Alkaline Mineral Soil Amendment: A Climate Change ‘Stabilization Wedge’?
Abstract
:1. Introduction
2. Carbon in Agricultural Soil
2.1. Soil Carbon Sequestration as SOC
2.2. Soil Carbon Sequestration as SIC via Enhanced Weathering
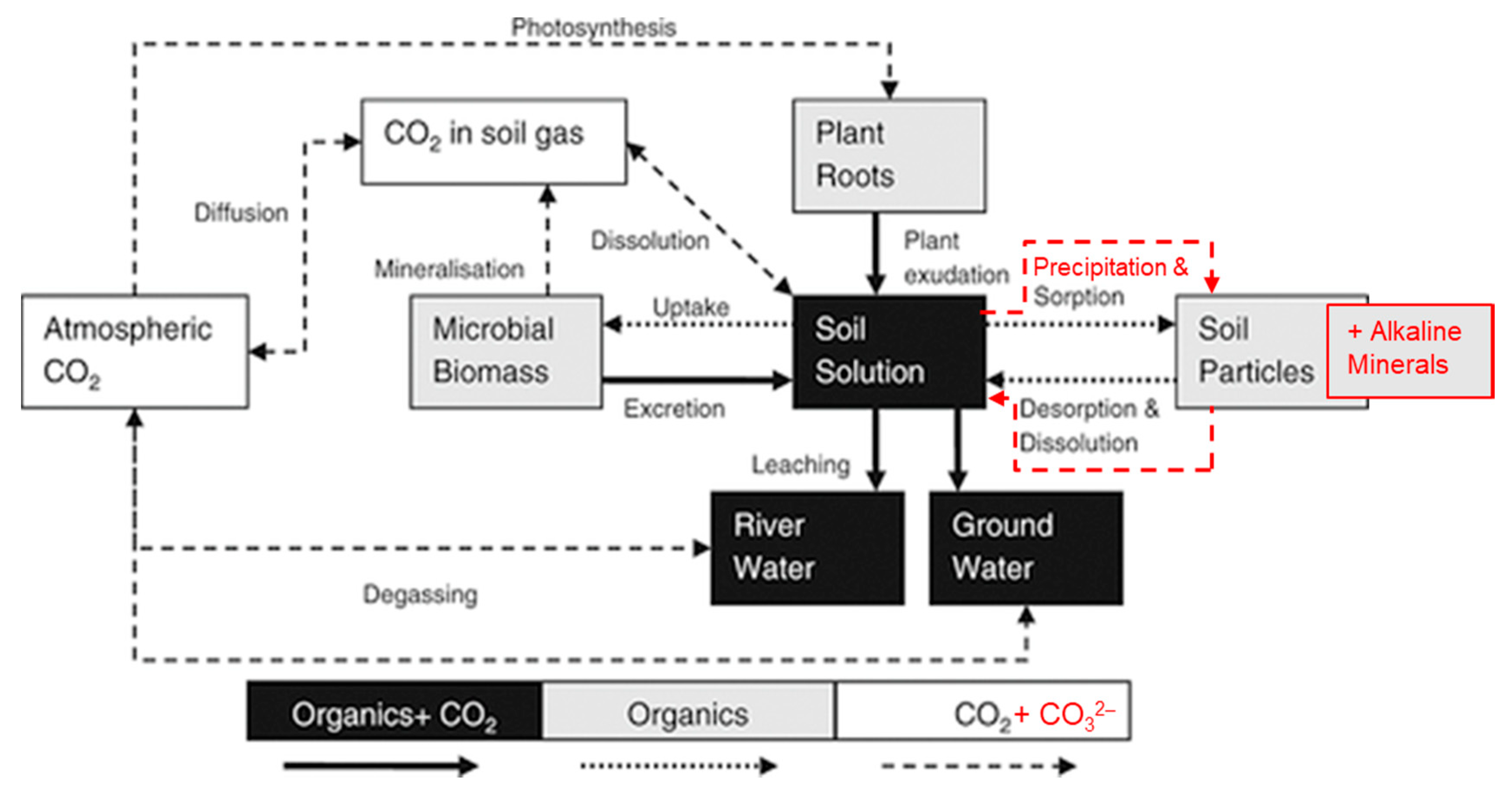
3. Alkaline Minerals as Soil Amendment
3.1. Soil Inorganic Carbon Accumulation
3.2. Other Effects of Alkaline Mineral Soil Amendment
4. Factors Controlling Mineral Weathering
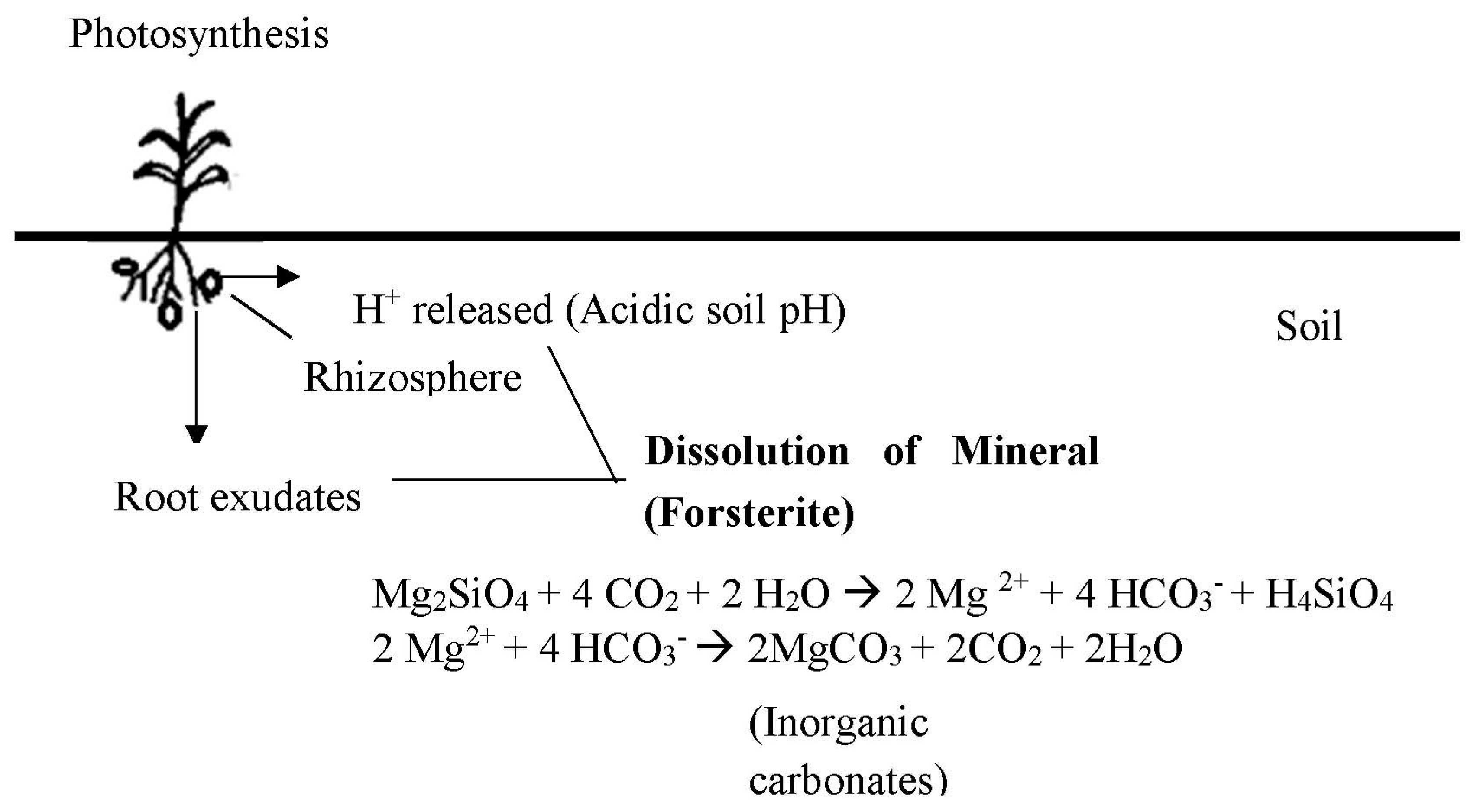
4.1. Role of Plants and Microbes
