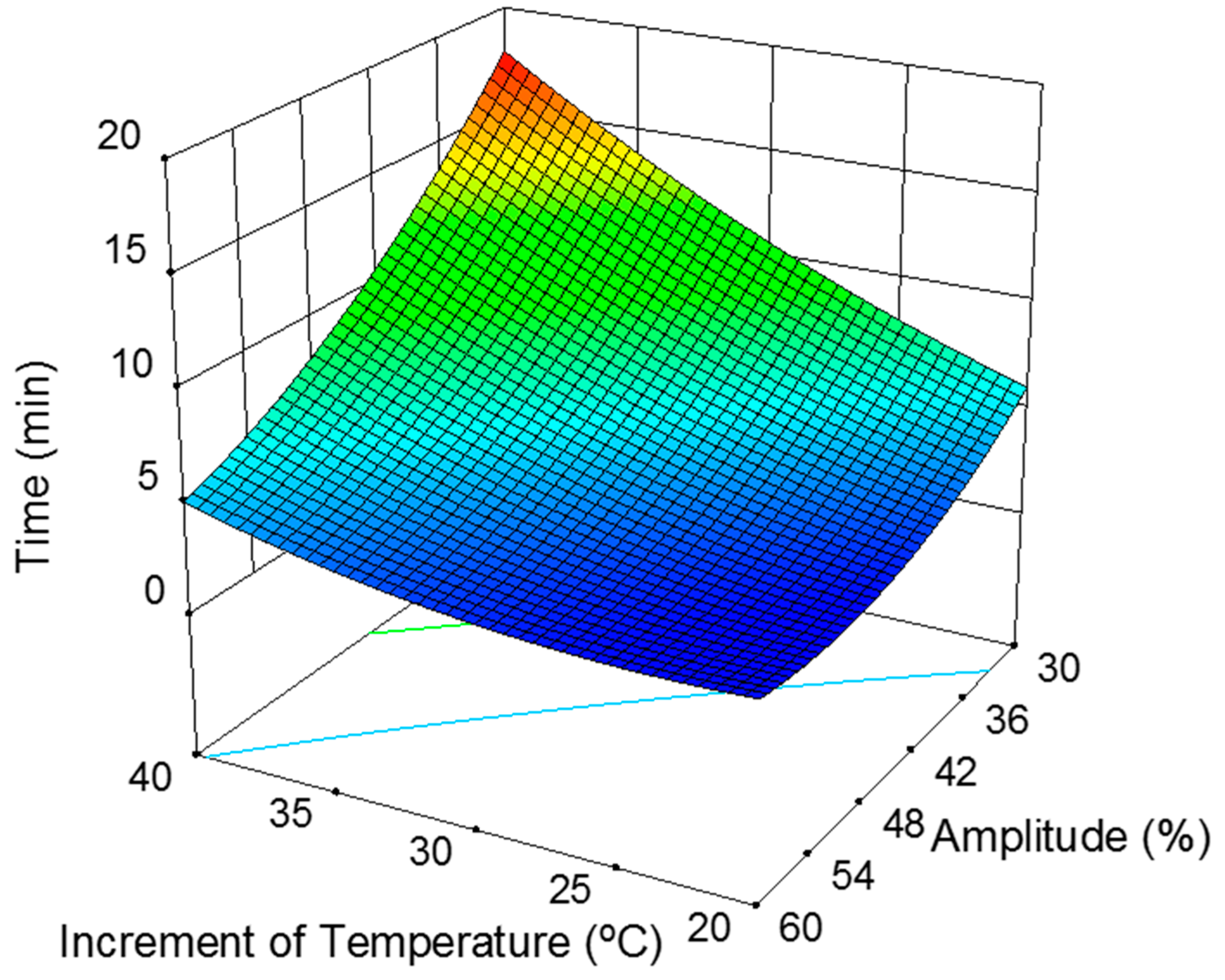
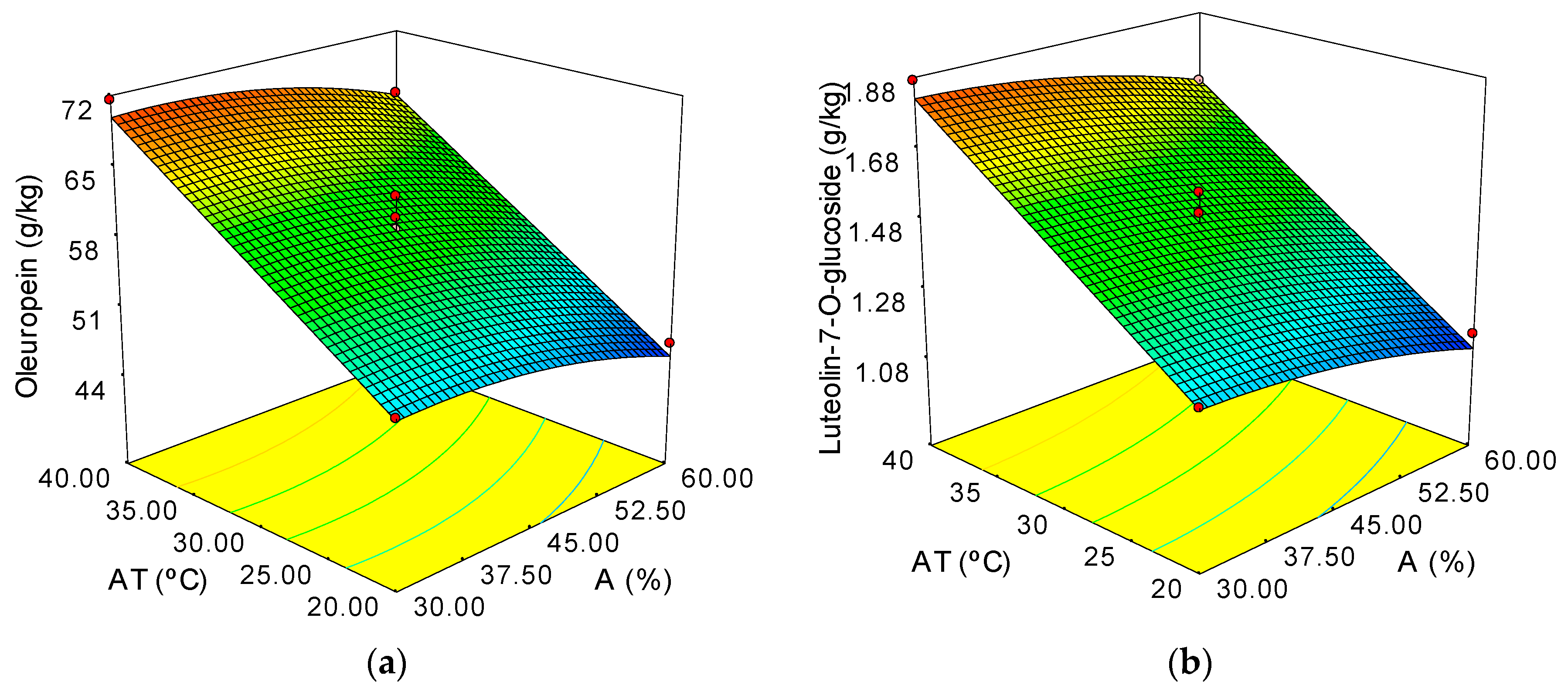
Increment of Temperature and Amplitude Influence
3.2. Optimization of the UAE Conditions
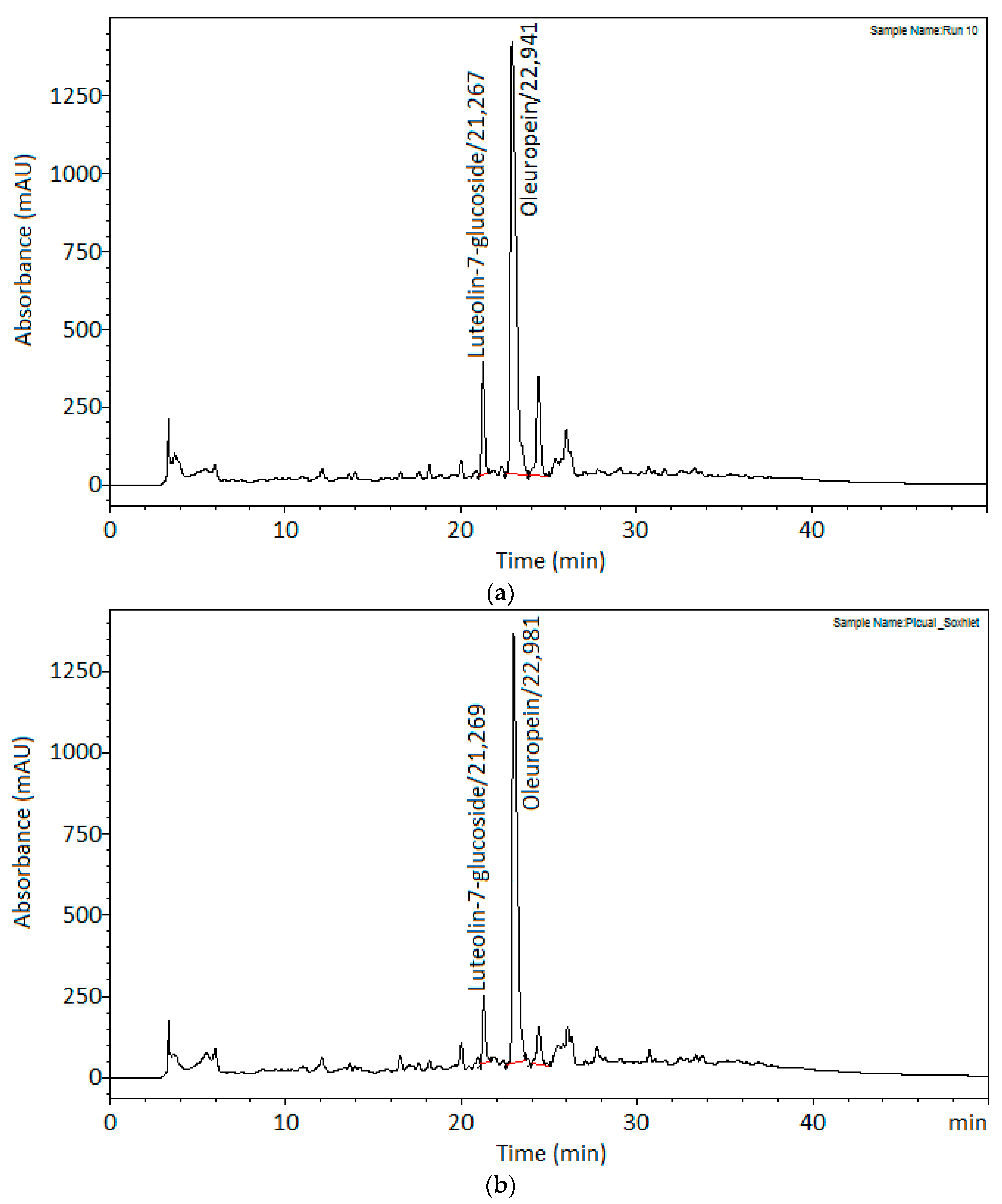
3.3. Comparison against Soxhlet Conventional Extraction Method
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahmanian, N.; Jafaric, S.M.; Wani, T.A. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends Food Sci. Technol. 2015, 42, 150–172. [Google Scholar] [CrossRef]
- Cara, C.; Romero, I.; Oliva, J.M.; Sáez, F.; Castro, E. Liquid hot water pretreatment of olive tree pruning residues. Appl. Biochem. Biotechnol. 2007, 136–140, 379–394. [Google Scholar]
- Xie, P.; Huang, L.; Zhang, C.; Zhang, Y. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure-activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego Capote, F. Extraction of oleuropein and related phenols from olive leaves and branches. In Olives and Olive Oil in Health and Disease Prevention, 1st ed.; Preedy, V., Watson, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1, pp. 259–273. [Google Scholar]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P.P. Olive by-products: Challenge application in cosmetic industry. Ind. Crop. Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Žugčić, T.; Abdelkebir, R.; Alcantara, C.; Collado, M.C.; García-Pérez, J.V.; Meléndez-Martínez, A.J.; Jambrak, A.R.; Lorenzo, J.M.; Barba, F.J. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Technol. 2019, 83, 63–77. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Vogel, P.; Machado, I.K.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Dal Bosco, S.M. Polyphenols benefits of olive leaf (Olea europaea L.) to human health. Nutr. Hosp. 2015, 31, 1427–1433. [Google Scholar] [CrossRef]
