Algal Biofuels: Current Status and Key Challenges
Abstract
1. Introduction
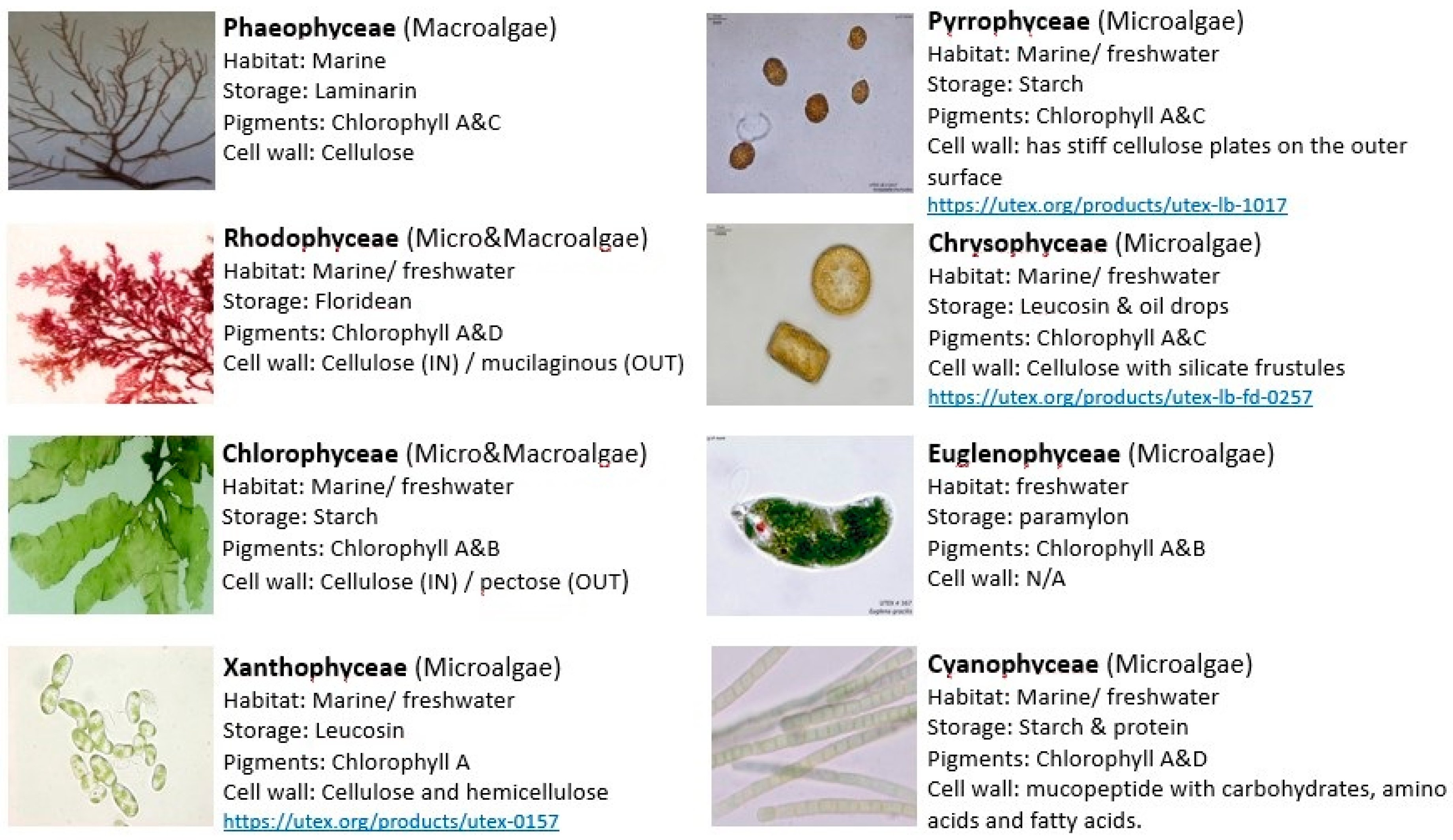
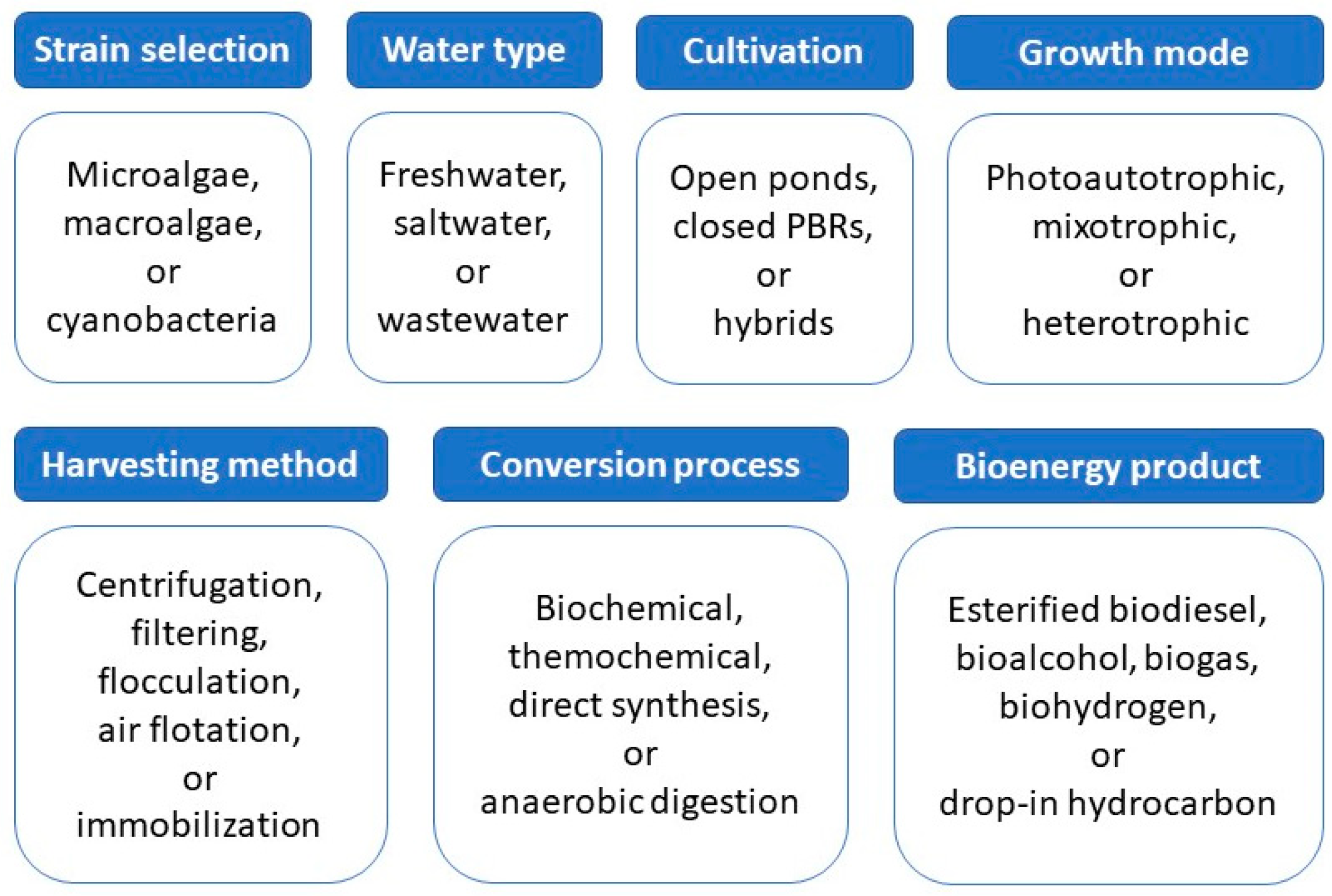
2. Algae
2.1. Microalgal Cultivation
2.2. Algal Harvesting
2.3. Algal Fuels
2.4. Conversion Techniques
2.4.1. Thermochemical Conversion
2.4.2. Biochemical Pathways
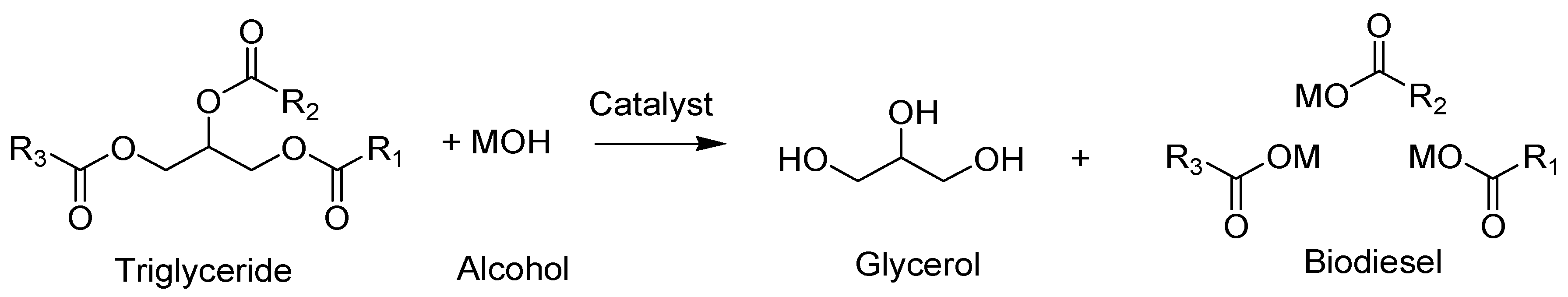
2.4.3. Transesterification
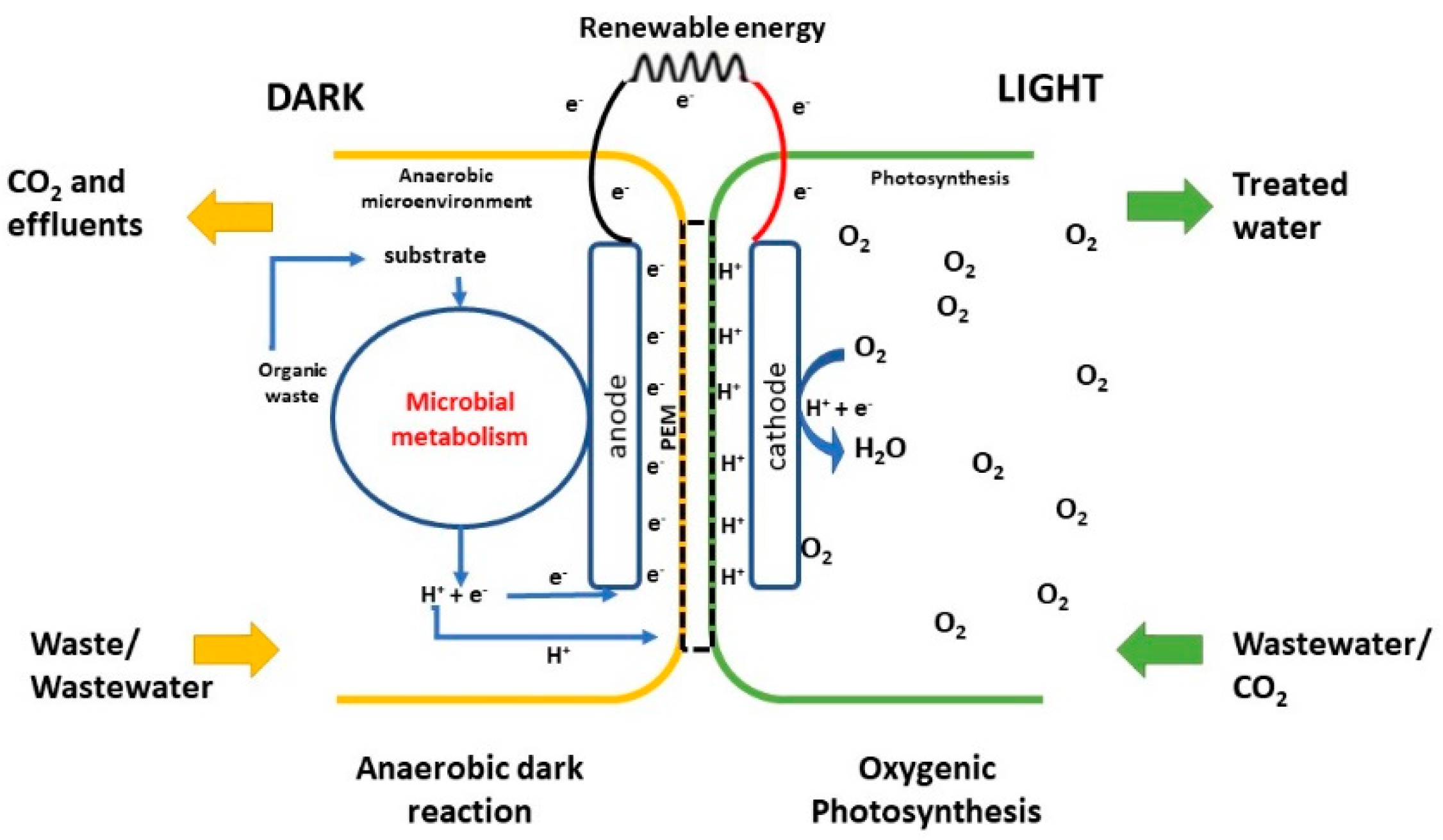
2.4.4. Photosynthetic Microbial Fuel Cell
3. Genetic Engineering Toward Biofuels
4. Current Status and Challenges
5. Biorefinery/Valorization
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhore, N. Energy Outlook: A View to 2040. Detroit Automotive Petroleum Forum: Detroit, MI, USA. Available online: https://www.api.org/~/media/files/certification/engine-oil-diesel/forms/whats-new/6-energy-outlook-view to 2040-nbhore-exxonmobil.pdf (accessed on 17 May 2019).
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Judge, D.; Earnshaw, D. The European Parliament; Palgrave: Basingstoke, UK, 2003. [Google Scholar]
- Office of Energy Efficiency & Renewable Energy. Biofuels Basics. Available online: https://www.energy.gov/eere/bioenergy/biofuels-basics (accessed on 17 May 2019).
