Numerical Simulation of Bubble-Liquid Two-Phase Turbulent Flows in Shallow Bioreactor
Abstract
:1. Introduction
2. Governing Equations of the Bubble-Liquid Flows
3. Numerical Simulation and Experiments
3.1. Simulation Algorithm
3.2. Experiments
4. Results and Discussion
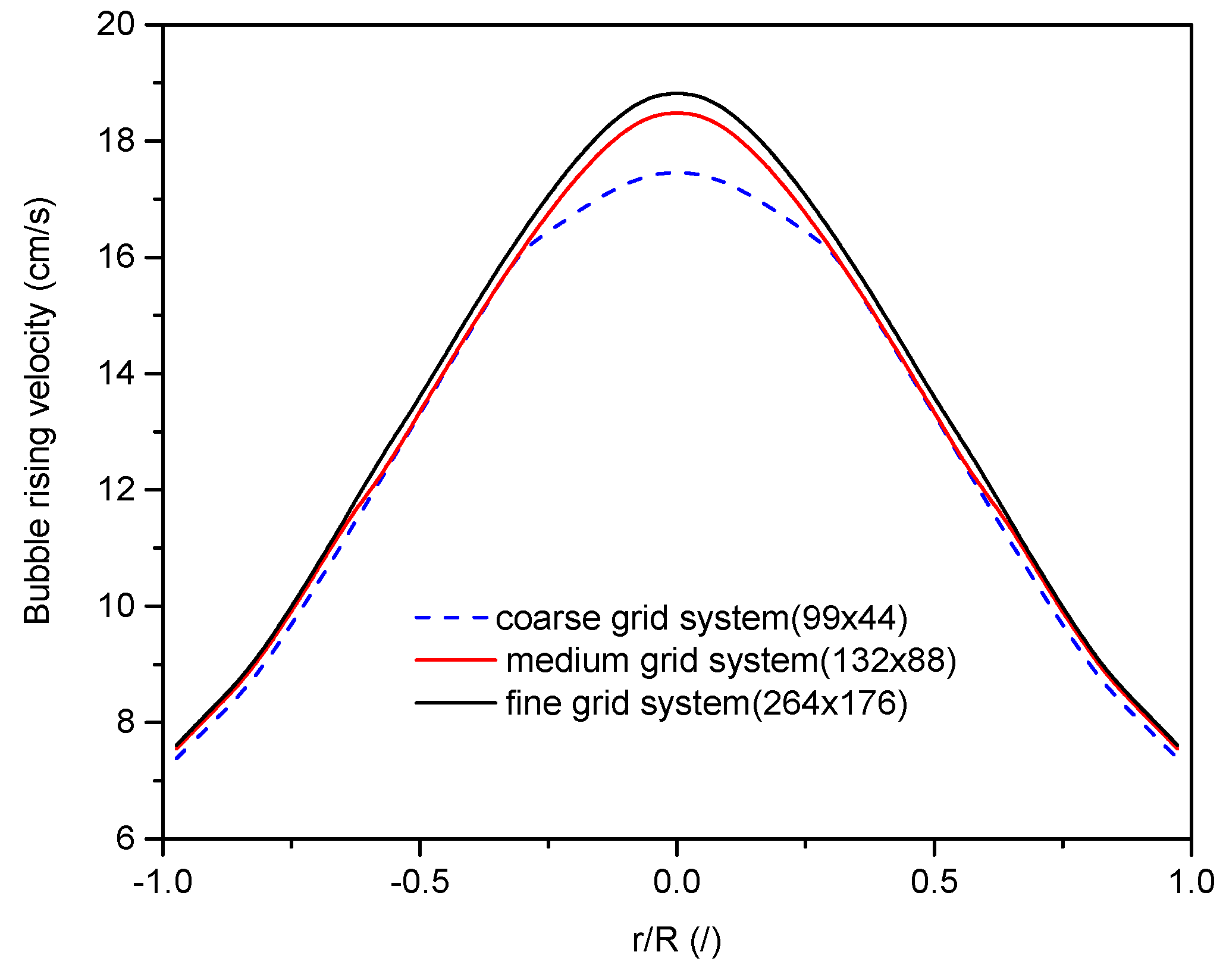
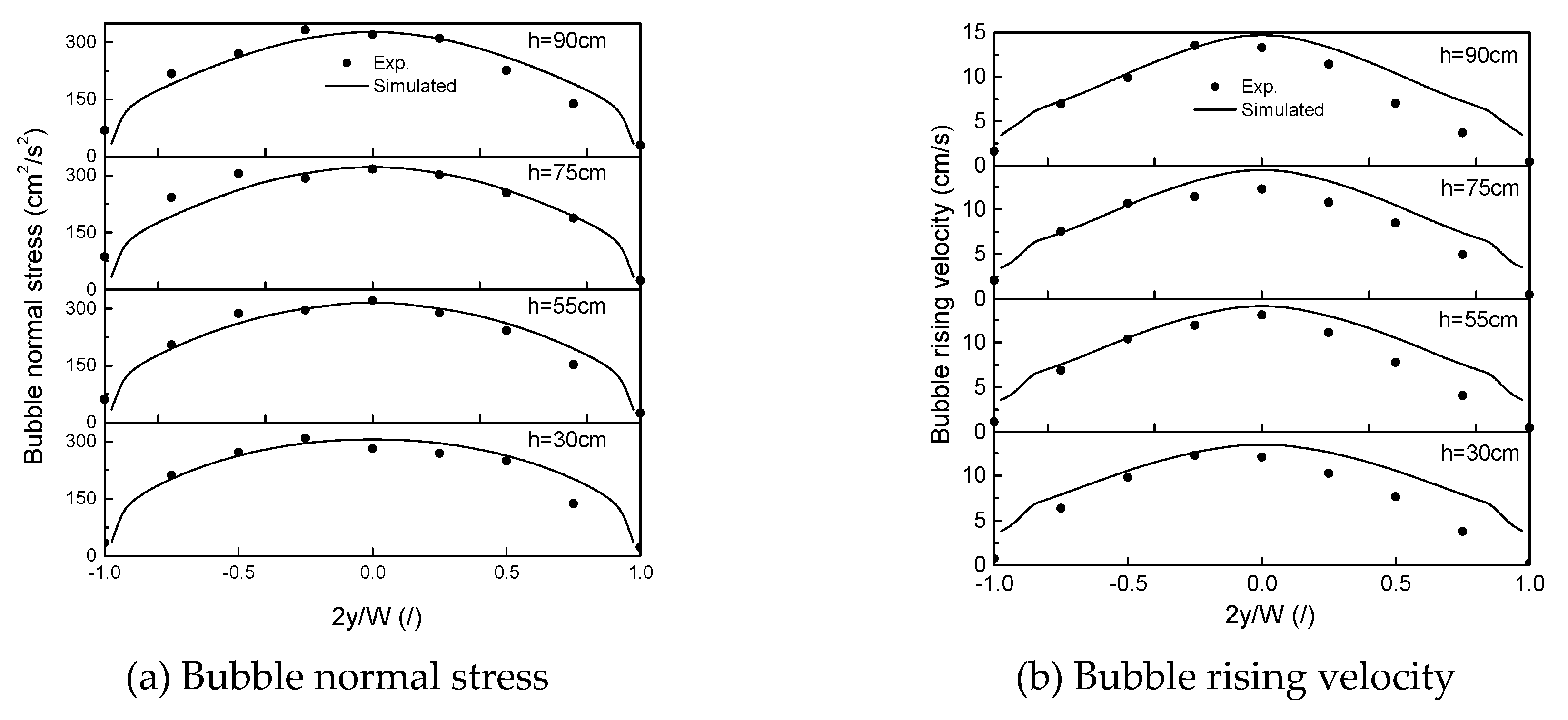
4.1. Grid Independence and Validation of Proposed Model
4.2. Key Bubble-Liquid Turbulent Hydrodynamics