- Şahin, S.; Elhussein, E.; Bilgin, M.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S. Effect of drying method on oleuropein, total phenolic content, flavonoid content, and antioxidant activity of olive (Olea europaea) leaf. J. Food Process. Pres. 2018, 1–10. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 70, 92–108. [Google Scholar] [CrossRef]
- Salem, M.B.; Affes, H.; Ksouda, K.; Sahnoun, Z.; Zeghal, K.M.; Hammami, S. Pharmacological activities of Olea europaea leaves. J. Food Process. Pres. 2015, 39, 3128–3136. [Google Scholar] [CrossRef]
- Hassen, I.; Casabianca, H.; Hosni, K. Biological activities of the natural antioxidant oleuropein: Exceeding the expectation—A mini-review. J. Funct. Foods 2015, 18, 926–940. [Google Scholar] [CrossRef]
- Samet, I.; Villareal, M.O.; Motojima, H.; Han, J.; Sayadi, S.; Isoda, H. Olive leaf components apigenin 7-glucoside and luteolin 7-glucoside direct human hematopoietic stem cell differentiation towards erythroid lineage. Differentiation 2015, 89, 146–155. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Carreres, J.E.; Micol, V.; García-Pérez, J.V. Influence of olive leaf processing on the bioaccessibility of bioactive polyphenols. J. Agric. Food Chem. 2014, 62, 6190–6198. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Brito, R.; Smirniotis, P.; Nikolaidou, Z.; Vieira, M.C. Extraction of bioactive compounds from olive leaves using emerging technologies. In Ingredients Extraction by Physicochemical Methods in Food, 1st ed.; Grumezescu, A., Holban, A.-M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 4, pp. 441–461. [Google Scholar]
- Daso, A.P.; Okonkwo, O.J. Conventional extraction techniques: Soxhlet and liquid-liquid extractions and evaporation. Anal. Sep. Sci. 2015, 5, 1437–1468. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef]
- Šoštarič, M.; Klinar, D.; Bricelj, M.; Golob, J.; Berovič, M.; Likozar, B. Growth, lipid extraction and thermal degradation of the microalga Chlorella vulgaris. New Biotechnol. 2012, 29, 325–331. [Google Scholar] [CrossRef]
