Solar Still Efficiency Enhancement by Using Graphene Oxide/Paraffin Nano-PCM
Abstract
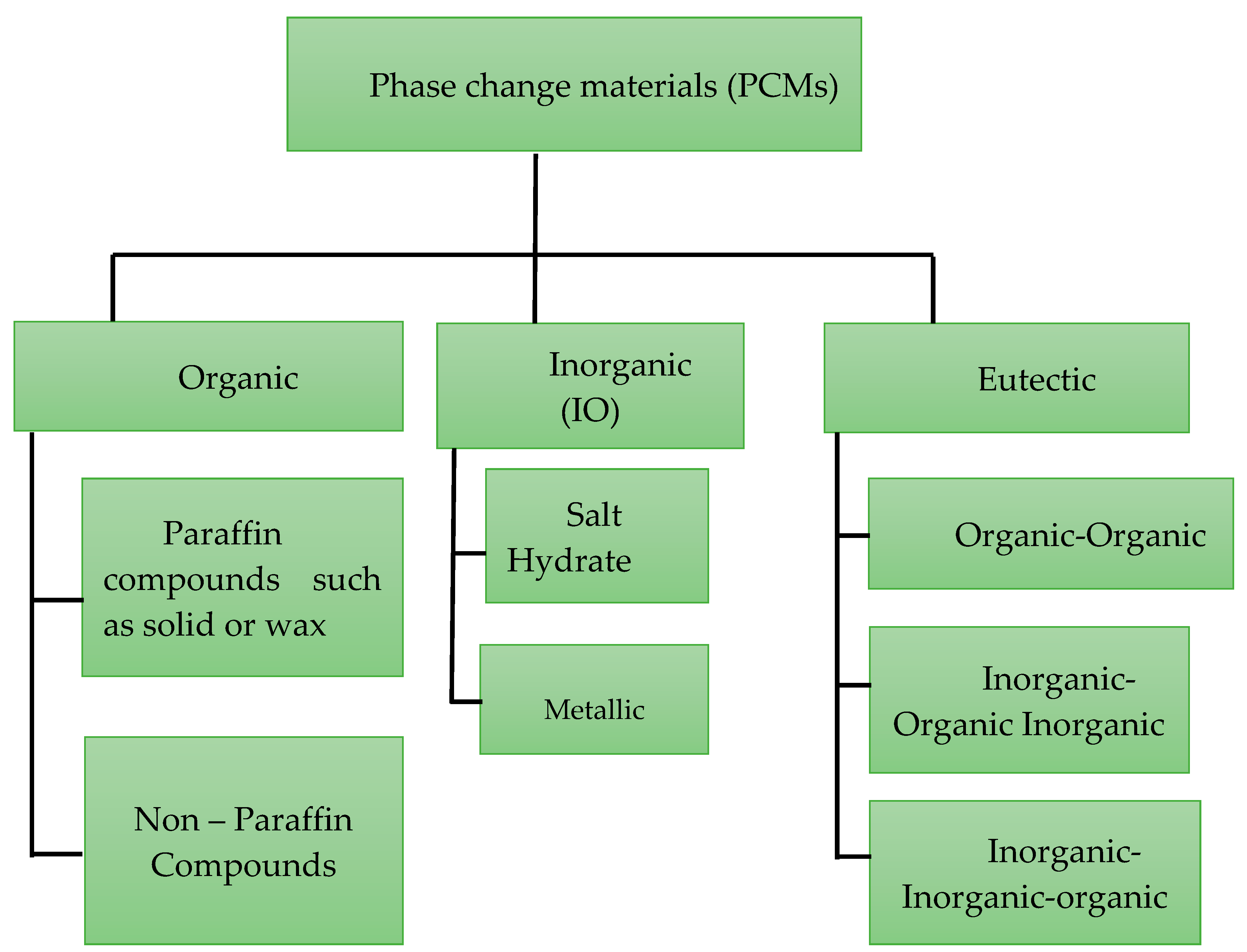
1. Introduction
2. Experimental
2.1. Thermophysical Properties
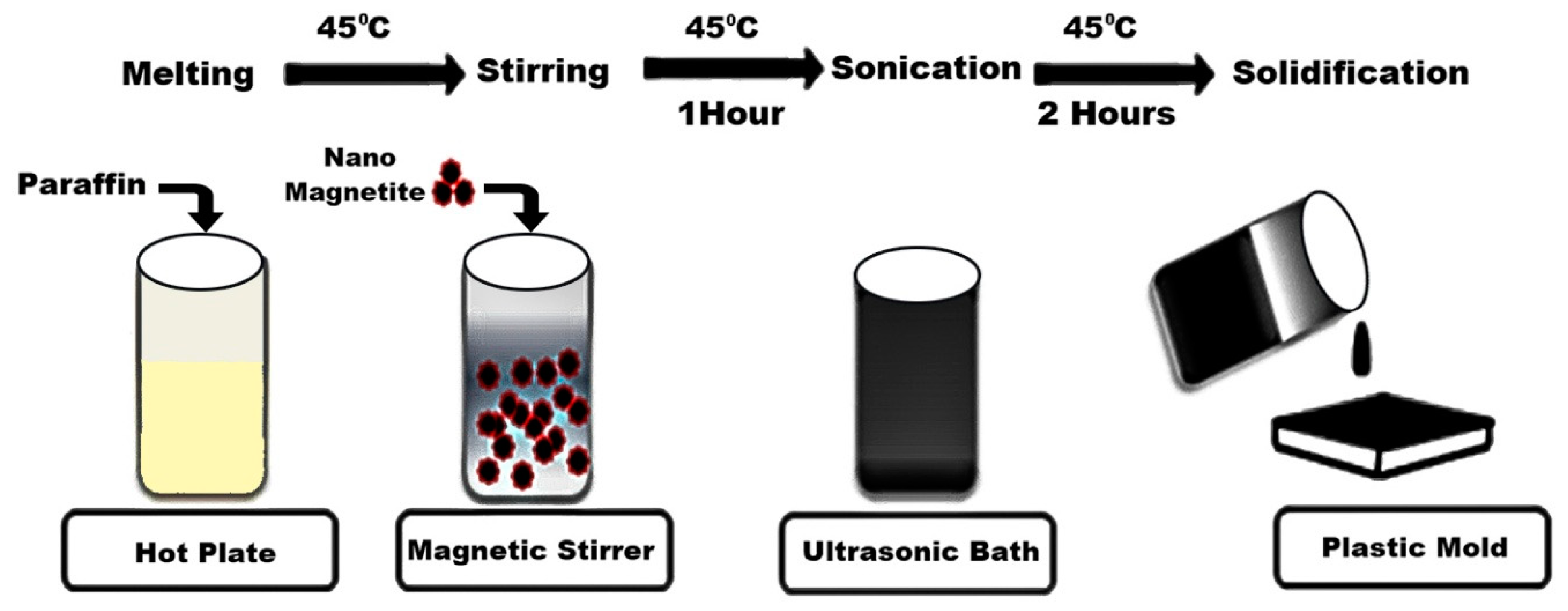
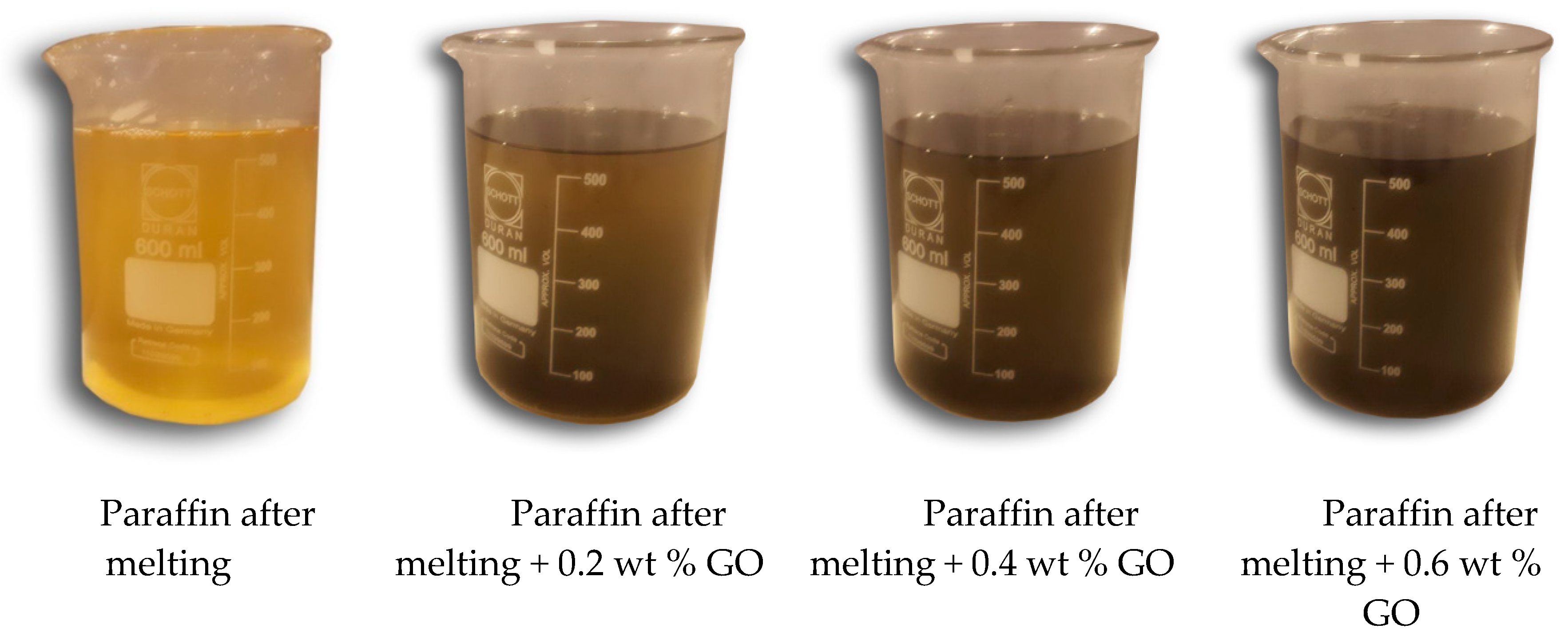
2.2. Preparation of the Nanocomposite
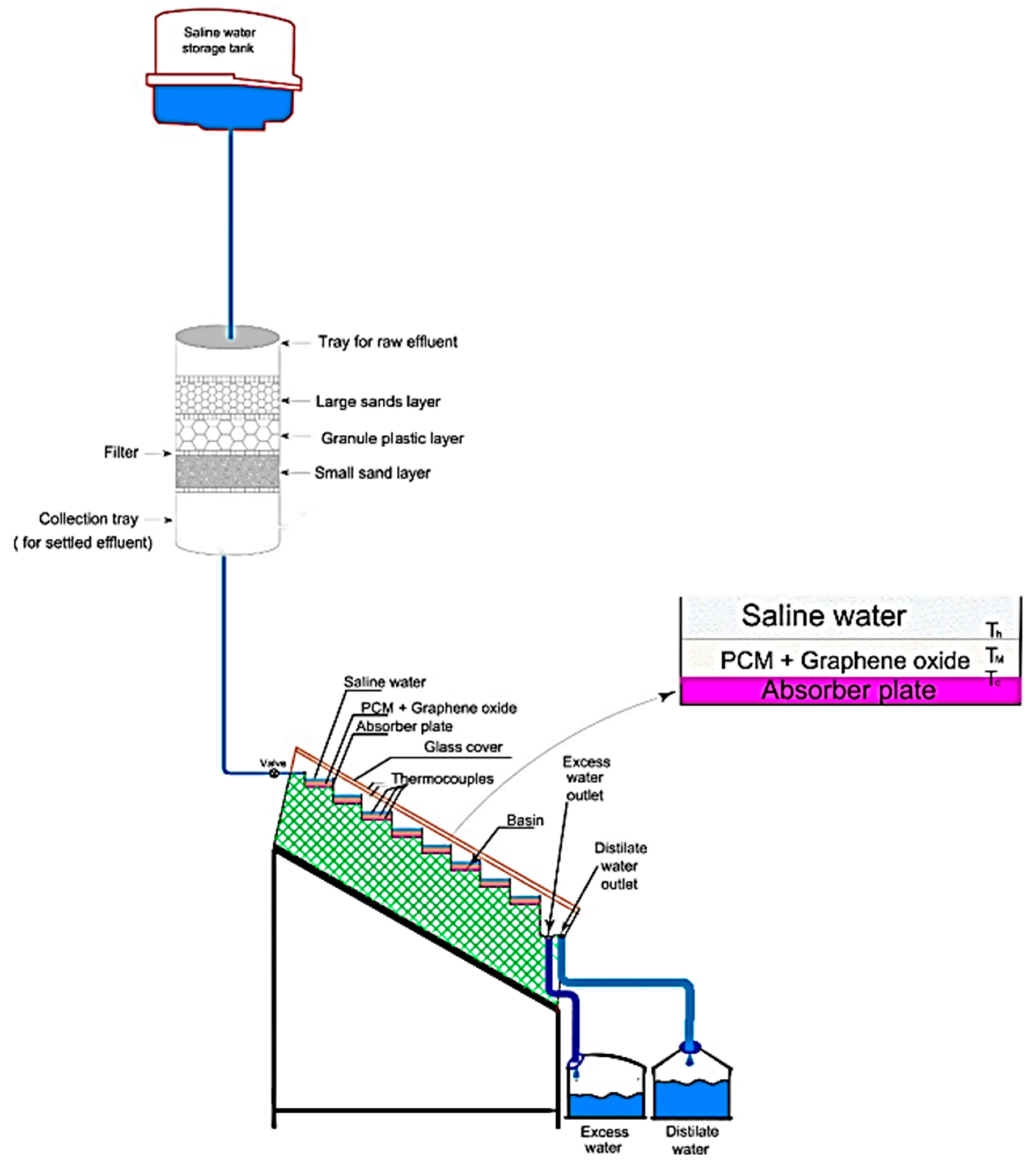
2.3. Experimental Apparatus
Uncertainties
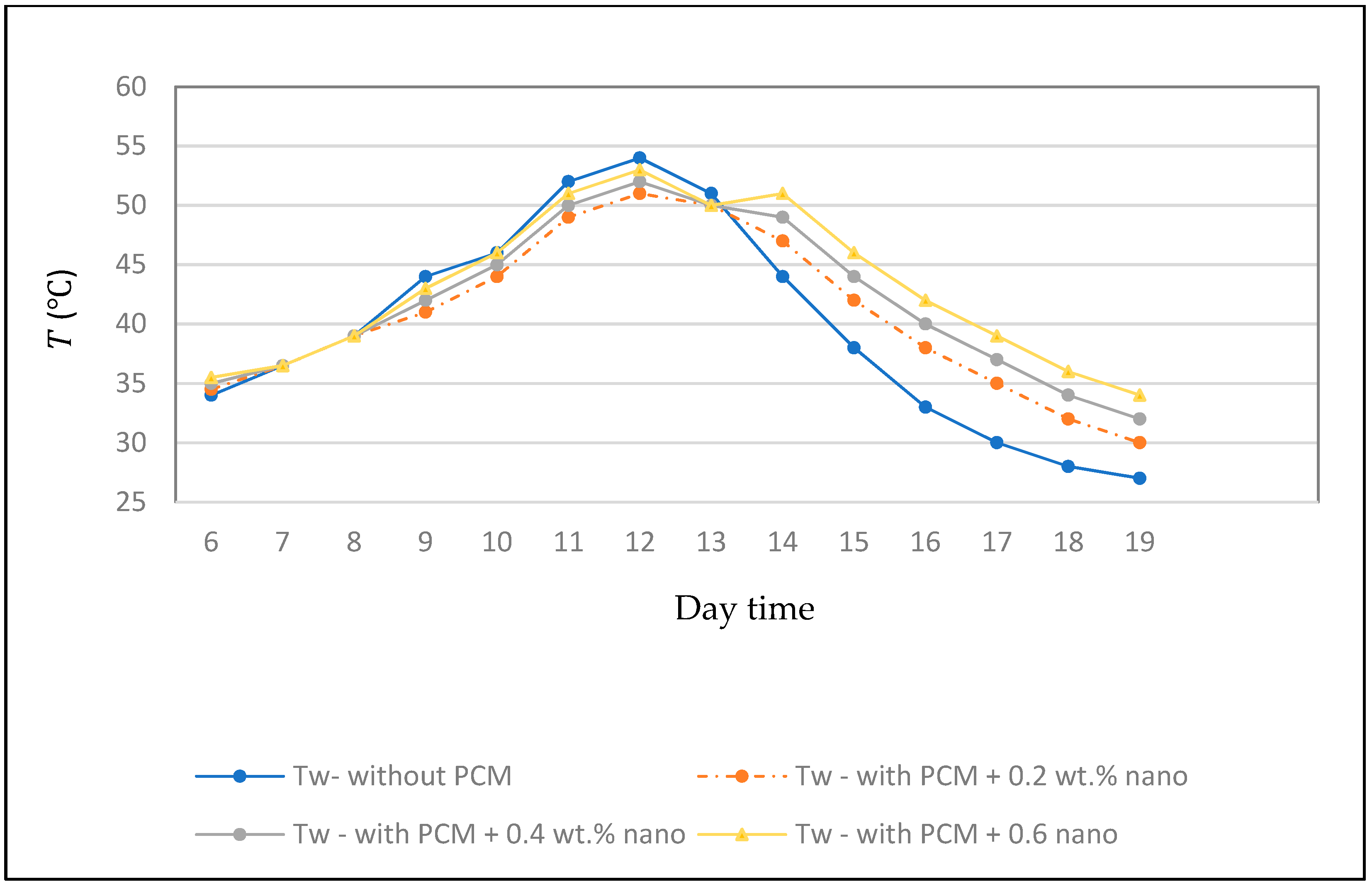
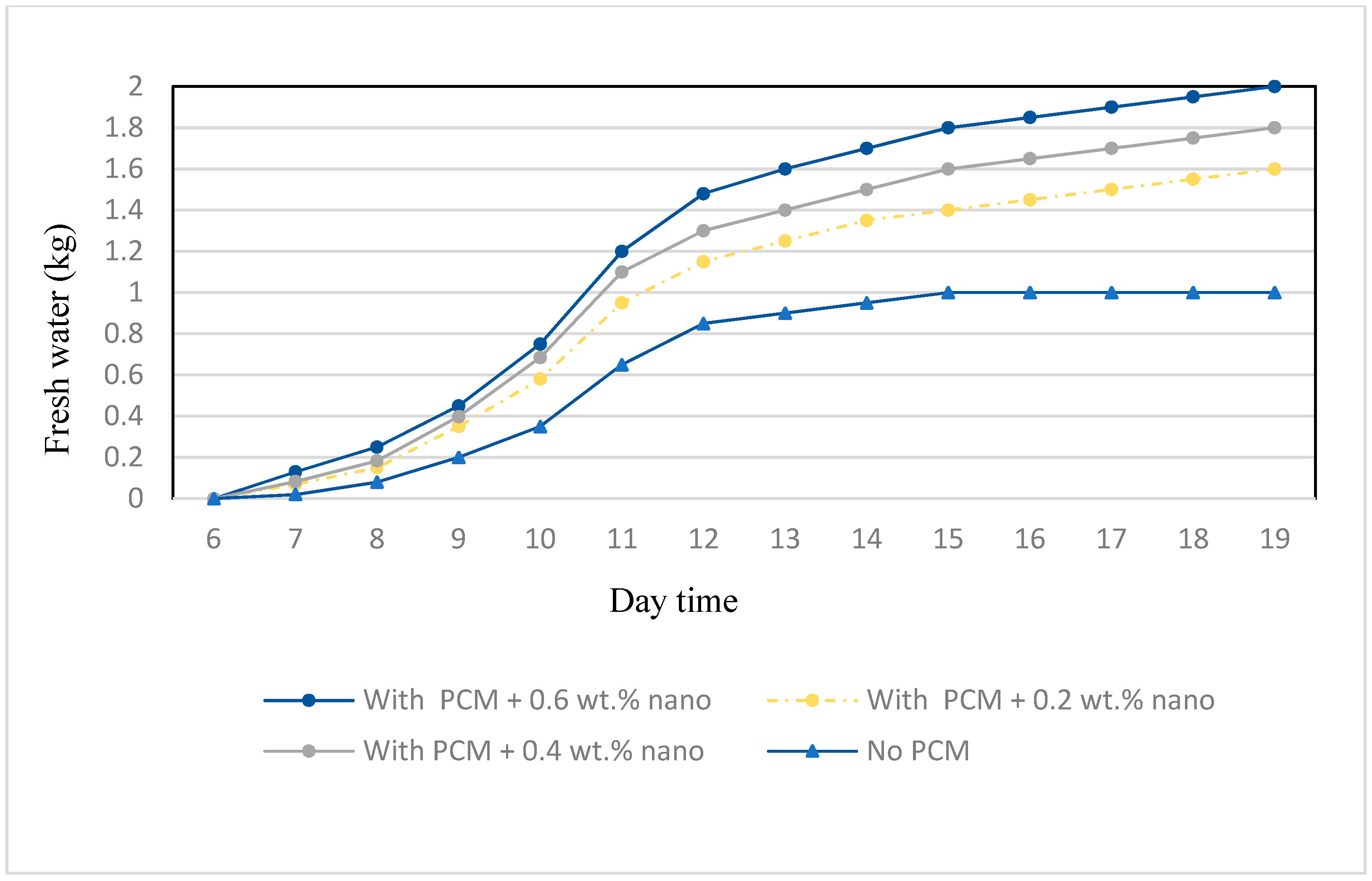
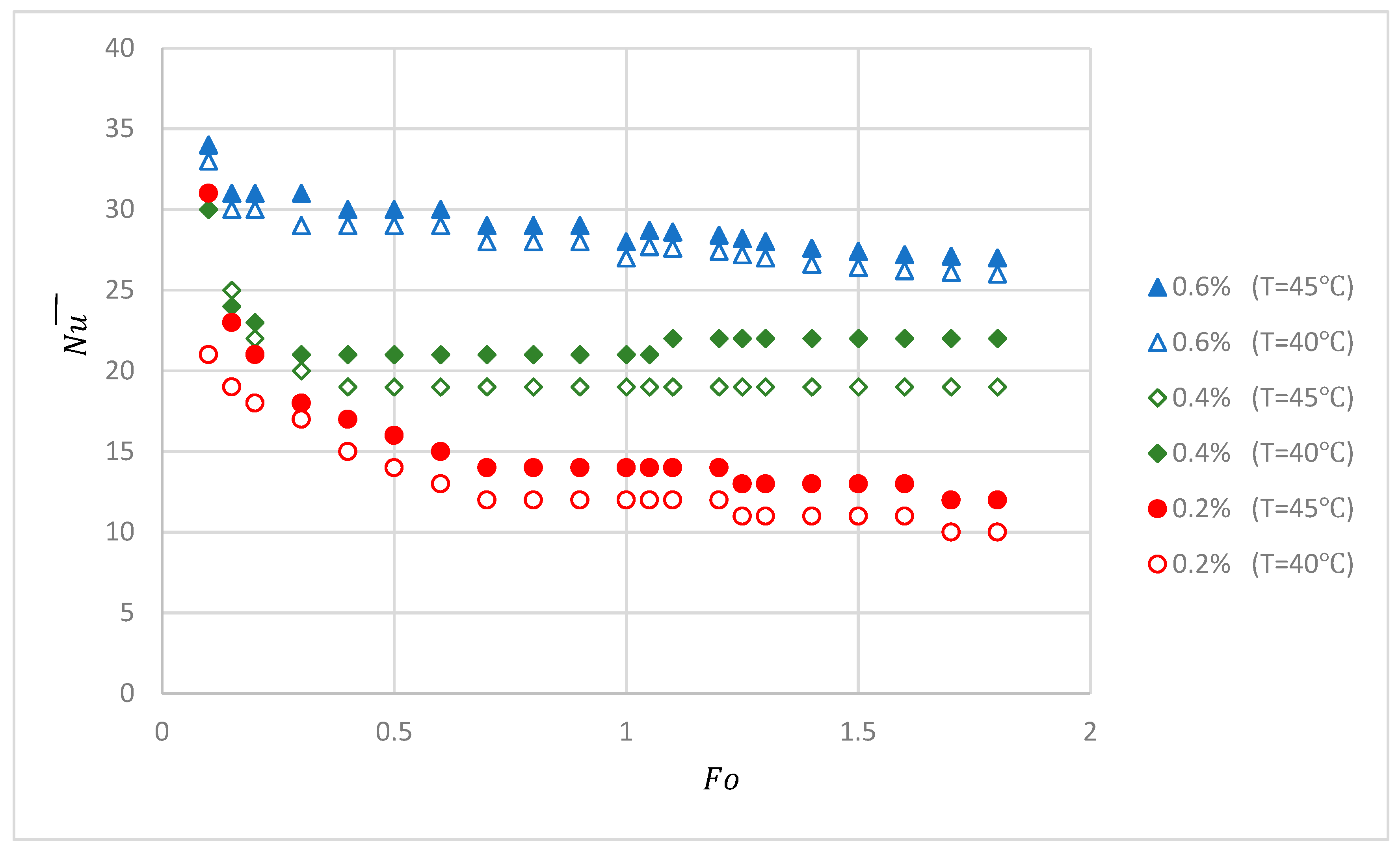
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | heat transfer area, m2 |
| B | Boltzmann constant, (1.3807 × J/K) |
| Cp | specific heat, J/(kg K) |
| Fof | Fourier number, |
| g | gravitational acceleration, m/s2 |
| hls | latent heat of fusion, kJ/kg |
| h | heat transfer coefficient in paraffin + nanomaterial (W/m2.K) |
| H | height, m |
| k | thermal conductivity, W/m k |
| L | depth, m |
| Nu | Nusselt number |
| qh | heat transfer rate at the hot wall, W |
| Raf | Rayleigh Number, g |
| Sb | subcooling parameter, ( |
| Stef | Stefan number,( |
| T | temperature, °C |
| W | width, m |
| GO | graphene oxide |
| PCM | phase-change material |
| Greek symbols | |
| thermal diffusivity, m2/s | |
| thermal expansion coefficient, 1/K | |
| dynamic viscosity Ns/m2 | |
| density, kg/m3 | |
| Φ | Percent of nanomaterial |
| correction factor (-) | |
| Subscripts | |
| air | air |
| C | cold wall |
| f | base fluid |
| h | hot surface |
| m | quantities for dispersed nanomaterial in the paraffin emulsion |