- European Biofuels Technology Platform. Strategic Research and Innovation Agenda 2016; European Biofuels Technology Platform: London, UK, 2016. [Google Scholar]
- Lü, J.; Sheahan, C.; Fu, P. Metabolic engineering of algae for fourth generation biofuels production. Energy Environ. Sci. 2011, 4, 2451–2466. [Google Scholar] [CrossRef]
- Rosenthal, E. UN report describes risks of inaction on climate change. The New York Times, 2007; 17. [Google Scholar]
- Brown, T.R.; Brown, R.C. A review of cellulosic biofuel commercial-scale projects in the United States. Biofuels Bioprod. Biorefin. 2013, 7, 235–245. [Google Scholar] [CrossRef]
- Nigam, G.; Singh, R.; Chaturvedi, A.K. Finite duration root nyquist pulses with maximum in-band fractional energy. IEEE Commun. Lett. 2010, 14, 797–799. [Google Scholar] [CrossRef]
- Dutta, K.; Daverey, A.; Lin, J. Evolution retrospective for alternative fuels: First to fourth generation. Renew. Energy 2014, 69, 114–122. [Google Scholar] [CrossRef]
- Sawin, J.L.; Martinot, E.; Sonntag-O’Brien, V.; McCrone, A.; Roussell, J.; Barnes, D.; Flavin, C.; Mastny, L.; Kraft, D.; Wang, S.; et al. Renewables 2010—Global Status Report; Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ) GmbH: Paris, France, 2013. [Google Scholar]
- Shuba, E.S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Beacham, T.A.; Sweet, J.B.; Allen, M.J. Large scale cultivation of genetically modified microalgae: A new era for environmental risk assessment. Algal Res. 2017, 25, 90–100. [Google Scholar] [CrossRef]
- Levitan, O.; Dinamarca, J.; Hochman, G.; Falkowski, P.G. Diatoms: A fossil fuel of the future. Trends Biotechnol. 2014, 32, 117–124. [Google Scholar] [CrossRef]
- Shafik, H.M.; Saad, M.G.; El-Serehy, H.A. Impact of nitrogen regime on fatty acid profiles of Desmodesmus quadricaudatus and Chlorella sp. and ability to produce biofuel. Acta Bot. Hung. 2015, 57, 205–218. [Google Scholar] [CrossRef]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae biofuel: Current status and future applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Tsukahara, K.; Sawayama, S. Liquid fuel production using microalgae. J. Jpn. Pet. Inst. 2005, 48, 251. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Rawat, I.; Kumar, R.R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Renewable and sustainable bioenergies production from palm oil mill effluent (POME): Win–win strategies toward better environmental protection. Biotechnol. Adv. 2011, 29, 124–141. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, T.; Yu, X.; Bates, P.D.; Dong, T.; Chen, S. High-density fed-batch culture of a thermotolerant microalga Chlorella sorokiniana for biofuel production. Appl. Energy 2013, 108, 281–287. [Google Scholar] [CrossRef]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Love, K.R.; Bagh, S.; Choi, J.; Love, J.C. Microtools for single-cell analysis in biopharmaceutical development and manufacturing. Trends Biotechnol. 2013, 31, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wang, Y.; Qin, J. Microalgal motility measurement microfluidic chip for toxicity assessment of heavy metals. Anal. Bioanal. Chem. 2012, 404, 3061–3069. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, J.; Worrall, C.; Vasilcanu, D.; Fryknäs, M.; Sulaiman, L.; Karimi, M.; Weng, W.H.; Lui, W.O.; Rudduck, C.; Axelson, M.; et al. Molecular characterization of acquired tolerance of tumor cells to picropodophyllin (PPP). PLoS ONE 2011, 6, e14757. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, J.Y.H.; Kwak, H.S.; Sim, S.J. Integrated microfluidic platform for multiple processes from microalgal culture to lipid extraction. Anal. Chem. 2014, 86, 8585–8592. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.L.; Kuo, M.Y.; Juang, Y.J. Development of flow through dielectrophoresis microfluidic chips for biofuel production: Sorting and detection of microalgae with different lipid contents. Biomicrofluidics 2014, 8, 064120. [Google Scholar] [CrossRef]
- Wang, K.; Brown, R.C.; Homsy, S.; Martinez, L.; Sidhu, S.S. Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production. Bioresour. Technol. 2013, 127, 494–499. [Google Scholar] [CrossRef]
- Abalde-Cela, S.; Gould, A.; Liu, X.; Kazamia, E.; Smith, A.G.; Abell, C. High-throughput detection of ethanol-producing cyanobacteria in a microdroplet platform. J. R. Soc. Interface 2015, 12, 20150216. [Google Scholar] [CrossRef]
- Hammar, P.; Angermayr, S.A.; Sjostrom, S.L.; van der Meer, J.; Hellingwerf, K.J.; Hudson, E.P.; Joensson, H.N. Single-cell screening of photosynthetic growth and lactate production by cyanobacteria. Biotechnol. Biofuels 2015, 8, 193. [Google Scholar] [CrossRef]
- Liao, Q.; Chang, J.S.; Herrmann, C.; Xia, A. Bioreactors for Microbial Biomass and Energy Conversion; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Lee, O.K.; Lee, E.Y. Sustainable production of bioethanol from renewable brown algae biomass September. Biomass Bioenergy 2016, 92, 70–75. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Leite, G.B.; Abdelaziz, A.E.M.; Hallenbeck, P.C. Bioresource technology algal biofuels: Challenges and opportunities. Bioresour. Technol. 2013, 145, 134–141. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Shaikh Abdur, R.; Saad Aldin, M.A.; Mohammad Mozahar, H.; Hugo, L. Biological CO2 fixation with production of microalgae in wastewater: A review. Renew. Sustain. Energy Rev. 2017, 76, 379–390. [Google Scholar]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C. Second generation biofuels: High-effjciency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Daliry, S.; Hallajsani, A.; Mohammadi Roshandeh, J.; Nouri, H.; Golzary, A. Investigation of optimal condition for Chlorella vulgaris microalgae growth. Glob. J. Environ. Sci. Manag. 2017, 3, 217–230. [Google Scholar]
- Chen, C.Y.; Durbin, E.G. Effects of pH on the growth and carbon uptake of marine phytoplankton. Mar. Ecol. Ser. 1994, 109, 83. [Google Scholar] [CrossRef]
- Huppe, H.C.; Turpin, D.H. Integration of carbon and nitrogen metabolism in plant and algal cells. Annu. Rev. Plant Biol. 1994, 45, 577–607. [Google Scholar] [CrossRef]
- Andrews, J.F. A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol. Bioenergy 1968, 10, 707–723. [Google Scholar] [CrossRef]
- Martinez, F.; Ascaso, C.; Orus, M.I. Morphometric and stereologic analysis of Chlorella vulgaris under heterotrophic growth conditions. Ann. Bot. 1991, 67, 239–245. [Google Scholar] [CrossRef]
- Scarsella, M.; Belotti, G.; De Filippis, P.; Bravi, M. Study on the optimal growing conditions of Chlorella vulgaris in bubble column photobioreactors. Chem. Eng. 2010, 20, 85–90. [Google Scholar]
- Butterfi, B.A.; Jones, J. Harvesting of algae grown in agricultural wastewaters. Trans. Geophys. Union. 1969, 50, 612. [Google Scholar]
- Chen, C.-L.; Chang, J.-S.; Lee, D.-J. Dewatering and drying methods for Microalgae. Dry. Technol. 2015, 33, 443–454. [Google Scholar] [CrossRef]
- Grima, E.M.; Belarbi, E.H.; Fernández, F.A.; Medina, A.R.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Lee, D.H.; Bae, C.Y.; Han, J.I.; Park, J.K. In situ analysis of heterogeneity in the lipid content of single green microalgae in alginate hydrogel microcapsules. Anal. Chem. 2013, 85, 8749–8756. [Google Scholar] [CrossRef]
- Divakaran, R.; Pillai, V.N. Flocculation of river silt using chitosan. Water Res. 2002, 36, 2414–2418. [Google Scholar] [CrossRef]
- Giovannoni, S.J.; DeLong, E.F.; Schmidt, T.M.; Pace, N.R. Tangential flow filtration and preliminary phylogenetic analysis of marine picoplankton. Appl. Environ. Microbiol. 1990, 56, 2572–2575. [Google Scholar] [PubMed]
- Bosma, R.; van Spronsen, W.A.; Tramper, J.; Wijffels, R.H. Ultrasound, a new separation technique to harvest microalgae. J. Appl. Phycol. 2003, 15, 143–153. [Google Scholar] [CrossRef]
- Gröschl, M. Ultrasonic Separation of Suspended Particles—Part I: Fundamentals. Acta Acust. United Acust. 1998, 84, 432–447. [Google Scholar]