4.2. Dissolution Rate
5. Modeling Enhanced Weathering
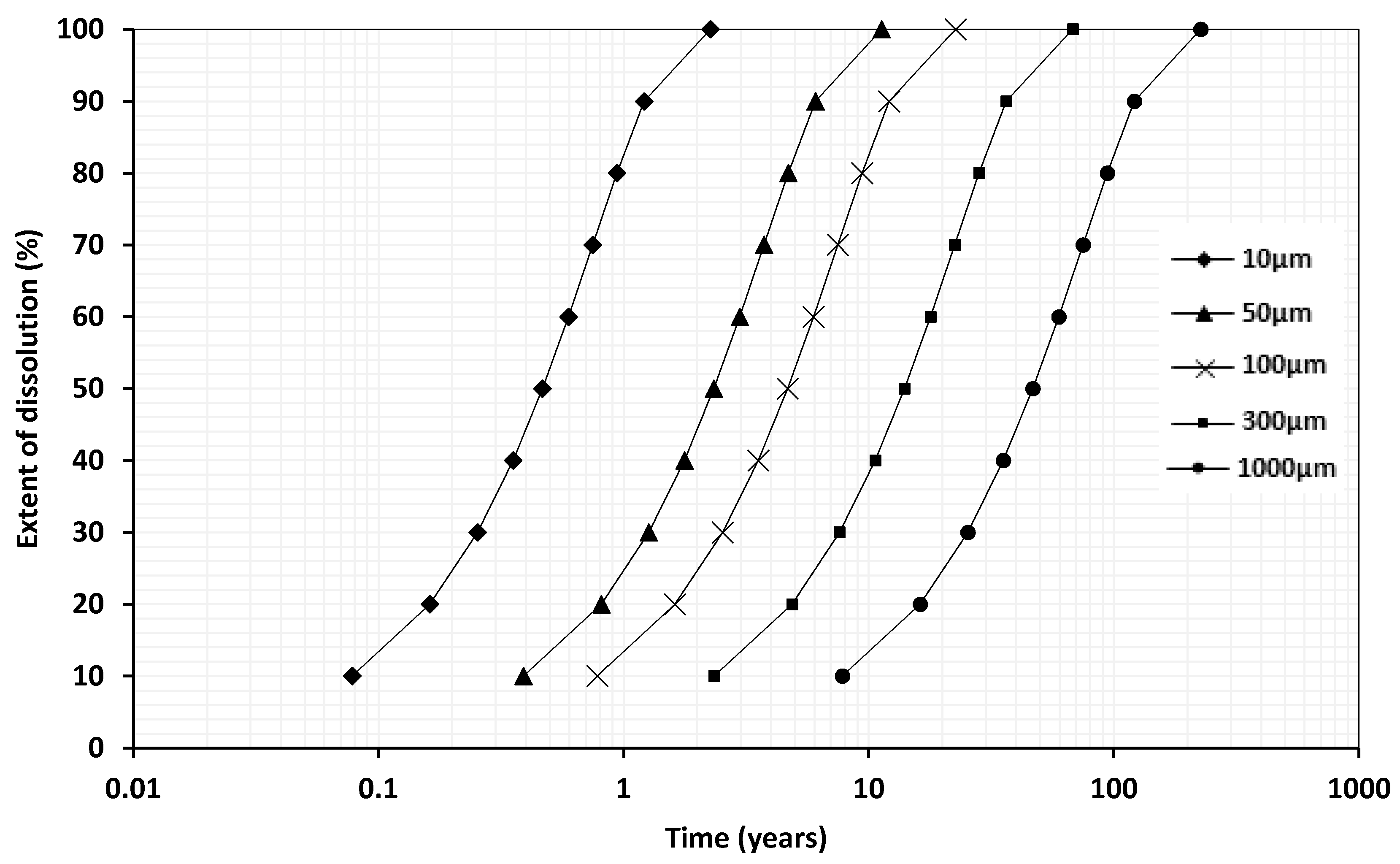
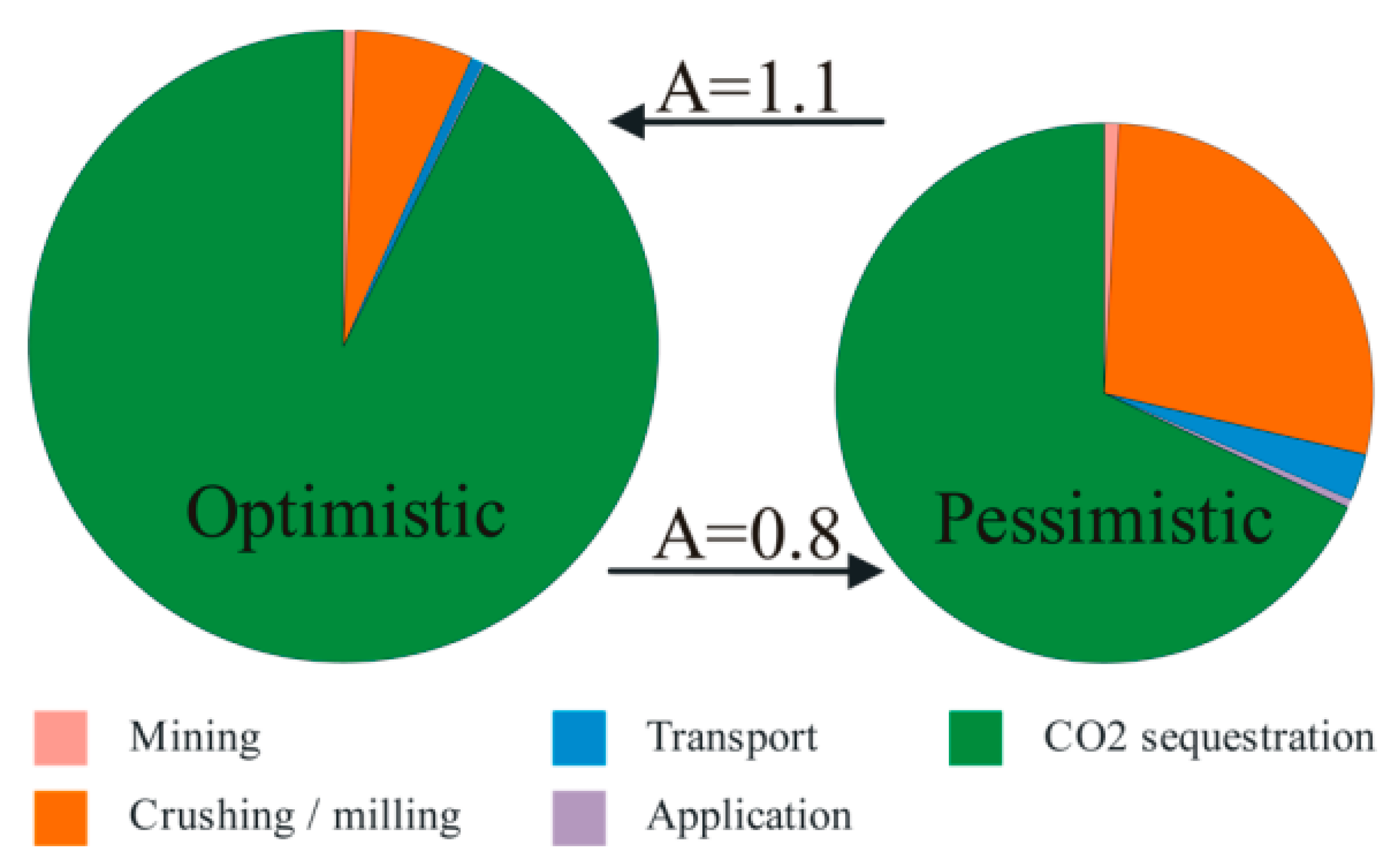
A Shrinking Core Model for Enhanced Weathering Estimation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Callendar, G.S. The artificial production of carbon dioxide and its influence on temperature. Q. J. R. Meteorol. Soc. 1938, 64, 223–240. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Singh, S.; Mankin, J.S.; Horton, D.E.; Swain, D.L.; Touma, D.; Charland, A.; Liu, Y.; Haugen, M.; Tsiang, M.; et al. Quantifying the influence of global warming on unprecedented extreme climate events. Proc. Natl. Acad. Sci. USA 2017, 114, 4881–4886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, W.; Haigh, J.D. The greenhouse effect and carbon dioxide. Weather 2013, 68, 100–105. [Google Scholar] [CrossRef]
- Mission Innovation. Joint Launch Statement. 2015. Available online: http://www.mission-innovation.net/wp-content/uploads/2015/11/Mission-Innovation-Joint-Launch-Statement.pdf (accessed on 2 January 2019).
