1. Introduction
Increasing the renewable energy fraction in transport is an urgent necessity to tackle global warming and air pollution. Recent pollution episodes in big cities have prompted the adoption of local and regional policies that will progressively (from the present to 2040–2050) ban internal combustion vehicles. Electricity from renewable sources will be decisive to achieve and maintain a fully sustainable mobility system as it has been analyzed in detail elsewhere [
1], but the short-and-mid-term attention must be focused on developing renewable and sustainable fuels, especially those with a more beneficial carbon balance [
2].
Advanced biofuels, including some non-crop and waste-based biofuels, possess important benefits such as higher GHG emission savings and the capacity not to strain food markets [
3]. European institutions agreed to specific targets that have been set in a new Renewable Energy Directive (2018/2001) [
4] published in December 2018, including 14% of renewable energy in rail and road transport by 2030. To achieve this, advanced biofuels will be double-counted, and their contribution must be at least 3.5% in 2030 (with a phase-in calendar from 2020). Moreover, crop-derived biofuels will be capped at levels achieved in 2020 and not higher than 7% whereas raw materials with a high risk of ILUC (indirect land-use change) must be fully abandoned by 2030.
In the case of diesel fuels, the pool of advanced biofuels is still wide. Alcohol-diesel blends, if the alcohol comes from lignocellulosic wastes, biodiesel fuels derived from residual oleaginous materials and/or paraffinic fuels such as BTL (Biomass-To-Liquid) or HVO (Hydrotreated Vegetable Oil) can be considered advanced biofuels.
So far, biodiesel fuels are the most studied but also the most controversial biofuels. The final product consists on a mix of a few fatty acid methyl esters (FAME); therefore, the fuel properties vary within narrow ranges around values generally close to those exhibited by conventional fossil diesel fuels. But the reduction of lifecycle GHG emissions of biodiesel fuels depends largely on the origin of the raw vegetable oil/animal fat. Even some biodiesel origins have been reported to increase GHG emissions [
5]. Two approaches, both treated in the present work, are gaining attention as a means to ensure a beneficial lifecycle GHG emission balance: (i) biodiesel fuels partially or totally derived from other industrial wastes and (ii) biodiesel routes that reduce the production of low valued byproducts (such as low-grade glycerol). The esterification of residual free fatty acids recovered from the palm oil industry is a way of dealing with a very abundant waste coming from the palm oil industry [
6]. Some works have been focused on the reactions involved in the production process and the selection of catalysts [
7,
8], whereas properties and engine emissions have not received much attention although they are expected to depend on the purity and quality of the final methyl ester product. In the second approach, esters of glycerol formal (FAGE) yielded from waste oils and glycerol as reagents have been proved as a reliable option. However, the high viscosity and poor cold flow properties of FAGE [
9] make desirable to blend FAGE and FAME to obtain a renewable biofuel with properties suitable for diesel applications. The reactions needed for both, FAGE and FAME, may be implemented jointly in current biodiesel plants thus making the whole process more economic. When used in diesel engines, benefits in carbon monoxide, hydrocarbons and particulate matter were found and supported based on their oxygen content [
10].
Alcohol-diesel blends are reported to reduce particle and other emissions mainly because of their oxygen content. Ethanol is the most conventional alcohol [
11,
12,
13] used for blending, but n-butanol has some advantages over ethanol, such as higher heating value, higher cetane number and better miscibility with diesel [
14,
15]. Recently, chemical and biological processes to produce n-butanol from renewable and waste sources have gained attention [
16]. Among them, bacterial fermentation may reduce greenhouse emissions on a life-cycle basis [
17] and, thus, is a promising technique [
18,
19].
Highly paraffinic fuels such as gas-to-liquid (GTL), biomass-to-liquid (BTL) and hydrotreated vegetable oil (HVO) have emerged as alternative diesel fuels with some outstanding diesel properties (mainly cetane number and heating values) that offer potential to reduce fuel consumption, and thus CO
2 emissions, and harmful emissions of diesel vehicles [
20]. Moreover, these fuels have been claimed to be fully compatible with current engine technologies. HVO has the best economic perspectives since the chemical process may be integrated in current oil refineries [
21,
22], using the existing reactors and hydrogen streams. HVO production technology removes the O-atoms and the double bonds of the raw material through a process called hydrotreating catalysis. The raw material is the same than in the case of biodiesel, but the chemical route and the final product are different. Many works have examined and compared both fuels, and even ternary blends (diesel-biodiesel-HVO) have been proposed to exploit the advantages of both biofuels.