| M | average temperature of PCM |
References
- Asbik, M.; Ansari, O.; Bah, A.; Zari, N.; Mimet, A.; El-Ghetany, H. Exergy analysis of solar desalination still combined with heat storage system using phase change material (PCM). Desalination 2016, 381, 26–37. [Google Scholar] [CrossRef]
- Faegh, M.; Shafii, M.B. Experimental investigation of a solar still equipped with an external heat storage system using phase change materials and heat pipes. Desalination 2017, 409, 128–135. [Google Scholar] [CrossRef]
- Pandey, A.; Hossain, M.; Tyagi, V.; Rahim, N.A.; Jeyraj, A.; Selvaraj, L.; Sari, A. Novel approaches and recent developments on potential applications of phase change materials in solar energy. Renew. Sustain. Energy Rev. 2018, 82, 281–323. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.; Xu, Y.; Dong, J. A review on supercooling of Phase Change Materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 905–919. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, P.-F.; Tzeng, C.-T.; Lai, C.-M. Thermal Performance of Microencapsulated Phase Change Material (mPCM) in Roof Modules during Daily Operation. Energies 2018, 11, 679. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Sun, Z. Numerical simulation and optimization of the melting process of phase change material inside horizontal annulus. Energies 2017, 10, 1249. [Google Scholar]