- Pacala, S.; Socolow, R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science 2004, 305, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Denman, K.L.; Brasseur, A.G.; Chidthaisong, P.; Ciais, P.M.; Cox, R.E.; Dickinson, D.; Hauglustaine, C.; Heinze, E.; Holland, D.; Jacob, U.; et al. Couplings between changes in the climate system and biogeochemistry. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S.D., Qin, M., Manning, Z., Chen, M., Marquis, K.B., Averyt, M., Tignor, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Zomer, R.J.; Bossio, D.A.; Sommer, R.; Verchot, L.V. Global sequestration potential of increased organic carbon in cropland soils. Sci. Rep. 2017, 7, 15554. [Google Scholar] [CrossRef]
- Machmuller, M.B.; Kramer, M.G.; Cyle, T.K.; Hill, N.; Hancock, D.; Thompson, A. Emerging land use practices rapidly increase soil organic matter. Nat. Commun. 2015, 6, 6995. [Google Scholar] [CrossRef]
- Lackner, K.S. A guide to CO2 sequestration. Science 2003, 300, 1677–1678. [Google Scholar] [CrossRef]
- Kwon, S.; Fan, M.; DaCosta, H.F.M.; Russell, A.G. Factors affecting the direct mineralization of CO2 with olivine. J. Environ. Sci. 2011, 23, 1233–1239. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- British Petroleum, B.P. Statistical Review of World Energy June 2017. Available online: https://web.archive.org/web/20180430082944/https://www.bp.com/content/dam/bp/en/corporate/pdf/energy-economics/statistical-review-2017/bp-statistical-review-of-world-energy-2017-full-report.pdf (accessed on 2 January 2019).
- Hartmann, J.; West, A.D.; Renforth, P.; Köhler, P.; De La Rocha, C.L.; Wolf-Gladrow, D.A.; Dürr, H.H.; Scheffran, J. Enhanced chemical weathering as a Geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 2013, 51, 113–149. [Google Scholar] [CrossRef]
- Moosdorf, N.; Renforth, P.; Hartmann, J. Carbon dioxide efficiency of terrestrial enhanced weathering. Environ. Sci. Technol. 2014, 48, 4809–4816. [Google Scholar] [CrossRef] [PubMed]
- Renforth, P.; Manning, D.A.C.; Lopez-Capel, E. Carbonate precipitation in artificial soils as a sink for atmospheric carbon dioxide. Appl. Geochem. 2009, 24, 1757–1764. [Google Scholar] [CrossRef]
- Tan, W.-F.; Zhang, R.; Cao, H.; Huang, C.-Q.; Yang, Q.-K.; Wang, M.-K.; Koopal, L.K. Soil inorganic carbon stock under different soil types and land uses on the loess plateau region of China. Catena 2014, 121, 22–30. [Google Scholar] [CrossRef]
- Monger, H.C.; Kraimer, R.A.; Khresat, S.; Cole, D.R.; Wang, X.; Wang, J. Sequestration of inorganic carbon in soil and groundwater. Geology 2015, 43, 375–378. [Google Scholar] [CrossRef]
- Dong, X.; Singh, B.P.; Li, G.; Lin, Q.; Zhao, X. Biochar increased field soil inorganic carbon content five years after application. Soil Tillage Res. 2019, 186, 36–41. [Google Scholar] [CrossRef]
- Hutchinson, J.J.; Campbell, C.A.; Desjardins, R.L. Some perspectives on carbon sequestration in agriculture. Agric. For. Meteorol. 2007, 142, 288–302. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Greenhouse gas mitigation in agriculture. Phil. Trans. R. Soc. B 2008, 363, 789–813. [Google Scholar] [CrossRef] [PubMed]
- Powlson, D.S.; Stirling, C.M.; Jat, M.L.; Gerard, B.G.; Palm, C.A.; Sanchez, P.A.; Cassman, K.G. Limited potential of no-till agriculture for climate change mitigation. Nat. Clim. Chang. 2014, 4, 678–683. [Google Scholar] [CrossRef]
- Bruce, J.P.; Frome, M.; Haites, E.; Janzen, H.; Lal, R.; Paustian, K. Carbon sequestration in soils. J. Soil Water Conserv. 1999, 54, 382–389. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Schuman, G.E.; Janzen, H.H.; Herrick, J.E. Soil carbon dynamics and potential carbon sequestration by rangelands. Environ. Pollut. 2002, 116, 391–396. [Google Scholar] [CrossRef]
- Schuman, G.E.; Reeder, J.D.; Manley, J.T.; Hart, R.H.; Manley, W.A. Impact of grazing management on the carbon and nitrogen balance of a mixed-grass rangeland. Ecol. Appl. 1999, 9, 65–71. [Google Scholar] [CrossRef]
- Janzen, H.H.; Angers, D.A.; Boehm, M.; Bolinder, M.; Desjardins, R.L.; Dyer, J.; Ellert, B.H.; Gibb, D.J.; Gregorich, E.G.; Helgason, B.L.; et al. A proposed approach to estimate and reduce net greenhouse gas emissions from whole farms. Can. J. Soil Sci. 2006, 86, 401–418. [Google Scholar] [CrossRef] [Green Version]