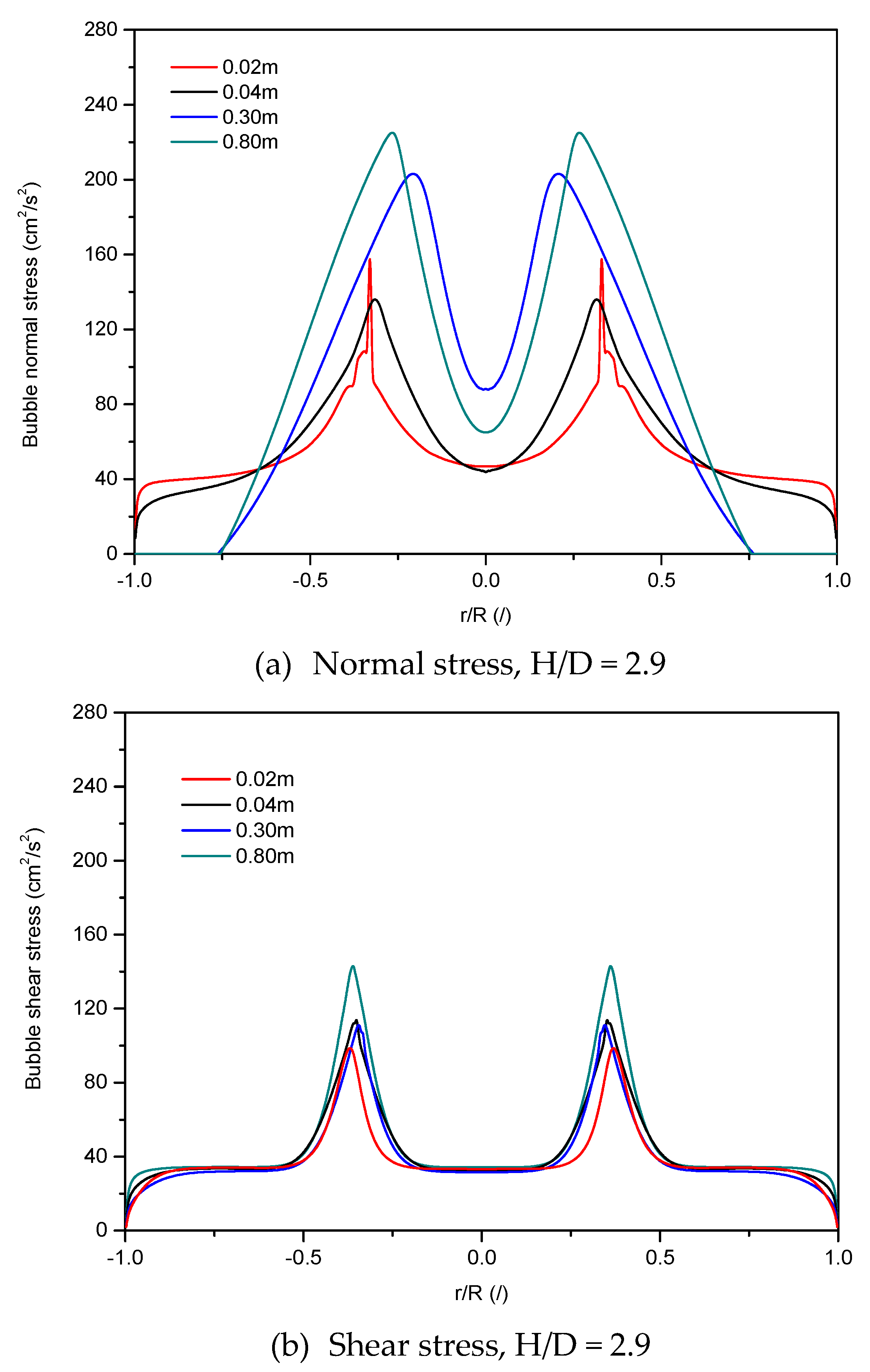
4.2.1. Normal and Shear Stresses of Bubble Phase
4.2.2. Turbulent Kinetic Energy of Bubble Phase
4.2.3. Turbulent Energy Dissipation Rate of Bubble Phase