The selection of fuels and blends should not be made ignoring the properties of the final blends. A correct blending proportion based on the property’s ranges set in current fuel quality standards may guarantee the absence of vehicle failures, such as those episodes encountered at some places when using certain biodiesel blends in cold ambient conditions. Besides, many of the fuel properties directly affect the engine-out emissions [
23]. The current European legislative framework imposes that any final diesel fuel (neat or blended) to be used in road transport must meet all the EN 590 requirements; besides, biodiesel and paraffinic fuels must fulfill, before blending in regular diesel, the additional requirements set in EN 14214 for biodiesel and EN 15940 for paraffinic fuels. In this frame, the present work examines the main fuel properties of feasible blends of four advanced biofuels in regular fossil diesel fuel and proposes ranges and target values for each biofuel. The selected blends will be tested in a diesel engine and/or vehicle, in a different study, to check whether the suggested blending proportions work as expected under real driving conditions.
2. Materials and Methods
Density was measured at 15 °C according to EN 3675 introducing an appropriate densimeter into the flask that contain the fuel tested. The flask is surrounded by water in a thermostatic bath from Fisher Scientific (Tamson TV 2000, with a temperature control precision of ± 0.1 K). Kinematic viscosity was measured using Cannon-Fenske viscometers introduced in the same bath. In this case, the temperature of the water during the viscosity tests was maintained at 40 °C, according to standard EN ISO 3104.
Higher heating value (HHV) analysis was conducted according to standard ASTM D4809, using a Parr 6100 calorimetric bomb. Lower heating value (LHV) was also calculated from the HHV value and the elemental analysis, which was carried out using a Carlo Erba EA1108 analyzer.
Lubricity was carried out according to EN 12156-1 in a high frequency reciprocating rig (HFRR) from PCS Instruments. The wear scar was measured with an Optika SZR1 microscope equipped with a 100x magnification lens. All lubricity tests were made inside a climatic chamber (provided by PCS Instruments) where the ambient temperature and humidity were controlled according to the limits set in EN 12156-1:2016 with the use of a potassium carbonate solution.
The Cold Filter Plugging Point (CFPP) and the Cloud Point (CP), representative of cold flow properties, were measured according to the standards EN 116/ASTM D6371 for CFPP and EN 23015/ASTM D2500 for CP. The device used to measure the CFPP was PACFPP 5Gs whereas the instrument used for measuring CP was PAC-CPP 5Gs.
Derived Cetane Number (DCN) was measured in the equipment Cetane ID510 by Herzog. This equipment consists of a constant-volume combustion chamber (0.473 L) and a common-rail diesel injector (operating at 1000 bar injection pressure) similar to those used in commercial engines (Bosch part no 0445110181) with 6 nozzles with orifice diameter of 0.17 mm. Synthetic air (21% O2 and 79% N2 v/v) was used as oxidant and the initial absolute pressure was kept constant at 21 bar. In every test, 15 fuel injections for each fuel were carried out. DCN is determined through the ignition and combustion delays according to ASTM D7668, both calculated from the pressure signal.
Finally, water content measurement was carried out by using Karl-Fischer 831 KF according to EN 12937.