- Janzen, H.H.; Desjardins, R.L.; Asselin, J.M.; Grace, B. The Health of Our Air: Toward Sustainable Agriculture in Canada. Research Branch, Agriculture and Agri-Food Canada; No. A53-1981; Exhibition Catalogue: New York, NY, USA, 1999. [Google Scholar]
- Campbell, C.A.; Janzen, H.H.; Paustian, K.; Gregorich, E.G.; Sherrod, L.; Liang, B.C.; Zentner, R.P. Carbon storage in soils of the North American great plains. Agron. J. 2005, 97, 349–363. [Google Scholar] [CrossRef]
- Schuman, G.E.; Herrick, J.E.; Janzen, H.H. The dynamics of soil carbon in rangelands. In The Potential of US Grazing Lands to Sequester Carbon and Mitigate the Greenhouse Effect; Follett, R.F., Kimble, J.M., Lal, R., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001; Chapter 11; pp. 267–290. [Google Scholar]
- Joseph, S.; Lehmann, J. Biochar for environmental management: An introduction. In Biochar for Environmental Management; Routledge: London, UK, 2015; pp. 33–46. [Google Scholar]
- Liu, L.; Shen, G.; Sun, M.; Cao, X.; Shang, G.; Chen, P. Effect of biochar on nitrous oxide emission and its potential mechanisms. J. Air Waste Manag. Assoc. 2014, 64, 894–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, M.; M’hamdi, N.; Dkhili, Z.; Brar, S.K.; Misra, K. Thermochemical transformation of agro-biomass into biochar: simultaneous carbon sequestration and soil amendment. In Biotransformation of Waste Biomass into High Value Biochemicals; Springer: New York, NY, USA, 2014; pp. 51–70. [Google Scholar]
- Haefele, S.M.; Konboon, Y.; Wongboon, W.; Amarante, S.; Maarifat, A.A.; Pfeiffer, E.M.; Knoblauch, C. Effects and fate of biochar from rice residues in rice-based systems. Field Crop. Res. 2011, 121, 430–440. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar stability in soil: Decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Balaria, A.; Johnson, C.E.; Groffman, P.M.; Fisk, M.C. Effects of calcium silicate treatment on the composition of forest floor organic matter in a northern hardwood forest stand. Biogeochemistry. 2015, 122, 313–326. [Google Scholar] [CrossRef]
- Balaria, A.; Johnson, C.E.; Groffman, P.M. Effects of calcium treatment on forest floor organic matter composition along an elevation gradient. Can. J. For. Res. 2014, 44, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Driscoll, C.T.; Johnson, C.E.; Siccama, T.G. Chemical changes in soil and soil solution after calcium silicate addition to a northern hardwood forest. Biogeochemistry 2010, 100, 3–20. [Google Scholar] [CrossRef]
- Hartmann, J.; Jansen, N.; Dürr, H.H.; Kempe, S.; Köhler, P. Global CO2-consumption by chemical weathering: What is the contribution of highly active weathering regions? Glob. Planet Chang. 2009, 69, 185–194. [Google Scholar] [CrossRef]
- Schlesinger, W.H. The formation of caliche in soils of the Mojave Desert, California. Geochim. Cosmochim. Acta 1985, 49, 57–66. [Google Scholar] [CrossRef]
- Washbourne, C.-L.; Lopez-Capel, E.; Renforth, P.; Ascough, P.L.; Manning, D.A.C. Rapid removal of atmospheric CO2 by urban soils. Environ. Sci. Technol. 2015, 49, 5434–5440. [Google Scholar] [CrossRef]
- Hangx, S.J.T.; Spiers, C.J. Coastal spreading of olivine to control atmospheric CO2 concentrations: A critical analysis of viability. Int. J. Greenh. Gas Control 2009, 3, 757–767. [Google Scholar] [CrossRef]
- Ten Berge, H.F.M.; van der Meer, H.G.; Steenhuizen, J.W.; Goedhart, P.W.; Knops, P.; Verhagen, J. Olivine weathering in soil, and its effects on growth and nutrient uptake in ryegrass (Lolium perenne L.): A pot experiment. PLoS ONE 2012, 7, e42098. [Google Scholar] [CrossRef]
- Köhler, P.; Hartmann, J.; Wolf-Gladrow, D.A. Geoengineering potential of artificially enhanced silicate weathering of olivine. Proc. Natl. Acad. Sci. USA 2010, 107, 20228–20233. [Google Scholar] [CrossRef] [Green Version]
- Renforth, P.; Manning, D.A.C. Laboratory carbonation of artificial silicate gels enhanced by citrate: Implications for engineered pedogenic carbonate formation. Int. J. Greenh. Gas Control 2011, 5, 1578–1586. [Google Scholar] [CrossRef] [Green Version]
