RETRACTED: Production of Hydrogen by Methane Steam Reforming Coupled with Catalytic Combustion in Integrated Microchannel Reactors
Abstract
1. Introduction
2. Model Development
2.1. Description of the Reaction System
2.2. Mathematical Model
2.3. Chemical Kinetic Model
2.4. Computation Scheme
3. Results
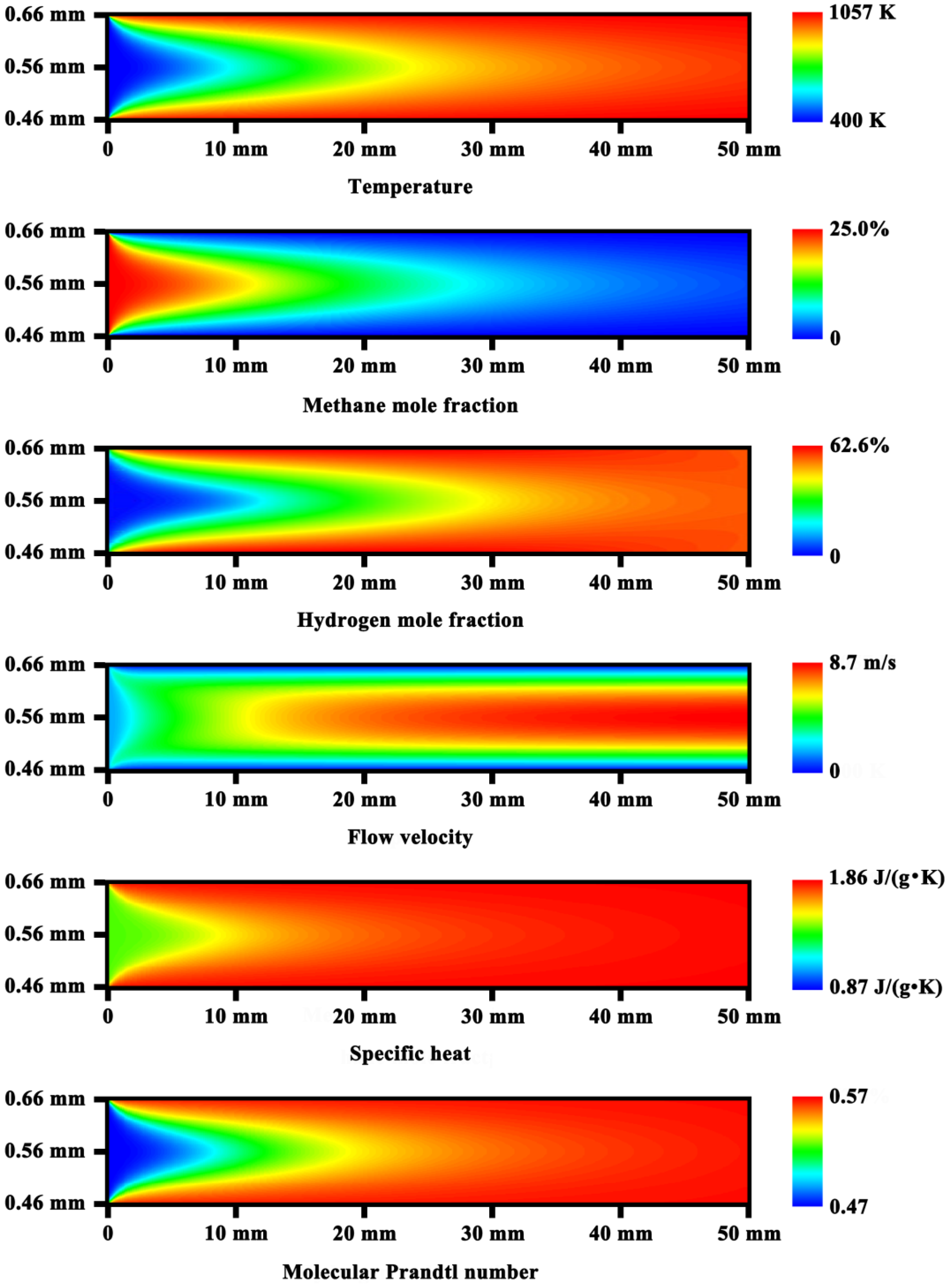
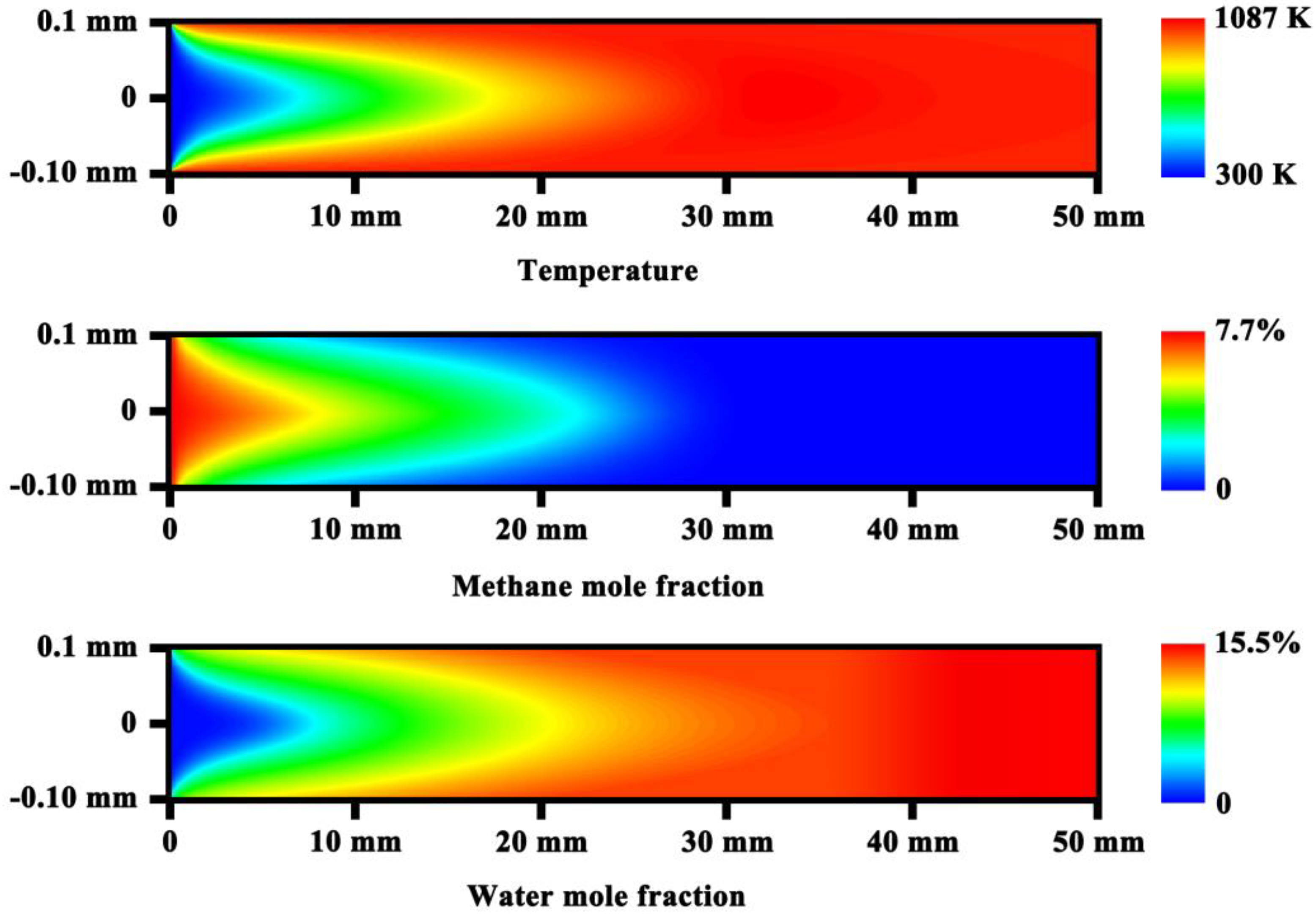
3.1. Analysis of Transport and Reaction Time Scales
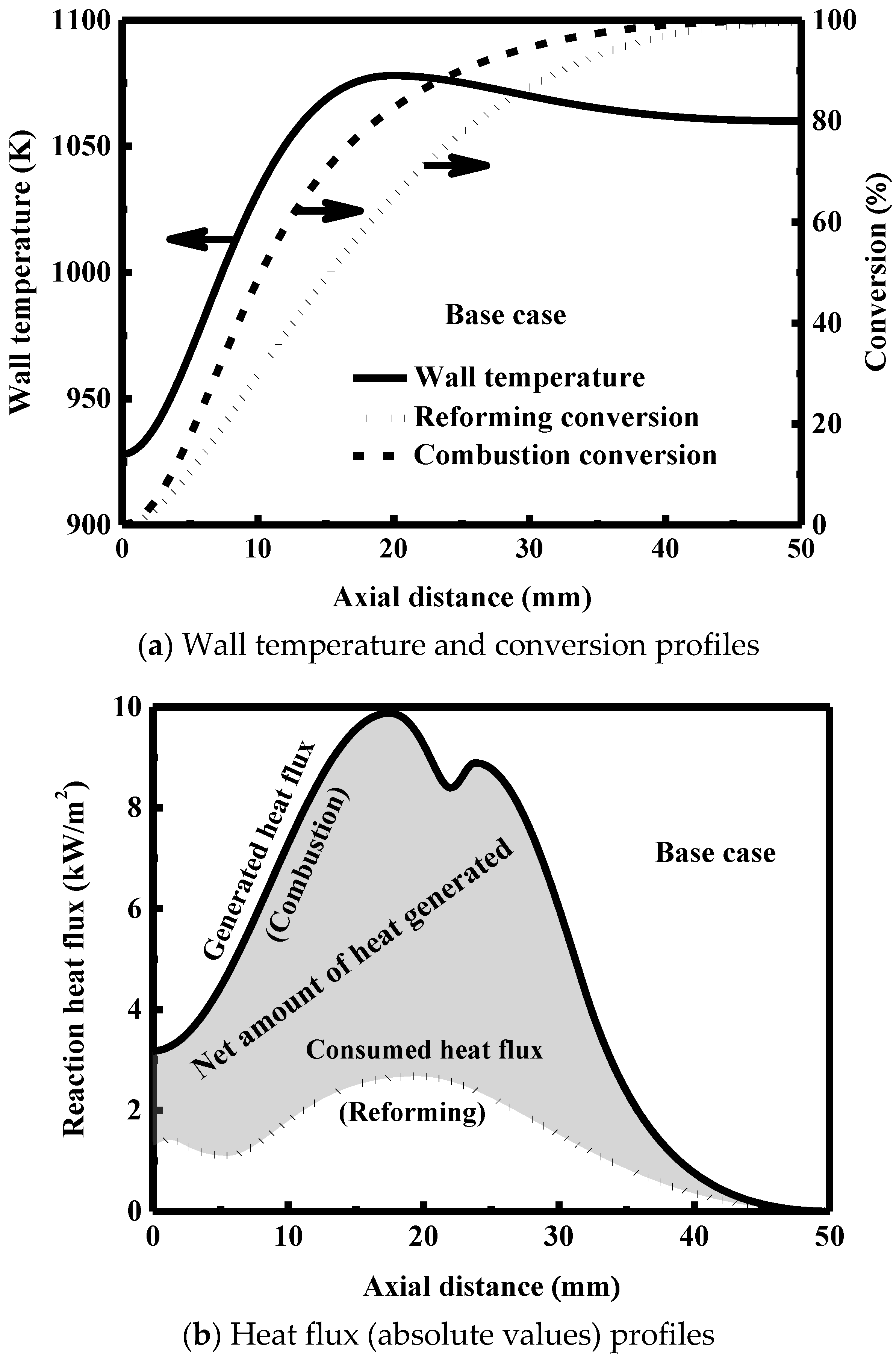
3.2. Heat Exchange Characteristics
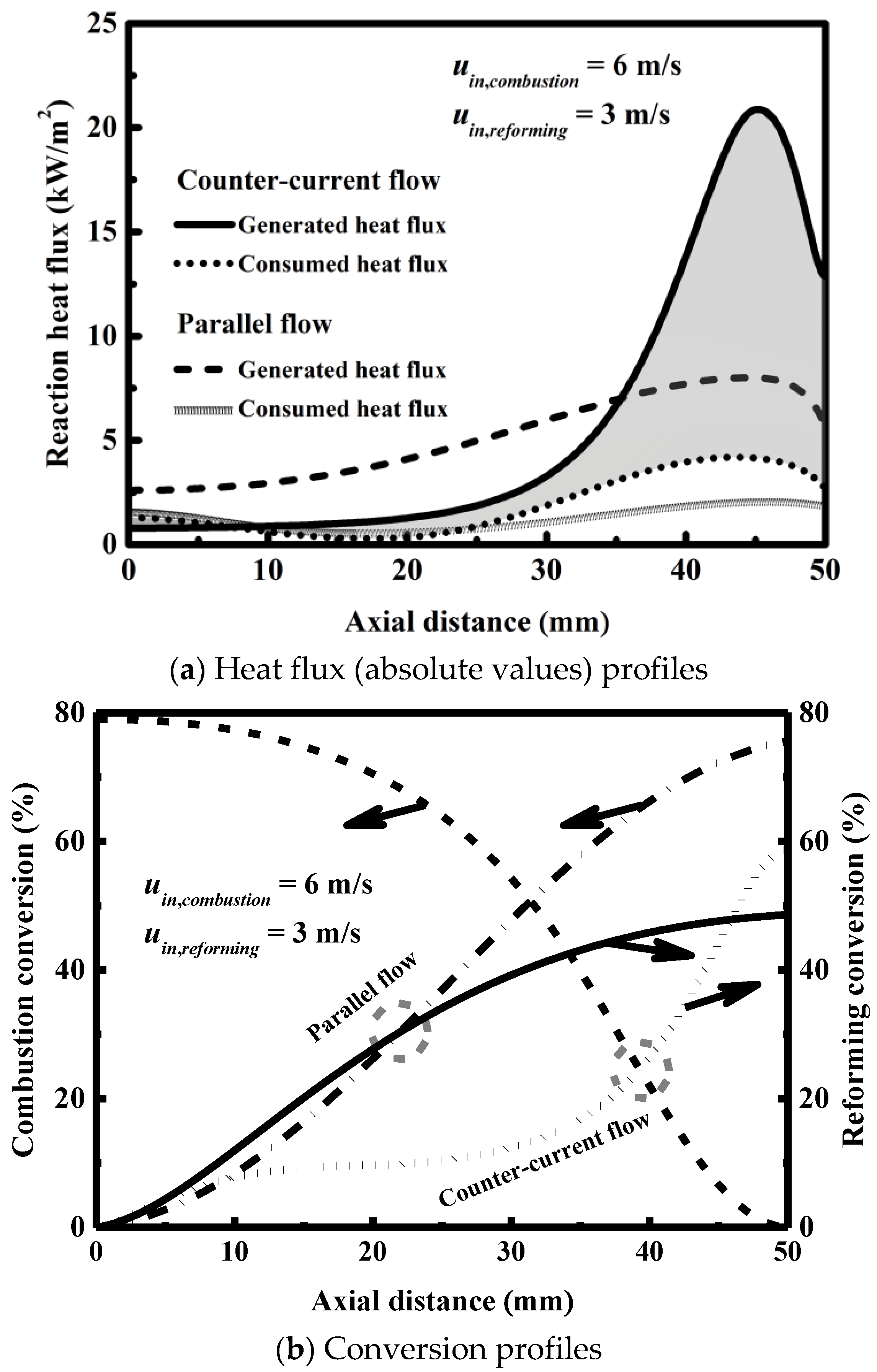
3.3. Effect of Flow Arrangement
3.4. Effect of Reactor Dimension
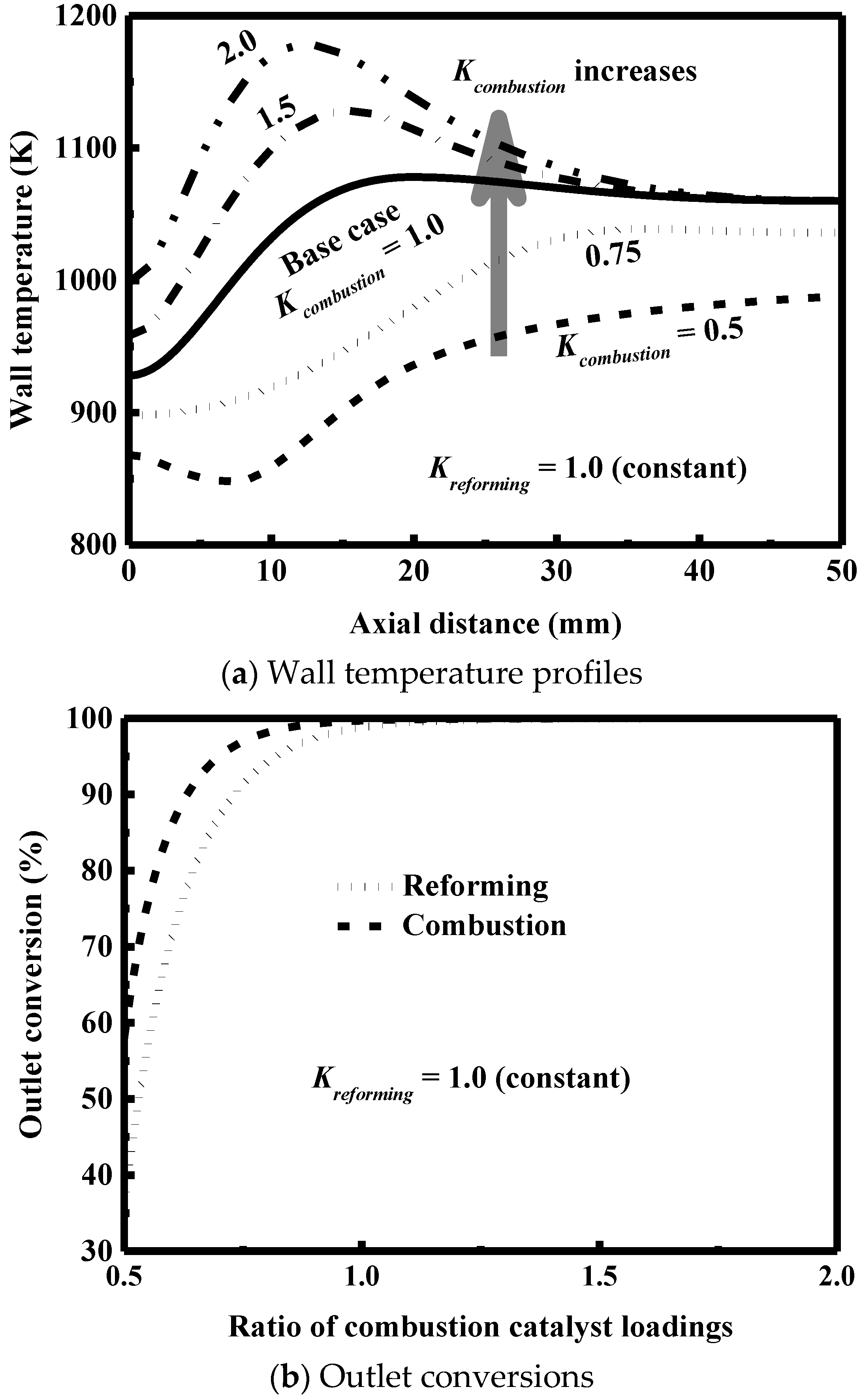
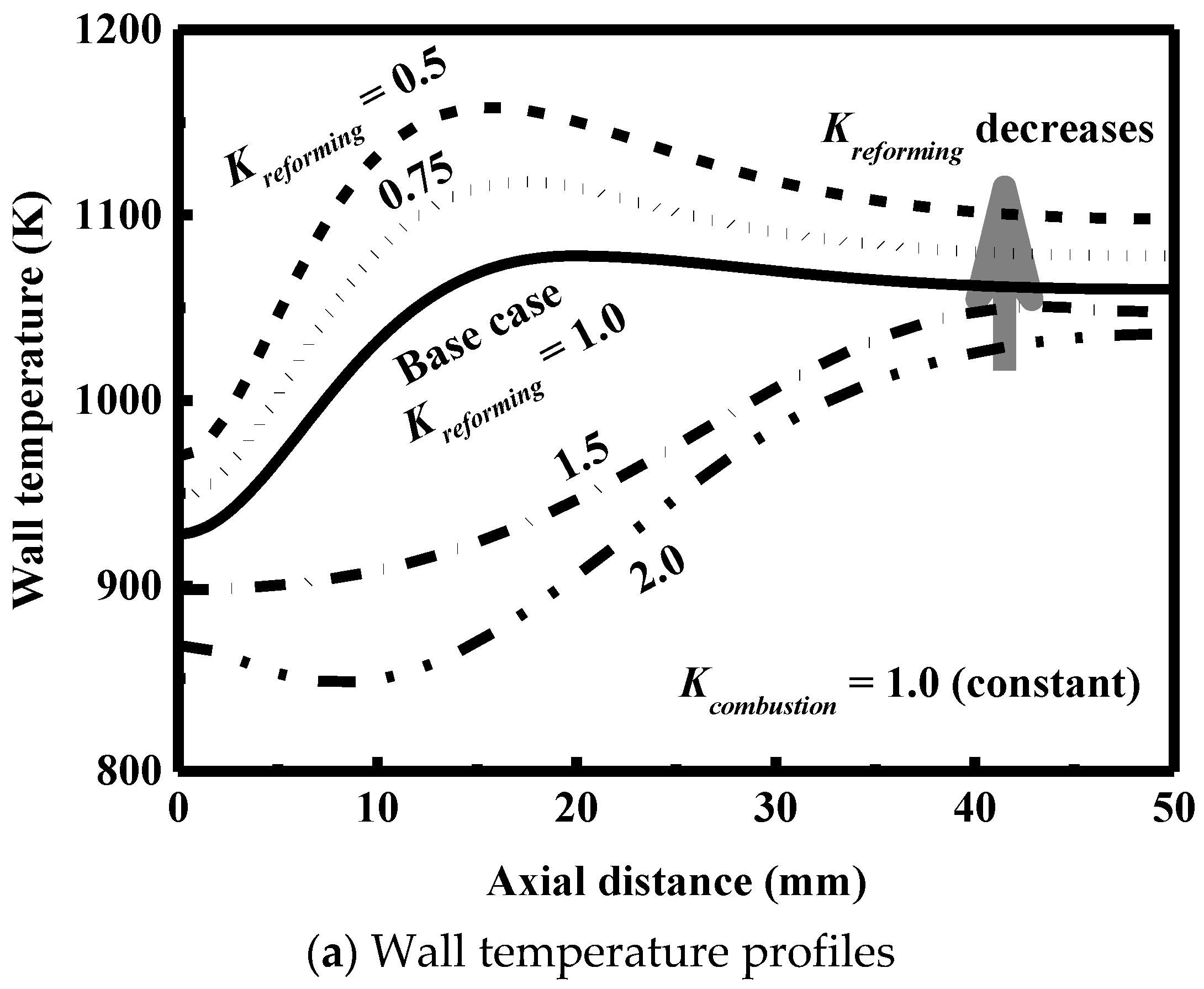
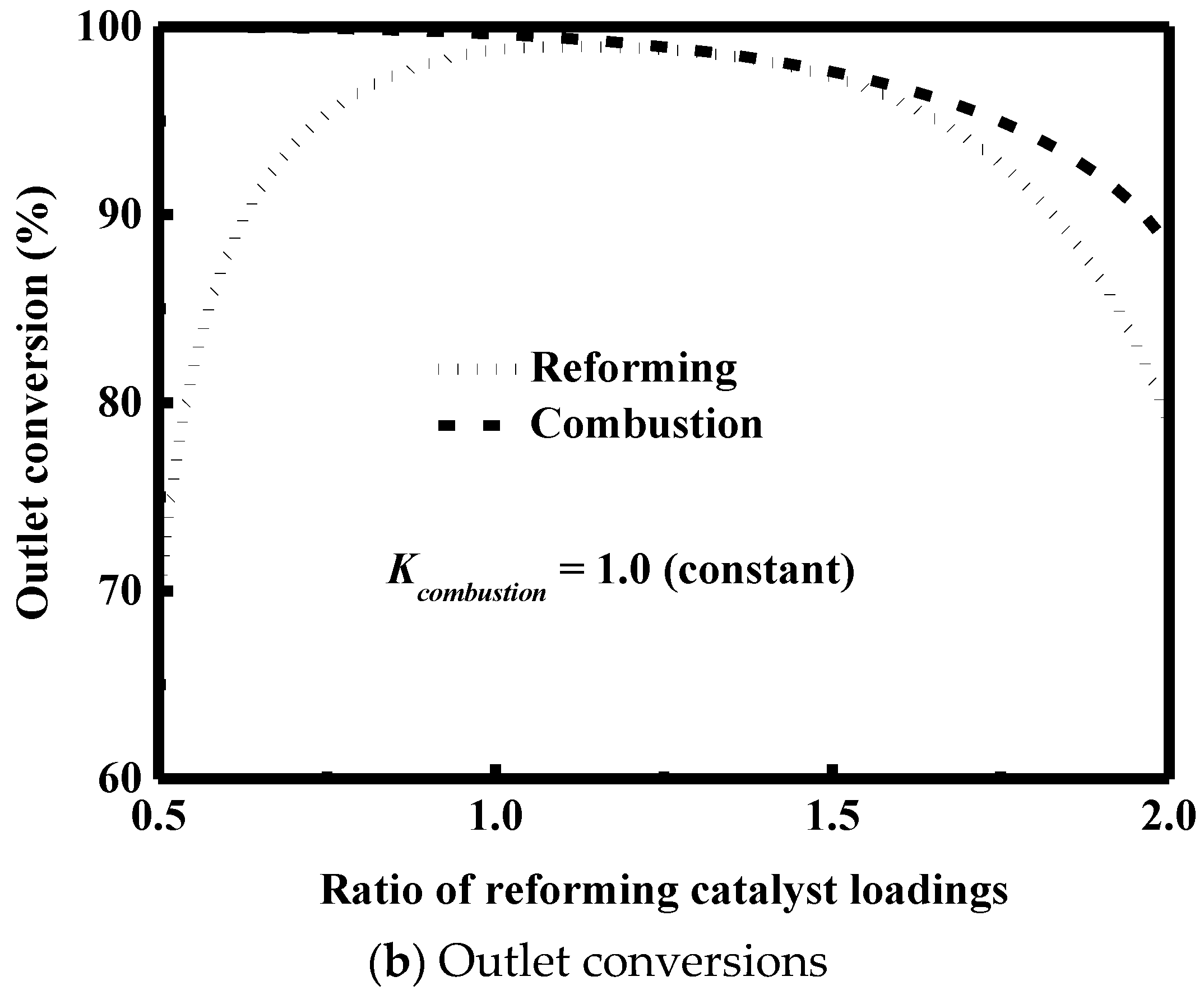
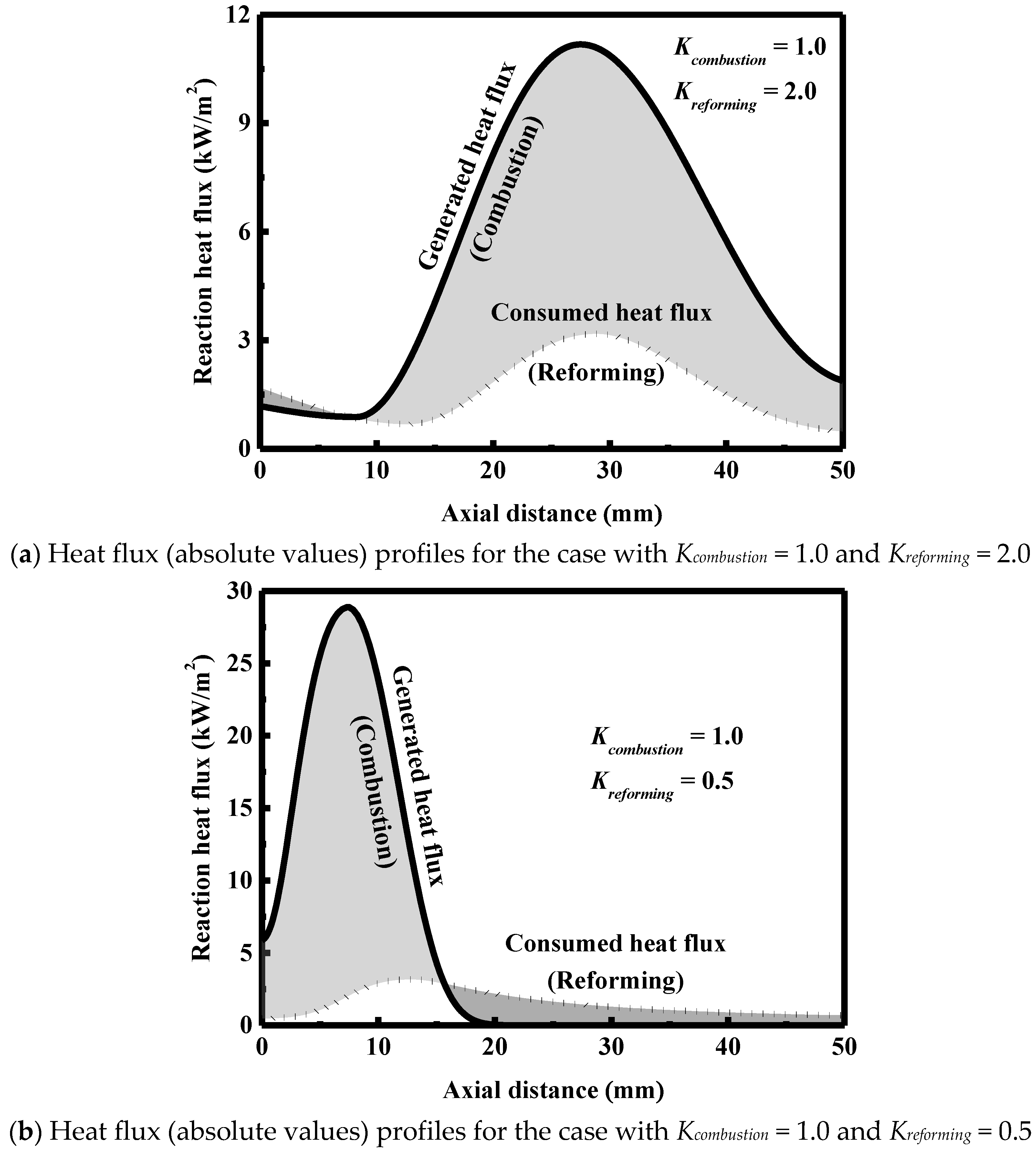
3.5. Effect of Catalyst Loading
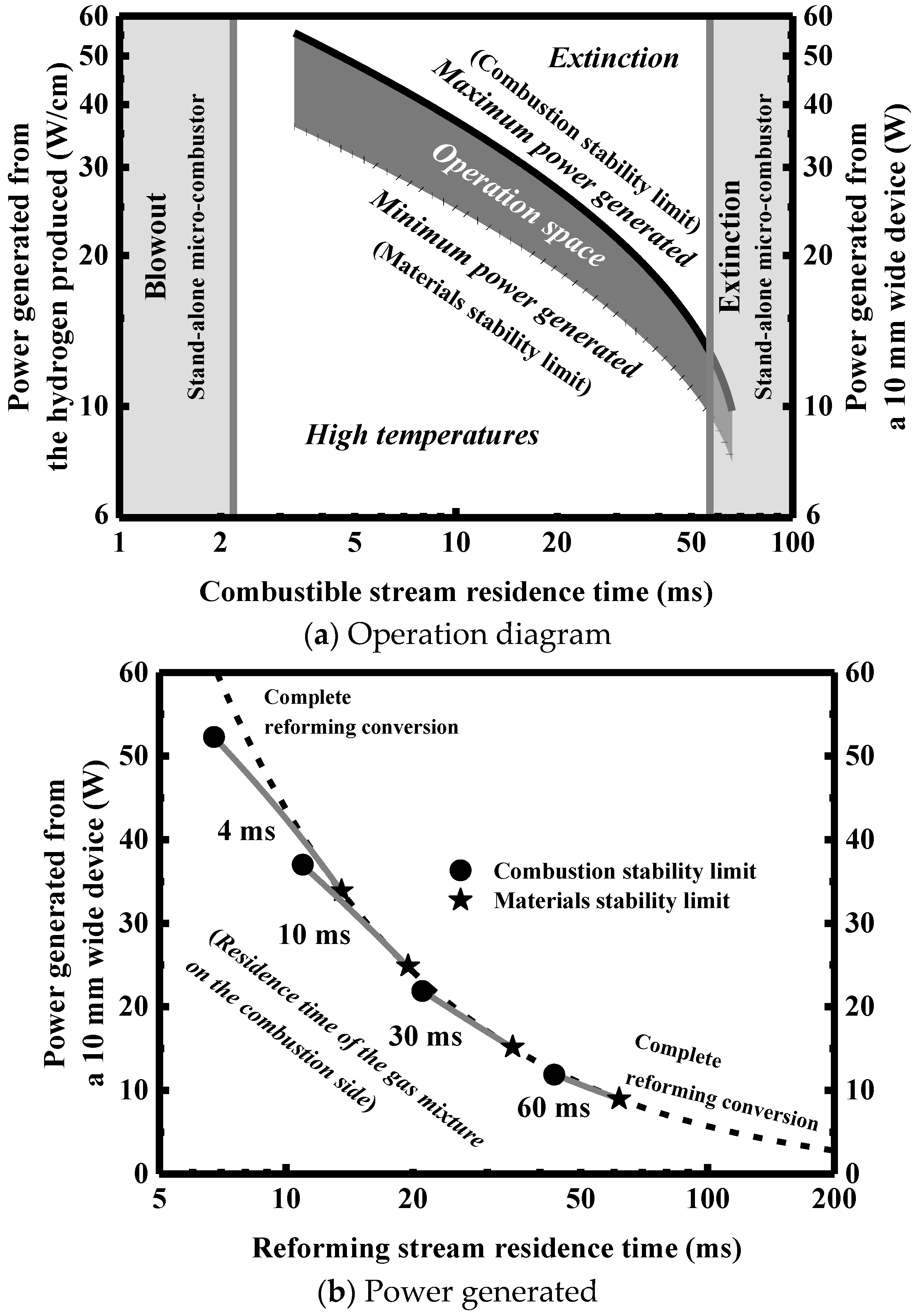
4. Engineering Maps
5. Conclusions
- The production of hydrogen can be achieved at millisecond contact times from methane steam reforming, but the flows, catalysts, and dimensions must be properly designed.
- The improvement in catalyst performance is necessary for the reforming process. High transport rates and a rapid reforming process must be symbiotic for achieving the efficient production of hydrogen.
- The dimension of the reactor is an important design parameter. Small dimensions are required to enable both fast transport rates and a rapid reforming process.
- The catalyst loading is an important variable to optimize the performance of the reactor and must be carefully designed to avoid the formation of hot spots and low outlet conversions.
- Countercurrent heat exchange can achieve slightly better reactor performance, but parallel heat exchange is recommended to ensure stable and reliable operation of the system.
- Thermal management is an effective way to improve the performance of the reactor. The flow rates should be carefully designed to meet the requirements of the materials and combustion stability.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | surface area |
| C | concentration |
| c | specific heat capacity |
| d | gap distance |
| dpore | mean pore diameter |
| D | diffusivity |
| DT | thermal diffusivity |
| Dm | mixture-averaged diffusivity |
| Day | transverse Damköhler number |
| Fcat/geo | catalyst/geometric surface area |
| Fo | Fourier number |
| F | view factor |
| h | specific enthalpy |
| kad | adsorption rate constant |
| ho | external heat loss coefficient |
| K | ratio of catalyst loadings |
| Kg | number of gaseous species |
| Ks | number of surface species |
| l | length |
| m | total number of gaseous and surface species |
| p | pressure |
| Pe | Péclet number |
| q | heat flux |
| R | ideal gas constant |
| s | sticking coefficient |
| rate of appearance of surface species m | |
| S | surface area |
| T | absolute temperature |
| u | streamwise velocity component |
| ū | average flow velocity |
| uin | inlet flow velocity |
| V | diffusion velocity |
| v | transverse velocity component |
| V’ | volume |
| W | relative molecular mass |
| x, y | streamwise and transverse reactor coordinates |
| Y | mass fraction |
| Greek Variables | |
| γ | active surface area per unit volume of the washcoat |
| δ | thickness |
| ε | emissivity |
| εp | catalyst porosity |
| η | effectiveness factor |