4. Results and Discussion
4.1. Density
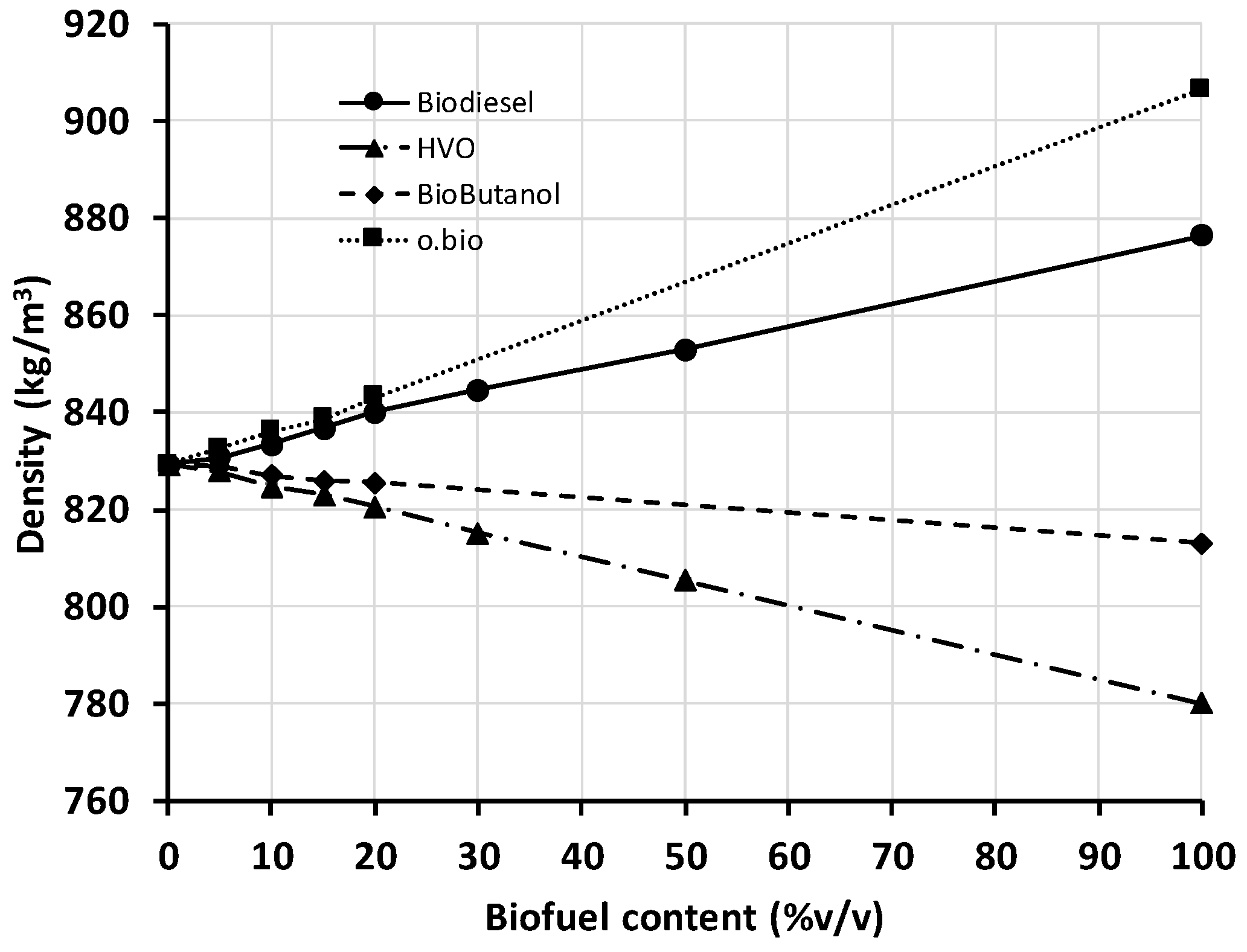
Figure 1 presents the density of all the blends and neat fuels tested. Each blend/fuel was tested three times and the average value is displayed in the figure. None of the replicated measurements deviated more than 2 kg/m
3 from the average value. Fuel dispensers in filling stations operate in volume units. In diesel vehicles, electronic injection adjusts the fuel mass introduced into the combustion chamber by modifying the injection time (i.e., the volume of fuel) and assuming a standard density set in the ECU cartography. In view of these, fuel quality standards impose a narrow density range, as suggested elsewhere [
24]. In the case of EN 590, density is specified at 820–845 kg/m
3.
Compared to the density of diesel fuel (biofuel content = 0%), blends with biodiesel (FAMEs) or o.bio
® (FAMEs and FAGEs, mainly) increase density, while blends with HVO or butanol have the opposite effect. The density of biobutanol is closer to that of diesel than that of ethanol (ethanol was not tested here, but it is the most common alcohol used for diesel blending despite its miscibility and hygroscopic problems), which is one of the advantages of biobutanol blending over ethanol. Considering the importance of limiting the variations of density, it would be possible to design a diesel blend with a selected composition of two of the biofuels here tested (biodiesel and HVO, for example, since the production processes of both biofuels have been suggested to be combined in a single plant [
22]) obtaining a ternary blend which keeps the density at the same value than diesel’s.
As expected, the trend of the density is quite linear with the blend composition, which indicates that there is no change of volume on mixing (or it is negligible). Therefore, the density of a blend at any other composition than tested here could be calculated with minor error by multiplying the density of the two components by the volume fractions; this can be extended to blends with more than two components. In order to fulfill the required regulated density range, the content of biofuel cannot exceed 20% (it could be higher in the case of butanol blends, though in this case high biobutanol contents are not debated in the diesel fuel market).
4.2. Lower Heating Value
The heating value of a fuel indicates its energy content per unit mass (MJ/kg) or volume (MJ/L). Each blend/fuel was tested three times, and the average deviation was 0.10 MJ/kg (the maximum difference between replications was 0.40 MJ/kg). Although the instruments used to perform heating value measurements usually provide mass units, volume units are preferred in the transport sector because of the reasons outlined previously (density section).
Although this property is not currently limited in diesel quality standards, its effect is meaningful. Low values lead to higher fuel consumption and a loss of engine power at full load. In modern vehicles, the lean NOx trap (LNT) purging and the Diesel Particle Filter (DPF) regeneration are accomplished by post-injecting fuel to increase exhaust temperature. The volume and timing of these post-injections are set in the electronic control unit (ECU) by the manufacturer engine calibration department. But if the heating value of the fuel used by a user during real driving is not high enough, it may derive in a lower exhaust temperature than needed and, therefore, in unsuccessful or incomplete regenerations and eventually vehicle failure [
25,
26].