- Manning, D.A.C.; Renforth, P.; Lopez-Capel, E.; Robertson, S.; Ghazireh, N. Carbonate precipitation in artificial soils produced from basaltic quarry fines and composts: An opportunity for passive carbon sequestration. Int. J. Greenh. Gas Control 2013, 17, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Haque, F.; Santos, R.M.; Dutta, A.; Thimmanagari, M.; Chiang, Y.W. Co-Benefits of wollastonite weathering in agriculture: CO2 sequestration and promoted plant growth. ACS Omega 2019, 4, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Amann, T.; Hartmann, J.; Struyf, E.; de Oliveira Garcia, W.; Fischer, E.K.; Janssens, I.; Meire, P.; Schoelynck, J. Constraints on enhanced weathering and related carbon sequestration—A cropland mesocosm approach. Biogeosci. Discuss. 2018. [Google Scholar] [CrossRef]
- Van Straaten, P. Farming with rocks and minerals: Challenges and opportunities. Anais da Academia Brasileira de Ciências 2006, 78, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Gillman, G.P.; Burkett, D.C.; Coventry, R.J. Amending highly weathered soils with finely ground basalt rock. Appl. Geochem. 2002, 17, 987–1001. [Google Scholar] [CrossRef]
- Barral Silva, M.T.; Silva, B.M.; García-Rodeja, E.; Vázquez Freire, N. Reutilization of granite powder as an amendment and fertilizer for acid soils. Chemosphere 2005, 61, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.M.; Van Audenaerde, A.; Chiang, Y.; Iacobescu, R.; Knops, P.; Van Gerven, T. Nickel extraction from olivine: Effect of carbonation pre-treatment. Metals 2015, 5, 1620–1644. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC PRESS: Boca Raton, FL, USA, 2011; pp. 1–534. [Google Scholar]
- Néel, C.; Soubrand-Colin, M.; Piquet-Pissaloux, A.; Bril, H. Mobility and bioavailability of Cr, Cu, Ni, Pb and Zn in a basaltic grassland: Comparison of selective extractions with quantitative approaches at different scales. Appl. Geochem. 2007, 22, 724–735. [Google Scholar] [CrossRef]
- Miranda, M.; Benedito, J.L.; Blanco-Penedo, I.; López-Lamas, C.; Merino, A.; López-Alonso, M. Metal accumulation in cattle raised in a serpentine-soil area: Relationship between metal concentrations in soil, forage and animal tissues. J. Trace Elem. Med. Biol. 2009, 23, 231–238. [Google Scholar] [CrossRef]
- Fernández, S.; Seoane, S.; Merino, A. Plant heavy metal concentrations and soil biological properties in agricultural serpentine soils. Commun. Soil Sci. Plant Anal. 1999, 30, 1867–1884. [Google Scholar] [CrossRef]
- Müller, G. Index of geoaccumulation in sediments of the rhine river. Geojournal 1969, 2, 108–118. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; Jan, T.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef] [PubMed]
- CCME. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health. 2007. Available online: http://ceqg-rcqe.ccme.ca/download/en/342/ (accessed on 2 January 2019).
- Hinsinger, P.; Fernandes Barros, O.N.; Benedetti, M.F.; Noack, Y.; Callot, G. Plant-induced weathering of a basaltic rock: Experimental evidence. Geochim. Cosmochim. Acta 2001, 65, 137–152. [Google Scholar] [CrossRef]
- Manning, D.A.C.; Renforth, P. Passive sequestration of atmospheric CO2 through coupled plant—Mineral reactions in urban soils. Environ. Sci. Technol. 2013, 47, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, O.S.; Schott, J. Forsterite surface composition in aqueous solutions: A combined potentiometric, electrokinetic, and spectroscopic approach. Geochim. Cosmochim. Acta 2000, 64, 3299–3312. [Google Scholar] [CrossRef]
- Epihov, D.Z.; Batterman, S.A.; Hedin, L.O.; Leake, J.R.; Smith, L.M.; Beerling, D.J. N2-fixing tropical legume evolution: a contributor to enhanced weathering through the Cenozoic? Proc. R. Soc. B Biol. Sci. 2017, 284, 20170370. [Google Scholar] [CrossRef]
- Nezat, C.A.; Blum, J.D.; Klaue, A.; Johnson, C.E.; Siccama, T.G. Influence of landscape position and vegetation on long-term weathering rates at the hubbard brook experimental forest, New Hampshire, USA. Geochim. Cosmochim. Acta 2004, 68, 3065–3078. [Google Scholar] [CrossRef]
- Jongmans, A.G.; Lundström, U.S.; van Breemen, N.; van Hees, P.A.W.; Finlay, R.D.; Srinivasan, M. Rock-eating fungi. Nature 1997, 389, 682–683. [Google Scholar] [CrossRef]
- Schuiling, R.D.; Krijgsman, P. Enhanced weathering: an effective and cheap tool to sequester CO2. Clim. Chang. 2006, 74, 349–354. [Google Scholar] [CrossRef]
- Ober, J.A. Mineral Commodity Summaries 2018; US Geological Survey: Reston, VA, USA, 2018.