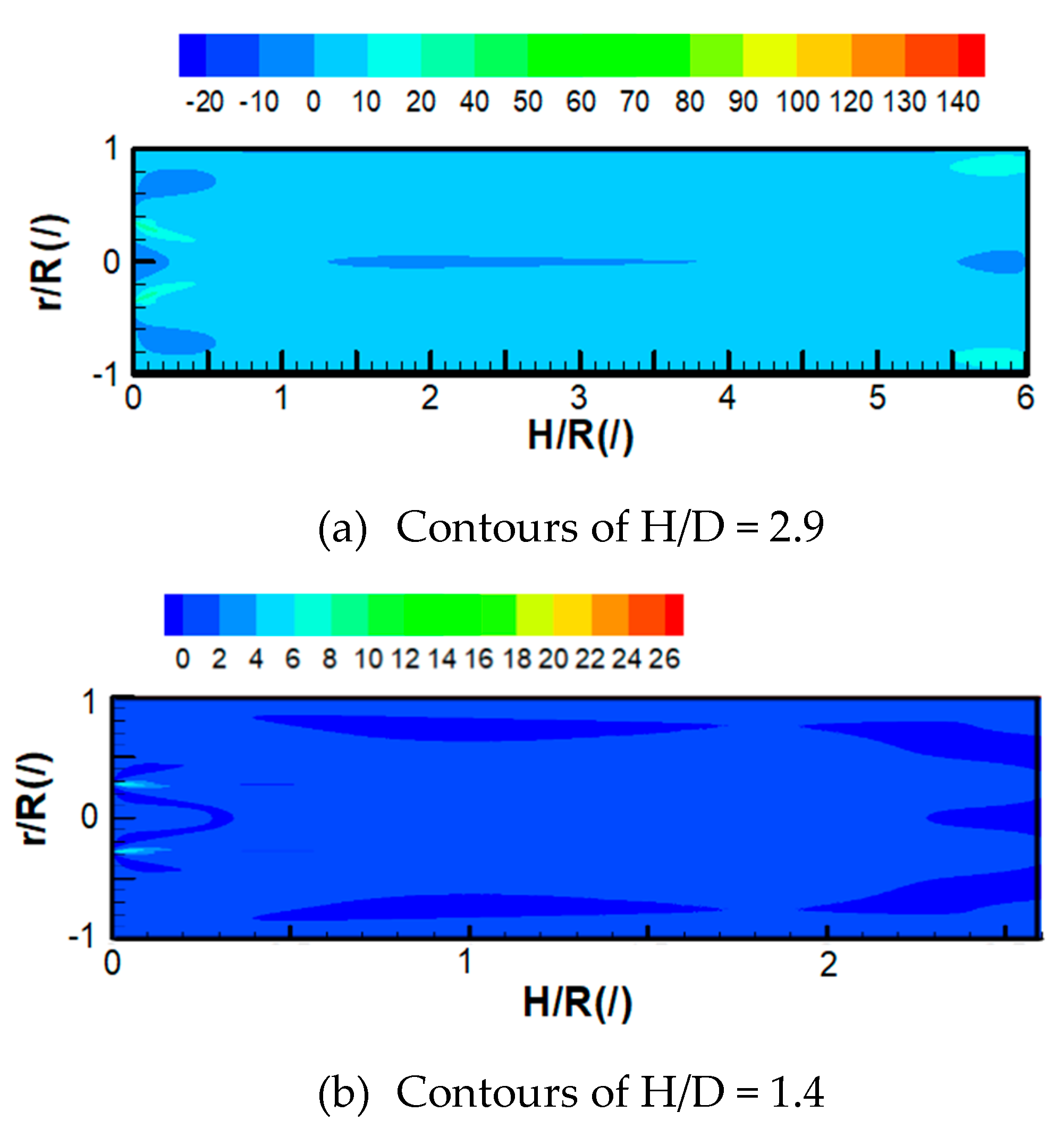
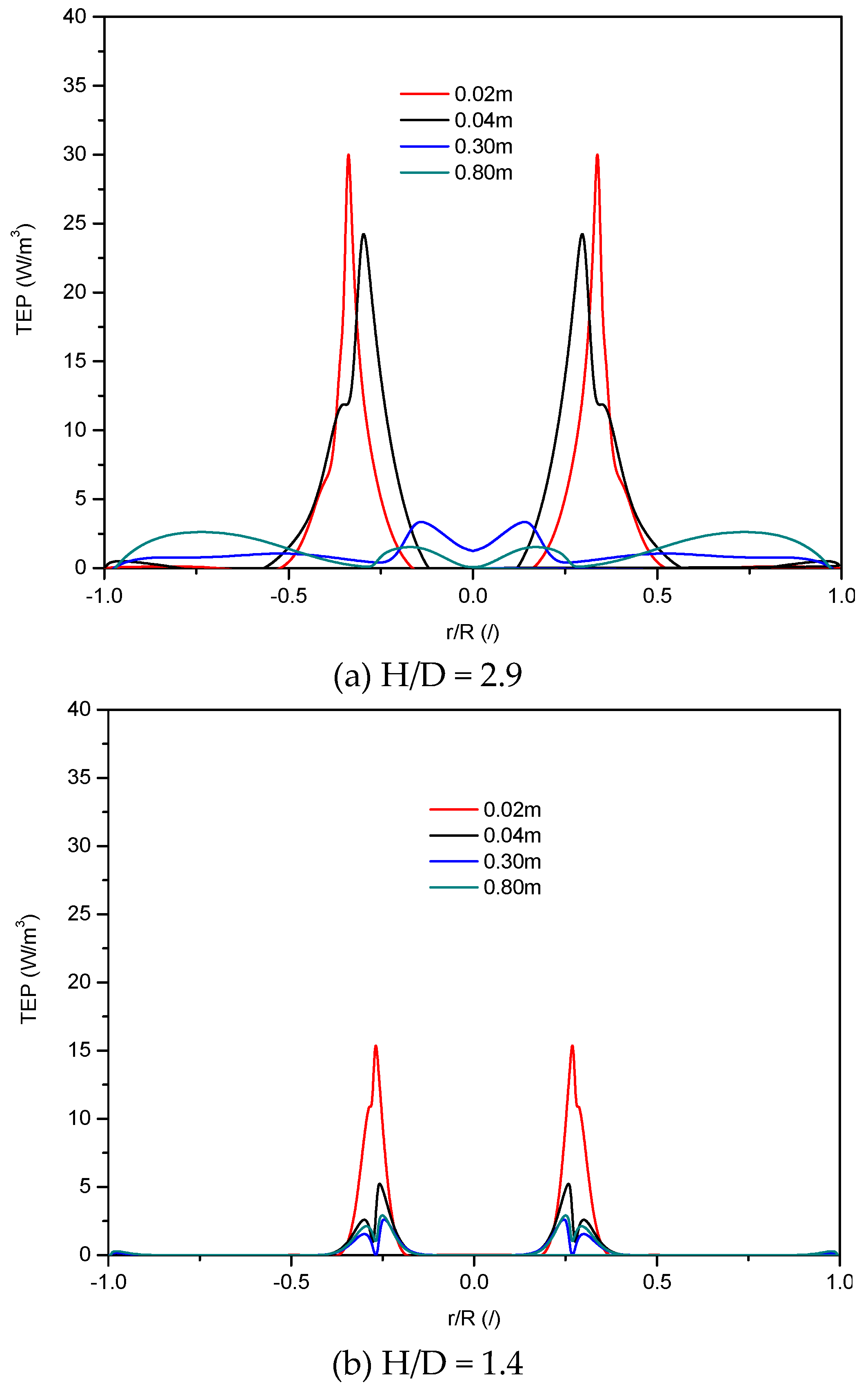
4.2.4. Turbulent Energy Production of Bubble-Liquid Two-Phase Flow
5. Conclusions
- The proposed second-order moment bubble-liquid two-phase turbulence model can well simulate the hydrodynamic parameters with good accuracy.
- The velocities and their fluctuations, and the normal and shear Reynolds stresses of bubble and liquid phases are distinctively anisotropic.