As in the case of density, the heating values of the blends tested changes quite linearly with the blend composition (
Figure 2). This confirms, together with the nil change of volume on mixing, that these fuel blends can be treated as ideal mixtures. The lower heating value of HVO in mass units is higher than that of the diesel fuel; on the contrary, in volume units, it is lower. This trend can be extrapolated to all paraffinic fuels (HVO, XTL), since paraffins in the C-range typical of diesel fuels usually display high heating values in mass units but lower densities than other hydrocarbons (such as aromatics). Nevertheless, the loss of volumetric heating value of HVO compared to diesel is only 3%; therefore, this property does not hinder the use of neat HVO in vehicles with no (or just minor) changes in the engine calibration. The opposite case is biobutanol, which heating value is unassumingly low. The content of biobutanol in diesel blends should be limited around 10% if admissible loss of heating value is to be set at 3%.
About o.bio® and biodiesel fuels, the heating values of both are close to each other, especially when given in volume units, as their components are quite similar (ester-based). Compared to diesel, the heating value of neat o.bio® and biodiesel is approximately 10% lower, in volume units, thus the recommended content in diesel blend should not be higher than 30%.
4.3. Lubricity
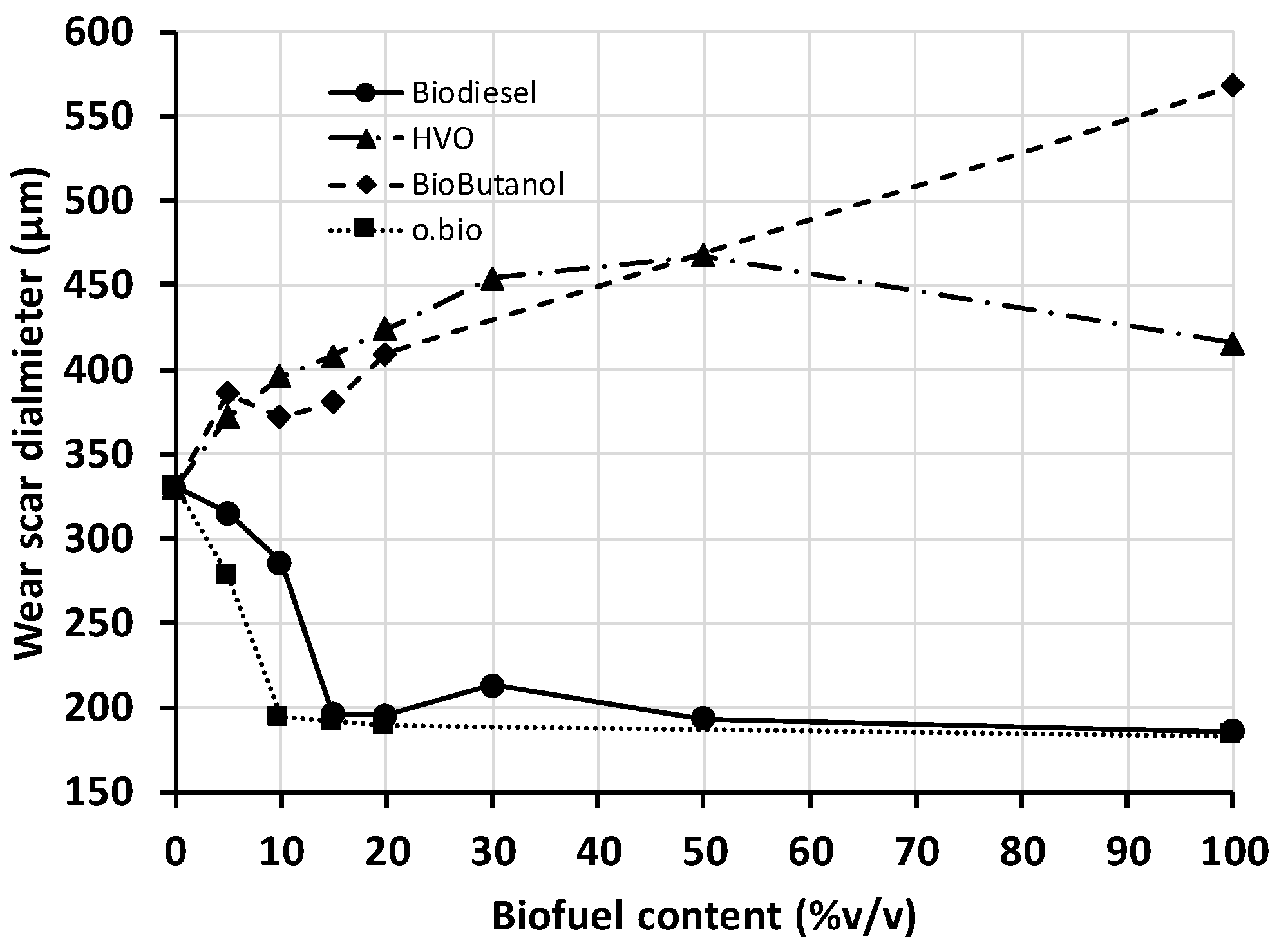
Fuel lubricity is essential in the injection system as it reduces wear and scuffing between parts in relative motion [
27], such as in the interior of diesel fuel pumps and fuel injectors. The concern about fuel lubricity has increased over the years due to the sulphur removal in the fuel (the process used in oil refineries for this task eliminates natural lubricant hydrocarbons) and the lower mechanical tolerances allowed in injection system components.