- Palandri, J.L.; Kharaka, Y.K. A Compilation of Rate Parameters of Water-Mineral Interaction Kinetics for Application to Geochemical Modeling. US Geological Survey Open File Report 2004-1068. Available online: http://www.dtic.mil/cgi-bin/GetTRDoc?Location=U2&doc=GetTRDoc.pdf&AD=ADA440035 (accessed on 2 January 2019).
- Renforth, P.; Pogge von Strandmann, P.A.E.; Henderson, G.M. The dissolution of olivine added to soil: Implications for enhanced weathering. Appl. Geochem. 2015, 61, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Béarat, H.; Mckelvy, M.J.; Chizmeshya, A.V.G.; Gormley, D.; Nunez, R.; Carpenter, R.W. Carbon sequestration via aqueous olivine mineral carbonation: Role of passivating layer formation. Environ. Sci. Technol. 2006, 40, 4802–4808. [Google Scholar] [CrossRef]
- Rimstidt, J.D.; Brantley, S.L.; Olsen, A.A. Systematic review of forsterite dissolution rate data. Geochim. Cosmochim. Acta 2012, 99, 159–178. [Google Scholar] [CrossRef]
- Peters, S.C.; Blum, J.D.; Driscoll, C.T.; Likens, G.E. Dissolution of wollastonite during the experimental manipulation of hubbard brook watershed. Biogeochemistry 2004, 67, 309–329. [Google Scholar] [CrossRef]
- Chen, Y.; Brantley, S.L. Dissolution of forsteritic olivine at 65 °C and 2 < pH <5. Chem. Geol. 2000, 165, 267–281. [Google Scholar] [CrossRef]
- Giammar, D.E.; Bruant, R.G.; Peters, C.A. Forsterite dissolution and magnesite precipitation at conditions relevant for deep saline aquifer storage and sequestration of carbon dioxide. Chem. Geol. 2005, 217, 257–276. [Google Scholar] [CrossRef]
- Golubev, S.V.; Pokrovsky, O.S.; Schott, J. Experimental determination of the effect of dissolved CO2 on the dissolution kinetics of Mg and Ca silicates at 25 °C. Chem. Geol. 2005, 217, 227–238. [Google Scholar] [CrossRef]
- Luce, R.W.; Bartlett, R.W.; Parks, G.A. Dissolution kinetics of magnesium silicates. Geochim. Cosmochim. Acta 1972, 36, 35–50. [Google Scholar] [CrossRef]
- Olsen, A.A.; Donald Rimstidt, J. Oxalate-promoted forsterite dissolution at low pH. Geochim. Cosmochim. Acta 2008, 72, 1758–1766. [Google Scholar] [CrossRef]
- Rosso, J.; Rimstidt, J. A high resolution study of forsterite dissolution rates. Geochim. Cosmochim. Acta 2000, 64, 797–811. [Google Scholar] [CrossRef]
- Wogelius, R.A.; Walther, J.V. Olivine dissolution at 25 °C: Effects of pH, CO2, and organic acids. Geochim. Cosmochim. Acta 1991, 55, 943–954. [Google Scholar] [CrossRef]
- Gbor, P.K.; Jia, C.Q. Critical evaluation of coupling particle size distribution with the shrinking core model. Chem. Eng. Sci. 2004, 59, 1979–1987. [Google Scholar] [CrossRef]
- Nori, L.L.C. A Blockchain-Based Marketplace for Removing Carbon Dioxide from the Atmosphere. Version 3.0.1. 2019. Available online: https://nori.com/white-paper (accessed on 9 May 2019).