| θfree | surface coverage of free sites |
| λ | thermal conductivity |
| ρ | density of the gas mixture |
| σ’ | Stefan–Boltzmann constant |
| σ | site occupancy |
| μ | dynamic viscosity |
| τ | time scale |
| τp | catalyst tortuosity factor |
| τreaction | intrinsic reaction time scale |
| τx | axial transfer time scale |
| τy | transverse transfer time scale |
| rate of appearance of gaseous species k | |
| Γ | density of catalyst surface sites |
| Φ | Thiele modulus |
| Subscripts | |
| g | gas |
| i, k, m | indexes of species, gaseous species, and surface species |
| s, w | solid and wall |
| x, y | streamwise and transverse components |
References
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Lei, L.; Keels, J.M.; Tao, Z.; Zhang, J.; Chen, F. Thermodynamic and experimental assessment of proton conducting solid oxide fuel cells with internal methane steam reforming. Appl. Energy 2018, 224, 280–288. [Google Scholar] [CrossRef]
- Hagos, F.Y.; Aziz, A.R.A.; Sulaiman, S.A.; Mamat, R. Engine speed and air-fuel ratio effect on the combustion of methane augmented hydrogen rich syngas in DI SI engine. Int. J. Hydrog. Energy 2018. [Google Scholar] [CrossRef]
- Nadaleti, W.C.; Przybyla, G. Emissions and performance of a spark-ignition gas engine generator operating with hydrogen-rich syngas, methane and biogas blends for application in southern Brazilian rice industries. Energy 2018, 154, 38–51. [Google Scholar] [CrossRef]
- Oechsler, B.F.; Dutra, J.C.S.; Bittencourt, R.C.P.; Pinto, J.C. Simulation and control of steam reforming of natural gas-reactor temperature control using residual gas. Ind. Eng. Chem. Res. 2017, 56, 2690–2710. [Google Scholar] [CrossRef]
- Kumar, A.; Baldea, M.; Edgar, T.F.; Ezekoye, O.A. Smart manufacturing approach for efficient operation of industrial steam-methane reformers. Ind. Eng. Chem. Res. 2015, 54, 4360–4370. [Google Scholar] [CrossRef]
- Castagnola, L.; Lomonaco, G.; Marotta, R. Nuclear systems for hydrogen production: State of art and perspectives in transport sector. Glob. J. Energy Technol. Res. Updates 2014, 1, 4–18. [Google Scholar]
- Hori, M.; Matsui, K.; Tashimo, M.; Yasuda, I. Synergistic hydrogen production by nuclear-heated steam reforming of fossil fuels. Prog. Nucl. Energy 2005, 47, 519–526. [Google Scholar] [CrossRef]
- Arku, P.; Regmi, B.; Dutta, A. A review of catalytic partial oxidation of fossil fuels and biofuels: Recent advances in catalyst development and kinetic modelling. Chem. Eng. Res. Des. 2018, 136, 385–402. [Google Scholar] [CrossRef]
- Sari, A. A theoretical study on high pressure partial oxidation of methane in Rh-washcoated monoliths. Chem. Eng. Res. Des. 2017, 121, 134–148. [Google Scholar] [CrossRef]
- Tonkovich, A.L.Y.; Yang, B.; Perry, S.T.; Fitzgerald, S.P.; Wang, Y. From seconds to milliseconds to microseconds through tailored microchannel reactor design of a steam methane reformer. Catal. Today 2007, 120, 21–29. [Google Scholar] [CrossRef]
- Stefanidis, G.D.; Vlachos, D.G.; Kaisare, N.S.; Maestri, M. Methane steam reforming at microscales: Operation strategies for variable power output at millisecond contact times. AIChE J. 2009, 55, 180–191. [Google Scholar] [CrossRef]
- Stefanidis, G.D.; Vlachos, D.G. Millisecond methane steam reforming via process and catalyst intensification. Chem. Eng. Technol. 2008, 31, 1201–1209. [Google Scholar] [CrossRef]
