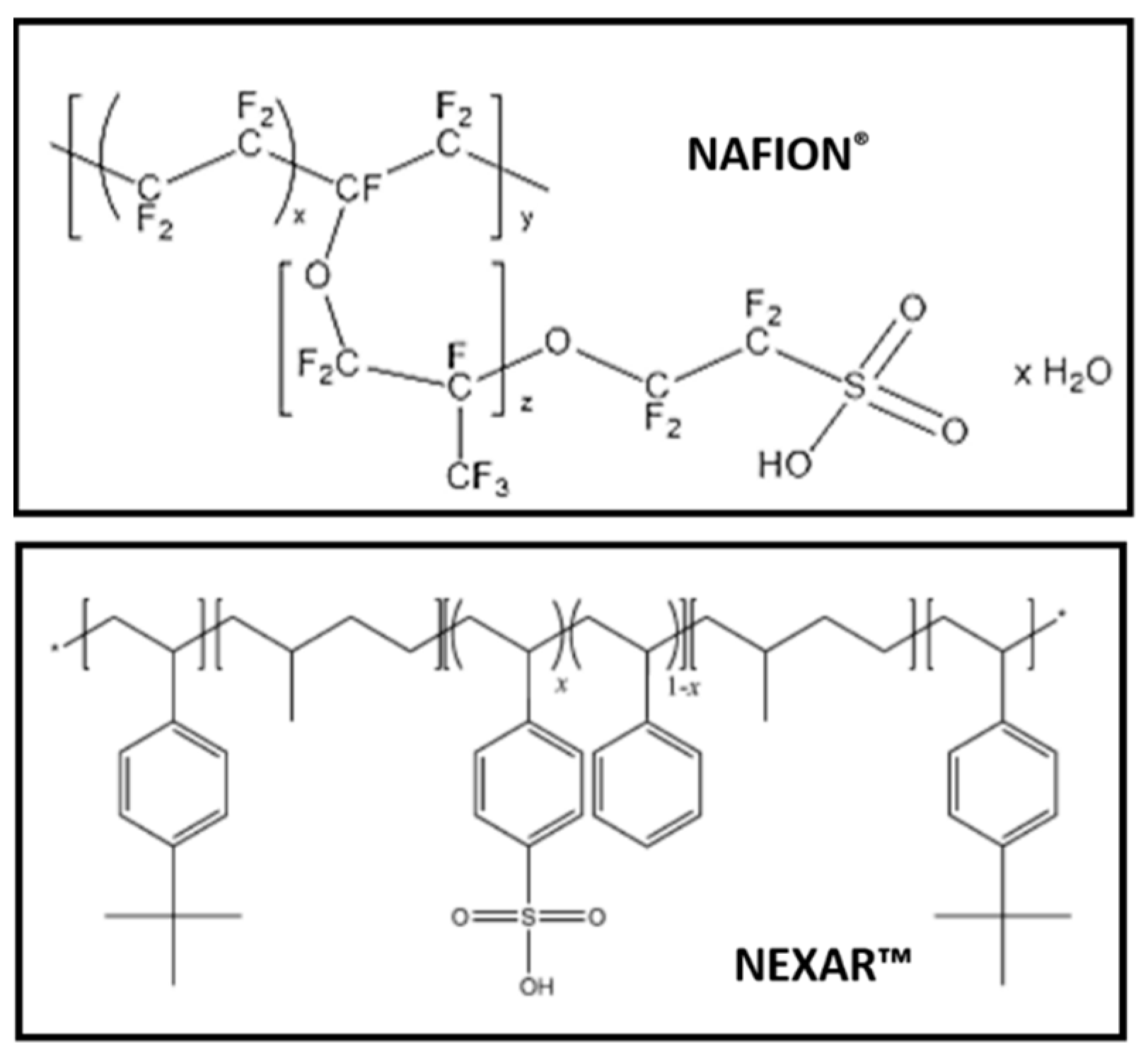
3.1. Water Uptake of Membranes
Water uptake is an important property for polymeric membranes that have to be used as PEM in water electrolysis; therefore, we measured this value for our Nafion and Nexar membranes. In particular, the water content value of each membrane was determined by using a microbalance and recorded as:
water uptake% = [(
mwet −
mdry)/
mdry] × 100, where
mdry is the mass of membrane dried in an oven at 60 °C for 2 h and then put to equilibrate in a desiccator before being weighed;
mwet is the weight of the membrane after immersion in deionized water at room temperature for at least 48 h. In order to measure the water uptake,
mwet was measured after quickly removing most of the free surface liquid on membrane surface with a paper tissue.
Table 1 reports the calculated water uptake for the two commercial films: Nexar shows a water uptake one order of magnitude higher than Nafion. This aspect is important in explaining the performance shown by this polymer as reported in the following results. The water uptake values were measured for three consecutive times, all showing the same values.
Furthermore, we report in
Table 1 λ, the number of water molecules per sulfonic acid group of ionomer based on the water uptake as:
where the number of moles of water is calculated by the water uptake and the number of moles of sulfonic groups is calculated considering the
EW (the number of grams of dry polymer per mole of sulfonic acid groups) for each polymer, i.e., 0.92 meq/g and 2 meq/g for Nafion and Nexar, respectively. The presence of sulfonilic groups was confirmed by the acquisition of IR spectra for both Nexar and Nafion commercial films. The IR spectra are reported in
Figure S1 of Supporting Information, also showing the higher hydrophilic character of Nexar film with respect to Nafion film, in agreement with
Table 1.
For Nafion NRE212, the swelling in thickness is 9% as reported in [
25]. In order to determine the swelling of the Nexar polymer, we measured the diameter increase (%) of a circular polymeric disc before and after water absorption. We observed a 33% increase of the diameter (from 2.7 cm to 3.6 cm), in agreement with the one measured by Geise in [
13,
14]. Knowing the diameter of the dry polymeric disc (
ddry) and its thickness (h = 50 μm), we calculated the volume of the cylinder with height corresponding to the thickness of the membrane in the dry state (V
in = πr
2h). After water absorption, the final volume (V
fin = V
in + V
H2O) is the sum of the one corresponding to the dry membrane and the volume of absorbed water molecules (calculated by the water uptake). Using the last volume and knowing the diameter of the wet disc
dwet, it was possible to calculate the thickness of the membrane after water absorption: this value is 92 μm (increase of 84%) for the Nexar membrane with water uptake of 168%.
Usually, Nafion membranes are subjected to a washing and regeneration process by rinsing in nitric acid and hydrogen peroxide to remove organic impurities, in sulphuric acid to remove any metallic impurities, and again in boiling deionized H
2O to remove excess acid [
7,
8,
9]. Concerning Nexar, such oxidizing treatments are not recommended by the Producers, since they can damage the polymeric structure. Therefore, an optimization of the activation processes for s-PBC membranes based on other acidic media such as HCl should be performed in the future.
For this reason, in this work, we just compare the two polymeric films as received by the two companies after only washing in Milli Q water (till neutral pH), without any further treatment.