- Kumar, T.S.; Jegadheeswaran, S.; Chandramohan, P. Performance investigation on fin type solar still with paraffin wax as energy storage media. J. Therm. Anal. Calorim. 2019, 136, 101–112. [Google Scholar] [CrossRef]
- Kabeel, A.E.; Abdelgaied, M. Improving the performance of solar still by using PCM as a thermal storage medium under Egyptian conditions. Desalination 2016, 383, 22–28. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.; Sahin, A. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Cerritelli, G.F.; Miliozzi, A.; Kenny, J.M. Effect of nanoparticles on heat capacity of nanofluids based on molten salts as PCM for thermal energy storage. Nanoscale Res. Lett. 2013, 8, 448. [Google Scholar] [CrossRef]
- Olia, H.; Torabi, M.; Bahiraei, M.; Ahmadi, M.H.; Goodarzi, M.; Safaei, M.R. Application of nanofluids in thermal performance enhancement of parabolic trough solar collector: state-of-the-art. Appl. Sci. 2019, 9, 463. [Google Scholar] [CrossRef]
- Sheikholeslami, M. Numerical simulation for solidification in a LHTESS by means of nano-enhanced PCM. J. Taiwan Inst. Chem. Eng. 2018, 86, 25–41. [Google Scholar] [CrossRef]
- Shahsavar, A.; Khanmohammadi, S.; Karimipour, A.; Goodarzi, M. A novel comprehensive experimental study concerned synthesizes and prepare liquid paraffin-Fe3O4 mixture to develop models for both thermal conductivity & viscosity: A new approach of GMDH type of neural network. Int. J. Heat Mass Transf. 2019, 131, 432–441. [Google Scholar]
- Rufuss, D.D.W.; Iniyan, S.; Suganthi, L.; Davies, P. Nanoparticles enhanced phase change material (NPCM) as heat storage in solar still application for productivity enhancement. Energy Procedia 2017, 141, 45–49. [Google Scholar] [CrossRef]