- Holladay, J.D.; Wang, Y. A review of recent advances in numerical simulations of microscale fuel processor for hydrogen production. J. Power Sources 2015, 282, 602–621. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, P.; Chen, H.; Pei, P. A review of automotive proton exchange membrane fuel cell degradation under start-stop operating condition. Appl. Energy 2018, 223, 249–262. [Google Scholar] [CrossRef]
- Chen, H.; Song, Z.; Zhao, X.; Zhang, T.; Pei, P.; Liang, C. A review of durability test protocols of the proton exchange membrane fuel cells for vehicle. Appl. Energy 2018, 224, 289–299. [Google Scholar] [CrossRef]
- Bassina, I.A.; Malkov, Y.P.; Molchanov, O.N.; Stepanov, S.G.; Troshchinenko, G.A.; Zasypkin, I.M. Thermodynamic study of characteristics of the converter with separated supply of hydrocarbon fuel for thermo-oxidative and steam reforming. Thermophys. Aeromech. 2018, 21, 249–254. [Google Scholar] [CrossRef]
- Mettler, M.S.; Stefanidis, G.D.; Vlachos, D.G. Enhancing stability in parallel plate microreactor stacks for syngas production. Chem. Eng. Sci. 2011, 66, 1051–1059. [Google Scholar] [CrossRef]
- Bawornruttanaboonya, K.; Devahastin, S.; Mujumdar, A.S.; Laosiripojana, N. A computational fluid dynamic evaluation of a new microreactor design for catalytic partial oxidation of methane. Int. J. Heat Mass Transfer 2017, 115, 174–185. [Google Scholar] [CrossRef]
- Carrera, A.; Pelucchi, M.; Stagni, A.; Beretta, A.; Groppi, G. Catalytic partial oxidation of n-octane and iso-octane: Experimental and modeling results. Int. J. Hydrogen Energy 2017, 42, 24675–24688. [Google Scholar] [CrossRef]
- Shigarov, A.B.; Fadeev, S.I.; Kirillov, V.A. Modeling of a heat-integrated catalytic reformer/combustor of methane: Fine balancing between hot spots and extinction. Chem. Eng. Technol. 2009, 32, 1367–1375. [Google Scholar] [CrossRef]
- Cremers, C.; Pelz, A.; Stimming, U.; Haas-Santo, K.; Görke, O.; Pfeifer, P.; Schubert, K. Micro-structured methane steam reformer with integrated catalytic combustor. Fuel Cells 2007, 7, 91–98. [Google Scholar] [CrossRef]
- Mettler, M.S.; Stefanidis, G.D.; Vlachos, D.G. Scale-out of microreactor stacks for portable and distributed processing: Coupling of exothermic and endothermic processes for syngas production. Ind. Eng. Chem. Res. 2010, 49, 10942–10955. [Google Scholar] [CrossRef]
- Irankhah, A.; Rahimi, M.; Rezaei, M. Performance research on a methane compact reformer integrated with catalytic combustion. Chem. Eng. Technol. 2014, 37, 1220–1226. [Google Scholar] [CrossRef]
- Van Grootel, P.W.; Hensen, E.J.M.; van Santen, R.A. The CO formation reaction pathway in steam methane reforming by rhodium. Langmuir 2010, 26, 16339–16348. [Google Scholar] [CrossRef] [PubMed]
- Al-Rifai, N.; Cao, E.; Dua, V.; Gavriilidis, A. Microreaction technology aided catalytic process design. Curr. Opin. Chem. Eng. 2013, 2, 338–345. [Google Scholar] [CrossRef]
- Tonkovich, A.Y.; Perry, S.; Wang, Y.; Qiu, D.; LaPlante, T.; Rogers, W.A. Microchannel process technology for compact methane steam reforming. Chem. Eng. Sci. 2004, 59, 4819–4824. [Google Scholar] [CrossRef]