Each fuel/blend was replicated twice, being the average deviation between replications lower than 10 μm, which is below the maximum deviation admitted in the test standard EN 12156-1:2016 (50 μm maximum in one case out of twenty).
Figure 3 presents the lubricity of the fuels and blends of this work. Neat diesel and HVO are commercial fuels, additivated with lubricity enhancers by the fuel manufacturers to fulfill the limit (lower than 460 μm) set in EN 590 (for automotive diesel fuels) and EN 15940 (for paraffinic fuels). The highest wear scar diameter (i.e., poorest lubricity) was found for biobutanol, though this alcohol is better lubricant than ethanol [
15]. The biobutanol tested has no lubricant additive as it was used as received from the chemical supplier. Both ester-based fuels, biodiesel, and o.bio
®, exhibited the best lubricity. The ester functionality present in FAME (biodiesel) molecules can be adsorbed on metal surfaces while the rest of the chain (long aliphatic chain) creates a film that prevents wear. The extremely good lubricant properties of biodiesel have been proved previously [
28,
29], but the results here presented confirm now the same result for FAGE/FAME blends.
The lubricity of the blends did not change linearly with the blend composition. In the case of the ester-based biofuels, the lubricity improved strongly with the addition of low biofuel content into diesel until reaching a “saturation” level. From this, no further lubricity gain was manifested even at higher biofuel concentrations. Comparing both biofuels, o.bio® (FAGE/FAME blend) was the most promising one since only 10% was needed to achieve the maximum reduction of the wear scar.
In the case of biobutanol and HVO blends in diesel, the lubricity deteriorated with the biofuel content and the lubricity loss was more significant in the low content range (0–10%). Low biofuel content is the most likely future scenario in the fuel market; therefore, this finding must be observed carefully by all involved agents. For example, in case the lubricity of the reference diesel fuel used for blending was too close to the permitted value, even blends with biobutanol or HVO at 10% or lower could lead to blends out of the lubricity specification.
All blends HVO/diesel tested fulfill the maximum limit set in quality standards, but the results in
Figure 3 evidence some synergistic interaction between both fuels that results in poorer lubricant capacity for 30 and 50% blends than for any of the two neat fuels. As both fuels were additivated in origin to enhance lubricity, this result could be a consequence of some incompatibility between both additives.
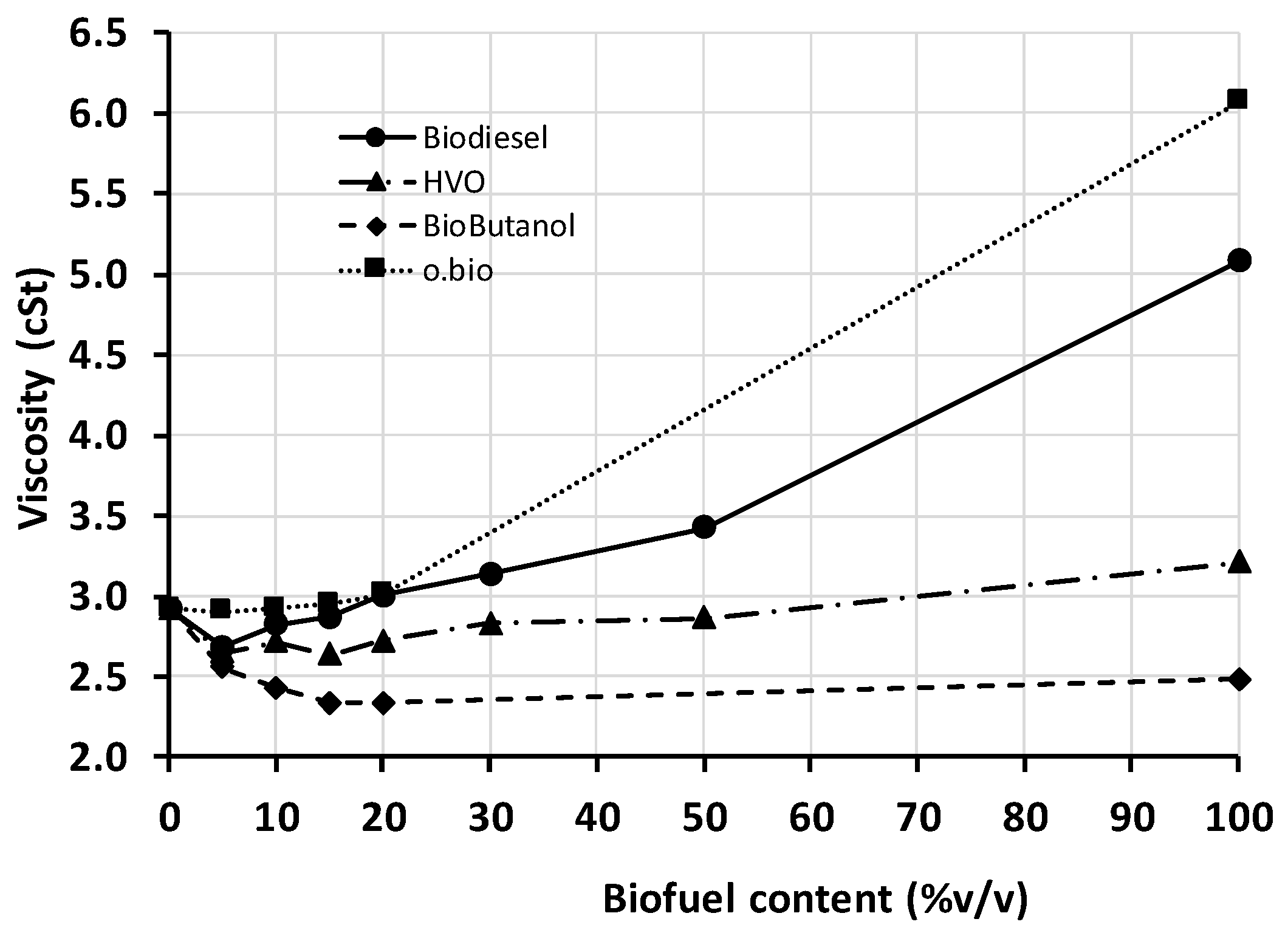
4.4. Kinematic Viscosity
Inside the combustion chamber, fuel viscosity affects the fuel jet features, fuel atomization and fuel-air mixing thus affecting combustion process and emissions. Outside the chamber, viscosity has effects on the injection system. Unlike other properties, neither too high nor too low values are desirable. Very high viscosity can reduce fuel flow rate and even damage the fuel pump. Very low viscosity increases leakages in the injection system, which is aggravated at increased ambient temperatures. Fuel quality standards are aware of these issues and fix a valid viscosity range: 2–4.5 cSt in EN 590 and EN 15940, and 3.5–5 cSt in EN 14214 (since methyl esters in biodiesel are more viscous substances).