- Şahan, N.; Fois, M.; Paksoy, H. Improving thermal conductivity phase change materials—A study of paraffin nanomagnetite composites. Sol. Energy Mater. Sol. Cells 2015, 137, 61–67. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, R.; Peng, F.; Fang, Y.; Akiyama, T.; Wang, S. Synthesis, characterization and thermal properties of paraffin microcapsules modified with nano-Al2O3. Appl. Energy 2015, 137, 731–737. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, J.; Song, G.; Liu, Y.; Tang, G. The experimental exploration of nano-Si3N4/paraffin on thermal behavior of phase change materials. Thermochim. Acta 2014, 597, 101–106. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Koh, W.-G. Magnetic nanoparticle-embedded PCM nanocapsules based on paraffin core and polyurea shell. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 46–51. [Google Scholar] [CrossRef]
- Fan, L.-W.; Fang, X.; Wang, X.; Zeng, Y.; Xiao, Y.-Q.; Yu, Z.-T.; Xu, X.; Hu, Y.-C.; Cen, K.-F. Effects of various carbon nanofillers on the thermal conductivity and energy storage properties of paraffin-based nanocomposite phase change materials. Appl. Energy 2013, 110, 163–172. [Google Scholar] [CrossRef]
- Jesumathy, S.; Udayakumar, M.; Suresh, S. Experimental study of enhanced heat transfer by addition of CuO nanoparticle. Heat Mass Transf. 2012, 48, 965–978. [Google Scholar] [CrossRef]