- Settar, A.; Nebbali, R.; Madani, B.; Abboudi, S. Numerical investigation on the wall-coated steam methane reformer improvement: Effects of catalyst layer patterns and metal foam insertion. Int. J. Hydrog. Energy 2015, 40, 8966–8979. [Google Scholar] [CrossRef]
- Yang, L.; Nieves-Remacha, M.J.; Jensen, K.F. Simulations and analysis of multiphase transport and reaction in segmented flow microreactors. Chem. Eng. Sci. 2017, 169, 106–116. [Google Scholar] [CrossRef]
- Van Gerven, T.; Stankiewicz, A. Structure, energy, synergy, time-the fundamentals of process intensification. Ind. Eng. Chem. Res. 2009, 48, 2465–2474. [Google Scholar] [CrossRef]
- Abiev, R. Process intensification by pulsations in chemical engineering: Some general principles and implementation. Ind. Eng. Chem. Res. 2017, 56, 13497–13507. [Google Scholar] [CrossRef]
- Suryawanshi, P.L.; Gumfekar, S.P.; Bhanvase, B.A.; Sonawane, S.H.; Pimplapure, M.S. A review on microreactors: Reactor fabrication, design, and cutting-edge applications. Chem. Eng. Sci. 2018, 189, 431–448. [Google Scholar] [CrossRef]
- Kolb, G. Review: Microstructured reactors for distributed and renewable production of fuels and electrical energy. Chem. Eng. Process. 2013, 65, 1–44. [Google Scholar] [CrossRef]
- Che, F.; Gray, J.T.; Ha, S.; McEwen, J.-S. Reducing reaction temperature, steam requirements, and coke formation during methane steam reforming using electric fields: A microkinetic modeling and experimental study. ACS Catal. 2017, 7, 6957–6968. [Google Scholar] [CrossRef]
- Vásquez Castillo, J.M.; Sato, T.; Itoh, N. Microkinetic analysis of the methane steam reforming on a ru-supported catalytic wall reactor. Ind. Eng. Chem. Res. 2017, 56, 8815–8822. [Google Scholar] [CrossRef]
- Cui, X.; Kær, S.K. Two-dimensional thermal analysis of radial heat transfer of monoliths in small-scale steam methane reforming. Int. J. Hydrogen Energy 2018, 43, 11952–11968. [Google Scholar] [CrossRef]
- Settar, A.; Abboudi, S.; Lebaal, N. Effect of inert metal foam matrices on hydrogen production intensification of methane steam reforming process in wall-coated reformer. Int. J. Hydrogen Energy 2018, 43, 12386–12397. [Google Scholar] [CrossRef]
- Ricca, A.; Palma, V.; Martino, M.; Meloni, E. Innovative catalyst design for methane steam reforming intensification. Fuel 2017, 198, 175–182. [Google Scholar] [CrossRef]
- Amjad, U.-E.S.; Vita, A.; Galletti, C.; Pino, L.; Specchia, S. Comparative study on steam and oxidative steam reforming of methane with noble metal catalysts. Ind. Eng. Chem. Res. 2013, 52, 15428–15436. [Google Scholar] [CrossRef]
- Gouveia Gil, A.; Wu, Z.; Chadwick, D.; Li, K. Microstructured catalytic hollow fiber reactor for methane steam reforming. Ind. Eng. Chem. Res. 2015, 54, 5563–5571. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Boeltken, T.; Piermartini, P.; Selinsek, M.; Loewert, M.; Dallmann, F.; Kreuder, H.; Cholewa, M.; Wunsch, A.; Belimov, M.; et al. Micro and micro membrane reactors for advanced applications in chemical energy conversion. Curr. Opin. Chem. Eng. 2017, 17, 108–125. [Google Scholar] [CrossRef]
- Sidhu, T.P.K.; Govil, A.; Roy, S. Optimal monolithic configuration for heat integrated ethanol steam reformer. Int. J. Hydrogen Energy 2017, 42, 7770–7785. [Google Scholar] [CrossRef]