3.2. Electrical Characterization of Membranes
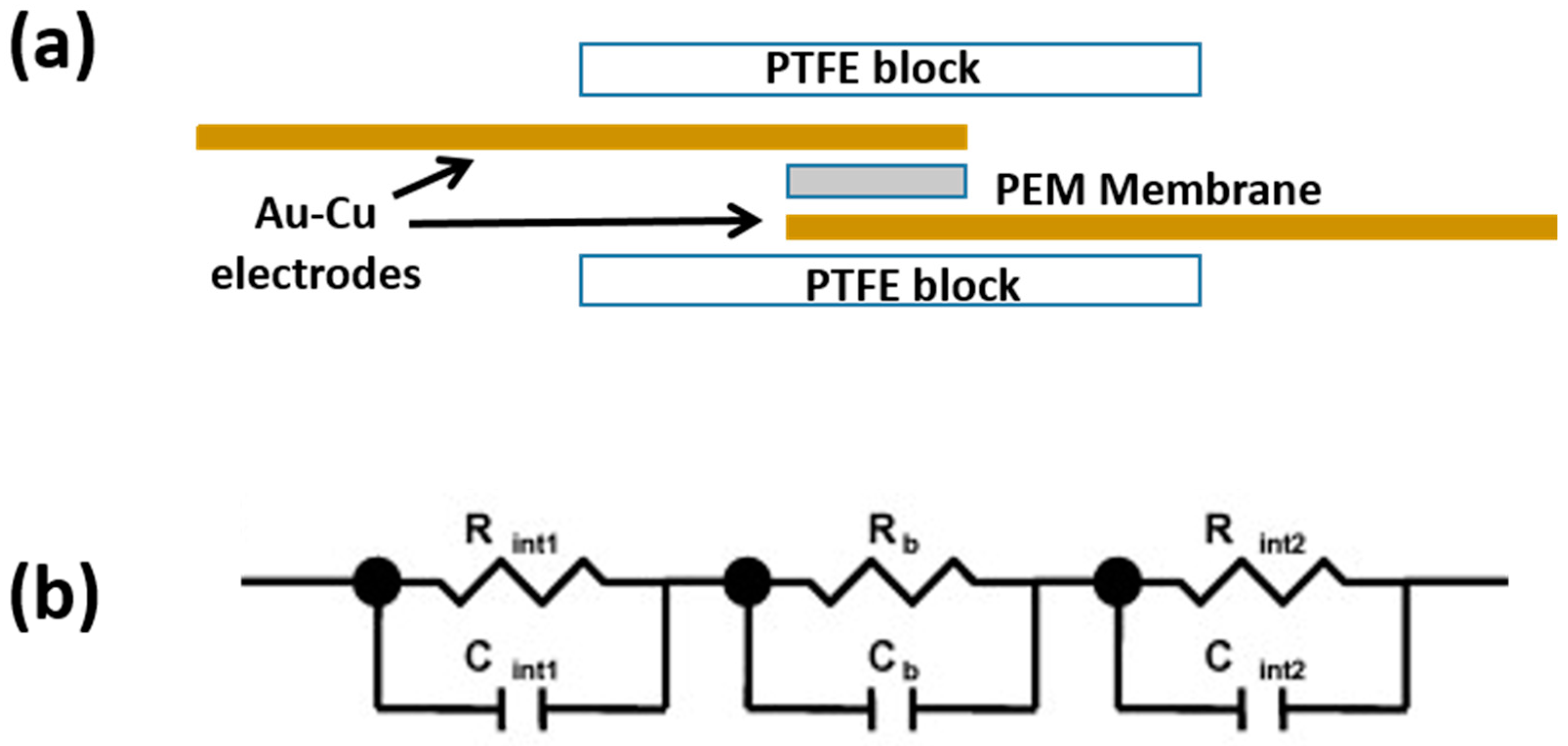
The membrane proton conduction was investigated using AC Impedance spectroscopy in the frequency range from 20 Hz to 2 MHz and with 100 mV small signal amplitude and 0 V bias. The through-plane conductivity was measured by placing the membrane between two gold-plated copper stripes (0.1 mm thick) used as electrodes and two PTFE blocks held together by screws, as illustrated in
Figure 2a. Before the measurements, membranes were immersed in water for some hours and during the test, the bottom part of the membrane in the cell was kept in deionized water in order to avoid its complete drying.
The LCR system was calibrated by measuring the impedance in open and short configuration. Conductivity σ can be calculated as:
where
L and
A correspond to membrane thickness and area, respectively, and
R is the bulk membrane resistance.
As reported in the literature [
26], such a system can be ideally schematized by an equivalent electrical circuit, as shown in
Figure 2b, considering the contribution of the bulk membrane (
Rb) in parallel with the bulk membrane capacitance (
Cb) and in series with the contribution of the interfaces between membrane and electrode. The latter contribution can be represented by a parallel combination of the capacitance (
Cint1 and
Cint2) and the resistance (
Rint1 and
Rint2) at the interfaces. Therefore, the impedance of the equivalent circuit of
Figure 2b can be written as:
The real part is resistive and the imaginary part is capacitive and the impedance spectra are expected to consist of (i) a semicircle at high frequencies, associated to the bulk membrane impedance, and (ii) another semicircle at low frequencies, associated to the membrane/electrode interfaces. In order to get the membrane resistance value, it is necessary to consider the high frequency region of the spectra and remove any inductance contribution. As described above, this can be done by LCR calibration.