- The established turbulent energy production successfully verifies the higher cell damage at the gas jetting inlet region.
- The shallow shape bioreactor is more favorable for cell living than the long and narrow-shaped reactor.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| c | Empirical constants |
| cd | Drag coefficient |
| D | Diffusion term |
| d | Bubble size |
| Eo | Eotvos number |
| g | Gravitational acceleration |
| k | Turbulent kinetic energy |
| m | Bubble mass |
| P | Production term |
| p | Pressure |
| Re | Reynolds number |
| S | Phase interaction term |
| u,v | Velocity components |
| Velocity vector coordination | |
| Greek Alphabets | |
| α | Volume fraction |
| β | Coefficients defined in the text |
| δ | Unit tensor |
| ε | Dissipation rate of turbulent kinetic energy |
| λ | Empirical constant |
| μ | Dynamic viscosity |
| ν | Kinematic viscosity |
| Π | Pressure strain term |
| ρ | Density |
| σ | Prandtl number; surface tension force |
| τrb | Bubble relaxation time |
| τij | Molecular viscous stress |
| Subscripts | |
| b | Bubble phase |
| e | Effective |
| g | Gas |
| i, j, k | Coordinate directions |
| l | Liquid phase |
| m | Bubble or liquid phase |
| t | Turbulent |
| Superscripts | |
| = | Mass weighted value |
| ″ | Fluctuating component of mass weighting |
| - | Time-averaged value |
| ′ | Fluctuation component of in time averaging |
References
- Harrison, D.; Grace, J.R. Fluidized Bed with Internal Baffles; Academic Press: New York, NY, USA, 1971; pp. 599–626. [Google Scholar]
- Virr, M.J.; Williams, H.W. Heat recovery by shallow fluidized beds. Chem. Eng. Prog. 1985, 81, 50–59. [Google Scholar]
- Yang, J.Y.; Liu, A.Y.; Squires, A.M. Pressure drop across shallow fluidized beds: Theory and experiment. Powder Technol. 1987, 53, 79–89. [Google Scholar] [CrossRef]
- Chisti, Y. Animal-cell damage in sparged bioreactors. Trends Biotechnol. 2000, 18, 420–432. [Google Scholar] [CrossRef]
- Bahadori, F.; Rahimi, R. Simulations of gas distributors in the design of shallow bubble column reactors. Chem. Eng. Technol. 2007, 30, 443–447. [Google Scholar] [CrossRef]
- Lau, R.; Sim, W.S.B. Effect of gas distributor on hydrodynamics in shallow bubble column reactors. Can. J. Chem. Eng. 2009, 87, 847–854. [Google Scholar] [CrossRef]
- Fife, J.P.; Derksen, R.C.; Ozkan, H.E. Evaluation of a contraction flow field on hydrodynamic damage to entomopathogenic nematodes-A biological pest control agent. Biotechnol. Bioeng. 2004, 5, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.J. Current opinion in chemical engineering, mixing, aeration and cell damage, 30+ years later: What we learned, how it affected the cell culture industry and what we would like to know more about. Curr. Opin. Chem. Eng. 2015, 10, 94–102. [Google Scholar] [CrossRef]
- Throat, B.N.; Joshi, J.B. Effect of sparger design and height to diameter ratio on fractional gas holdup in bubble column. Trans. Inst. Chem. Eng. 1998, 76, 823–834. [Google Scholar] [CrossRef]
- Veera, P.; Joshi, J.B. Measurement of gas hold up profiles by gamma ray tomography: Effect of sparger design and height of dispersion in bubble columns. Trans. Inst. Chem. Eng. 1999, 77, 303–317. [Google Scholar] [CrossRef]
- Dhotre, M.T.; Ekambara, K.; Joshi, J.B. CFD simulation of sparger design and height to diameter ratio on gas hold-up profiles in bubble column reactors. Exp. Therm. Fluid Sci. 2004, 28, 407–421. [Google Scholar] [CrossRef]