In the present work, each sample was measured three times, and the deviation between replications never exceeded 0.02 cSt. The viscosity values are illustrated in
Figure 4. The neat biofuels ranked as follows: biobutanol showed the lowest value; followed by HVO, which viscosity was close to that of diesel fuel; and biodiesel and o.bio
® finally, the latter with the highest value. Glycerol esters (FAGE) are characterized by higher viscosities than methyl esters (FAME); therefore, if FAGE is blended in FAME as in the present o.bio
® fuel and the final FAGE/FAME blend is desired to meet the current biodiesel standard, a low viscosity FAME is preferred to enlarge the feasible FAGE content window.
The viscosity of the biofuel/diesel blends changed non-linearly with the composition; in general, when any of the biofuels examined is blended in diesel, the viscosity of the least viscous fuel prevails in the blend. This makes the viscosity of the blends to be lower than expected if this property were linear with composition. All biofuel/diesel blends fall in the permitted viscosity range, but the viscosity of all the blends (especially at up to 20% HVO) is clearly lower than those of neat diesel or HVO. Once more, if the viscosity of any of these two neat fuels was closer to the minimum limit, a blend could exhibit lower viscosity than allowed in the standards. Biodiesel/diesel and o.bio
® /diesel blends up to 20% do not change substantially the viscosity of the diesel fuel (a slight decrease is observed for biodiesel, at has been reported elsewhere [
30]); therefore, this blending range is recommended.
Finally, viscosity and lubricity in fuel are often associated in discussions (the higher the viscosity, the better the lubricity). Comparing
Figure 3 and
Figure 4, it is obvious that better lubricity is not necessarily expected in a blend with higher viscosity. Indeed, the HFRR test used to evaluate lubricity works in the boundary lubrication region [
31], where the friction coefficient is not dependent on the fluid viscosity as demonstrated in basic rheology [
32] (unlike the hydrodynamic lubrication region, where a liquid film separates the parts in relative motion and fluid viscosity plays a role).
4.5. Cold Flow Properties
Assuring enough fuel fluidity is necessary to move the fuel along the supply system (passing through the filter/s) and preventing irregular idling and startability problems. Cold flow properties are a hot issue at present as there have been some recent episodes related to insufficient values even though the fuels involved did meet the requirements in the in-force standards. The suspicions point out towards some minor components (saturated monoglycerides, sterol glucosides, soaps) present in the biodiesel fraction of current automotive fuels.
