Pyrolysis Kinetics of the Arid Land Biomass Halophyte Salicornia Bigelovii and Phoenix Dactylifera Using Thermogravimetric Analysis
Abstract
:1. Introduction
2. Results and Discussion
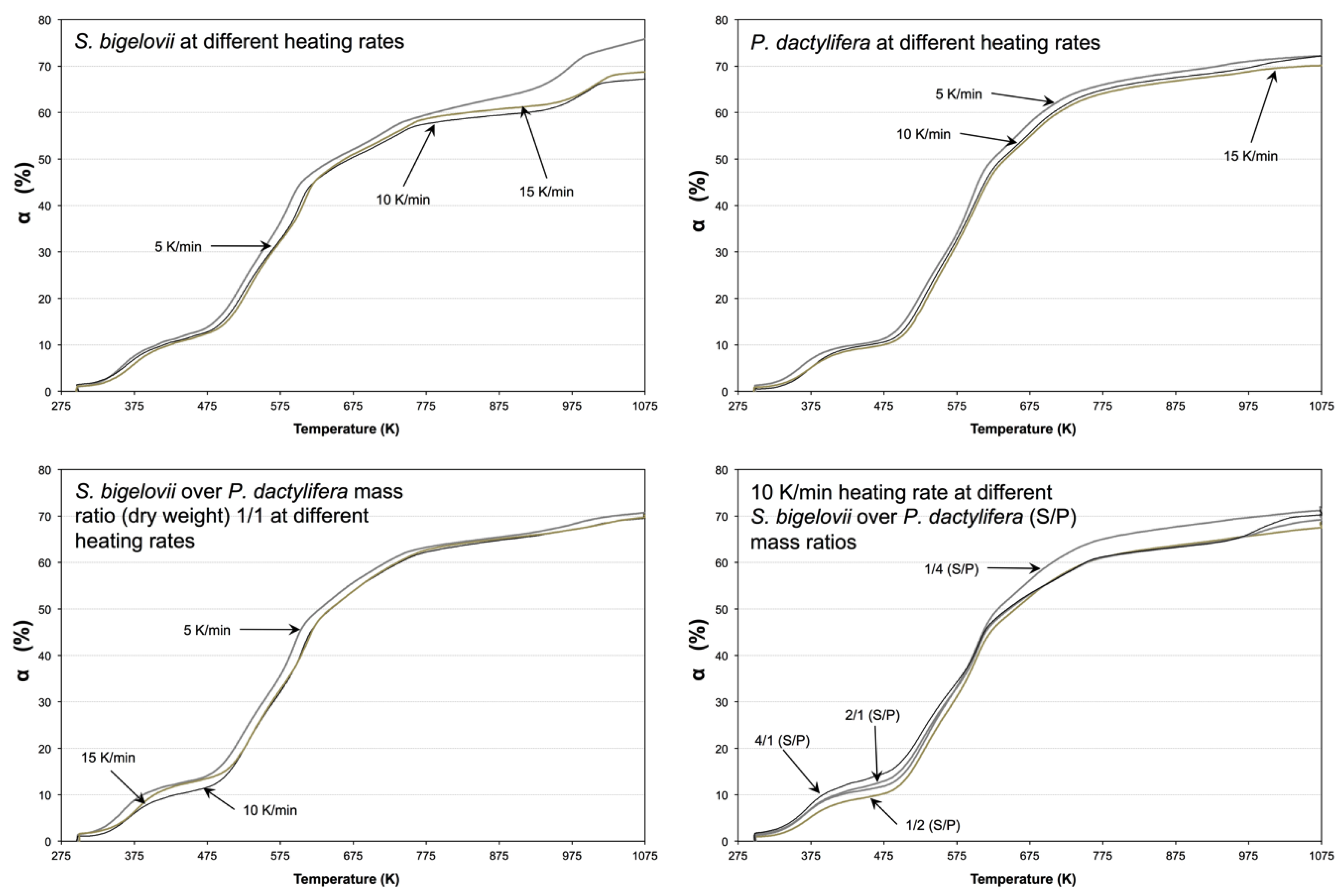