- Kawasaki, H.; Tanaka, H. Correlation of volumetric mass transfer coefficient with gas hold-up in the calming section in a bubble column with a single hole orifice. J. Chem. Eng. Jpn. 1996, 29, 365–367. [Google Scholar] [CrossRef]
- Griffiths, J.B. Animal cell products, overview. In Encyclopedia of Cell Technology; Spier, R.E., Ed.; Wiley: New York, NY, USA, 2000; Volume 1, pp. 71–76. [Google Scholar]
- Catapano, G.; Czermak, P.; Eibl, R.; Eibl, D.; Portner, R. Bioreactor design and scale-up. In Cell and Tissue Reaction Engineering; Springer: Berlin, Germany, 2009. [Google Scholar]
- Camarasa, E.; Vial, C.; Poncin, S.; Wild, G.; Midoux, N.; Bouillard, J. Influence of coalescence behaviors of the liquid and of gas sparging on hydrodynamics and bubble characteristics in bubble column. Chem. Eng. Process. 1999, 38, 329–344. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.L.; Liu, W.T.; Zhao, Y.H. Model and simulation of gas liquid flow with wide size distributions in circulating fluidized bed. J. Chem. Indust. Eng. 2003, 54, 1065–1071. [Google Scholar]
- Pfleger, C.P.; Becker, S. Modeling and simulation of the dynamic flow behavior in bubble column. Chem. Eng. Sci. 2001, 56, 1737–1747. [Google Scholar] [CrossRef]
- Gregoriades, N.; Clay, J.; Ma, N.; Koelling, K.; Chalmers, J.J. Cell damage of microcarrier culture as a function of local energy dissipation created by a rapid extensional flow. Biotechnol. Bioeng. 2000, 69, 171–182. [Google Scholar] [CrossRef]
- Mollet, M.; Ma, N.N.; Zhao, Y.; Brodkey, B.; Taticek, R.; Chalmers, J.J. Bioprocess equipment: Characterization of energy dissipation rate and its potential to damage cells. Biotechnol. Prog. 2004, 20, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Williams-Dalson, W.; Keshavarz-Moore, E.; Shamlou, P.A. Computational-fluid-dynamic (CFD) analysis of mixing and gas–liquid mass transfer in shake flasks. Biotechnol. Appl. Biochem. 2005, 41, 1–8. [Google Scholar]
- Rajaram, S.G.; Ashwin, W.P.; Jyeshtharaj, B.J. Combined effect of hydrodynamic and interfacial flow parameters on Lysozyme deactivation in a stirred tank bioreactor. Biotechnol. Prog. 2006, 22, 660–672. [Google Scholar]
- Kelly, W.J. Using computational fluid dynamics to characterize and improve bioreactor performance. Biotechnol. Appl. Biochem. 2008, 49, 225–238. [Google Scholar] [CrossRef]
- Mollet, M.; Godoy, R.; Berdugo, C.; Chalmers, J.J. Computer simulations of the energy dissipation rate in a fluorescence-activated cell sorter: Implications to cells. Biotechnol. Bioeng. 2008, 100, 260–272. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, K.; Fan, S. CFD simulation coupled with population balance equations for aerate stirred bioreactors. Eng. Life Sci. 2009, 9, 421–430. [Google Scholar] [CrossRef]
- Oncul, A.A.; Kalmbach, A.; Genzel, Y.; Reichl, U.; Thevenin, D. Characterization of the flow conditions in 2 L and 20 L wave bioreactors using computational fluid dynamics. Biotechnol. Prog. 2010, 26, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Chris, J.; Venkatesh, N.; Chris, A. Verification of energy dissipation rate scalability in pilot and production scale bioreactors using computational fluid dynamics. Biotechnol. Prog. 2014, 30, 760–764. [Google Scholar]
- Liu, Y.; Hu, W.W.; Wiltberger, K. Effects of bubble–liquid two-phase turbulent hydrodynamics on cell damage in sparged bioreactor. In Proceedings of the 245th ACS National Meeting and Exposition, New Orleans, LA, USA, 7–11 April 2014. [Google Scholar]
