1. Introduction
Salinity gradient energy, also known as blue energy, is a sustainable and renewable source of energy. The energy is obtained by mixing two aqueous solutions with different salinities, and applying membrane-based technologies [
1,
2,
3,
4,
5]. The energy generated can be significant, depending on the relative salt concentrations of the streams [
1]. The energy of river water mixing with the sea water is approximately equal to 2 kJ per liter river water, which is equivalent to the energy of a waterfall with the height of 200 m [
6,
7]. Several researchers studied membrane-based technologies such as pressure retarded osmosis (PRO), reverse electrodialysis (RED) and capacitive mixing (CapMix) to control and harness this energy [
4,
5,
6,
8,
9,
10,
11]. Among the mentioned methods, RED is more robust due to earlier return on invested capital (ROIC), better tunability, easier maintenance, longer life time, less sensitivity to bio-fouling and smaller physical footprint [
1]. Reverse electrodialysis relies on selective-permeable membranes known as ion exchange membranes [
1,
5,
12]. Kingsbury et al. [
12] and Li et al. [
13] reported that energy can be stored and generated through electrodialysis (ED) and reverse electrodialysis (RED) as an opposite process of ED. Therefore, a concentration battery, which is highly scalable in both small scale and large scale [
13], can be established by combining the two processes. A schematic of a RED stack is shown in
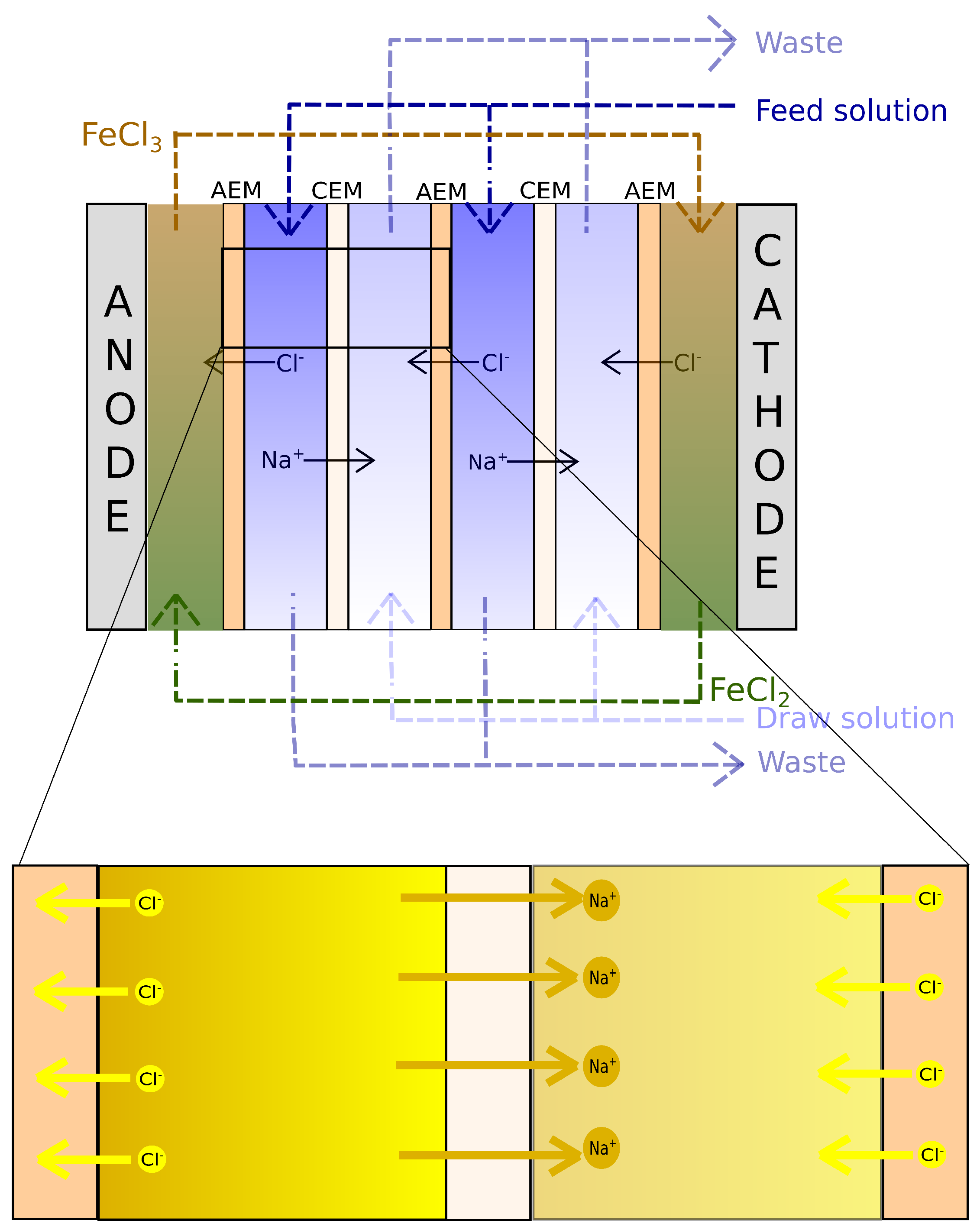
Figure 1. Concentrated and dilute solutions are fed through series of channels separated by ion-selective membranes. An ionic current is produced due to the chemical potential difference between the concentrated and dilute solutions, resulting in anion migration through the anion exchange membrane (AEM) towards the anode, and cation migration through the cation exchange membrane (CEM) towards the cathode. In the compartments containing electrodes, a redox reaction occurs, converting the ionic current into an electric, allowing for the energy to be harvested [