In this work, the measured properties were cloud point—CP (temperature at which some of the fuel components start to crystallize) and cold filter plugging point—CFPP (temperature at which the fuel does not pass through a filter in a required time, under standardized instrument and operating conditions). Each sample was replicated three times, and the maximum deviation between replications was 1 °C for CFPP and 0.4 °C for CP. The average results are presented in
Figure 5.
Both properties evolved quite similarly, and the values were very close. Biobutanol was not measured as its CP and CFPP values, around −90 °C (the freezing point of n-butanol), are outside the instrument range. The CP/CFPP values for o.bio® were better than for biodiesel, this being a consequence of the acetal content in o.bio®, which acts as a cold flow improver. Moreover, neat biodiesel is out of the POFF specification in EN 14214 (−10 °C in winter and 0 °C in summer, in Spain), which should be corrected by increasing the flow improvers dose.
Despite the low freezing point of n-butanol, blends up to 20% biobutanol content did not decrease the diesel CP/CFPP values, which indicates that, for diesel/biobutanol blends, the cold flow properties are exclusively determined by the diesel fraction. Diesel/HVO blends behaved linearly, being the HVO properties better than those of diesel fuel. In the case of neat HVO, the difference between CP and CFPP is larger than for the other fuels. Larger differences between CP and CFPP are typical in fuels that have been largely additivated with flow improvers, since these additives are not effective in depleting CP as they are in CFPP.
The cold flow properties of biodiesel and o.bio® blends in diesel did not change linearly, and the values exhibited by the blends resulted worse than expected if the tendency had been linear. In the case of biodiesel/diesel blends, blends up to 20% biodiesel content meet the specification for winter, POFF lower than −10 °C (comparatively, if the CFPP of these blends had evolved linearly with the biodiesel content, up to 35% biodiesel would have been admissible).
4.6. Cetane Number
Cetane number evaluates the autoignition quality of diesel fuel. The higher the cetane number, the shorter the ignition delay time of the fuel (defined as the elapsed time between the fuel injection and the start of the fuel combustion) when injected into the combustion chamber. Historically, high cetane numbers are preferred to improve cold startability and reduce combustion noise (also emissions have been reported to depend on cetane number [
24]), but the use of high injection pressures, downsized engines and multi-injection strategies have made cetane number less critical. Even in new combustion modes and dual fuel concepts, a lower cetane number could be beneficial [
33]. Nevertheless, fuel standards in Europe set a minimum value of 51 (diesel, biodiesel) and 70 (paraffinic fuels).
Each sample was measured once, but the given value is the average of 15 fuel injection events in the measuring instrument. Diesel fuel was replicated twice, and the deviation was less than 0.5 units. The results, presented in
Figure 6, show that HVO fuel has a very high cetane number as usual in paraffinic fuels, while neat biobutanol has an extremely low value (pure alcohols are more appropriate for spark ignition than for compression ignition engines). For diesel automotive applications, biobutanol/diesel blends should be limited at 10% biobutanol content to maintain a cetane number higher than 51. Sometimes the addition of biodiesel to alcohol-diesel blends has been explored to compensate for the low cetane number of alcohols [
34].
The cetane number of the o.bio® fuel was close to that of diesel, therefore all blends tested displayed similar values as well. In the case of the biodiesel fuel, the cetane number was higher than that of diesel fuel on account of its high saturated ester content. Cetane number does not impede the use of o.bio®/diesel and biodiesel/diesel blends at any concentration, although high concentrations may not fulfill the cetane number limit if other more unsaturated sources are used for biofuel production.