Proton conductivity is a fundamental factor to evaluate the performance of proton exchange membranes and PEM cells. Commonly, proton conductivity is measured along the plane of the membrane [
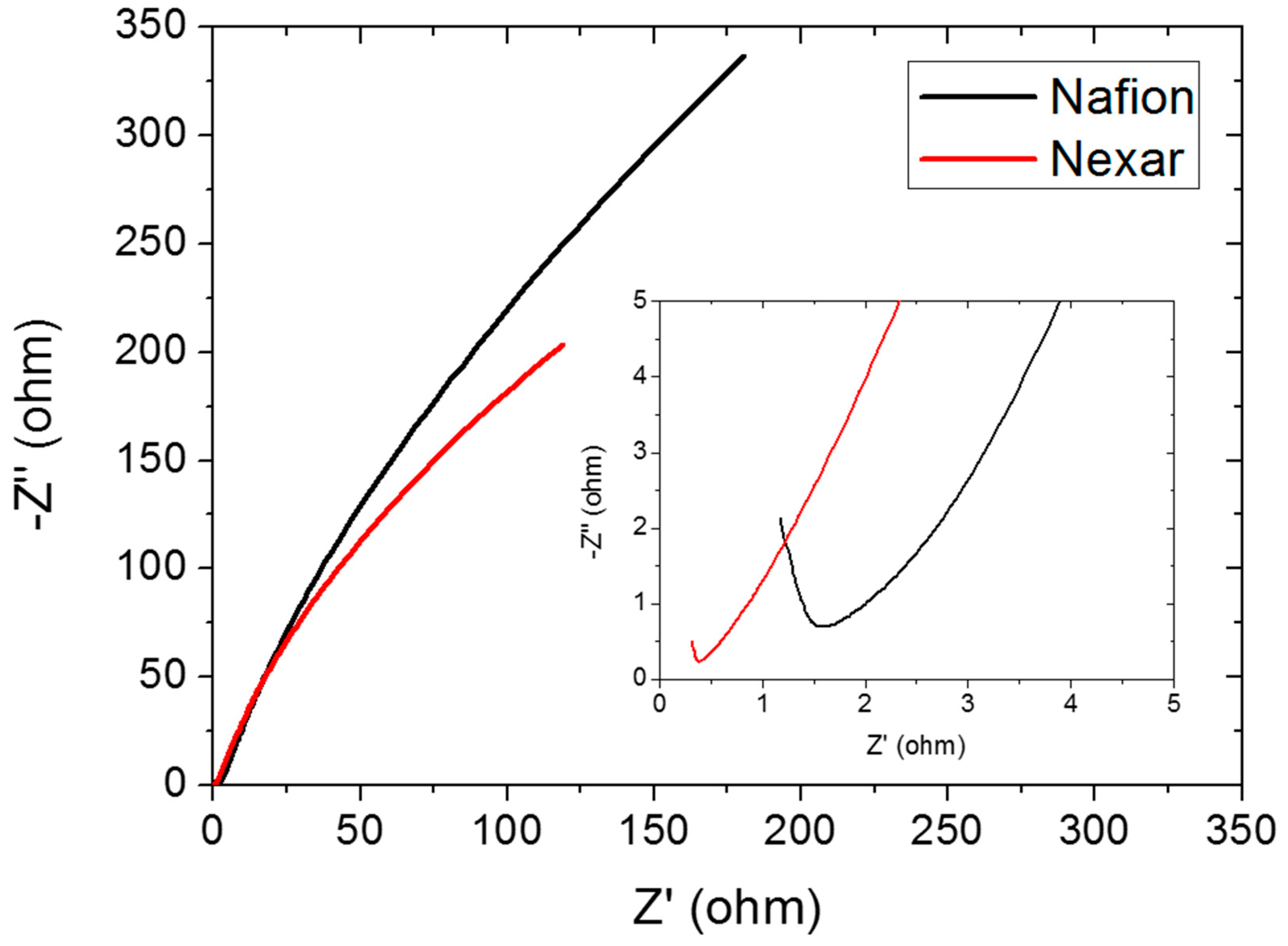
26]. However, in the specific application of water electrolysis cell, protons must be transported from the anode to the cathode through the membrane and, considering that materials like s-PBC can exhibit morphological anisotropy, it is evident how relevant the measurement of the through-plane conductivity is for studying proton exchange membranes. The impedance curves recorded for the Nafion and Nexar membranes are shown in
Figure 3 and in the inset, a magnification of the same impedance curves at higher frequencies is reported. Each impedance curve is composed of a contribution at low frequencies (right of the graph) due to the electrode-membrane interfaces and a contribution at high frequencies (left part of the graph) related to the bulk membrane impedance [
26]. The onset of the semicircle observed in the magnification of the impedance curves is attributed to the bulk membrane resistance.
Concerning the impedance measurements, we observed that for both Nafion and Nexar it is necessary to repeat the measurement for some minutes in order to achieve stability (improvement of contact between membrane and electrodes). Afterwards, several measurements were performed consecutively for about 1 hour, showing that the Z” vs Z’ curve remained stable with a variability of the calculated R value within ±0.05 Ω for Nafion and Nexar.
Table 2 reports the calculated values of resistance and conductivity for the two membranes for an area of 5 cm
2 and considering the thickness value for two extreme conditions: 50 μm for dry membranes or a larger value for wet membranes proportional to the swelling of their thickness after water absorption as calculated in the previous paragraph (54.5 μm and 92 μm, respectively, for Nafion and Nexar).
From the impedance curves recorded at 0 V shown in
Figure 3, Nexar™ shows a resistance value lower than for Nafion
®, i.e., 0.38 Ω instead of 1.55 Ω, calculated by linear extrapolation of the linear portion of the semicircle at lower frequencies. The calculated conductivities according to Equation (1) are also reported in
Table 2. The value for Nafion is in agreement with that reported in the literature in the case of low humidity [
27]. Nexar shows higher proton conductivity with respect to Nafion; in particular, it is about four times higher than the value of Nafion for dry membranes and seven times higher for wet membranes, as shown in
Table 2. This is fundamental in explaining the improved performance of Nexar with respect to Nafion in the cell, as shown in the last section of the paper. It is mandatory to underline that these values are obtained for membranes without activation processes and for low humidity content, so higher values can be expected in the case of fully hydrated membranes.
The differences observed at low frequencies between the two polymers may depend on their superficial morphologies (flat or disordered surfaces) and the surface hydrophilicity, which affect the electrical contact between the electrodes and the membranes. In particular, at very low frequencies, the impedance curve for Nexar shows a smaller slope with respect to Nafion, i.e., the Nexar capacity at the interfaces is higher than the Nafion one, probably due to a higher amount of water molecules present at the interface between the Nexar surface and the electrodes (at low frequencies the contribution of the bulk material capacitance can be neglected).