- Kshetrimayum, K.S.; Jung, I.; Na, J.; Park, S.; Lee, Y.; Park, S.; Lee, C.-J.; Han, C. CFD simulation of microchannel reactor block for Fischer-Tropsch synthesis: Effect of coolant type and wall boiling condition on reactor temperature. Ind. Eng. Chem. Res. 2016, 55, 543–554. [Google Scholar] [CrossRef]
- Herdem, M.S.; Mundhwa, M.; Farhad, S.; Hamdullahpur, F. Multiphysics modeling and heat distribution study in a catalytic microchannel methanol steam reformer. Energy Fuels 2018, 32, 7220–7234. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, D.-E.; Lee, Y.-J.; Kwak, G.; Jun, K.-W.; Park, M.-J. CFD modeling of a thermally efficient modular reactor for Fischer-Tropsch synthesis: Determination of the optimal size for each module. Ind. Eng. Chem. Res. 2016, 55, 9416–9425. [Google Scholar] [CrossRef]
- Jung, I.; Kshetrimayum, K.S.; Park, S.; Na, J.; Lee, Y.; An, J.; Park, S.; Lee, C.-J.; Han, C. Computational fluid dynamics based optimal design of guiding channel geometry in U-type coolant layer manifold of large-scale microchannel Fischer-Tropsch reactor. Ind. Eng. Chem. Res. 2016, 55, 505–515. [Google Scholar] [CrossRef]
- Rodríguez-Guerra, Y.; Gerling, L.A.; López-Guajardo, E.A.; Lozano-García, F.J.; Nigam, K.D.P.; Montesinos-Castellanos, A. Design of micro- and milli-channel heat exchanger reactors for homogeneous exothermic reactions in the laminar regime. Ind. Eng. Chem. Res. 2016, 55, 6435–6442. [Google Scholar] [CrossRef]
- Jiwanuruk, T.; Putivisutisak, S.; Vas-Umnuay, P.; Bumroongsakulsawat, P.; Cheng, C.K.; Assabumrungrat, S. Modeling of thermally-coupled monolithic membrane reformer for vehicular hydrogen production. Int. J. Hydrogen Energy 2017, 42, 26308–26319. [Google Scholar] [CrossRef]
- Hayes, R.E.; Mmbaga, J.P. Introduction to Chemical Reactor Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4665-8053-4. [Google Scholar]
- Izurieta, E.M.; Borio, D.O.; Pedernera, M.N.; López, E. Parallel plates reactor simulation: Ethanol steam reforming thermally coupled with ethanol combustion. Int. J. Hydrogen Energy 2017, 42, 18794–18804. [Google Scholar] [CrossRef]
- Sari, A.; Sabziani, J. Modeling and 3D-simulation of hydrogen production via methanol steam reforming in copper-coated channels of a mini reformer. J. Power Sources 2017, 352, 64–76. [Google Scholar] [CrossRef]
- Italiano, C.; Luchters, N.T.J.; Pino, L.; Fletcher, J.V.; Specchia, S.; Fletcher, J.C.Q.; Vita, A. High specific surface area supports for highly active Rh catalysts: Syngas production from methane at high space velocity. Int. J. Hydrogen Energy 2018, 43, 11755–11765. [Google Scholar] [CrossRef]
- Thalinger, R.; Götsch, T.; Zhuo, C.; Hetaba, W.; Wallisch, W.; Stöger-Pollach, M.; Schmidmair, D.; Klötzer, B.; Penner, S. Rhodium-catalyzed methanation and methane steam reforming reactions on rhodium-perovskite systems: Metal-support interaction. ChemCatChem 2016, 8, 2057–2067. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Wilcox, J.; Basile, A. Advances on methane steam reforming to produce hydrogen through membrane reactors technology: A review. Cat. Rev. Sci. Eng. 2016, 58, 1–35. [Google Scholar] [CrossRef]
- Bergman, T.L.; Lavine, A.S.; Incropera, F.P.; DeWitt, D.P. Fundamentals of Heat and Mass Transfer, Enhanced eText, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; ISBN 978-1-119-32042-5. [Google Scholar]
- ANSYS Fluent User’s Guide; Release 16.0; ANSYS Inc.: Canonsburg, PA, USA, 2014.