- Muñoz, R.; Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Hossain, A.; Salleh, A.; Boyce, A.; Chowdhury, P.; Naqiuddin, M. Biodiesel fuel production from microalgae as renewable energy. Am. J. Biochem. Biotechnol. 2008, 4, 250–254. [Google Scholar]
- Ward, A.J.; Lewis, D.M.; Green, F.B. Anaerobic digestion of algae biomass: A review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Oncel, S.S.; Kose, A.; Faraloni, C.; Imamoglu, E.; Elibol, M.; Torzillo, G.; Vardar Sukan, F. Biohydrogen production from model microalgae Chlamydomonas reinhardtii: A simulation of environmental conditions for outdoor experiments. Int. J. Hydrogen Energy 2015, 40, 7502–7510. [Google Scholar] [CrossRef]
- RangaRao, A.; Ravishankar, G.A. Infmuence of CO2 on growth and hydrocarbon production in Botryococcus braunii. J. Microbiol. Biotechnol. 2007, 17, 414–419. [Google Scholar]
- Buxy, S.; Diltz, R.; Pullammanappallil, P. Biogasification of Marine Algae Nannochloropsis Oculata; Wicks, G., Simon, J., Zidan, R., Brigmon, R., Fischman, G., Arepalli, S., Norris, A., McCluer, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Ghasemi, Y.; Rasoul-Amini, S.; Naseri, A.T.; Montazeri-Najafabady, N.; Mobasher, M.A. Microalgae biofuel potentials. Appl. Biochem. Microbiol. 2012, 48, 126–144. [Google Scholar] [CrossRef]
- Archana, T.; Anjana, P. Cyanobacterial hydrogen production: A step towards clean environment. Int. J. Hydrogen Energy 2012, 37, 139–150. [Google Scholar]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Zhao, X.Q.; Yen, H.W.; Ho, S.H.; Cheng, C.L.; Lee, D.J.; Bai, F.W.; Chang, J.S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal biomass as a fermentation feedstock for bioethanol production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; van Hooijdonk, G.; Faaij, A.P.C. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Ehimen, E.A.; Sun, Z.F.; Carrington, C.G. Variables affecting the in situ transesterification of microalgae lipids. Fuel 2010, 89, 677–684. [Google Scholar] [CrossRef]
- Huang, J.; Xia, J.; Jiang, W.; Li, Y.; Li, J. Biodiesel production from microalgae oil catalyzed by a recombinant lipase. Bioresour. Technol. 2015, 180, 47–53. [Google Scholar] [CrossRef]
- Tang, H.; Abunasser, N.; Garcia, M.E.D.; Chen, M.; Ng, K.Y.S.; Salley, S.O. Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Appl. Energy 2011, 88, 3324–3330. [Google Scholar] [CrossRef]
- Carvalho Júnior, R.M.; Vargas, J.V.C.; Ramos, L.P.; Marino, C.E.B.; Torres, J.C.L. Microalgae biodiesel via in situ methanolysis. J. Chem. Technol. Biotechnol. 2011, 86, 1418–1427. [Google Scholar] [CrossRef]
- El-Shimi, H.I.; Attia, N.K.; El-Sheltawy, S.T.; El-Diwani, G.I. Biodiesel production from spirulina-platensis microalgae by in-situ transesterification process. J. Sustain. Bioenergy Syst. 2013, 3, 224–233. [Google Scholar] [CrossRef]
- Johnson, M.B.; Wen, Z. Production of biodiesel fuel from the Microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuels 2009, 23, 5179–5183. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; Shalaby, E.A.; Shanab, S.M.M. Enhancement of biodiesel production from different species of algae. Grasas y Aceites 2010, 61, 416–422. [Google Scholar]
- Saad, M.G.; Shafik, H.M.; Mekki, L.; El-Kholy, R. Impact of different nitrogen concentrations on biomass productivity, lipid content and target fatty acids within Chlorella sp. and Desmodesmus quadricaudatus to enhance biodiesel production. Int. J. Sci. Technol. Res. 2018, 7, 123–130. [Google Scholar]
- Saad, M.G.; Shafik, H.M. The challenges of biodiesel production from Oscillatoria sp. J. Int. J. Adv. Res. 2017, 5, 1316–1322. [Google Scholar] [CrossRef]
- Choi, S.P.; Nguyen, M.T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef]
- Nayak, B.K.; Roy, S.; Das, D. Biohydrogen production from algal biomass (Anabaena sp. PCC 7120) cultivated in airlift photobioreactor. Int. J. Hydrogen Energy 2014, 39, 7553–7560. [Google Scholar] [CrossRef]
- Miyamoto, K.; Hallenbeck, P.C.; Benemann, J.R. Hydrogen production by the thermophilic alga Mastigocladus laminosus: Hydrogen production by the thermophilic alga Mastigocladus laminosus: Effects of nitrogen, temperature, and inhibition of photosynthesis. Appl. Environ. Microbiol. 1979, 38, 440–446. [Google Scholar]
- Barreiro, D.L.; Prins, W.; Ronsse, F.; Brilman, W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B.; Skill, S.C.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C.A. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Eboibi, B.E.; Lewis, D.M.; Ashman, P.J.; Chinnasamy, S. Effect of operating conditions on yield and quality of biocrude during hydrothermal liquefaction of halophytic microalga Tetraselmis sp. Bioresour Technol. 2014, 170, 20–29. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xie, J.; Liu, H.; Yin, X.; Wu, C. Bio-oil production from hydrothermal liquefaction of high-protein high-ash microalgae including wild Cyanobacteria sp. and cultivated Bacillariophyta sp. Fuel 2016, 183, 9–19. [Google Scholar] [CrossRef]
- Alba, L.G.; Torri, C.; Samorì, C.; van der Spek, J.; Fabbri, D.; Kersten, S.R.A.; Brilman, D.W.F. (Wim) Hydrothermal treatment (HTT) of Microalgae: Evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuels 2012, 26, 642–657. [Google Scholar] [CrossRef]
- Hirano, A.; Ueda, R.; Hirayama, S.; Ogushi, Y. CO2 fixation and ethanol production with microalgal photosynthesis and intracellular anaerobic fermentation. Energy 1997, 22, 137–142. [Google Scholar] [CrossRef]
- Wu, Q.; Shiraiwa, Y.; Takeda, H.; Sheng, G.; Fu, J. Liquid-saturated hydrocarbons resulting from pyrolysis of the marine coccolithophores Emiliania huxleyi and Gephyrocapsa oceanica. Mar. Biotechnol. 1999, 1, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Zamalloa, C.; Boon, N.; Verstraete, W. Anaerobic digestibility of Scenedesmus obliquus and Phaeodactylum tricornutum under mesophilic and thermophilic conditions. Appl. Energy 2012, 92, 733–738. [Google Scholar] [CrossRef]
- Inglesby, A.E.; Fisher, A.C. Enhanced methane yields from anaerobic digestion of Arthrospira maxima biomass in an advanced flow-through reactor with an integrated recirculation loop microbial fuel cell. Energy Environ. Sci. 2012, 5, 7996–8006. [Google Scholar] [CrossRef]
- Nguyen, T.; Roddick, F.A.; Fan, L. Impact of green algae on the measurement of Microcystis aeruginosa populations in lagoon-treated wastewater with an algae online analyser. Environ. Technol. 2015, 36, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.M.; Prussi, M.; Bettucci, L.; Libelli, I.M.; Chiaramonti, D. Characterization of microalga Chlorella as a fuel and its thermogravimetric behavior. Appl. Energy 2013, 102, 24–31. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q.; Yang, C. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis 2004, 71, 855–863. [Google Scholar] [CrossRef]
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; O’Connor, P.; Seshan, K. Catalytic pyrolysis of microalgae to high-quality liquidbio-fuels. Biomass Bioenergy 2011, 35, 3199–3207. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Ma, F.; Xuan, L.; Xu, Y.; Huo, H.; Zhou, D.; Dong, S. Optimization of Chlorella vulgaris and bioflocculant-producing bacteria co-culture: Enhancing microalgae harvesting and lipid content. Lett. Appl. Microbiol. 2015, 60, 497–503. [Google Scholar] [CrossRef]
- Grierson, S.; Strezov, V.; Ellem, G.; Mcgregor, R.; Herbertson, J. Thermal characterisation of microalgae under slow pyrolysis conditions. J. Anal. Appl. Pyrolysis 2009, 85, 118–123. [Google Scholar] [CrossRef]
- Pan, P.; Hu, C.; Yang, W.; Li, Y.; Dong, L.; Zhu, L.; Tong, D.; Qing, R.; Fan, Y. The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils. Bioresour. Technol. 2010, 101, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Grierson, S.; Strezov, V.; Shah, P. Properties of oil and char derived from slow pyrolysis of Tetraselmis chui. Bioresour. Technol. 2011, 102, 8232–8240. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Lea-Langton, A.R.; Ross, A.B.; Williams, P.T. Catalytic hydrothermal gasification of algae for hydrogen production: Composition of reaction products and potential for nutrient recycling. Bioresour. Technol. 2013, 127, 72–80. [Google Scholar] [CrossRef]