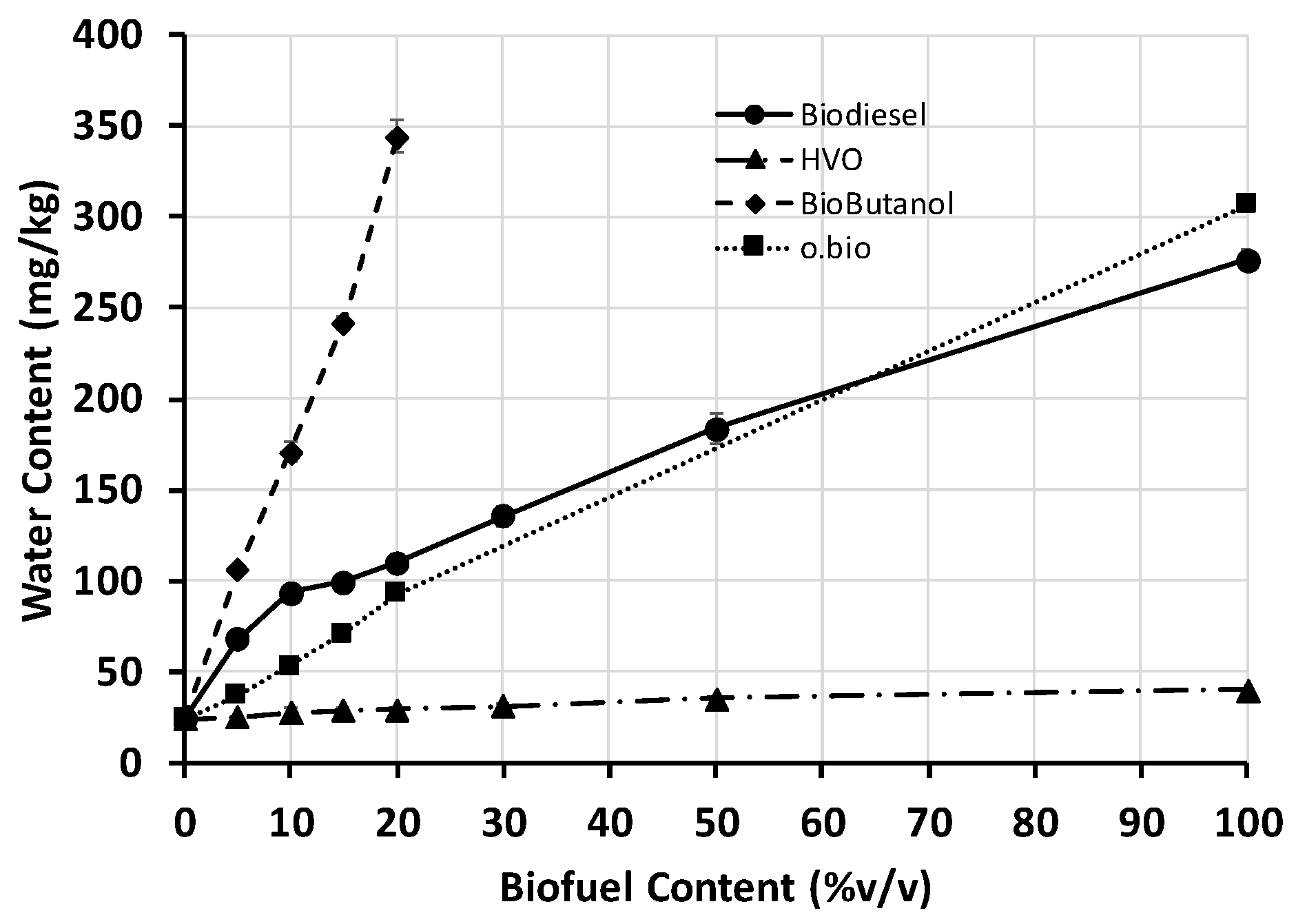
4.7. Water Content
The water content of a fuel is an important property to be controlled since a high presence of water could lead to corrosion issues, microbial growth and decrease fuel lubricity. To prevent these, European Standards EN 590 and EN 15940 limit the water content under 200 mg/kg.
Figure 7 shows the water content of the samples tested in this work. All the fuels and blends were measured three times and deviation never exceed 20 mg/kg between replications. Neat diesel and HVO showed water contents below 50 mg/kg. Both fuels meet their respective quality standards, and therefore all HVO/diesel blends. Biodiesel presented a higher water content than HVO and diesel, but still below the limit of the standard EN 14214 (500 mg/kg). O.Bio
® fuel presented similar values of water content than biodiesel, which proves the higher hygroscopicity of esters compared to hydrocarbons.
5. Conclusions
The work presented measured the main fuel properties of four selected advanced biofuels and their blends with a fossil diesel fuel. Analyzing the properties and comparing the values with the limits imposed in the involved fuel quality standards, the feasibility of some blends at low biofuel content has been proved. Biofuel properties, along with other features (sustainability, greenhouse gas balance, economic prospective), must be considered when selecting the most adequate biofuels and blending ratios to be accepted by all stakeholders involved.
In the case of HVO, waste acid-derived biodiesel and o.bio® (a blend of fatty acid methyl esters and fatty acid glycerol formal methyl esters), blends up to 20% content in biofuel have been suggested here. For HVO blends, higher blending ratios could provoke a lubricity loss if the HVO was not additivated with lubricity enhancers. For the ester-based biofuels, higher blending ratios could derive in poor cold flow properties and density and heating values too different from those assumed when calibrating the cartography of the engine. Biobutanol blends must be further restricted to 10% butanol content to avoid excessive water content and very low cetane number and heating value.
Though not evaluated here, the results found anticipate the potential of ternary blends prepared with diesel and two of the biofuels here explored. The effect of one biofuel in one particular property can be compensated with the reverse effect of other biofuels, resulting in a neutral blend with the same property value than that of the reference diesel fuel. This would be the case of density of diesel/biodiesel/HVO blends, which could be produced in a single plant and whose composition could be selected to have, in the final blend, the same density as the diesel fuel used for blending.