14,
15,
16,
17]. In the electrode compartments, the rinse solutions are recirculated continuously to provide the medium for the redox reactions.
The net power density represents the energy generation performance of a RED system as defined by Equation (
1) [
19]:
The net power density depends on the stack resistance, pressure loss in the channels due to the flowing solutions and the potential difference between the two solutions, described by Equation (
2) [
3,
12]:
where
represents the pump power density,
is the open circuit potential,
is the current density,
is the number of membranes and
is the stack resistance.
and
are concentrations of the concentrated and dilute solutions, respectively. F is Faraday’s constant, T is temperature, R is the universal gas constant and
is the average perm-selectivity, which expresses the membranes ability to transport a specific type of ion [
20]. The stack resistance is equal to the load resistance when the power density is maximum and can be calculated by Equation (
3) [
19,
21]:
where
and
are the resistances of the AEM and CEM, respectively.
is the mask fraction or shadow factor of the spacer [
12] and
is the spacer porosity.
and
are the channel heights of dilute and concentrated solutions compartments, respectively, and
and
are the corresponding conductivities of the concentrated and dilute solutions.
Ramon et al. [
5] and Post et al. [
1] documented that the RED power density can be maximized by increasing the difference in salinity between the streams, while at the same time reducing the stack resistivity. Tedesco et al. [
22] suggested that this is realized by a reduction of the channel height, as shown in Equation (
3). The reduction in channel height is however limited by the size of the spacers, which keep the CEM and AEM separated.