- Khoo, H.H.; Koh, C.Y.; Shaik, M.S.; Sharratt, P.N. Bioenergy co-products derived from microalgae biomass via thermochemical conversion—Life cycle energy balances and CO2 emissions. Bioresour. Technol. 2013, 143, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Duman, G.; Uddin, M.A.; Yanik, J. Hydrogen production from algal biomass via steam gasification. Bioresour. Technol. 2014, 166, 24–30. [Google Scholar] [CrossRef]
- Sanchez-Silva, L.; López-González, D.; Garcia-Minguillan, A.M.; Valverde, J.L. Pyrolysis, combustion and gasification characteristics of Nannochloropsis gaditana microalgae. Bioresour. Technol. 2013, 130, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Stucki, S.; Vogel, F.; Ludwig, C.; Haiduc, A.G.; Brandenberger, M. Catalytic gasification of algae in supercritical water for biofuel production and carbon capture. Energy Environ. Sci. 2009, 2, 535–541. [Google Scholar] [CrossRef]
- Alghurabie, I.K.; Hasan, B.O.; Jackson, B.; Kosminski, A.; Ashman, P.J. Fluidized bed gasification of Kingston coal and marine microalgae in a spouted bed reactor. Chem. Eng. Res. Des. 2013, 91, 1614–1624. [Google Scholar] [CrossRef]
- Woertz, I.C.; Benemann, J.R.; Du, N.; Unnasch, S.; Mendola, D.; Mitchell, B.G.; Lundquist, T.J. Life cycle GHG emissions from microalgal biodiesel–a CA-GREET model. Environ. Sci. Technol. 2014, 48, 6060–6068. [Google Scholar] [CrossRef]
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M. U.S. Department of Energy, Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks (Leads); Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016. [Google Scholar]
- Raheem, A.; Azlina, W.W.; Yap, Y.T.; Danquah, M.K.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Chaiwong, K.; Kiatsiriroat, T.; Vorayos, N.; Thararax, C. Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass Bioenergy 2013, 56, 600–606. [Google Scholar] [CrossRef]
- Campanella, A.; Muncrief, R.; Harold, M.P.; Griffith, D.C.; Whitton, N.M.; Weber, R.S. Thermolysis of microalgae and duckweed in a CO2-swept fixed-bed reactor: Bio-oil yield and compositional effects. Bioresour. Technol. 2012, 109, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, P.; Liu, S.; Fan, L.; Zhou, N.; Min, M.; Cheng, Y.; Peng, P.; Anderson, E.; Wang, Y.; et al. Microwave-assisted pyrolysis of biomass for bio-oil production. In Pyrolysis; IntechOpen: London, UK, 2017; pp. 129–166. [Google Scholar]
- Brand, S.; Hardi, F.; Kim, J.; Suh, D.J. Effect of heating rate on biomass liquefaction: Differences between subcritical water and supercritical ethanol. Energy 2014, 68, 420–427. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Huang, M.Y.; Chang, J.S. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Kaleem, I.; Chun, L.; Tong, J. Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy 2010, 35, 5406–5411. [Google Scholar] [CrossRef]
- Ross, A.B.; Biller, P.; Kubacki, M.L.; Li, H.; Lea-Langton, A.; Jones, J.M. Hydrothermal processing of microalgae using alkali and organic acids. Fuel 2010, 89, 2234–2243. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Rizzo, A.M.; Pari, L. Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production. Appl. Energy 2017, 185, 963–972. [Google Scholar] [CrossRef]
- Raza, H. Aspen Simulation of Hydrothermal Liquefaction Process for the Conversion of Algae to Renewable Fuels and Chemicals; Lamar University-Beaumont: Beaumont, TX, USA, 2014. [Google Scholar]
- Dote, Y.; Sawayama, S.; Inoue, S.; Minowa, T.; Yokoyama, S.Y. Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction. Fuel 1994, 73, 1855–1857. [Google Scholar] [CrossRef]
- Zou, S.; Wu, Y.; Yang, M.; Li, C.; Tong, J. Bio-oil production from sub-and supercritical water liquefaction of microalgae Dunaliella tertiolecta and related properties. Energy Environ. Sci. 2010, 3, 1073–1078. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Hydrothermal liquefaction of a microalga with heterogeneous catalysts. Ind. Eng. Chem. Res. 2010, 50, 52–61. [Google Scholar] [CrossRef]
- Ramos-Suárez, J.L.; Carreras, N. Use of microalgae residues for biogas production. Chem. Eng. J. 2014, 242, 86–95. [Google Scholar] [CrossRef]
- Hidaka, T.; Inoue, K.; Suzuki, Y.; Tsumori, J. Growth and anaerobic digestion characteristics of microalgae cultivated using various types of sewage. Bioresour. Technol. 2014, 170, 83–89. [Google Scholar] [CrossRef]
- Costa, J.C.; Gonçalves, P.R.; Nobre, A.; Alves, M.M. Biomethanation potential of macroalgae Ulva spp. and Gracilaria spp. and in co-digestion with waste activated sludge. Bioresour. Technol. 2012, 114, 320–326. [Google Scholar] [CrossRef]
- Passos, F.; Solé, M.; García, J.; Ferrer, I. Biogas production from microalgae grown in wastewater: Effect of microwave pretreatment. Appl. Energy 2013, 108, 168–175. [Google Scholar] [CrossRef]
- Marsolek, M.D.; Kendall, E.; Thompson, P.L.; Shuman, T.R. Thermal pretreatment of algae for anaerobic digestion. Bioresour. Technol. 2014, 151, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Laurens, L.M.L.; Nagle, N.; Davis, R.; Sweeney, N.; Van Wychen, S.; Lowell, A.; Pienkos, P.T. Acid-catalyzed algal biomass pretreatment for integrated lipid and carbohydrate-based biofuels production. Green Chem. 2015, 17, 1145–1158. [Google Scholar] [CrossRef]
- Aikawa, S.; Joseph, A.; Yamada, R.; Izumi, Y.; Yamagishi, T.; Matsuda, F.; Kawai, H.; Chang, J.S.; Hasunuma, T.; Kondo, A. Direct conversion of Spirulina to ethanol without pretreatment or enzymatic hydrolysis processes. Energy Environ. Sci. 2013, 6, 1844–1849. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Fasahati, P.; Woo, H.C.; Liu, J. Industrial-scale bioethanol production from brown algae: Effects of pretreatment processes on plant economics. Appl. Energy 2015, 1139, 175–187. [Google Scholar] [CrossRef]
- Teixeira, A.C.R.; Sodré, J.R.; Guarieiro, L.L.N.; Vieira, E.D.; de Medeiros, F.F.; Alves, C.T. A Review on Second and Third Generation Bioethanol Production; SAE International: Warrendale, PA, USA, 2016; pp. 1–7. [Google Scholar]
- Fan, X.; Wang, H.; Guo, R.; Yang, D.; Zhang, Y.; Yuan, X.; Qiu, Y.; Yang, Z.; Zhao, X. Comparative study of the oxygen tolerance of Chlorella pyrenoidosa and Chlamydomonas reinhardtii CC124 in photobiological hydrogen production. Algal Res. 2016, 16, 240–244. [Google Scholar] [CrossRef]
- Antal, T.K.; Krendeleva, T.E.; Tyystjärvi, E. Multiple regulatory mechanisms in the chloroplast of green algae: Relation to hydrogen production. Photosynth. Res. 2015, 125, 357–381. [Google Scholar] [CrossRef]
- Das, A.A.; Esfahani, M.M.; Velev, O.D.; Pamme, N.; Paunov, V.N. Artificial leaf device for hydrogen generation from immobilised C. reinhardtii microalgae. J. Mater. Chem. A 2015, 3, 20698–20707. [Google Scholar] [CrossRef]
- Machado, I.M.; Atsumi, S. Cyanobacterial biofuel production. J. Biotechnol. 2012, 162, 50–56. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Gude, V.G.; Mannarswamy, A.; Deng, S.; Cooke, P.; Munson-McGee, S.; Rhodes, I.; Lammers, P.; Nirmalakhandan, N. Optimization of direct conversion of wet algae to biodiesel under supercritical methanol conditions. Bioresour. Technol. 2011, 102, 118–122. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Kang, S.W.; Song, Y.S.; Park, C.; Han, S.O.; Kim, S.W. Biodiesel production by a mixture of Candida rugosa and Rhizopus oryzae lipases using a supercritical carbon dioxide process. Bioresour. Technol. 2011, 102, 2105–2108. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G. Fuel properties of highly polyunsaturated fatty acid methyl esters. Prediction of fuel properties of algal biodiesel. Energy Fuel 2012, 26, 5265–5273. [Google Scholar] [CrossRef]