- Babosa, M.J.; Albrecht, M.; Wijffels, R.H. Hydrodynamic stress and lethal events in sparged microalgae cultures. Biotechnol. Bioeng. 2003, 83, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Koelling, K.; Chalmers, J.J. The fabrication and use of a transient contraction flow device to quantify the sensitivity of mammalian and insect cells to hydrodynamic forces. Biotechnol. Bioeng. 2002, 80, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Venkat, R.V.; Stock, L.R.; Chalmers, J.J. Study of hydrodynamics in microcarrier culture spinner vessels: Particle tracking velocimetry approach. Biotechnol. Bioeng. 1996, 49, 456–466. [Google Scholar] [CrossRef]
- Sucosky, P.; Osorio, D.F.; Brown, J.B.; Neitzel, G.P. Fluid mechanics of a spinner-flask bioreactor. Biotechnol. Bioeng. 2003, 85, 34–42. [Google Scholar] [CrossRef]
- Xia, J.Y.; Wang, S.J.; Zhang, S.L.; Zhong, J.J. Computational investigation of fluid dynamics in a recently developed centrifugal impeller bioreactor. Biochem. Eng. J. 2008, 38, 406–413. [Google Scholar] [CrossRef]
- Ma, N.; Xie, L.; Chalmers, J.J.; Aunins, J.G. Quantitative studies of cell damage in sparged bioreactors using a bubble collector to segregate bubble rupture events. Biotechnol. Prog. 2004, 20, 1183–1191. [Google Scholar] [CrossRef]
- Meier, S.J.; Hatton, T.A.; Wang, D.I. Cell death from bursting bubbles: Role of cell attachment to rising bubbles in sparged reactors. Biotechnol. Bioeng. 1999, 62, 468–478. [Google Scholar] [CrossRef]
- Zhu, Y.; Cuenca, V.Y.; Zhou, W.C.; Varma, A. NS0 cell damage by high gas velocity sparging in protein-free and cholesterol-free cultures. Biotechnol. Bioeng. 2008, 101, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Jiang, L.X. Numerical prediction effects of particle-particle collisions on gas-particle flows in swirl chamber. Energy Convers. Manag. 2011, 3, 1748–1754. [Google Scholar] [CrossRef]
- Zhou, L.X.; Yang, M.; Lian, C.Y.; Fan, L.S.; Lee, D.J. On the second-order moment turbulence model for simulating a bubble column. Chem. Eng. Sci. 2002, 57, 3269–3281. [Google Scholar] [CrossRef]
- Zhou, L.X.; Yang, M.; Fan, L.S. A second-order moment three-phase turbulence model for simulating gas–liquid–solid flows. Chem. Eng. Sci. 2005, 60, 647–653. [Google Scholar] [CrossRef]
- Lin, T.J.; Reese, J.; Hong, T.; Fan, L.S. Quantitative analysis and computation of two-dimensional bubble columns. AIChE J. 1996, 42, 301–318. [Google Scholar] [CrossRef]
- Gudin, C.; Chaumont, D. Cell fragility—The key problem of microalgae mass production in closed photobioreactors. Bioresour. Technol. 1991, 38, 145–151. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhou, L.; Zhang, Y. Numerical Simulation of Bubble-Liquid Two-Phase Turbulent Flows in Shallow Bioreactor. Energies 2019, 12, 2269. https://doi.org/10.3390/en12122269
Liu Y, Zhou L, Zhang Y. Numerical Simulation of Bubble-Liquid Two-Phase Turbulent Flows in Shallow Bioreactor. Energies. 2019; 12(12):2269. https://doi.org/10.3390/en12122269
Chicago/Turabian StyleLiu, Yang, Lixing Zhou, and Youngju Zhang. 2019. "Numerical Simulation of Bubble-Liquid Two-Phase Turbulent Flows in Shallow Bioreactor" Energies 12, no. 12: 2269. https://doi.org/10.3390/en12122269
APA StyleLiu, Y., Zhou, L., & Zhang, Y. (2019). Numerical Simulation of Bubble-Liquid Two-Phase Turbulent Flows in Shallow Bioreactor. Energies, 12(12), 2269. https://doi.org/10.3390/en12122269