- Kee, R.J.; Dixon-lewis, G.; Warnatz, J.; Coltrin, M.E.; Miller, J.A.; Moffat, H.K. A Fortran Computer Code Package for the Evaluation of Gas-Phase, Multicomponent Transport Properties; Report No. SAND86-8246B; Sandia National Laboratories: Livermore, CA, USA, 1998. [Google Scholar]
- Gerken, I.; Brandner, J.J.; Dittmeyer, R. Heat transfer enhancement with gas-to-gas micro heat exchangers. Appl. Therm. Eng. 2016, 93, 1410–1416. [Google Scholar] [CrossRef]
- Vera, M.; Quintero, A.E. On the role of axial wall conduction in mini/micro counterflow heat exchangers. Int. J. Heat Mass Transfer 2018, 116, 840–857. [Google Scholar] [CrossRef]
- Seetharamu, K.N.; Leela, V.; Kotloni, N. Numerical investigation of heat transfer in a micro-porous-channel under variable wall heat flux and variable wall temperature boundary conditions using local thermal non-equilibrium model with internal heat generation. Int. J. Heat Mass Transfer 2017, 112, 201–215. [Google Scholar] [CrossRef]
- Yang, Y.; Morini, G.L.; Brandner, J.J. Experimental analysis of the influence of wall axial conduction on gas-to-gas micro heat exchanger effectiveness. Int. J. Heat Mass Transfer 2014, 69, 17–25. [Google Scholar] [CrossRef]
- Deutschmann, O. Modeling of the interactions between catalytic surfaces and gas-phase. Catal. Lett. 2015, 145, 272–289. [Google Scholar] [CrossRef]
- Koop, J.; Deutschmann, O. Detailed surface reaction mechanism for Pt-catalyzed abatement of automotive exhaust gases. Appl. Catal. B 2009, 91, 47–58. [Google Scholar] [CrossRef]
- Karadeniz, H.; Karakaya, C.; Tischer, S.; Deutschmann, O. Numerical modeling of stagnation-flows on porous catalytic surfaces: CO oxidation on Rh/Al2O3. Chem. Eng. Sci. 2013, 104, 899–907. [Google Scholar] [CrossRef]
- Deutschmann, O. Modeling and Simulation of Heterogeneous Catalytic Reactions: From the Molecular Process to the Technical System; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 978-3-527-32120-9. [Google Scholar]
- Mladenov, N.; Koop, J.; Tischer, S.; Deutschmann, O. Modeling of transport and chemistry in channel flows of automotive catalytic converters. Chem. Eng. Sci. 2010, 65, 812–826. [Google Scholar] [CrossRef]
- Welty, J.; Rorrer, G.L.; Foster, D.G. Fundamentals of Momentum, Heat, and Mass Transfer, Revised 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; ISBN 978-1-119-03439-1. [Google Scholar]
- Tolmachoff, E.D.; Allmon, W.; Waits, C.M. Analysis of a high throughput n-dodecane fueled heterogeneous/homogeneous parallel plate microreactor for portable power conversion. Appl. Energy 2014, 128, 111–118. [Google Scholar] [CrossRef]
- Ahn, J.; Eastwood, C.; Sitzki, L.; Ronney, P.D. Gas-phase and catalytic combustion in heat-recirculating burners. Proc. Combust. Inst. 2005, 30, 2463–2472. [Google Scholar] [CrossRef]
- Karagiannidis, S.; Mantzaras, J.; Jackson, G.; Boulouchos, K. Hetero-/homogeneous combustion and stability maps in methane-fueled catalytic microreactors. Proc. Combust. Inst. 2007, 31, 3309–3317. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Benedetto, A.; Di Sarli, V.; Landi, G.; Pirone, R. High-pressure methane combustion over a perovskyte catalyst. Ind. Eng. Chem. Res. 2012, 51, 7547–7558. [Google Scholar] [CrossRef]
- Landi, G.; Di Benedetto, A.; Barbato, P.S.; Russo, G.; Di Sarli, V. Transient behavior of structured LaMnO3 catalyst during methane combustion at high pressure. Chem. Eng. Sci. 2014, 116, 350–358. [Google Scholar] [CrossRef]
- Barbato, P.S.; Di Sarli, V.; Landi, G.; Di Benedetto, A. High pressure methane catalytic combustion over novel partially coated LaMnO3-based monoliths. Chem. Eng. J. 2015, 259, 381–390. [Google Scholar] [CrossRef]
- Di Sarli, V.; Barbato, P.S.; Di Benedetto, A.; Landi, G. Start-up behavior of a LaMnO3 partially coated monolithic combustor at high pressure. Catal. Today 2015, 242, 200–210. [Google Scholar] [CrossRef]
- Landi, G.; Barbato, P.S.; Di Sarli, V.; Di Benedetto, A. Multifuel catalytic combustion in the presence of carbon dioxide over fully and partially perovskite-coated monoliths. Ind. Eng. Chem. Res. 2017, 56, 4920–4928. [Google Scholar] [CrossRef]
- Hughes, K.J.; Turányi, T.; Clague, A.R.; Pilling, M.J. Development and testing of a comprehensive chemical mechanism for the oxidation of methane. Int. J. Chem. Kinet. 2001, 33, 513–538. [Google Scholar] [CrossRef]
- Turányi, T.; Zalotai, L.; Dóbé, S.; Bérces, T. Effect of the uncertainty of kinetic and thermodynamic data on methane flame simulation results. Phys. Chem. Chem. Phys. 2002, 4, 2568–2578. [Google Scholar] [CrossRef]
- Deutschmann, O.; Maier, L.I.; Riedel, U.; Stroemman, A.H.; Dibble, R.W. Hydrogen assisted catalytic combustion of methane on platinum. Catal. Today 2000, 59, 141–150. [Google Scholar] [CrossRef]
- DETCHEM. Detailed Chemistry in CFD; DETCHEM: Karlsruhe, Germany, 2017; Available online: http://www.detchem.com/ (accessed on 5 August 2018).