- James, G.O.; Hocart, C.H.; Hillier, W.; Price, G.D.; Djordjevic, M.A. Temperature modulation of fatty acid profiles for biofuel production in nitrogen deprived Chlamydomonas reinhardtii. Bioresour. Technol. 2013, 127, 441–447. [Google Scholar] [CrossRef]
- Dunn, R.O.; Bagby, M.O. Low-temperature properties of triglyceride-based diesel fuels: Transesterified methyl esters and petroleum middle distillate/Ester Blends. JAOCS 1995, 72, 895–904. [Google Scholar] [CrossRef]
- Antczak, M.S.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic biodiesel synthesis–key factors affecting efficiency of the process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, M.Z.; Prasad, S.; Banerjee, U.C. Prospects of biodiesel production from microalgae in India. Renew. Sustain. Energy Rev. 2009, 13, 2361–2372. [Google Scholar] [CrossRef]
- Bajhaiya, A.K.; Mandotra, S.K.; Suseela, M.R.; Toppo, K.; Ranade, S. ALGAL BIODIESEL: The next generation biofuel for India. Asian J. Exp. Biol. Sci 2010, 1, 728–739. [Google Scholar]
- Mohan, S.V.; Srikanth, S.; Chiranjeevi, P.; Arora, S.; Chandra, R. Algal biocathode for in situ terminal electron acceptor (TEA) production: Synergetic association of bacteria—Microalgae metabolism for the functioning of biofuel cell. Bioresour. Technol. 2014, 166, 566–574. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domıguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef]
- Saba, B.; Christy, A.D.; Yu, Z.; Co, A.C. Sustainable power generation from bacterio-algal microbial fuel cells (MFCs): An overview. Renew. Sustain. Energy Rev. 2017, 73, 75–84. [Google Scholar] [CrossRef]
- Cucu, A.; Costache, T.A.; Tiliakos, A. Microalgae as native oxygen suppliers in bicameral microbial fuel cells. Dig. J. Nanomater. Biostruct. 2013, 8, 1301–1312. [Google Scholar]
- Powell, E.E.; Evitts, R.W.; Hill, G.A. A microbial fuel cell with a photosynthetic microalgae cathodic half cell coupled to a yeast anodic half cell. Energy Sources Part A 2011, 33, 440–448. [Google Scholar] [CrossRef]
- Commault, A.S.; Lear, G.; Novis, P. Photosynthetic biocathode enhances the power output of a sediment-type microbial fuel cell. N. Z. J. Bot. 2014, 52, 48–59. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Liu, J.; Lee, H.; Li, C.; Li, N.; Ren, N. Sequestration of CO2 discharged from anode by algal cathode in microbial carbon capture cells (MCCs). Biosens. Bioelectron. 2010, 25, 2639–2643. [Google Scholar] [CrossRef]
- Juang, D.F.; Lee, C.H.; Hsueh, S.C.; Chou, H.Y. Power generation capabilities of microbial fuel cells with different oxygen supplies in the cathodic chamber. Appl. Biochem. Biotechnol. 2012, 167, 714–731. [Google Scholar] [CrossRef]
- Wu, X.Y.; Song, T.S.; Zhu, X.J.; Wei, P.; Zhou, C. Construction and operation of microbial fuel cell with Chlorella vulgaris biocathode for electricity generation. Appl. Biochem. Biotechnol. 2013, 171, 2082–2092. [Google Scholar] [CrossRef]
- Dunahay, T.G.; Jarvis, E.E.; Dais, S.S.; Roessler, P.G. Manipulation of microalgal lipid production using genetic engineering. Appl. Biochem. Biotechnol. 1996, 57, 223–231. [Google Scholar] [CrossRef]
- Courchesne, N.M.; Parisien, A.; Wang, B.; Lan, C.Q. Enhancement of lipid production using biochemical, genetic and transcription factor engineering approaches. J. Biotechnol. 2009, 141, 31–41. [Google Scholar] [CrossRef]
- Siloto, R.M.P.; Truksa, M.; Brownfield, D.; Good, A.G.; Weselake, R.J. Directed evolution of acyl-CoA: Diacylglycerol acyltransferase: Development and characterization of Brassica napus DGAT1 mutagenized libraries. Plant Physiol. Biochem. 2009, 47, 456–461. [Google Scholar] [CrossRef]
- Posewitz, M.C.; King, P.W.; Smolinski, S.L.; Smith, R.D.; Ginley, A.R.; Ghirardi, M.L.; Seibert, M. Identification of genes required for hydrogenase activity in Chlamydomonas reinhardtii. Biochem. Soc. Trans. 2005, 33, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Posewitz, M.C.; Smolinski, S.L.; Kanakagiri, S.; Melis, A.; Seibert, M.; Ghirardi, M.L. Hydrogen photoproduction is attenuated by disruption of an isoamylase gene in Chlamydomonas reinhardtii. Plant Cell 2004, 16, 2151–2163. [Google Scholar] [CrossRef] [PubMed]
- Ramazanov, A.; Ramazanov, Z. Isolation and characterization of a starchless mutant of Chlorella pyrenoidosa STL-PI with a high growth rate, and high protein and polyunsaturated fatty acid content. Phycol. Res. 2006, 54, 255–259. [Google Scholar] [CrossRef]
- Beer, L.L.; Boyd, E.S.; Peters, J.W.; Posewitz, M.C. Engineering algae for biohydrogen and biofuel production. Curr. Opin. Biotechnol. 2009, 20, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Melis, A.; Seibert, M.; Ghirardi, M.L. Hydrogen fuel production by transgenic microalgae. Adv. Exp. Med. Biol. 2007, 616, 110. [Google Scholar] [PubMed]
- Kruse, O.; Rupprecht, J.; Bader, K.P.; Thomas-Hall, S.; Schenk, P.M.; Finazzi, G.; Hankamer, B. Improved photobiological H2 production in engineered green algal cells. J. Biol. Chem. 2005, 280, 34170–34177. [Google Scholar] [CrossRef] [PubMed]
- Surzycki, R.; Cournac, L.; Peltier, G.; Rochaix, J.D. Potential for hydrogen production with inducible chloroplast gene expression in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2007, 104, 17548–17553. [Google Scholar] [CrossRef] [PubMed]
- Mussgnug, J.H.; Thomas-Hall, S.; Rupprecht, J.; Foo, A.; Klassen, V.; McDowall, A.; Schenk, P.M.; Kruse, O.; Hankamer, B. Engineering photosynthetic light capture: Impacts on improved solar energy to biomass conversion. Plant Biotechnol. J. 2007, 5, 802–814. [Google Scholar] [CrossRef]
- Rasala, B.A.; Gimpel, J.A.; Tran, M.; Hannon, M.J.; Miyake-stoner, S.J.; Specht, E.A.; Mayfield, S.P. Genetic engineering to improve algal biofuels production. In Algae for Biofuels and Energy; Springer: Dordrecht, The Netherlands, 2013; pp. 99–113. ISBN 9789400754799. [Google Scholar]
- Chen, G.Q.; Chen, F. Growing phototrophic cells without light. Biotech. Lett. 2006, 28, 607. [Google Scholar] [CrossRef]
- Fischer, H.; Robl, I.; Sumper, M.; Kröger, N. Targeting and covalent modification of cell wall and membrane proteins heterologously expressed in the diatom Cylindrotheca fusiformis (Bacillariophyceae). J. Phycol. 1999, 35, 113–120. [Google Scholar] [CrossRef]
- Hallmann, A.; Sumper, M. The Chlorella hexose/H + symporter is a useful selectable marker and biochemical reagent when expressed in Volvox. Proc. Natl. Acad. Sci. USA 1996, 93, 669–673. [Google Scholar] [CrossRef]
- Doebbe, A.; Rupprecht, J.; Beckmann, J.; Mussgnug, J.H.; Hallmann, A.; Hankamer, B.; Kruse, O. Functional integration of the HUP1 hexose symporter gene into the genome of C. reinhardtii: Impacts on biological H2 production. J. Biotechnol. 2007, 131, 27–33. [Google Scholar] [CrossRef]
- Zaslavskaia, L.A.; Lippmeier, J.C.; Shih, C.; Ehrhardt, D.; Grossman, A.R.; Apt, K.E. Trophic conversion of an obligate photoautotrophic organism through metabolic engineering. Science 2001, 292, 2073–2075. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; Brand, J. Principles of bioprospecting for microalgae. In Proceedings of the Algae Biomass Summit, Orlando, FL, USA, 30 September–3 October 2013. [Google Scholar]
- Saladini, F.; Patrizi, N.; Pulselli, F.M.; Marchettini, N.; Bastianoni, S. Guidelines for emergy evaluation of first, second and third generation biofuels. Renew. Sustain. Energy Rev. 2016, 66, 221–227. [Google Scholar] [CrossRef]
- Tomei, J.; Helliwell, R. Food versus fuel? Going beyond biofuels. Land Use Policy 2016, 56, 320–326. [Google Scholar] [CrossRef]
- Wise, T.A.; Cole, E. Mandating Food Insecurity: The Global Impacts of Rising Biofuel Mandates and Targets; Global Development and Environment Institute: Medford, MA, USA, 2015. [Google Scholar]
- Office of Energy Efficiency and Renewable Energy. National Algal Biofuels Technology Roadmap; U.S. Department of Energy: Washington, DC, USA, 2010.