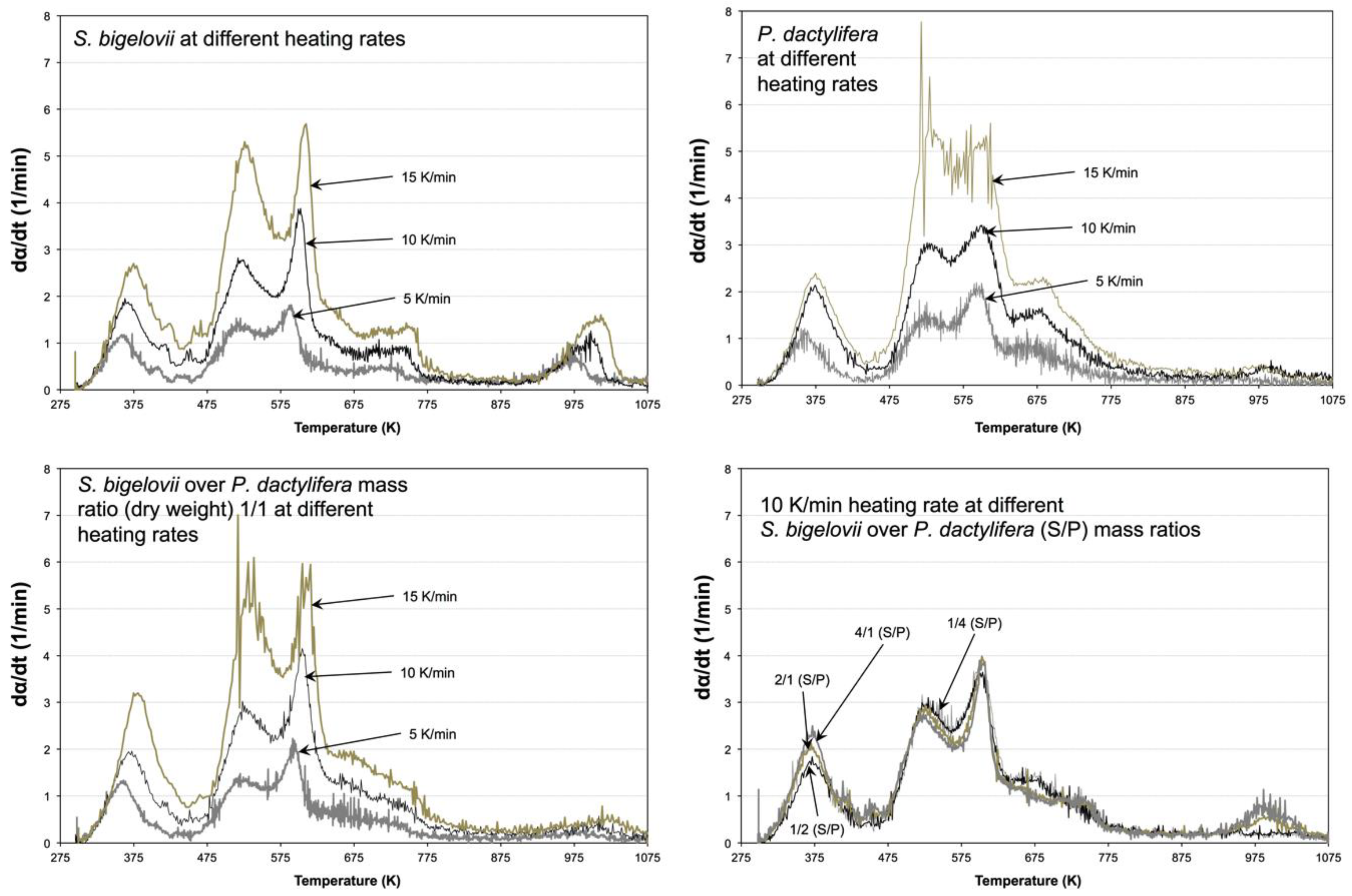
2.1. Effect of Heating Rate on Conversion and Reaction Rate
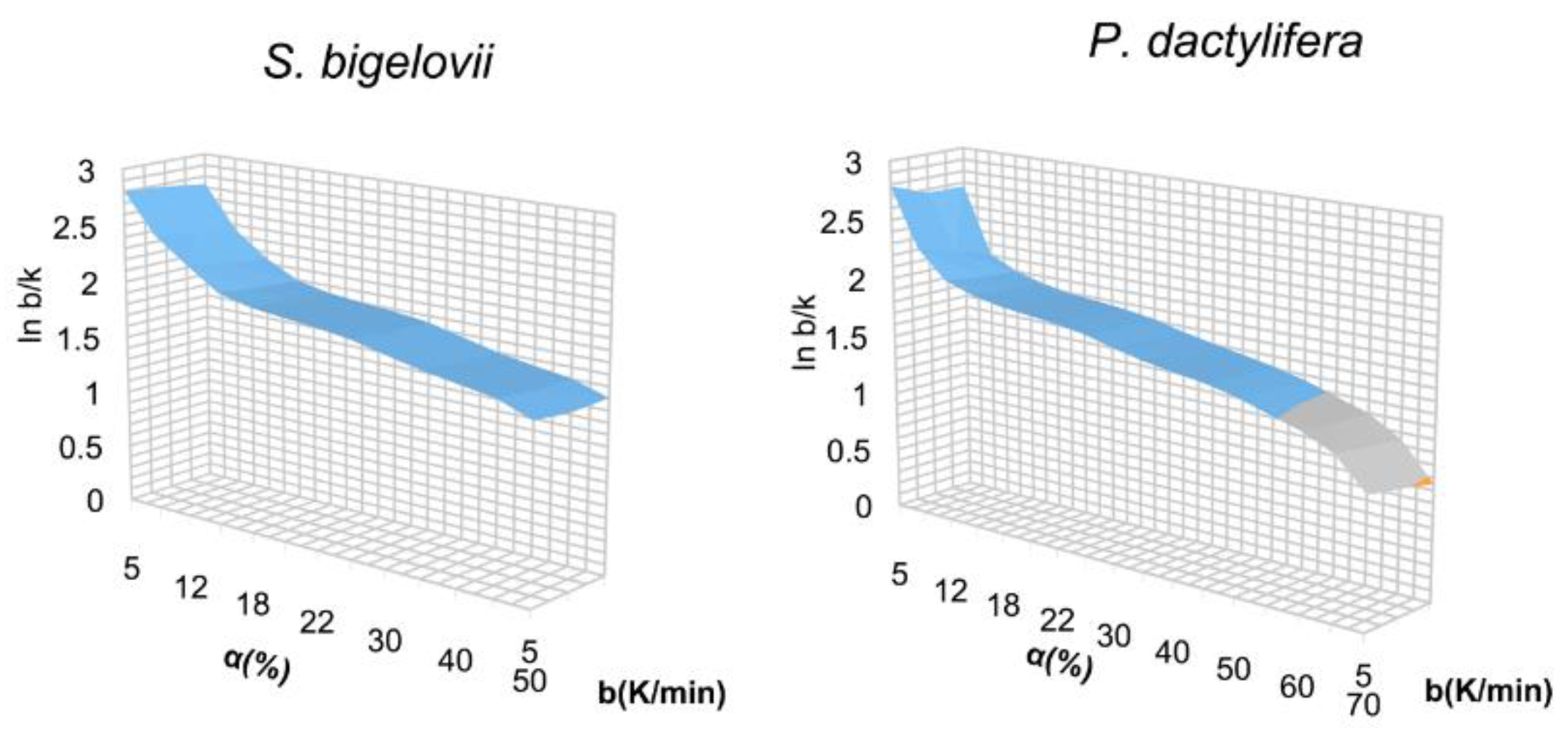
2.2. Kinetic Analysis
2.2.1. Kinetic Analysis-S. bigelovii
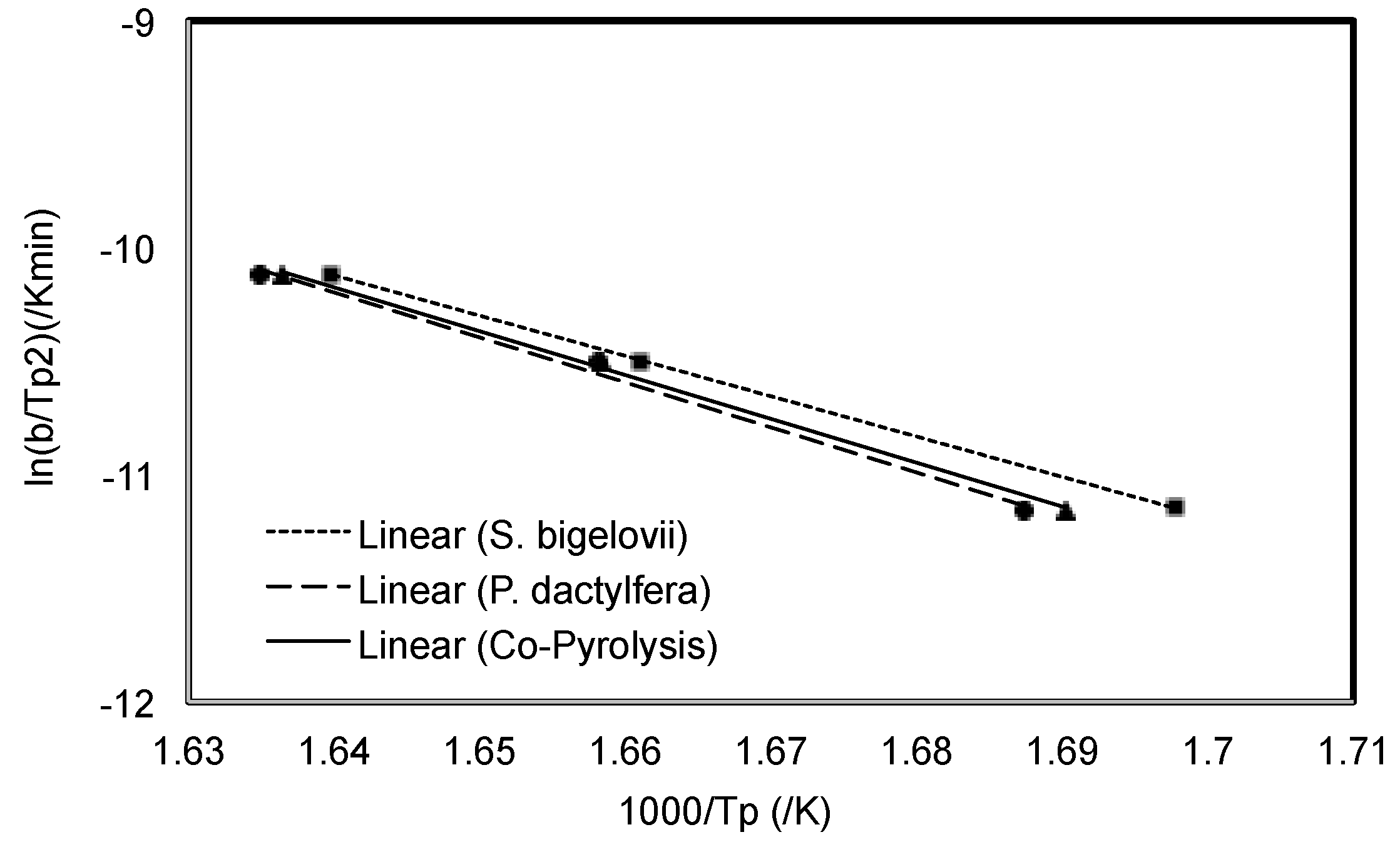
Kissinger Method