- Schädel, B.T.; Duisberg, M.; Deutschmann, O. Steam reforming of methane, ethane, propane, butane, and natural gas over a rhodium-based catalyst. Catal. Today 2009, 142, 42–51. [Google Scholar] [CrossRef]
- Karakaya, C.; Maier, L.; Deutschmann, O. Surface reaction kinetics of the oxidation and reforming of CH4 over Rh/Al2O3 catalysts. Int. J. Chem. Kinet. 2016, 48, 144–160. [Google Scholar] [CrossRef]
- Kee, R.J.; Rupley, F.M.; Meeks, E.; Miller, J.A. CHEMKIN-III: A Fortran Chemical Kinetics Package for the Analysis of Gasphase Chemical and Plasma Kinetics; Report No. SAND96-8216; Sandia National Laboratories: Livermore, CA, USA, 1996. [Google Scholar]
- Coltrin, M.E.; Kee, R.J.; Rupley, F.M.; Meeks, E. SURFACE CHEMKIN-III: A Fortran Package for Analyzing Heterogeneous Chemical Kinetics at a Solid-Surface-Gas-Phase Interface; Report No. SAND96-8217; Sandia National Laboratories: Livermore, CA, USA, 1996. [Google Scholar]
- Maier, L.; Schädel, B.; Delgado, K.H.; Tischer, S.; Deutschmann, O. Steam reforming of methane over nickel: Development of a multi-step surface reaction mechanism. Top. Catal. 2011, 54, 845–858. [Google Scholar] [CrossRef]
- Gordon, S.; McBride, B.J. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications I. Analysis; Report No. NASA RP-1311; National Aeronautics and Space Administration, Lewis Research Center: Cleveland, OH, USA, 1994. [Google Scholar]
- McBride, B.J.; Gordon, S. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications II. User’s Manual and Program Description; Report No. NASA RP-1311-P2; National Aeronautics and Space Administration, Lewis Research Center: Cleveland, OH, USA, 1996. [Google Scholar]
- Naqiuddin, N.H.; Saw, L.H.; Yew, M.C.; Yusof, F.; Ng, T.C.; Yew, M.K. Overview of micro-channel design for high heat flux application. Renew. Sust. Energ. Rev. 2018, 82, 901–914. [Google Scholar] [CrossRef]
- Cengel, Y.; Ghajar, A. Heat and Mass Transfer: Fundamentals and Applications, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2015; ISBN 9781259173295. [Google Scholar]
- Kolios, G.; Frauhammer, J.; Eigenberger, G. Efficient reactor concepts for coupling of endothermic and exothermic reactions. Chem. Eng. Sci. 2002, 57, 1505–1510. [Google Scholar] [CrossRef]
- Regatte, V.R.; Kaisare, N.S. Hydrogen generation in spatially coupled cross-flow microreactors. Chem. Eng. J. 2013, 215–216, 876–885. [Google Scholar] [CrossRef]
- Quintero, A.E.; Vera, M. Laminar counterflow parallel-plate heat exchangers: An exact solution including axial and transverse wall conduction effects. Int. J. Heat Mass Transfer 2017, 104, 1229–1245. [Google Scholar] [CrossRef]
- Ranganayakulu, C.; Seetharamu, K.N. Compact Heat Exchangers: Analysis, Design and Optimization Using FEM and CFD Approach; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; ISBN 978-1-119-42437-6. [Google Scholar]
- Rahimpour, M.R.; Dehnavi, M.R.; Allahgholipour, F.; Iranshahi, D.; Jokar, S.M. Assessment and comparison of different catalytic coupling exothermic and endothermic reactions: A review. Appl. Energy 2012, 99, 496–512. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 5th ed.; Pearson Education, Inc.: London, UK, 2016; ISBN 978-0-13-388751-8. [Google Scholar]
- Hessel, V. Novel process windows—Gate to maximizing process intensification via flow chemistry. Chem. Eng. Technol. 2009, 32, 1655–1681. [Google Scholar] [CrossRef]