- Cabanelas, I.T.D.; Ruiz, J.; Arbib, Z.; Chinalia, F.A.; Garrido-Pérez, C.; Rogalla, F.; Nascimento, I.A.; Perales, J.A. Comparing the use of different domestic wastewaters for coupling microalgal production and nutrient removal. Bioresour. Technol. 2013, 131, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Patzek, T.W. Ethanol production using corn, switchgrass, and wood; Biodiesel production using soybean and sunflower. Nat. Resour. Res. 2005, 14, 65–76. [Google Scholar] [CrossRef]
- Mutanda, T.; Ramesh, D.; Karthikeyan, S.; Kumari, S.; Anandraj, A.; Bux, F. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 2011, 102, 57–70. [Google Scholar] [CrossRef]
- Savage, P. Algae under pressure and in hot water. Science 2012, 338, 1039. [Google Scholar] [CrossRef]
- Hall, C.A.S.; Dale, B.E.; Pimentel, D. Seeking to understand the reasons for different energy return on investment (EROI) estimates for biofuels. Sustainability 2011, 3, 2413–2432. [Google Scholar] [CrossRef]
- Sills, D.L.; Paramita, V.; Franke, M.J.; Johnson, M.C.; Akabas, T.M.; Greene, C.H.; Tester, J.W. Quantitative uncertainty analysis of life cycle assessment for algal biofuel production. Environ. Sci. Technol. 2012, 47, 687–694. [Google Scholar] [CrossRef]
- Youngs, H.; Somerville, C.R. California’s Energy Future. The Potential for Biofuels; California Council on Science and Technology: Sacramento, CA, USA, 2013. [Google Scholar]
- Gonzalez, L.M.; Kinnin, L.A.; Blikslager, A.T. Characterization of discrete equine intestinal epithelial cell lineages. Am. J. Vet. Res. 2015, 76, 358–366. [Google Scholar] [CrossRef]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.; Kleinegris, D.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Rizwan, M.; Lee, J.H.; Gani, R. Optimal design of microalgae-based biorefinery: Economics, opportunities and challenges. Appl. Energy 2015, 150, 69–79. [Google Scholar] [CrossRef]
- Vanthoor-koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Pradhan, R.R.; Pradhan, R.R.; Das, S.; Dubey, B.; Dutta, A. Bioenergy combined with carbon capture potential by microalgae at flue gas-based carbon sequestration plant of NALCO as accelerated carbon sink. In Carbon Utilization; Springer: Singapore, 2017; pp. 231–244. [Google Scholar]
- Li, Y.Q.; Horsman, M.; Wang, B.; Wu, N.; Lan, C.Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 2008, 81, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Langley, N.M.; Harrison, S.T.L.; Van Hille, R.P. A critical evaluation of CO2 supplementation to algal systems by direct injection. Biochem. Eng. J. 2012, 68, 70–75. [Google Scholar] [CrossRef]

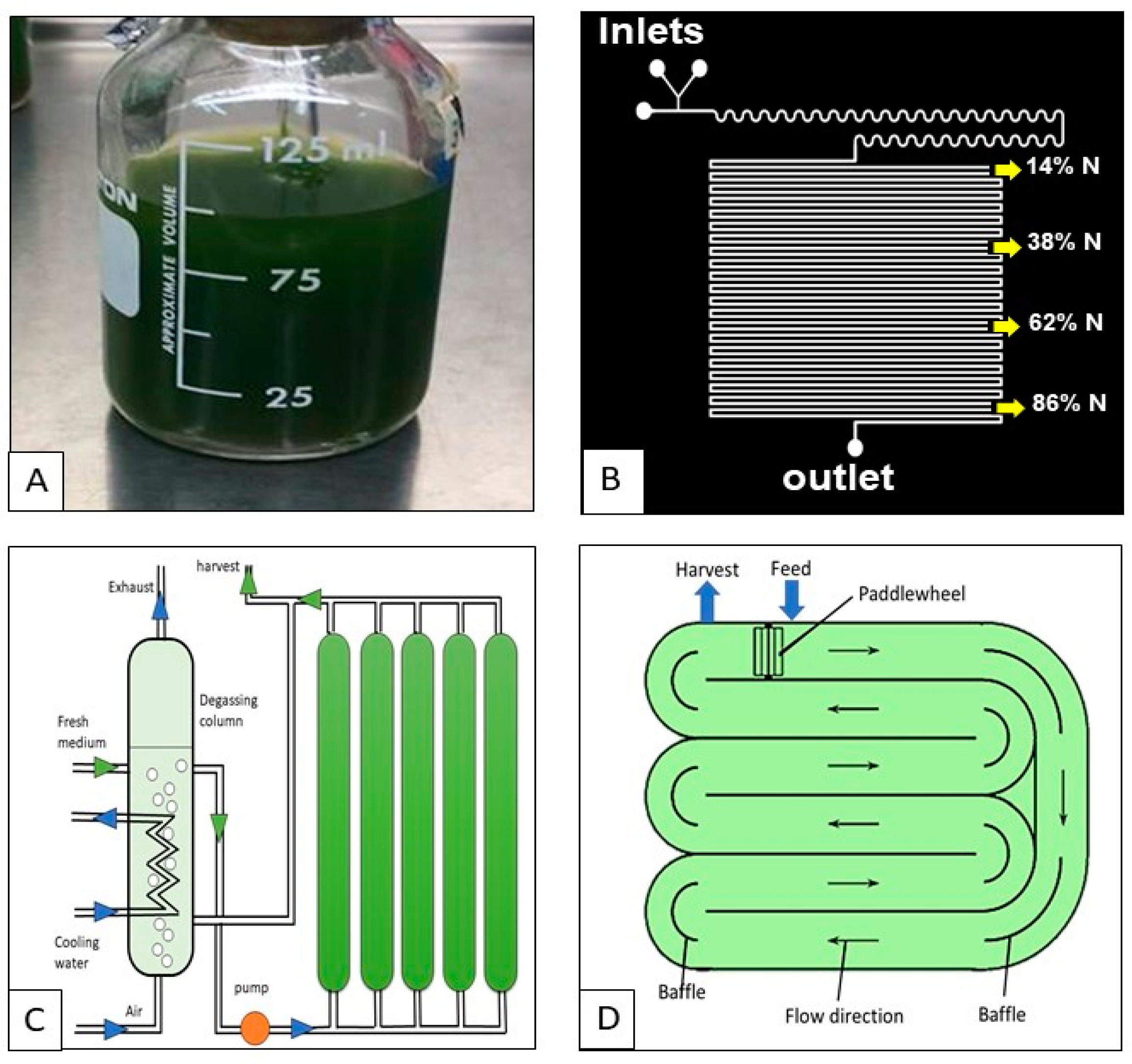
| Cultivation Technique | Type | Advantages | Issues |
|---|---|---|---|
| Photoautotrophic cultivation | Closed photobioreactors |
|
|
| Open ponds |
|
| |
| Heterotrophic cultivation | - |
|
|
| Technique | Theory | Advantages | Disadvantage | Reference |
|---|---|---|---|---|
| Flocculation | Cells are aggregated by increasing their size using a flocculant which can be chemicals (ferric sulfate, ferric chloride, and ammonium sulfate), bioagents (chitosan), or microbes (bacteria). | Time-saving |
| [34,49,50,51] |
| Filtration | Large cells (size >70 µm) can be filtered under pressure or suction whereas smaller cells (size <30 µm) require ultrafilters to be harvested. Ceramic-coated membrane sheets can substitute conventional membranes. |
|
| [52] |
| Flotation | Trapping algal cells by bubbling air |
|
| [34] |
| Sonication | Pumping organisms continuously into a resonator chamber due to acoustic forces. |
|
| [53,54] |
| Centrifugation | Sedimentation based on the velocity, cell size, and density |
|
| [34,40,55] |
| Precipitation | Some algae are self-precipitated. They settle at the bottom after stopping circulation. |
|
| [3] |
| Biofuel Type | Algal Species | Experimental Conditions | Reference |
|---|---|---|---|
| Biodiesel | Chlorella sp. | 60 °C, 4 h, H2SO4, MeOH | [67] |
| Chlorella pyrenoidosa | 90 °C, 2 h, H2SO4, MeOH | [68] | |
| Dunaliella tertiolecta | 110 °C, 5 h, H2SO4, MeOH-THF | [69] | |
| Nannochloropsis oculata | 80 °C, 2 h, NaOH, MeOH chloroform (10:1) | [70] | |
| Spirulina sp. | Catalyst concentration, methanol = 80 mL, reaction time = 8 h, at 65 °C and 650 rpm | [71] | |
| Schizochytrium limacinum | 90 °C, 40 min, H2SO4, MeOH/chloroform | [72] | |
| Dictyochloropsis splendida | 110 °C, 5 h, NaOH, MeOH | [73] | |
| Desmodesmus quadricaudatus and Chlorella sp. | Pure batch cultures, BG-11 standard and nitrogen-free medium, hexane-ether, methanol | [15] | |