- Borić, M.; Puliyalil, H.; Novak, U.; Likozar, B. An intensified atmospheric plasma-based process for the isolation of the chitin biopolymer from waste crustacean biomass. Green Chem. 2018, 20, 1199–1204. [Google Scholar] [CrossRef]
- Bajić, M.; Jalšovec, H.; Travan, A.; Novak, U.; Likozar, B. Chitosan-based films with incorporated supercritical CO2 hop extract: Structural, physicochemical, and antibacterial properties. Carbohydr. Polym. 2019, 219, 261–268. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Cifá, D.; Skrt, M.; Pittia, P.; Di Mattia, C.; Ulrih, N.P. Enhanced yield of oleuropein from olive leaves using ultrasound-assisted extraction. Food Sci. Nutr. 2018, 6, 1128–1137. [Google Scholar] [CrossRef]
- Shirzad, H.; Niknam, V.; Taheri, M.; Ebrahimzadeh, H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: A nutraceutical study using RSM and LC–ESI–DAD–MS. J. Food Sci. Technol. 2017, 54, 2361–2371. [Google Scholar] [CrossRef]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crop. Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Žlabur, J.Š.; Castro, E. Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology. Ultrason. Sonochem. 2019, 51, 487–495. [Google Scholar] [CrossRef]
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT-Food Sci. Technol. 2018, 89, 284–290. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Di Khanh, N. Advances in the extraction of anthocyanin from vegetables. J. Food Nutr. Sci. 2015, 3, 126–134. [Google Scholar] [CrossRef]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Development of dummy molecularly imprinted based on functionalized silica nanoparticles for determination of acrylamide in processed food by matrix solid phase dispersion. Food Chem. 2016, 210, 78–84. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Şahin, S.; Şamlı, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction withresponse surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