3.3. Modification of GDLs
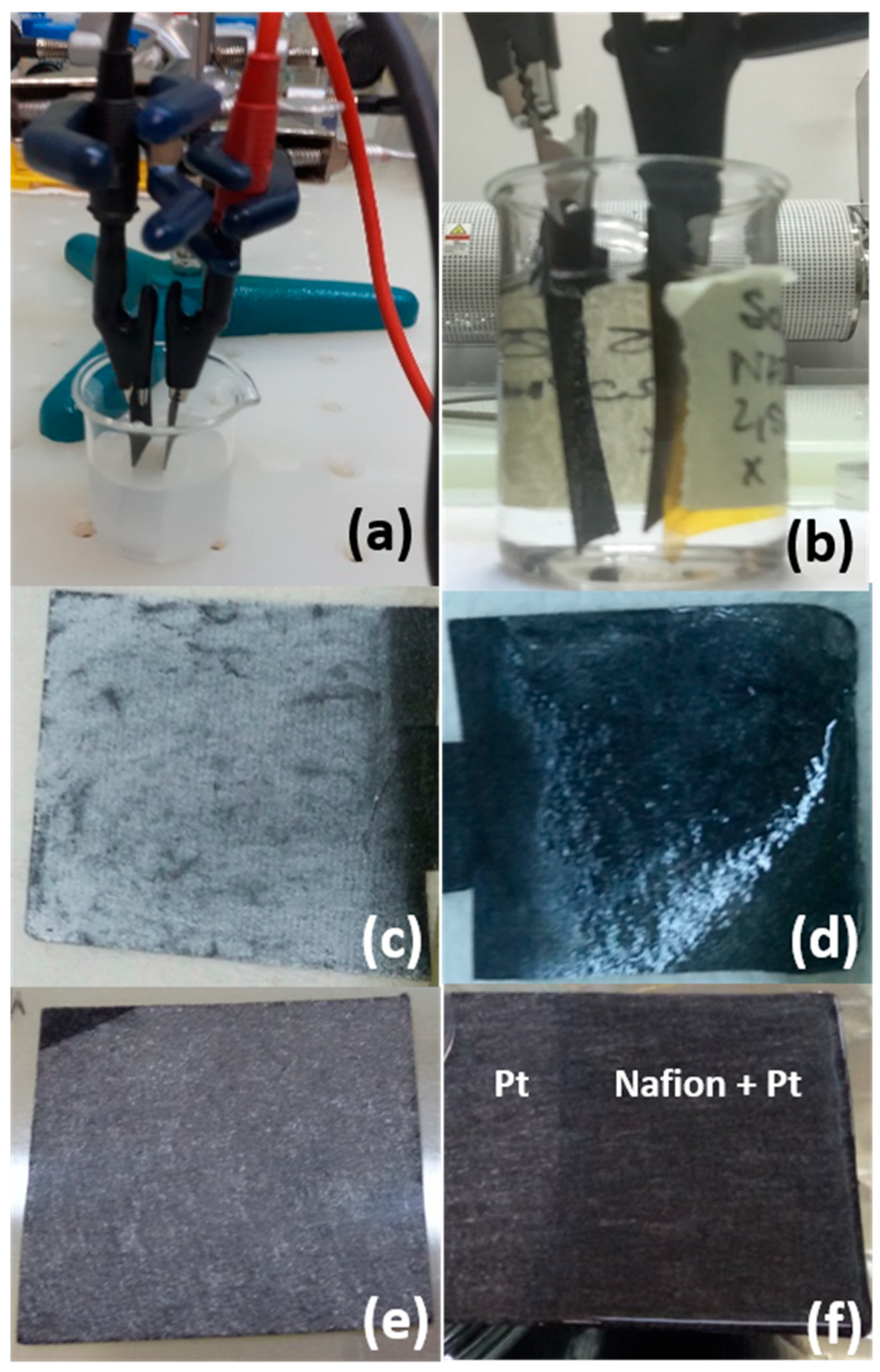
In
Figure 4a–d we report the photos showing the electrophoretic depositions of TiO
2 nanoparticles in ethanol (a) and Nafion solution (b) and the GDLs covered by TiO
2 (c) and, afterwards, by Nafion (d).
Pt was deposited by sputtering with a nominal thickness of 40 nm. The photos of the GDLs covered by Pt after the sputtering process and the same covered by Nafion after the electrophoretic deposition are reported in
Figure 4e,f. The weight of the GDLs was measured before and after the deposition, in order to get an estimation of the weight of the different materials (Pt, TiO
2, Nafion) deposited per unit area (mg/cm
2). The amount of deposited catalysts and Nafion layers in mg/cm
2 for the modified GDLs used in this study are reported in
Table 3: GDLs covered only by Nafion are referred as N1 and N2, while those containing titanium dioxide or Pt and Nafion are called N-Ti and N-Pt, respectively. As shown in the table, within the same experimental conditions, the deposition of the polymeric films on different substrates is quite reproducible.
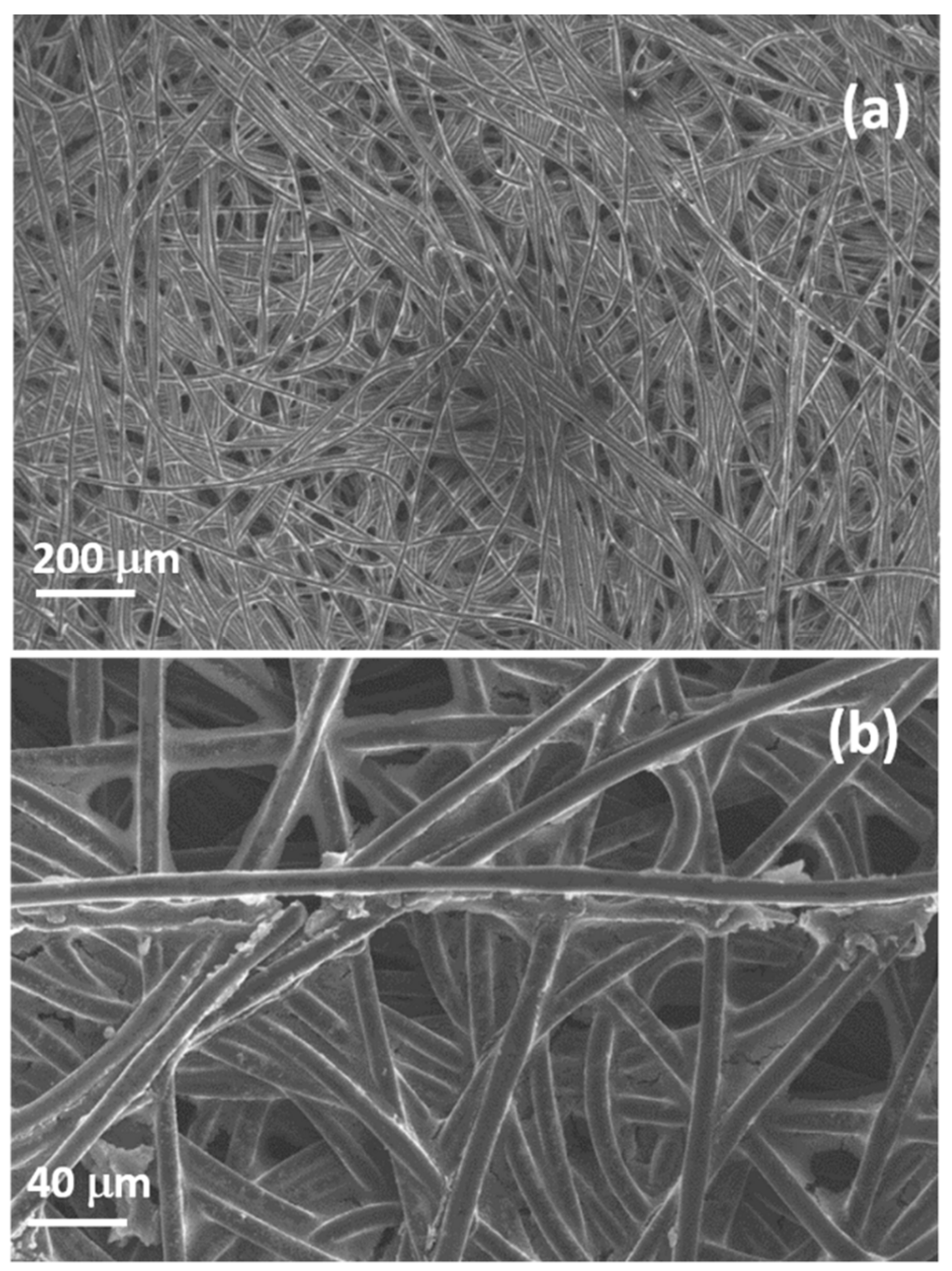
Commercial GDL is formed by carbon fibers of diameters randomly distributed around 10 µm, forming a porous structure. The fibers are prevalently composed of carbon with a small amount of fluorine due to PTFE that is added during production to increase their hydrophobicity. The morphology and composition are confirmed by SEM and EDX characterization reported in
Figure 5 and
Figure S2 of the Supporting Information, respectively.