- Nikačević, N.M.; Huesman, A.E.M.; Van den Hof, P.M.J.; Stankiewicz, A.I. Opportunities and challenges for process control in process intensification. Chem. Eng. Process. 2012, 52, 1–15. [Google Scholar] [CrossRef]
- Amani, E.; Alizadeh, P.; Moghadam, R.S. Micro-combustor performance enhancement by hydrogen addition in a combined baffle-bluff configuration. Int. J. Hydrogen Energy 2018, 43, 8127–8138. [Google Scholar] [CrossRef]
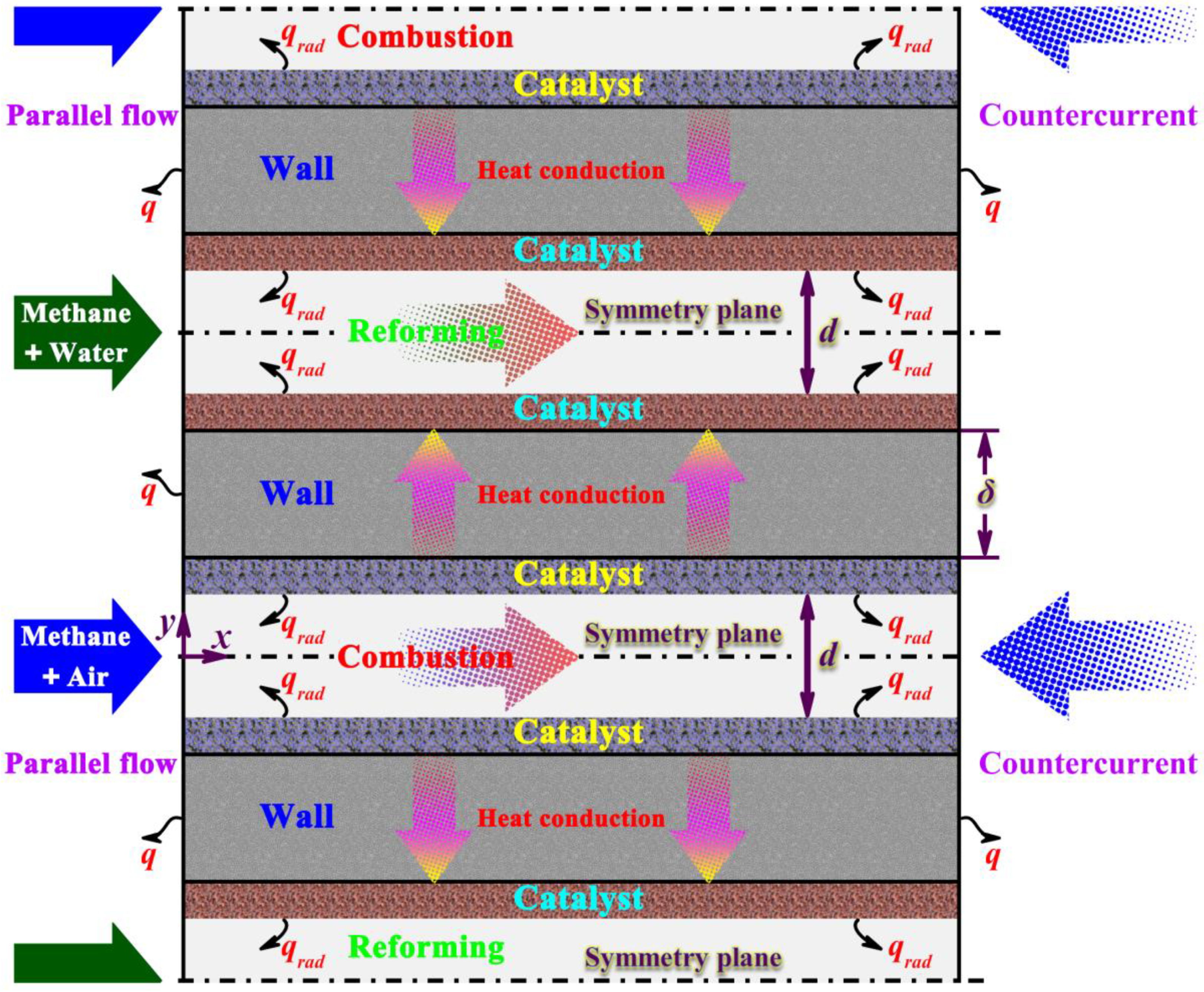
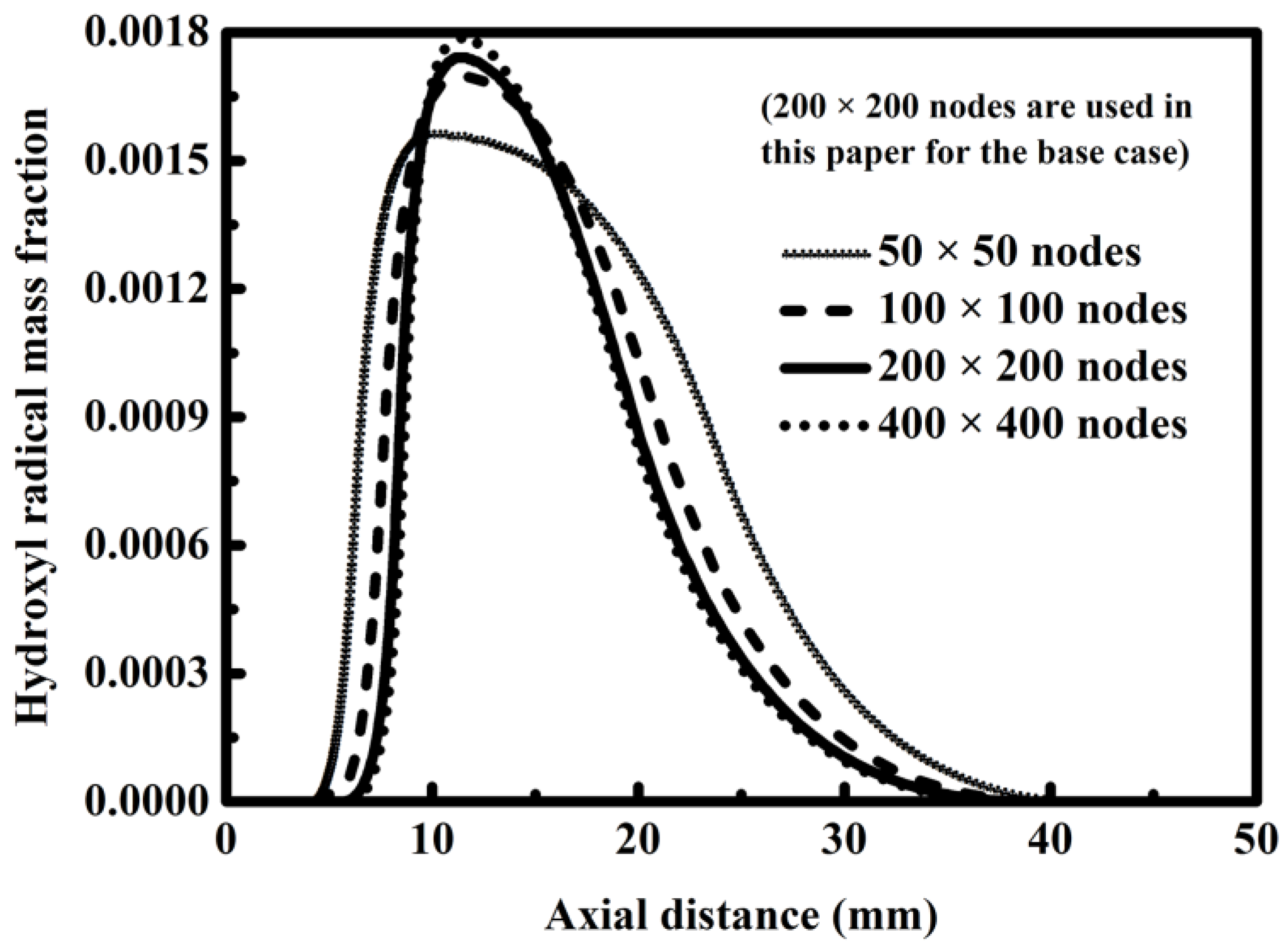
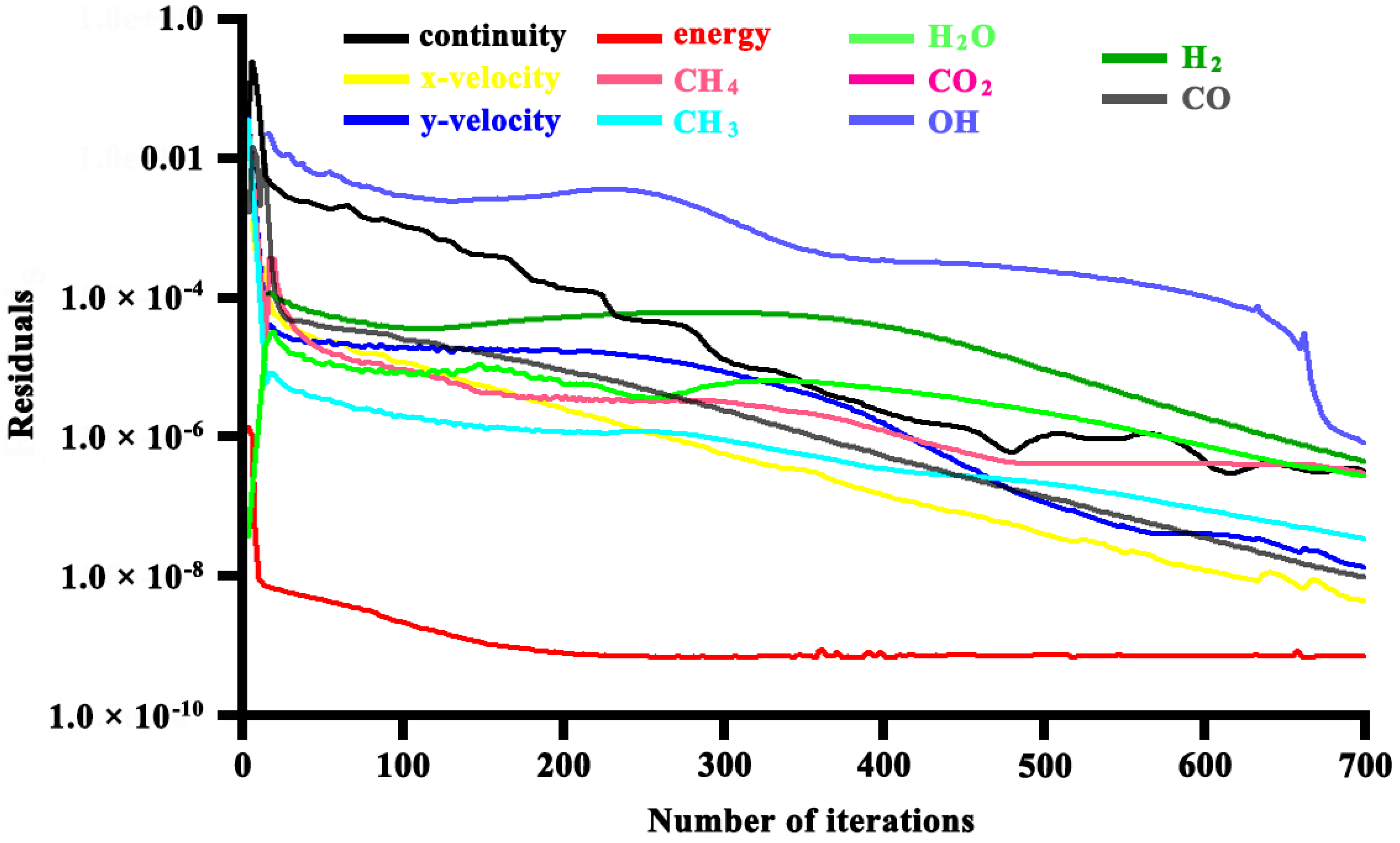
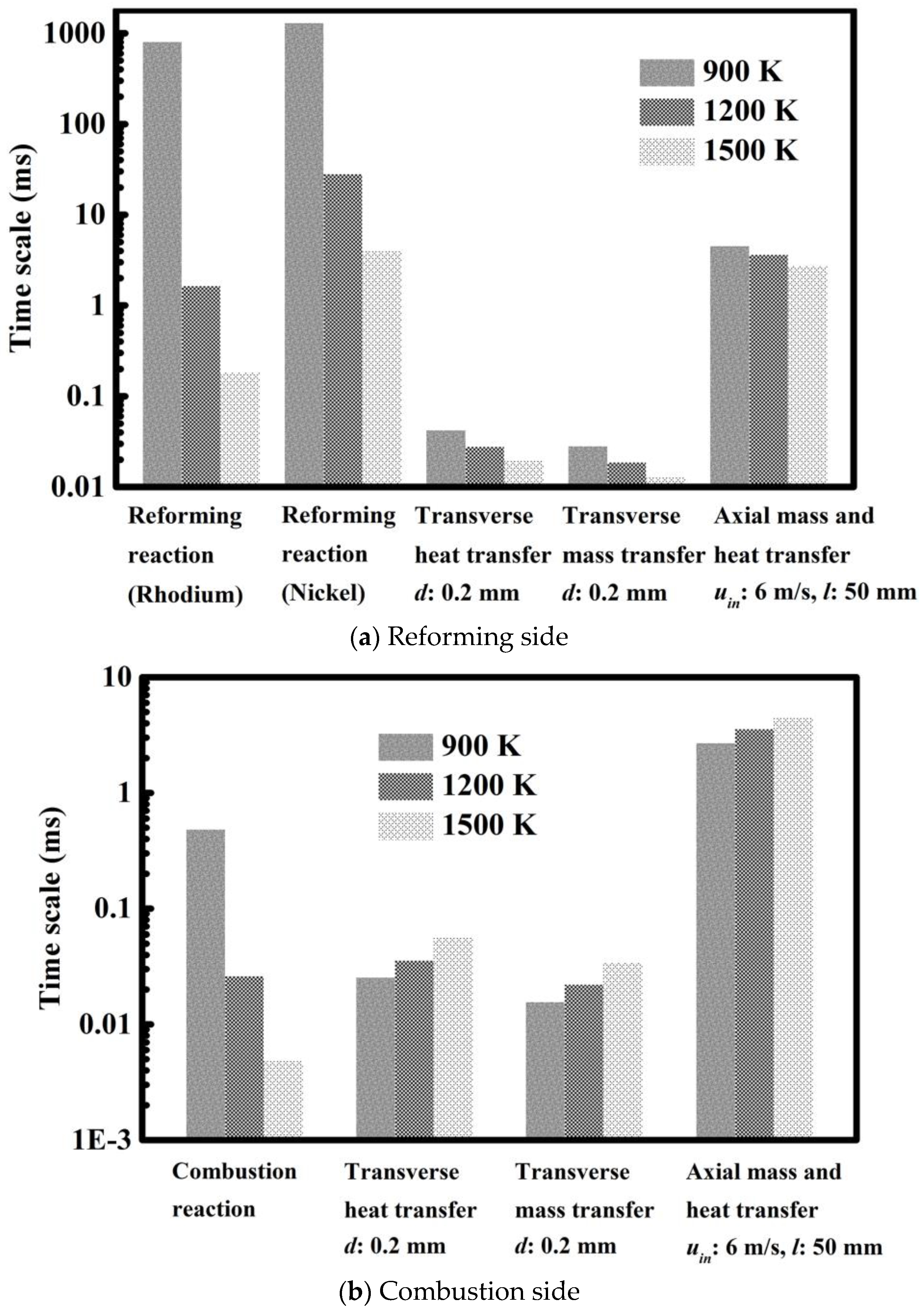

| Parameters and Conditions | Combustion Side | Reforming Side | Unit |
|---|---|---|---|
| Geometry | |||
| Channel height | 0.2 | mm | |
| Channel length | 50.0 | mm | |
| Solid wall | |||
| Thickness | 0.2 | mm | |
| Thermal conductivity | 80.0 | W/(m·K) | |
| External heat loss coefficient | 20 | W/(m2·K) | |
| Gas phase | |||
| Inlet molar steam-to-carbon ratio | - | 3.0 | - |
| Inlet equivalence ratio | 0.8 | - | - |
| Inlet flow velocity | 6.0 | 2.0 | m/s |
| Inlet pressure | 0.1 | 0.1 | MPa |
| Inlet temperature | 300 | 400 | K |
| Catalysts | |||
| Surface site density | 2.72 × 10−9 (Pt) | 2.66 × 10−9 (Ni) 2.72 × 10−9 (Rh) | mol/cm2 |
| Porosity | 0.5 | 0.6 | - |
| Tortuosity factor | 3 | 3 | - |
| Thickness | 0.08 | 0.08 | mm |
| Mean pore diameter | 20 | 20 | nm |
| Catalyst/geometric surface area | 20 | 20 | - |
| Reactor Performance | Parallel | Countercurrent |
|---|---|---|
| Flow velocity at the inlet of the reformer | 3.0 m/s | 3.0 m/s |
| Flow velocity at the inlet of the combustor | 6.0 m/s | 6.0 m/s |
| Conversion at the outlet of the reformer | 48.6 | 58.7 |
| Conversion at the outlet of the combustor | 75.6 | 79.0 |
| Maximum wall temperature | 1047 K | 1160 K |
| Minimum wall temperature | 870 K | 848 K |
| Fluid temperature difference | ||
| Maximal value appeared in the transverse direction | 570 K | 687 K |
| Minimum value appeared in the transverse direction | −66 K | −180 K |
| Ratio of overall generated and consumed heat | 3.78 | 3.26 |
| Ratio of local generated and consumed heat | ||
| At the reformer entrance | 1.6 | 0.6 |
| At the reformer outlet | 2.8 | 4.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liu, B.; Gao, X.; Xu, D. RETRACTED: Production of Hydrogen by Methane Steam Reforming Coupled with Catalytic Combustion in Integrated Microchannel Reactors. Energies 2018, 11, 2045. https://doi.org/10.3390/en11082045
Chen J, Liu B, Gao X, Xu D. RETRACTED: Production of Hydrogen by Methane Steam Reforming Coupled with Catalytic Combustion in Integrated Microchannel Reactors. Energies. 2018; 11(8):2045. https://doi.org/10.3390/en11082045
Chicago/Turabian StyleChen, Junjie, Baofang Liu, Xuhui Gao, and Deguang Xu. 2018. "RETRACTED: Production of Hydrogen by Methane Steam Reforming Coupled with Catalytic Combustion in Integrated Microchannel Reactors" Energies 11, no. 8: 2045. https://doi.org/10.3390/en11082045
APA StyleChen, J., Liu, B., Gao, X., & Xu, D. (2018). RETRACTED: Production of Hydrogen by Methane Steam Reforming Coupled with Catalytic Combustion in Integrated Microchannel Reactors. Energies, 11(8), 2045. https://doi.org/10.3390/en11082045