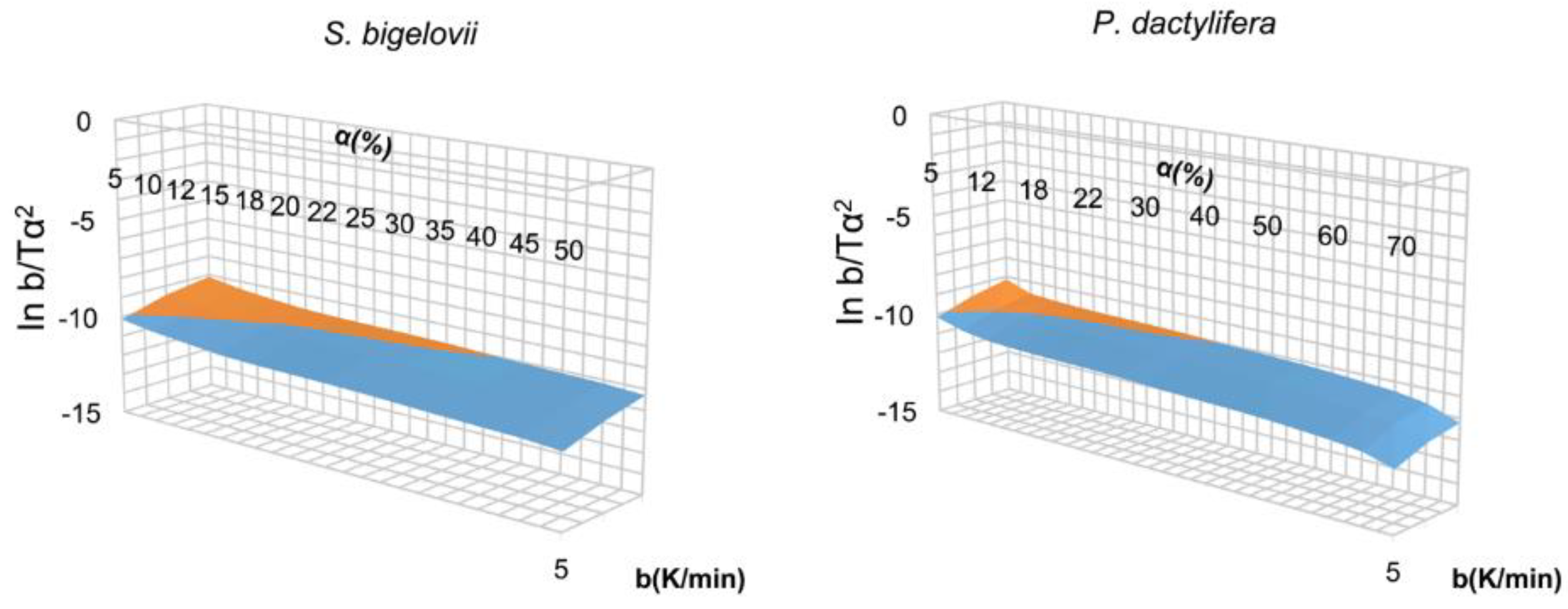
FWO Method
KAS Method
2.2.2. Summary of Parameters
3. Materials and Methods
3.1. Isoconversional Methods for Kinetic Parameter Estimation-Theory
3.1.1. Kissinger Method
3.1.2. FWO Method
3.1.3. KAS Method
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bastidas-Oyanedel, J.R.; Fang, C.; Almardeai, S.; Javid, U.; Yousuf, A.; Schmidt, J.E. Waste biorefinery in arid/semi-arid regions. Bioresour. Technol. 2016, 215, 21–28. [Google Scholar] [CrossRef] [PubMed]
- SBRC Pilot Facility Integrated Seawater Energy and Agriculture System. Available online: https://sbrc.masdar.ac.ae/index.php/projects/seas/item/76-the-seawater-energy-and-agriculture-system (accessed on 20 June 2011).