- İlbay, Z.; Şahin, S.; Büyükkabasakal, K. A novel approach for olive leaf extraction through ultrasound technology: Response surface methodology versus artificial neural networks. Korean J. Chem. Eng. 2014, 31, 1661–1667. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of ultrasound-assisted extraction of flavonoids from olive (Olea europaea) leaves, and evaluation of their antioxidant and anticancer activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M.D. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1108, 76–82. [Google Scholar] [CrossRef]
- Vidal, A.M.; Alcalá, S.; Ocaña, M.T.; De Torres, A.; Espínola, F.; Moya, M. Modeling of volatile and phenolic compounds and optimization of the process conditions for obtaining balanced extra virgin olive oils. Grasas Aceites 2018, 69, e250. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Vázquez-Roncero, A.; Janer del Valle, C.; Janer del Valle, M.L. Determinación de los polifenoles totales del aceite de oliva. Grasas Aceites 1973, 24, 350–357. [Google Scholar]
- Giacometti, J.; Žauhar, G.; Žuvić, M. Optimization of ultrasonic-assisted extraction of major phenolic compounds from olive leaves (Olea europaea L.) using response surface methodology. Foods 2018, 7, 149. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef]
- Bilgin, M.; Şahin, S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. Chem. E. 2013, 44, 8–12. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Cánovas, J.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; García-Pérez, J.V. Kinetic and compositional study of phenolic extraction from olive leaves (var. Serrana) by using power ultrasound. Innov. Food Sci. Emerg. Technol. 2013, 17, 120–129. [Google Scholar] [CrossRef]
| Run | Space Type | Increment of Temperature (∆T) | Maximum Temperature (°C) | Amplitude (A) | ||
|---|---|---|---|---|---|---|