| Desmodesmus quadricaudatus and Chlorella sp. | 70 °C, 180 min, H2SO4, MeOH | [74] | |
| Oscillatoria sp. | BG-11 medium with different nitrate concentrations; (1500, 375, 186, 94, 47, 23 and 0.0 mgL−1 NaNO3 | [75] | |
| Bioethanol | Chlamydomonas reinhardtii | Enzyme pretreatment, 70–100 °C, 30 min, S. cerevisiae S288C cultured anaerobically at 30 °C for 40 h, rotation of 160 rpm | [76] |
| Chlorococcum sp. | Yeast powder, 30 °C, 200 rpm, 60 h, no pretreatment | [65] | |
| Biohydrogen | Anabaena cylindrical | pretreatment with amylase followed by thermophilic fermentation under light intensity of 120 mmol/m2/s | [77] |
| Mastigocladus laminosus | Sparging the cultures with a gas mixture of 0.2 to 0.4% N2, 0.6% CO2, and balance argon, gas flow rate = 3 L/h | [78] | |
| Chlamydomonas reinhardtii | Aerobic and anaerobic phases, light intensity (70 × 2 mmol/m2/s), mixing speed of 170 ± 10 rpm/2.5 min | [58] | |
| Bio-oil | Chlorella sp. | 300 °C, 90 min | [79] |
| Chlorella vulgaris | 300 °C, 60 min | [80] | |
| Chlorogloeopsis fritschii | 300 °C, 60 min | [80] | |
| Nannochloropsis sp. | 300 °C, 90 min | [79] | |
| Nannochloropsis oculata | 350 °C, 60 min | [80] | |
| Nannochloropsis gaditana | 375 °C, 5 min | [81] | |
| Spirulina platensis | 300 °C, 60 min | [80] | |
| Tetraselmis sp. | 350 °C, 5 min | [82] | |
| Bacillariophyta sp. | 325 °C, 60 min | [83] | |
| Cyanobacteria sp. | 325 °C, 45 min | [83] | |
| Desmodesmus sp. | 375 °C, 5 min | [84] | |
| Scenedesmus dimorphus | 350 °C, 60 min | [80] | |
| Porphyridium cruentum | 350 °C, 60 min | [81] | |
| Phaeodactylum tricornutum | 375 °C, 5 min | [79] | |
| Ethanol | Chlorella vulgaris | Pellet washed with methanol (95%), incubated with α- amylase (100 °C and pH 6) and glucoamylase (60 °C and pH 4.5), fermented by Saccharomyces cerevisiae (IFO 309), pretreatment (ultrasonic radiation). | [85] |
| Gas | Emiliania huxleyi | Pyrolysis, batch cultivation, fixed bed, 400 °C | [86] |
| Methane | Chlorella vulgaris | 308 °C, 30 days, Batch culture, pretreatment (Thermal 40 min/alkali) | [60] |
| Spirulina sp. | 308 °C, 28 days, Batch, no pretreatment | [87] | |
| Scenedesmus obliquus | 306 °C, 20 days, Anaerobic Membrane Bioreactor, no pretreatment | [87] | |
| Arthrospira maxima | 308 °C, 2–4 days, Continuous Flow Stirred-Tank Reactor, pretreatment (Magnetic stirred and dried) | [88] | |
| Euglena gracilis | 30 °C, 150 mmol/m2/s | [89] | |
| Oil | Chlorella protothecoides | Slow pyrolysis, tubular reactor, 550 °C | [90] |
| Microcystis aeruginosa | Fast pyrolysis, 10 °C/min, 500 °C | [91] | |
| Oil/gas | Chlorella sp. | Pyrolysis, fixed bed reactor, 450 °C | [92] |
| Oil/gas/char | Chlorella vulgaris | Closed tubular photobioreactor. Fast pyrolysis, fluidized bed, 500 °C | [93] |
| Dunaliella tertiolecta | Pyrolysis, fluidized bed, 10 °C/min, 500 °C | [94] | |
| Nannochloropsis sp. | Pyrolysis, fixed bed reactor with/without HZSM-5, 10 °C/min, 400 °C | [95] | |
| Synechococcus | Pyrolysis, 500 °C, 10 °C/min | [94] | |
| Tetraselmis Chuii | IR-pyrolysis, fixed bed, 500 °C, 10 °C/min | [96] | |
| Syngas | Chlorella vulgaris | 450 °C, 30 min, Batch reactor | [97] |
| Nannochloropsis sp. | Fixed bed, 700–1000 °C, 1e10 bar, 10,000 °C/1 min, | [98] | |
| Nannochloropsis oculata | Fixed bed reactor, 850 °C, 15 min, Fe2O3, CO2 | [99] | |
| Nannochloropsis gaditana | 850 °C, TGA | [100] | |
| Spirulina platensis | Ru/ZrO2; Ru/C, >400 °C | [101] | |
| Saccharina latissimi | 450 °C, 30min, NaOH, Ni Batch reactor | [97] | |
| Tetraselmis sp. | Fixed bed reactor, 850 °C, co-gasification (10% algae and 90% coal) | [102] |
| Dimension | Issues |
|---|---|
| Environmental dimension |
|
| Economic dimension |
|
| Social dimension |
|
| Cultural dimension |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal Biofuels: Current Status and Key Challenges. Energies 2019, 12, 1920. https://doi.org/10.3390/en12101920
Saad MG, Dosoky NS, Zoromba MS, Shafik HM. Algal Biofuels: Current Status and Key Challenges. Energies. 2019; 12(10):1920. https://doi.org/10.3390/en12101920
Chicago/Turabian StyleSaad, Marwa G., Noura S. Dosoky, Mohamed S. Zoromba, and Hesham M. Shafik. 2019. "Algal Biofuels: Current Status and Key Challenges" Energies 12, no. 10: 1920. https://doi.org/10.3390/en12101920
APA StyleSaad, M. G., Dosoky, N. S., Zoromba, M. S., & Shafik, H. M. (2019). Algal Biofuels: Current Status and Key Challenges. Energies, 12(10), 1920. https://doi.org/10.3390/en12101920