- Shi, J.-N.; Ger, M.-D.; Liu, Y.-M.; Fan, Y.-C.; Wen, N.-T.; Lin, C.-K.; Pu, N.-W. Improving the thermal conductivity and shape-stabilization of phase change materials using nanographite additives. Carbon 2013, 51, 365–372. [Google Scholar] [CrossRef]
- Nourani, M.; Hamdami, N.; Keramat, J.; Moheb, A.; Shahedi, M. Thermal behavior of paraffin-nano-Al2O3 stabilized by sodium stearoyl lactylate as a stable phase change material with high thermal conductivity. Renew. Energy 2016, 88, 474–482. [Google Scholar] [CrossRef]
- Li, M. A nano-graphite/paraffin phase change material with high thermal conductivity. Appl. Energy 2013, 106, 25–30. [Google Scholar] [CrossRef]
- Warzoha, R.J.; Weigand, R.M.; Fleischer, A.S. Temperature-dependent thermal properties of a paraffin phase change material embedded with herringbone style graphite nanofibers. Appl. Energy 2015, 137, 716–725. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R. Diurnal thermal evaluation of an evacuated tube solar collector (ETSC) charged with graphene nanoplatelets-methanol nano-suspension. Renew. Energy 2019, 142, 364–372. [Google Scholar] [CrossRef]
- Sarafraz, M.; Tlili, I.; Abdul Baseer, M.; Safaei, M.R. Potential of Solar Collectors for Clean Thermal Energy Production in Smart Cities using Nanofluids: Experimental Assessment and Efficiency Improvement. Appl. Sci. 2019, 9, 1877. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, L.; Ma, Z.; Gonzalez-Diaz, A.; Wang, Y.; Roskilly, A.P. Comparative Analysis of Small-Scale Organic Rankine Cycle Systems for Solar Energy Utilisation. Energies 2019, 12, 829. [Google Scholar] [CrossRef]