| Actual (°C) | Coded | Actual (%) 1 | Coded | |||
| 1 | Center | 30 | 0 | 50 | 45 | 0 |
| 2 | Axial | 30 | 0 | 50 | 23.8 | −1.413 |
| 3 | Factorial | 20 | −1 | 40 | 60 | 1 |
| 4 | Axial | 30 | 0 | 50 | 66.2 | 1.413 |
| 5 | Factorial | 40 | 1 | 60 | 30 | −1 |
| 6 | Axial | 15.9 | −1.410 | 35.9 | 45 | 0 |
| 7 | Center | 30 | 0 | 50 | 45 | 0 |
| 8 | Center | 30 | 0 | 50 | 45 | 0 |
| 9 | Factorial | 20 | −1 | 40 | 30 | −1 |
| 10 | Axial | 44.1 | 1.410 | 64.1 | 45 | 0 |
| 11 | Factorial | 40 | 1 | 60 | 60 | 1 |
| 12 | Center | 30 | 0 | 50 | 45 | 0 |
| 13 | Center | 30 | 0 | 50 | 45 | 0 |
| Run | Ultrasonic Time (min) | R (g/kg) | TPC (mmol GAE/kg) | TFC (mmol RE/kg) | DPPH (mmol TE/kg) | Oleuropein (g/kg) | Luteolin-7-Glucoside (g/kg) |
|---|---|---|---|---|---|---|---|
| 1 | 5.05 | 244.2 1 | 178.1 | 211.0 | 182.9 | 58.2 | 1.46 |
| 2 | 14.45 | 242.1 | 180.4 | 211.8 | 179.6 | 58.8 | 1.51 |
| 3 | 2.00 | 190.7 | 146.1 | 170.2 | 146.2 | 47.3 | 1.15 |
| 4 | 3.00 | 227.9 | 160.1 | 191.3 | 158.1 | 52.3 | 1.31 |
| 5 | 17.93 | 292.9 | 212.8 | 261.0 | 213.8 | 71.5 | 1.87 |
| 6 | 2.42 | 188.5 | 138.7 | 157.8 | 135.9 | 44.9 | 1.08 |
| 7 | 5.13 | 256.0 | 186.3 | 223.4 | 183.2 | 62.2 | 1.56 |
| 8 | 5.55 | 248.1 | 177.9 | 212.1 | 175.0 | 58.9 | 1.46 |
| 9 | 5.82 | 209.5 | 156.3 | 185.6 | 156.8 | 51.0 | 1.26 |
| 10 | 9.27 | 312.4 | 215.9 | 266.1 | 214.6 | 71.6 | 1.88 |
| 11 | 4.98 | 283.5 | 197.2 | 239.4 | 198.8 | 65.3 | 1.67 |
| 12 | 5.05 | 247.6 | 182.8 | 218.7 | 185.5 | 60.1 | 1.50 |
| 13 | 5.07 | 221.5 | 164.3 | 195.9 | 166.2 | 52.3 | 1.33 |
| Response | Models | R2 | CV (%) |
|---|---|---|---|
| Time (min) | 5.17 + 3.64·∆T − 4.12·A − 2.28·∆TA + 1.01·∆T2 + 1.69 A2 ± 0.33 | 0.997 1 | 5.13 2 |
| R (g/kg) | 249.86 + 44.00 ∆T − 6.05·A − 6.87·A2 ± 3.82 | 0.992 | 1.56 |
| TPC (mmol GAE/kg) | 180.53 + 27.13·∆T − 6.83·A − 4.24·A2 ± 3.55 | 0.984 | 2.00 |