- Power, I.; McCutcheon, J.; Harrison, A.; Wilson, S.; Dipple, G.; Kelly, S.; Southam, C.; Southam, G. Strategizing carbon-neutral mines: A case for pilot projects. Minerals 2014, 4, 399–436. [Google Scholar] [CrossRef]

| Practice | Example | CCS (t CO2 ha−1 year−1) | Acceptance a | Agreement b | Net GHG Emission (t CO2 ha−1 year−1) | Effect |
|---|---|---|---|---|---|---|
| Cropland Management | Reduced tillage | 0.41 | H | M | 0.44 | Reduced decomposition and weed control. |
| Crop rotation | 0.59 | H | H | 0.69 | Reduced reliance on N inputs. | |
| Eliminate summer fallow | 0.17 | H | - | - | Reduces SOM decay. | |
| Nutrient management | 0.27 | M | H | 0.48 | Control on N2O release. | |
| Water management | 1.14 | L | L | 1.14 | Improves aeration. | |
| Increased productivity (e.g., fertilization, irrigation) | 0.30 | M | M | - | Stimulate N2O emission. | |
| Grassland management | Grazing intensity improvement | 0.45 | L | L | 0.46 | Influence crop growth. |
| Land restoration | Restore permanent grass or woodland | 2.57 | L | H | 3.72 | Improves soil fertility |
| Organic soil management | Use organic residues (manure, biosolids, crop residues) | 1.83 | M | H | 2.17 | High density C source |
| Organic soil restoration | 55.0 | M | H | 51.8 | ||
| Bioenergy | Energy crop plantation | 0.42 | M | H | 0.44 | CO2 neutral sources |
| Treatment | pH | Increase in Mg2+ (%) | Increase in K+ (%) | Reference |
|---|---|---|---|---|
| KIES 1 | 4.9 | 15.7 | 27.6 | [44] |
| KIES 2 | 5.04 | 30.6 | 55.2 | |
| OLIV 1 | 4.99 | 68.9 | 20.9 | |
| OLIV 2 | 5.1 | 132.8 | 16.0 | |
| OLIV 3 | 5.34 | 257.6 | 54.0 | |
| OLIV 4 | 5.96 | 691.2 | 130.7 | |
| Soil Treated with Basalt (25 t/ha) | Increase in Ca2+ (%) | Increase in Mg2+ (%) | Increase in K+ (%) | Reference |
| Haplorthox on basalt | 6.8 | 32.4 | 109.1 | [51] |
| Haplorthox on basalt fan | 44.2 | 128.0 | 140.0 | |
| Tropudult | 39.0 | 215.4 | 266.7 | |
| Haplorthox on metamorphic rock | 88.5 | 200.0 | 63.6 | |
| Dystropept on basalt alluvium | 11.2 | 53.5 | 90.9 | |
| Dystropept on granite alluvium | 16.6 | 133.3 | 50.0 | |
| Haplorthod | 110.8 | 335.7 | 171.4 |
| Treatment | Ni (mg/kg) | Igeo Ni |
|---|---|---|
| OLIV 1 | 0.00013 | −18.99 |
| OLIV 2 | 0.00026 | −17.99 |
| OLIV 3 | 0.00056 | −16.78 |
| OLIV 4 | 0.00138 | −15.57 |
| Class | Mineral | log Wr (moles/m2s) pH 5.1–7.7 | log Wr (moles/m2s) pH 2.0–4.0 |
|---|---|---|---|
| Feldspars | Albite | −12.56 | −10.16 |
| Oligoclase | −11.84 | −6.97 | |
| Andesine | −11.47 | −8.88 | |
| Labradorite | −10.91 | −7.87 | |
| K-feldspar | −12.41 | −10.06 | |
| Feldspathoids | Nepheline | −8.56 | −2.73 |
| Leucite | −9.2 | −6 | |
| Orthosilicates | Forsterite | −10.64 | −6.85 |
| Fayalite | −12.8 | −4.8 | |
| Almandine | −10.7 | −5.2 | |
| Grossular | −10.7 | −5.1 | |
| Andradite | −10.7 | −5.2 | |
| Staurolite | −12.22 | −6.9 | |
| Zoisite | −11.2 | −7.5 | |
| Kyanite | −17.44 | −10.17 | |
| Epidote | −11.99 | −10.6 | |
| Cyclosilicate | Cordierite | −11.2 | −3.8 |
| Tourmaline | −11.2 | −6.5 | |
| Inosilicates | Augite | −11.97 | −6.82 |
| Bronzite | −11.7 | −8.3 | |
| Diopside | −11.11 | −6.36 | |
| Enstatite | −12.72 | −9.02 | |
| Wollastonite | −8.88 | −5.37 | |
| Amphibole | Anthophyllite | −14.24 | −11.94 |
| Glaucophane | −10.1 | −5.6 | |
| Tremolite | −10.6 | −8.4 | |
| Phyllosilicates | Biotite | −12.55 | −9.84 |
| Glauconite | −9.1 | −4.8 |
| X (vol %) Mineral Dissolution | ||||||
|---|---|---|---|---|---|---|
| 25% | 50% | 100% | ||||
| CO2 Sequestered (tonne CO2/tonne mineral) | 0.313 | 0.625 | 1.25 | |||
| Grain Size (µm) | Time (years) at pH 4.5 a | Time (years) at pH 8.2 b | Time (years) at pH 4.5 a | Time (years) at pH 8.2 b | Time (years) at pH 4.5 a | Time (years) at pH 8.2 b |
| 10 | 0.21 | 2 | 0.47 | 5 | 2.3 | 23 |
| 50 | 1.0 | - | 2.3 | - | 11 | - |
| 100 | 2.1 | 21 | 4.7 | 48 | 23 | 233 |
| 300 | 6.2 | 64 | 14 | 144 | 68 | 700 |
| 1000 | 21 | 213 | 47 | 481 | 226 | 2333 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, F.; Chiang, Y.W.; Santos, R.M. Alkaline Mineral Soil Amendment: A Climate Change ‘Stabilization Wedge’? Energies 2019, 12, 2299. https://doi.org/10.3390/en12122299
Haque F, Chiang YW, Santos RM. Alkaline Mineral Soil Amendment: A Climate Change ‘Stabilization Wedge’? Energies. 2019; 12(12):2299. https://doi.org/10.3390/en12122299
Chicago/Turabian StyleHaque, Fatima, Yi Wai Chiang, and Rafael M. Santos. 2019. "Alkaline Mineral Soil Amendment: A Climate Change ‘Stabilization Wedge’?" Energies 12, no. 12: 2299. https://doi.org/10.3390/en12122299
APA StyleHaque, F., Chiang, Y. W., & Santos, R. M. (2019). Alkaline Mineral Soil Amendment: A Climate Change ‘Stabilization Wedge’? Energies, 12(12), 2299. https://doi.org/10.3390/en12122299