- Rashidi, S.; Akar, S.; Bovand, M.; Ellahi, R. Volume of fluid model to simulate the nanofluid flow and entropy generation in a single slope solar still. Renew. Energy 2018, 115, 400–410. [Google Scholar] [CrossRef]
- Bareschino, P.; Pepe, F.; Roselli, C.; Sasso, M.; Tariello, F. Desiccant-Based Air Handling Unit Alternatively Equipped with Three Hygroscopic Materials and Driven by Solar Energy. Energies 2019, 12, 1543. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Rashidi, S.; Karimi, N.; Ellahi, R. Convection of heat and thermodynamic irreversibilities in two-phase, turbulent nanofluid flows in solar heaters by corrugated absorber plates. Adv. Powder Technol. 2018, 29, 2243–2254. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Park, J.Y. Thermo-Fluid Dynamic Effects of the Radial Location of the Baffle Installed in a Solar Updraft Tower. Energies 2019, 12, 1340. [Google Scholar] [CrossRef]
- Abdollahzadeh, M.; Esmaeilpour, M. Enhancement of phase change material (PCM) based latent heat storage system with nano fluid and wavy surface. Int. J. Heat Mass Transf. 2015, 80, 376–385. [Google Scholar] [CrossRef]
- Sheikholeslami, M. Numerical modeling of nano enhanced PCM solidification in an enclosure with metallic fin. J. Mol. Liq. 2018, 259, 424–438. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K.; Namburu, P.K. Numerical study of fluid dynamic and heat transfer performance of Al2O3 and CuO nanofluids in the flat tubes of a radiator. Int. J. Heat Fluid Flow 2010, 31, 613–621. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Nsofor, E.C. Solidification of a PCM with nanoparticles in triplex-tube thermal energy storage system. Appl. Therm. Eng. 2016, 108, 596–604. [Google Scholar] [CrossRef]
- Arasu, A.V.; Mujumdar, A.S. Numerical study on melting of paraffin wax with Al2O3 in a square enclosure. Int. Commun. Heat Mass Transf. 2012, 39, 8–16. [Google Scholar] [CrossRef]
- Shukla, A.K.; Sudhakar, K.; Baredar, P. Design, simulation and economic analysis of standalone roof top solar PV system in India. Sol. Energy 2016, 136, 437–449. [Google Scholar] [CrossRef]
- Sint, N.K.C.; Choudhury, I.; Masjuki, H.H.; Aoyama, H. Theoretical analysis to determine the efficiency of a CuO-water nanofluid based-flat plate solar collector for domestic solar water heating system in Myanmar. Sol. Energy 2017, 155, 608–619. [Google Scholar] [CrossRef]
- Kermani, M.; Goudarzi, G.; Shahsavani, A.; Dowlati, M.; Asl, F.B.; Karimzadeh, S.; Jokandan, S.F.; Aghaei, M.; Kakavandi, B.; Rastegarimehr, B. Estimation of short-term mortality and morbidity attributed to fine particulate matter in the ambient air of eight Iranian cities. Ann. Glob. Health 2018, 84. [Google Scholar] [CrossRef]
- Llorens, D. Which Direction Should Solar Panels Face? Available online: https://solarpowerrocks.com/solar-basics/which-direction-should-solar-panels-face/ (accessed on 31 March 2019).