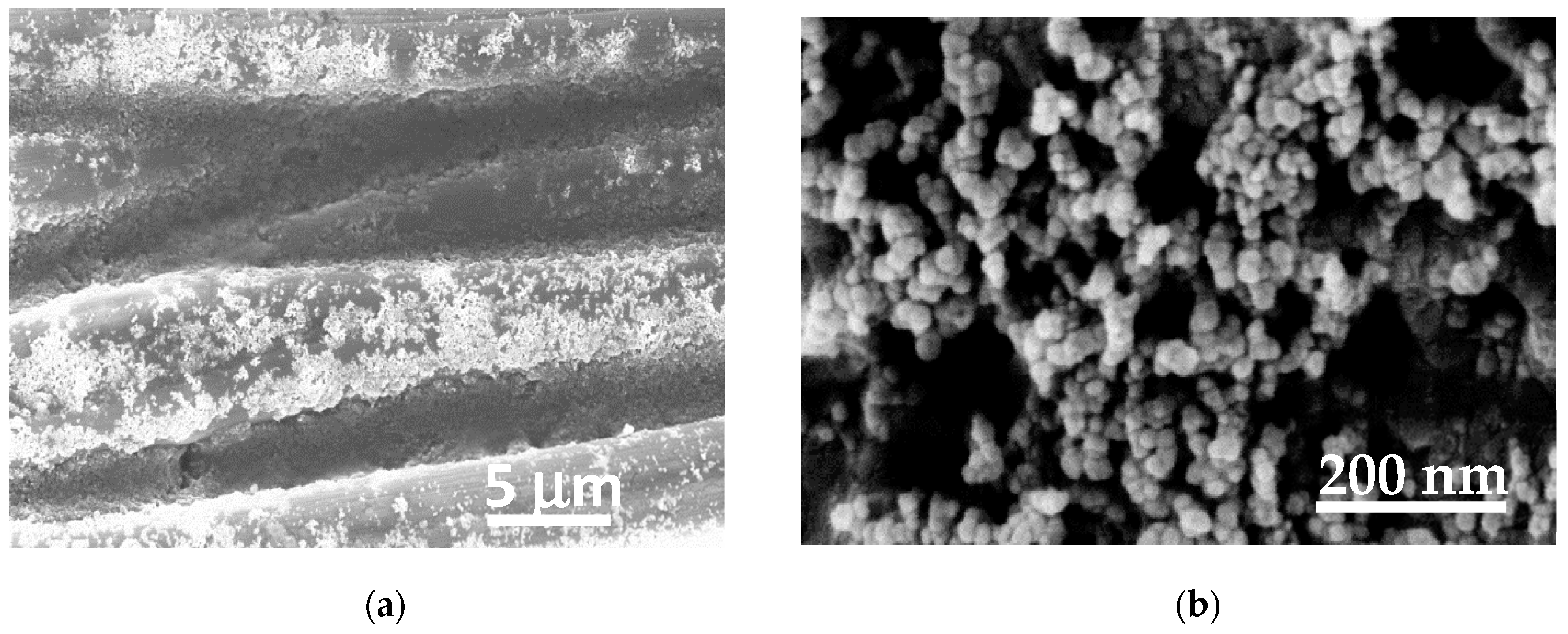
The GDL used on the cathodic HER side was modified by sputtering deposition of a Pt layer.
Figure 6 shows SEM images (at different magnifications) of the carbon fibers covered by a Pt layer formed by nanoparticles tens of nanometers in diameter.
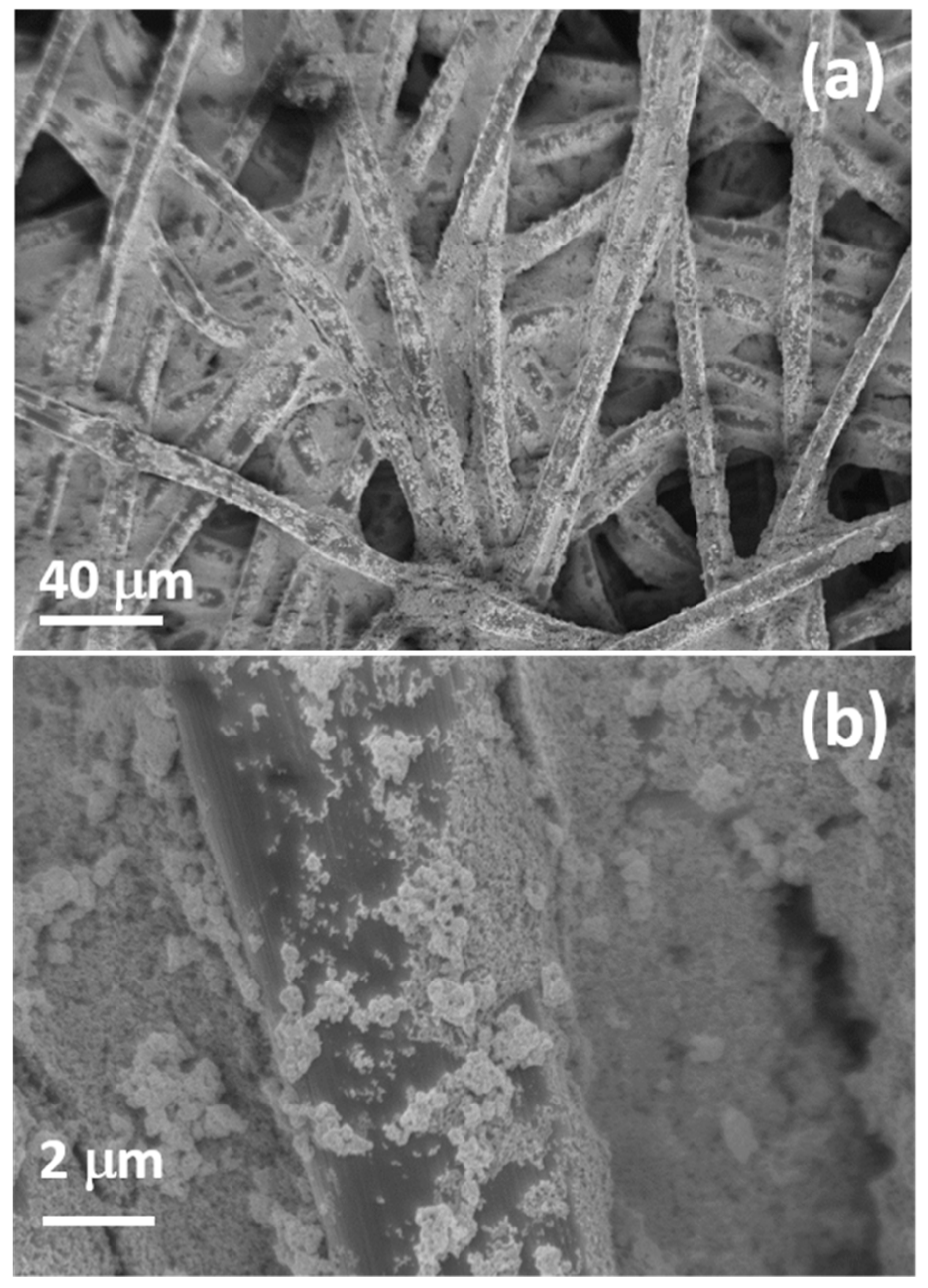
On the GDL used for the OER side, TiO2 nanoparticles were deposited by electrophoretic deposition (EPD).