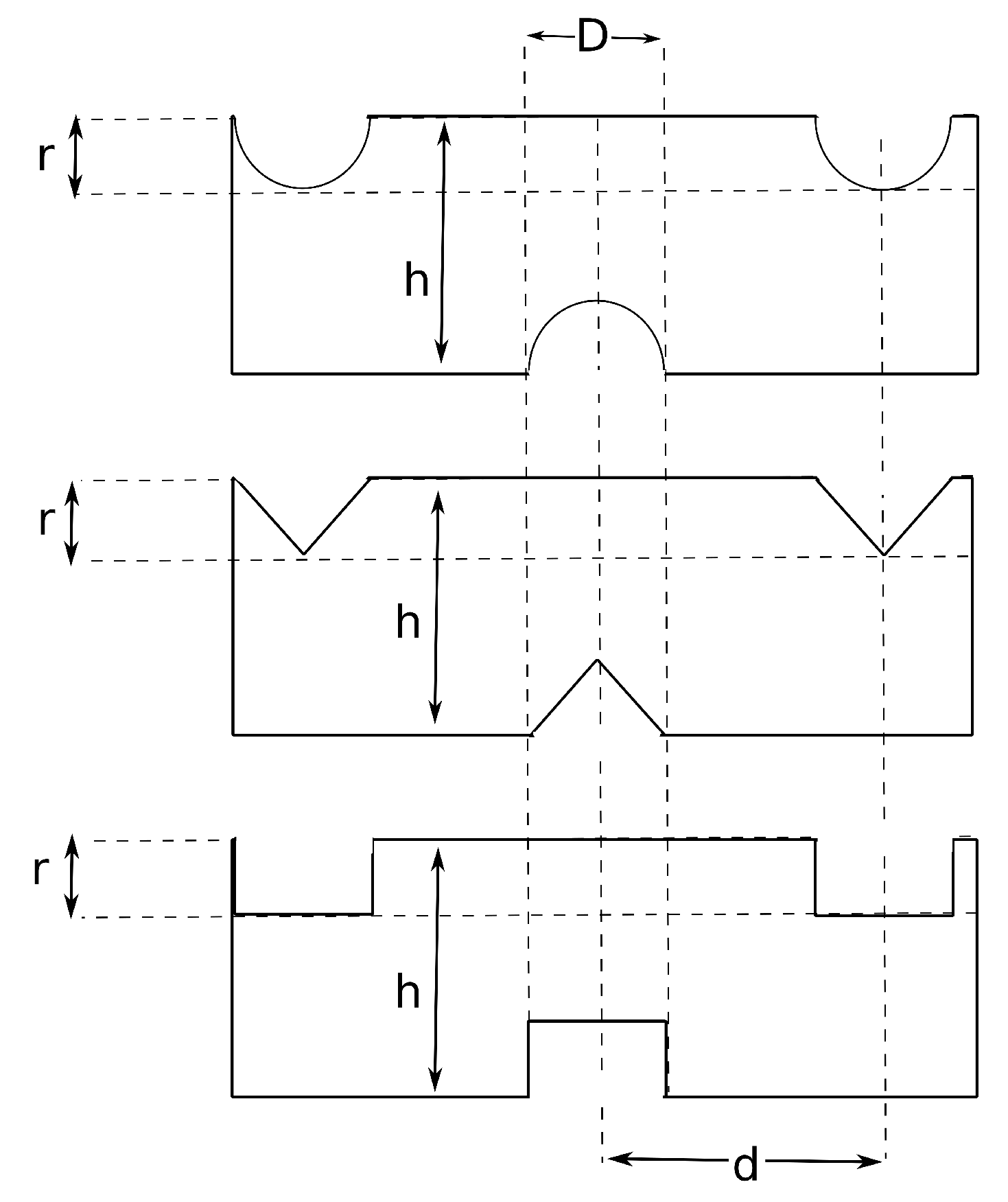
Spacers are typically composed of non-conductive polymeric filaments which reduce the available membrane area by hindering of ionic transport from the membrane to the solution. This is called the spacer shadow effect and increases the Ohmic resistance [
23,
24,
25,
26,
27]. These types of spacers will be named as inactive spacers in this article. Dlugolecki et al. [
24] reported that applying commercial available conductive spacer can increase the power density of RED around 30–40% compared to a system adopting inactive spacers.
Recent developments in membrane design provides an opportunity to omit inactive spacers by utilizing profiled (corrugated) membranes, also termed as membranes with integrated spacers. In this paper, the profiled (corrugated) membranes are named as active spacers for simplicity. Active spacers have number of advantages compared to inactive spacers. Active spacers increase the available membrane area for ionic transport by eliminating the inactive spacer shadow effect, thus enhancing the net power output [
24]. Moreover, simpler membrane module manufacture can be realized as the spacer already is present on the membrane surface [
28]. Larchet et al. [
29] investigated experimentally the performance of stacks with both active and inactive spacers for a wide range of concentrations and compared the results to a flat channel. They documented that active spacers may enhance mass transfer rate up to four times than that for a flat channel [
29].