- Sampathkumar, K.; Senthilkumar, P. Utilization of solar water heater in a single basin solar still—An experimental study. Desalination 2012, 297, 8–19. [Google Scholar] [CrossRef]
- Ho, C.J.; Gao, J.Y. An experimental study on melting heat transfer of paraffin dispersed with Al2O3 nanoparticles in a vertical enclosure. Int. J. Heat Mass Transf. 2013, 62, 2–8. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ebadi, S.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Melting of nano-PCM inside a cylindrical thermal energy storage system: Numerical study with experimental verification. Energy Convers. Manag. 2018, 166, 241–259. [Google Scholar] [CrossRef]

| Author | Used Nanomaterials | Results |
|---|---|---|
| Rufuss et al. [15] | Copper (II) oxide | 35% improvement in the productivity. |
| Sahan et al. [16] | Iron (II,III) oxide | Thermal conductivity of paraffin raised by 48% and 60%, by employing 10% and 20% wt.% of Fe3O4 nanoparticles. |
| Jiang et al. [17] | Aluminum oxide | 15% latent heat decrement and 27% thermal conductivity increment. |
| Yang et al. [18] | Silicon Nitride | Thermal conductivity rising by 35%, utilizing 10% nanoparticles. |
| Park et al. [19] | Iron (II,III) oxide | 45% latent heat decrement and 45% thermal conductivity enhancement by dispersing 6.6% nanoparticles in the base PCM. |
| Fan et al. [20] | Graphene | 164% thermal conductivity increment, by dispersing 5% nanomaterials in the paraffin. |
| Jesumatty et al. [21] | Copper (II) oxide | 6%, 6.7%, and 7.8% thermal conductivity augmentation, by dispersing 2%, 5%, and 10% nanoparticles in the base PCM. |
| Shi et al. [22] | Graphite nanoplatelets | Augmenting the thermal conductivity of PCM from 0.25 to 2.7, by dispersing 10% of nanomaterial in the base PCM. |
| Nourani et al. [23] | Aluminum oxide | Adding 10% nanoparticles to the base paraffin afford a 0.09 W/m °C thermal conductivity improvement. |
| Li [24] | Graphite | Adding 10% graphite nanomaterial to the base PCM, cause a 640.43% thermal conductivity enhancement. |
| Warzoha et al. [25] | Graphite nanofibers | 180% thermal conductivity increase and 10% latent heat decrease by adding 11.4% graphite nanofibers to the base paraffin. |
| Thermophysical Property | Paraffin | Graphene Oxide |
|---|---|---|
| Density (kg/m3) | 802 | 3600 |
| Specific heat (J/kg K) | 2320 (liquid) | 765 |
| Thermal conductivity (W/m K) | 0.23 (liquid) | 3000 |
| Dynamic viscosity (kg/m s) | 1.3 × 10−3 | - |
| Thermal expansion coefficient (1/K) | 9.1 × 10−4 | 1.25 × 10−5 |
| Latent heat (kJ/kg) | 226 | - |
| Melting temperature (°C | 44 | - |
| Parameters | Dimensions |
|---|---|
| Length of tank | 600 mm |
| Width of tank | 400 mm |
| Height of tank | 2800 mm |
| Inclination angles | 32.5° |
| Length of the glass covers | 1300 mm |
| Width of the glass covers | 1000 mm |
| Thickness of the glass covers | 4 mm |
| No. | Instrument | Range | Accuracy | % Error |
|---|---|---|---|---|
| 1 | Solarimeter | 0–900 W/m2 | ±4 W/m2 | 5 |
| 2 | Thermocouple | 0–300 °C | ±1 °C | 4 |
| 3 | Thermometer | 0–95 °C | ±0.1 °C | 0.4 |
| 4 | Anemometer | 0–12 m/s | ±0.1 m/s | 10 |
| 5 | Measuring jar | 0–1000 mL | ±9 mL | 9 |
| Φ | a | b | c | Average Deviation (%) | Parameter Ranges |
|---|---|---|---|---|---|
| 0% | 0.6 | 0.3 | −0.2 | 4.23 | Sb = 0.075–0.85; |
| 0.2% | 0.65 | 0.35 | −0.23 | 7 | Sb = 0.075–0.85; |
| 0.4% | 0.7 | 0.4 | −0.3 | 9 | Sb = 0.075–0.85; |
| 0.6% | 0.8 | 0.5 | −0.4 | 10.5 | Sb = 0.075–0.85; . |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safaei, M.R.; Goshayeshi, H.R.; Chaer, I. Solar Still Efficiency Enhancement by Using Graphene Oxide/Paraffin Nano-PCM. Energies 2019, 12, 2002. https://doi.org/10.3390/en12102002
Safaei MR, Goshayeshi HR, Chaer I. Solar Still Efficiency Enhancement by Using Graphene Oxide/Paraffin Nano-PCM. Energies. 2019; 12(10):2002. https://doi.org/10.3390/en12102002
Chicago/Turabian StyleSafaei, Mohammad Reza, Hamid Reza Goshayeshi, and Issa Chaer. 2019. "Solar Still Efficiency Enhancement by Using Graphene Oxide/Paraffin Nano-PCM" Energies 12, no. 10: 2002. https://doi.org/10.3390/en12102002
APA StyleSafaei, M. R., Goshayeshi, H. R., & Chaer, I. (2019). Solar Still Efficiency Enhancement by Using Graphene Oxide/Paraffin Nano-PCM. Energies, 12(10), 2002. https://doi.org/10.3390/en12102002