Figure 7 shows SEM images of the GDL fibers homogeneously covered by a layer of titanium dioxide nanoparticles, as confirmed by the EDX elemental maps (not shown here).
After a water splitting test (see
Figure S3), damage of the fibers was observed, also confirmed by the acquisition of elemental maps, maybe due to local increase of current during the electrochemical process. To avoid catalyst layer damage, which strongly affects the efficiency of the water splitting cell, both the cathode and the anode GDLs were covered by a thin polymeric layer.
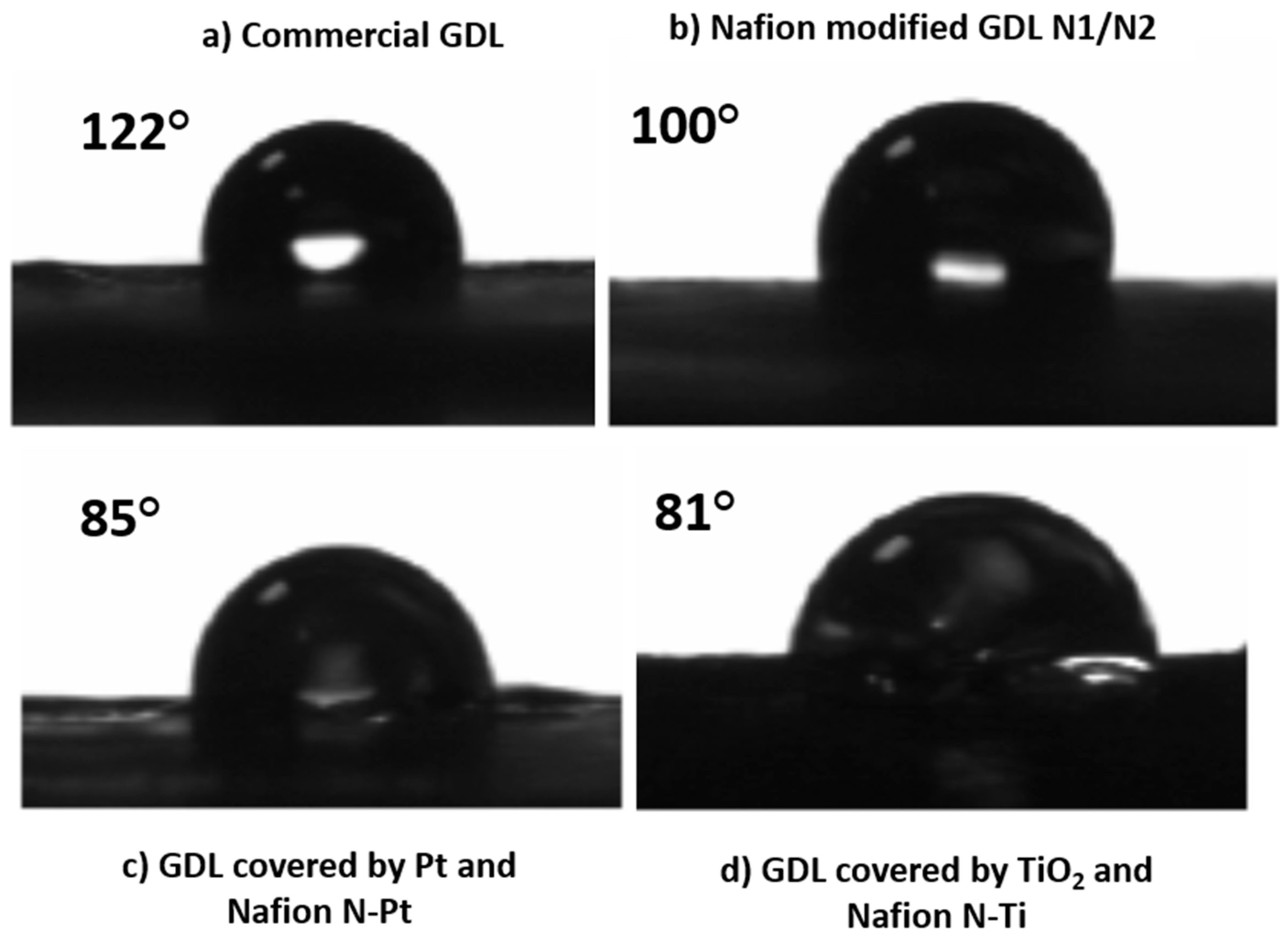
The EPD process was used for the deposition of the polymeric layers coating the Pt and titanium dioxide nanoparticles. In this case, the EPD is a simpler, more tunable, and lower cost method for polymer deposition than other methods (e.g., electrospray). According to the parameters reported in the experimental section, a Nafion layer was deposited on GDLs on the side precovered by Pt or titanium dioxide nanoparticles to protect them and the fibers during the water splitting reaction. Moreover, in this way, we increase the hydrophilicity of the GDL surface (as shown in the next section), allowing a better electrical contact and adaptation between membrane and GDL. A smooth homogeneous layer covering the fibers is then achieved, as shown by SEM analysis.
In
Figure 8, we report a SEM image obtained on a GDL after TiO
2 and Nafion deposition. The elemental maps were also acquired, confirming the presence of the catalysts, while the presence of sulfur confirmed the deposition of the Nafion layer. In
Figure S4 of the Supporting Information, we report a SEM image and the corresponding chemical maps of GDL covered by Nafion and TiO
2, showing a hole in the Nafion layer and allowing us to acquire the EDX signals of Ti and O alongside the other elements present on the modified GDL: C, F, S.
In
Figure S5, we report a SEM image of the Pt-containing GDL after Nafion deposition and the EDX analysis acquired on the same region, showing the presence of C, F, Pt, O, and S. It is worth mentioning that the presence of the Nafion protective layer allowed us to avoid any damage of the material or detachment of the catalyst during the electrolytic process in the water electrolysis cell.
Currently, catalyst loadings for the cathode side usually range between 0.5–1 mg/cm
2 [
11]. It is important to underline that, in this work, we have tested very low catalytic loadings (i.e., 0.03 mg/cm
2 for Pt) with respect to the literature. For TiO
2 nanoparticles, we used the minimum amount (i.e., 0.11 mg/cm
2) sufficient to observe an effect on the current produced in the water electrolysis test.