- Isahak, W.N.R.W.; Hisham, M.W.M.; Yarmo, M.A.; Hin, T.-Y.Y. A review on bio-oil production from biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2012, 16, 5910. [Google Scholar] [CrossRef]
- Czernik, S.; Scahill, J.; Diebold, J. The Production of Liquid Fuel by Fast Pyrolysis of Biomass. J. Sol. Energy Eng. 1995, 117, 2. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Demirbas, A. Determination of calorific values of bio-chars and pyro-oils from pyrolysis of beech trunkbarks. J. Anal. Appl. Pyrolysis 2004, 72, 215–219. [Google Scholar] [CrossRef]
- Uçar, S.; Karagöz, S. The slow pyrolysis of pomegranate seeds: The effect of temperature on the product yields and bio-oil properties. J. Anal. Appl. Pyrolysis 2009, 84, 151–156. [Google Scholar] [CrossRef]
- Solar, J.; de Marco, I.; Caballero, B.M.; Lopez-Urionabarrenechea, A.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of temperature and residence time in the pyrolysis of woody biomass waste in a continuous screw reactor. Biomass Bioenergy 2016, 95, 416–423. [Google Scholar] [CrossRef]
- Sait, H.H.; Hussain, A.; Salema, A.A.; Ani, F.N. Pyrolysis and combustion kinetics of date palm biomass using thermogravimetric analysis. Bioresour. Technol. 2012, 118, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Scano, G.; Boufala, J. Bio-oils from arid land plants: Flash pyrolysis of Euphorbia characias bagasse. Biomass Bioenergy 1994, 7, 291–296. [Google Scholar] [CrossRef]
- Putun, A.; Gercel, H.; Kockar, O.; Ege, O.; Snape, C.; Putun, E. Oil production from an arid-land plant fixed bed pyrolysis and hydropyrolysis. Fuel 1996, 75, 1307–1312. [Google Scholar] [CrossRef]
- Warshay, B.; Brown, J.J.; Sgouridis, S. Life cycle assessment of integrated seawater agriculture in the Arabian (Persian) Gulf as a potential food and aviation biofuel resource. Int. J. Life Cycle Assess. 2017, 22, 1033. [Google Scholar] [CrossRef]
- Abideen, Z.; Ansari, R.; Gul, B.; Khan, M.A. The place of halophytes in Pakistan’s biofuel industry. Biofuels 2012, 3, 211–220. [Google Scholar] [CrossRef]
- Buratti, C.; Barbanera, M.; Bartocci, P.; Fantozzi, F. Thermogravimetric analysis of the behavior of sub-bituminous coal and cellulosic ethanol residue during co-combustion. Bioresour. Technol. 2015, 186, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Gasparovic, L.; Korevnova, Z.; Jelemensky, L. Kinetic study of wood chips decomposition by TGA. Chem. Pap. 2010, 64, 174–181. [Google Scholar] [CrossRef]
- Bartocci, P.; Anca-Couce, A.; Slopiecka, K.; Nefkens, S.; Evic, N.; Retschitzegger, S.; Barbanera, M.; Buratti, C.; Cotana, F.; Bidini, G.; et al. Pyrolysis of pellets made with biomass and glycerol: Kinetic analysis and evolved gas analysis. Biomass Bioenergy 2017, 97, 11–19. [Google Scholar] [CrossRef]
- Kongkaew, N.; Pruksakit, W.; Patumsawad, S. Thermogravimetric Kinetic Analysis of the Pyrolysis of Rice Straw. Energy Procedia 2015, 79, 663–670. [Google Scholar] [CrossRef]
- Starink, M.J. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
| Method | S. bigelovii | P. dactylifera | ||
|---|---|---|---|---|
| E (KJ/mol) | A (min−1) | E (KJ/mol) | A (min−1) | |
| Kissinger | 147.6 | 3.13 × 109 | 164.7 | 9.55 × 1010 |
| FWO | 146.7 | 2.92 × 1020 | 204.3 | 1.93 × 1037 |
| KAS | 147.3 | 3.57 × 1014 | 201.4 | 8.54 × 1031 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzidzienyo, P.; Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Pyrolysis Kinetics of the Arid Land Biomass Halophyte Salicornia Bigelovii and Phoenix Dactylifera Using Thermogravimetric Analysis. Energies 2018, 11, 2283. https://doi.org/10.3390/en11092283
Dzidzienyo P, Bastidas-Oyanedel J-R, Schmidt JE. Pyrolysis Kinetics of the Arid Land Biomass Halophyte Salicornia Bigelovii and Phoenix Dactylifera Using Thermogravimetric Analysis. Energies. 2018; 11(9):2283. https://doi.org/10.3390/en11092283
Chicago/Turabian StyleDzidzienyo, Prosper, Juan-Rodrigo Bastidas-Oyanedel, and Jens Ejbye Schmidt. 2018. "Pyrolysis Kinetics of the Arid Land Biomass Halophyte Salicornia Bigelovii and Phoenix Dactylifera Using Thermogravimetric Analysis" Energies 11, no. 9: 2283. https://doi.org/10.3390/en11092283
APA StyleDzidzienyo, P., Bastidas-Oyanedel, J.-R., & Schmidt, J. E. (2018). Pyrolysis Kinetics of the Arid Land Biomass Halophyte Salicornia Bigelovii and Phoenix Dactylifera Using Thermogravimetric Analysis. Energies, 11(9), 2283. https://doi.org/10.3390/en11092283