| TFC (mmol RE/kg) | 216.01 + 37.27·∆T − 8.25·A − 5.47·A2 ± 5.43 | 0.980 | 2.56 |
| DPPH (mmol TE/kg) | 180.48 + 27.66·∆T − 7.00·A − 4.41·A2 ± 4.75 | 0.973 | 2.67 |
| Oleuropein (g/kg) | 59.60 + 9.55·∆T − 2.39·A − 1.63·A2 ± 1.53 | 0.977 | 2.61 |
| Luteolin-7-glucoside (g/kg) | 1.50 + 0.28·∆T − 0.074·A − 0.033·A2 ± 0.040 | 0.982 | 2.68 |
| Response | Soxhlet Extraction | Ultrasound-Assisted Extraction 1 |
|---|---|---|
| Time (min) | 240 | 17.91 ± 0.33 |
| R (g/kg) | 368.77 ± 0.14 2 | 293.06 ± 3.82 |
| TPC (mmol GAE/kg) | 251.91 ± 2.13 | 210.25 ± 3.55 |
| TFC (mmol RE/kg) | 274.97 ± 4.39 | 256.07 ± 5.43 |
| DPPH (mmol TE/kg) | 291.42 ± 18.80 | 210.73 ± 4.75 |
| Oleuropein (g/kg) | 65.57 ± 0.70 | 69.91 ± 1.53 |
| Luteolin-7-glucoside (g/kg) | 1.32 ± 0.03 | 1.82 ± 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lama-Muñoz, A.; Contreras, M.d.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Optimization of Oleuropein and Luteolin-7-O-Glucoside Extraction from Olive Leaves by Ultrasound-Assisted Technology. Energies 2019, 12, 2486. https://doi.org/10.3390/en12132486
Lama-Muñoz A, Contreras MdM, Espínola F, Moya M, Romero I, Castro E. Optimization of Oleuropein and Luteolin-7-O-Glucoside Extraction from Olive Leaves by Ultrasound-Assisted Technology. Energies. 2019; 12(13):2486. https://doi.org/10.3390/en12132486
Chicago/Turabian StyleLama-Muñoz, Antonio, María del Mar Contreras, Francisco Espínola, Manuel Moya, Inmaculada Romero, and Eulogio Castro. 2019. "Optimization of Oleuropein and Luteolin-7-O-Glucoside Extraction from Olive Leaves by Ultrasound-Assisted Technology" Energies 12, no. 13: 2486. https://doi.org/10.3390/en12132486
APA StyleLama-Muñoz, A., Contreras, M. d. M., Espínola, F., Moya, M., Romero, I., & Castro, E. (2019). Optimization of Oleuropein and Luteolin-7-O-Glucoside Extraction from Olive Leaves by Ultrasound-Assisted Technology. Energies, 12(13), 2486. https://doi.org/10.3390/en12132486