3.5. Water Electrolysis Cell Test
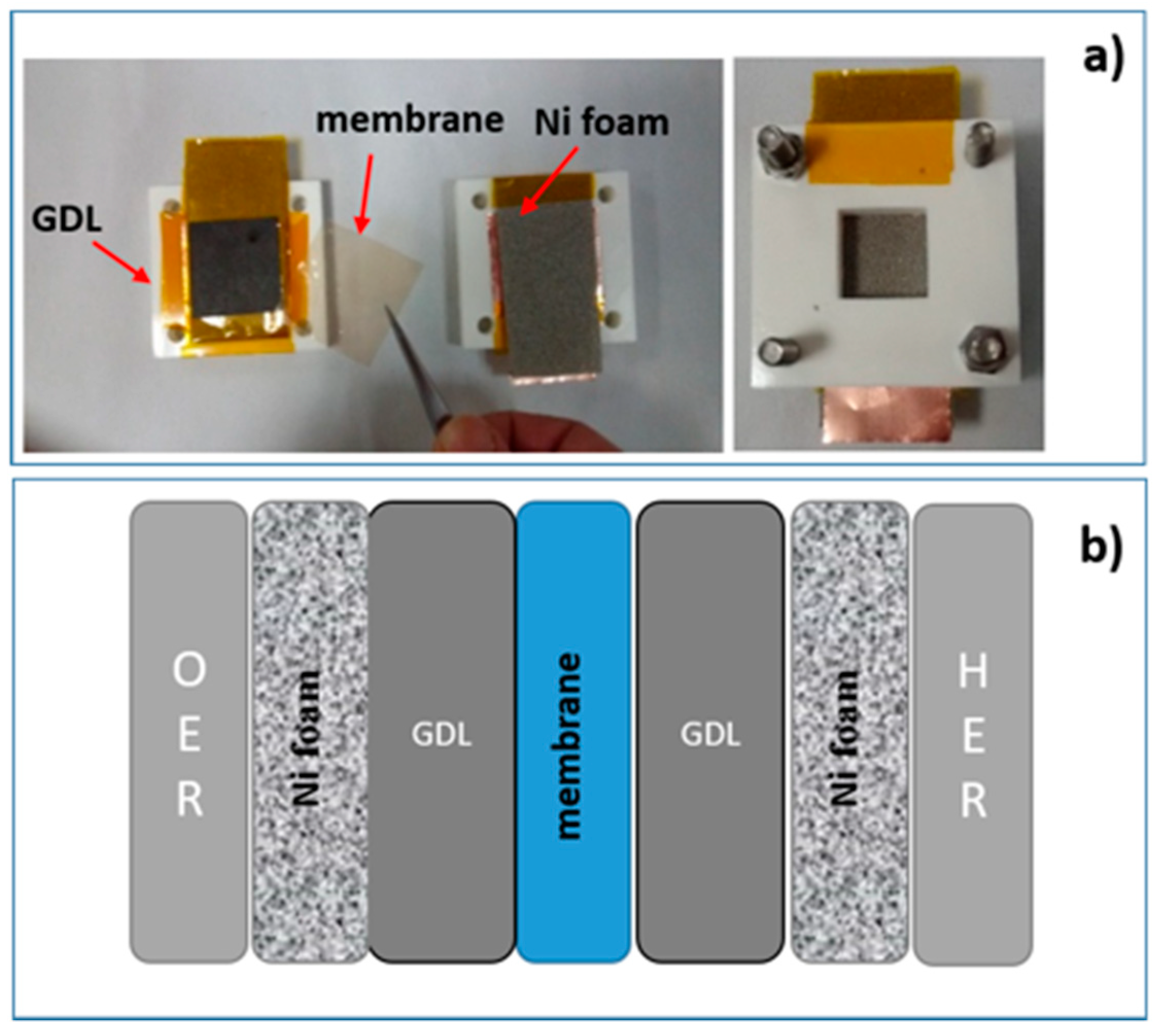
The electrochemical tests of different GDL/membrane/GDL configurations were performed using a home-made cell, as reported in
Figure 10, where we can insert, exchange, and test GDLs and membranes easily.
The membranes were sandwiched between two as-received or modified GDLs and the sandwich was put in contact with a Ni foam on the anode side and another one on the cathode side in such a way that the produced gases flow through them. The current is collected by two copper stripes that work as electrodes. The anode and cathode electrodes are insulated by a Kapton foil (orange films in the image). All the components are kept together by two PLA blocks joined by screws; in each block, a 2 cm × 2 cm square-shaped hole allows the produced gases to flow outside.
The current–voltage measurements have been performed varying the applied potential from 0 to 3 V. The experiments were performed at room temperature and under ambient light. Both the anode and cathode electroactive areas were 7 cm2.
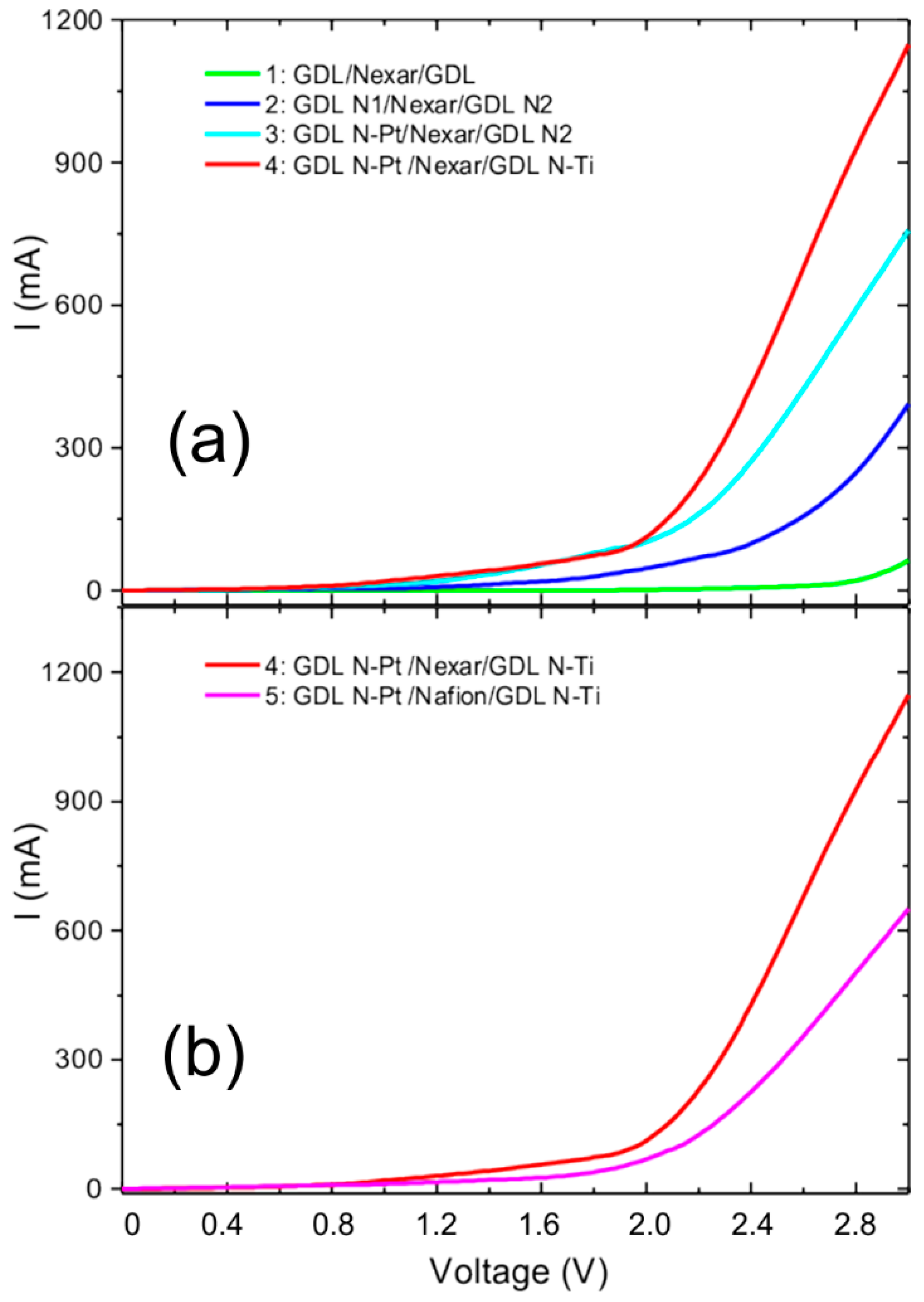
Figure 11 reports the I–V curves resulting from different water splitting tests using the following cell configurations:
1. GDL/Nexar/GDL: Nexar membrane between two unmodified gas diffusion layers.
2. GDL N1/Nexar/GDL N2: Nexar membrane between two gas diffusion layers with the surface covered by a Nafion layer.
3. GDL N-Pt/Nexar/GDL N2: Nexar membrane between modified GDLs. The GDL on the cathode side has been modified by a sputter-deposited Pt layer (0.03 mg/cm2) and then covered by a Nafion layer. The GDL on the anode side was covered only by a Nafion layer.
4. GDL N-Pt /Nexar/GDL N-Ti: Nexar membrane between modified GDLs. The GDL on the cathode side has been modified by a sputter-deposited Pt layer (0.03 mg/cm2) and then covered by a Nafion layer. The GDL on the anode side was modified by depositing TiO2 particles (0.11 mg/cm2) and, afterwards, a Nafion layer.
5. GDL N-Pt /Nafion/GDL N-Ti: Nafion membrane between the two GDLs used in the previous configuration (4).
All the I–V curves were acquired with the wet membranes, equilibrated in deionized water, but without a water flow in the cell during measurement. However, the bottom part of it is kept in deionized water during electrolysis, in order to replace the water consumption due to electrolysis, as reported in [
28]. Therefore, there is no liquid water mixed with the produced hydrogen and oxygen gases. The gases form at the interface between the membrane and the Pt- or TiO
2-loaded GDLs and then they can flow outside through the porous GDL-Ni foam and the squared holes.
The I–V measurements are performed using the cell assembly described in the “Water electrolysis cell test” Section and shown in
Figure 10. In this case, the cell assembly can affect (reduce) the measured current, since there are several interface resistances (membrane-GDL-Ni foam-Cu electrode-external electrical wiring) that can cause voltage drops. Therefore, the measured current is an underestimation of the value that could be reached in an optimized (from the electrical wiring point of view) cell. The main factor affecting the measurements is the degradation of the GDL during operation. We have observed that while the GDLs are in good condition, the measurements are perfectly reproducible. As soon as the GDLs deteriorate (on the OER side by oxidation and on the HER side by Pt loss), the current drops. A large improvement on the durability of GDLs was achieved by covering the GDLs with a thin Nafion layer and adding TiO
2 on the OER side. We have repeated the measurements for the different configurations as a function of time and observed that for bare GDLs, the measured current decreases with consecutive tests immediately. This is due to damages induced on both the HER side (Pt detachment) and the OER side (oxidation of carbon fibers) already after two tests. On the contrary, GDLs covered with thin Nafion layers and, on the OER side, with TiO
2 nanoparticles maintained their efficiency for several (more than 20) consecutive tests and no variation of their weights was observed (no Pt, TiO
2, or polymer layer detachment).
We have reported the tests made with the minimum amount of Pt and TiO2 sufficient to give a significant effect. It is possible to increase the amount of Pt to improve the catalytic effect, but it has to be noted that an excess of Pt deposited by sputtering will create a compact layer, with smaller efficiency due to lower surface/volume ratio. Similarly, the total amount of TiO2 particles can be increased, but we have observed that for a TiO2 concentration two times higher than the one reported in this work, a reduction of the total amount of measured current occurred and this effect can be ascribed to a blocking effect of the produced gases by the TiO2 particles, reducing the carbon fiber porosity.
Our results reported in
Figure 11a show that covering GDLs with Nafion (configuration 2) allows a large improvement in terms of current (from 60 to 390 mA at 3 V) compared to unmodified GDL (configuration 1). As expected, the addition of Pt (configuration 3), even at very low concentration (0.03 mg/cm
2), induces a general increase of the current (up to 760 mA at 3 V), and a lowering of the onset potential.
A further increase of the generated current (up to 1140 mA at 3 V) was obtained by the addition of TiO2 on the GDL on the anode side (configuration 4).
An estimation of the onset of I–V curves reported in
Figure 11 can be obtained by considering the intersection point between the tangent at maximum slope of current (linear part at higher voltage) and the background current (linear part at lower voltage). Therefore, we obtain the following results: 2.4 V for configuration 2, 2.2 V for configurations 3, and 2.1 V for the configurations 4 and 5. We also observed a variation of the slope by adding Pt. The addition of TiO
2 has a very small effect on the onset of the curve but increases the total measured current significantly, due to the effects discussed above: the TiO
2 (in this amount) has the role of increasing the hydrophilicity of the system, favoring the O
2 to flow out and retarding the oxidation of carbon fibers. For the measurement performed in the first configuration with bare GDLs, it is not possible to estimate the onset since we do not observe any reaction occurring in the considered voltage range, as expected, due to the absence of catalysts.
An absolute comparison with I–V measurements reported on commercial electrolytic cells cannot be done since the final electrical characteristics strongly depend on the cell’s assembly (electrical connections, contacts, catalyst content on both anode and cathode sides, water flow, etc.). However, in order to simply compare the two membranes, we kept configuration 4, which gave the best performance, and exchanged the Nexar membrane with the Nafion one (configuration 5). The comparison, reported in
Figure 11b, shows that Nexar has higher efficiency than Nafion: at 3 V, the measured current is 1140 mA for Nexar and 650 mA for Nafion.