Figure 2 illustrates the active and inactive spacer-filled channels.
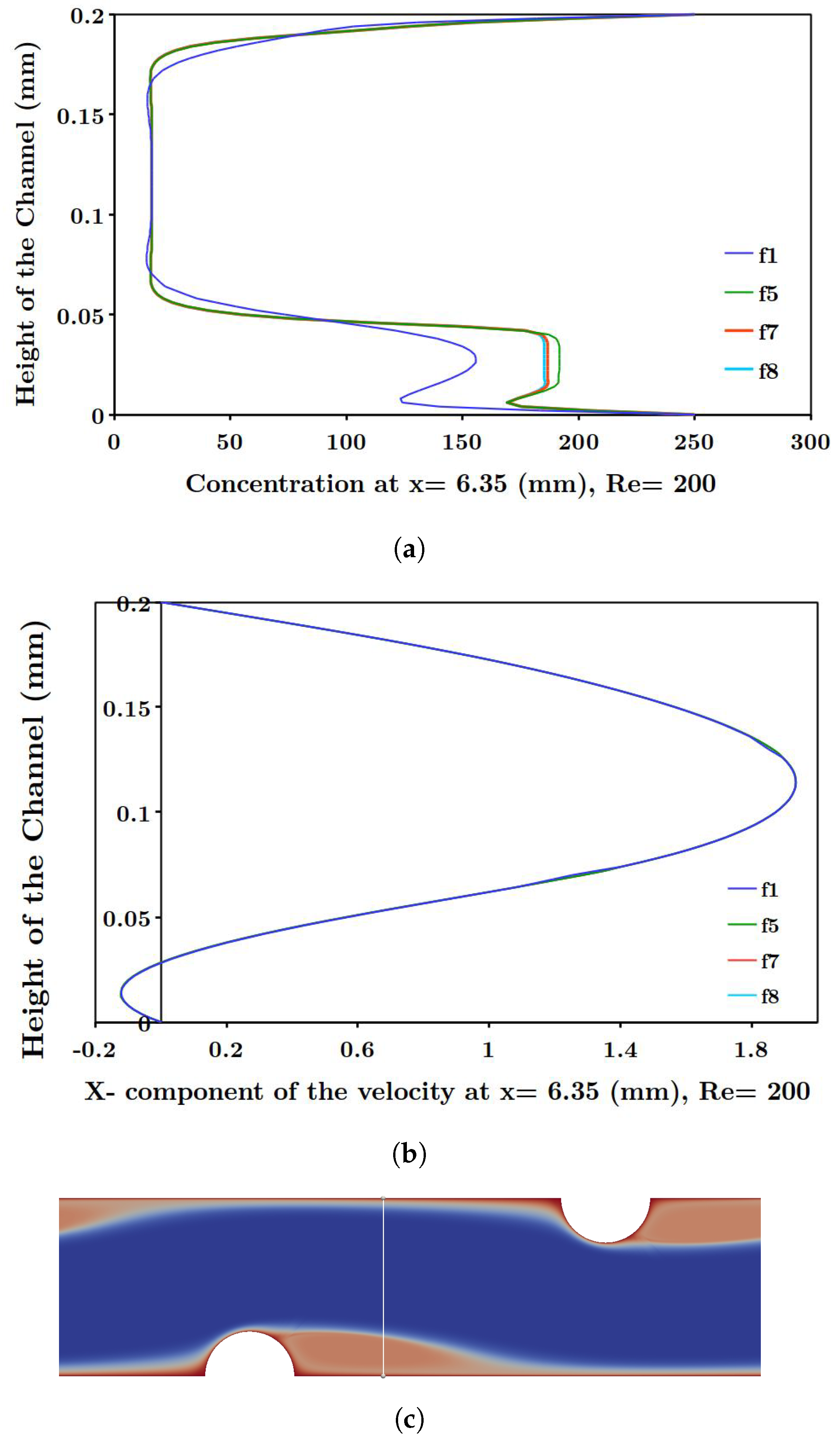
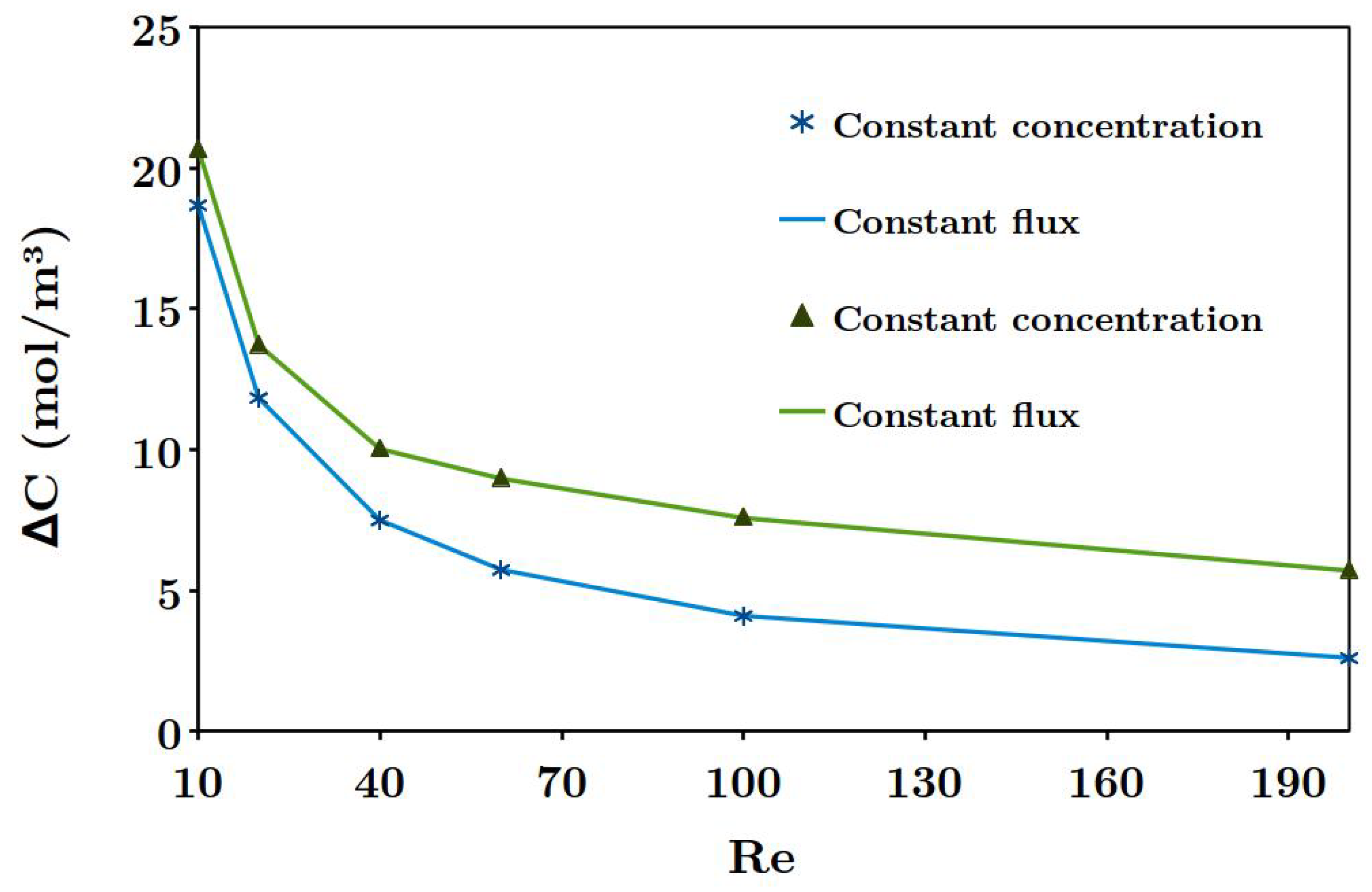
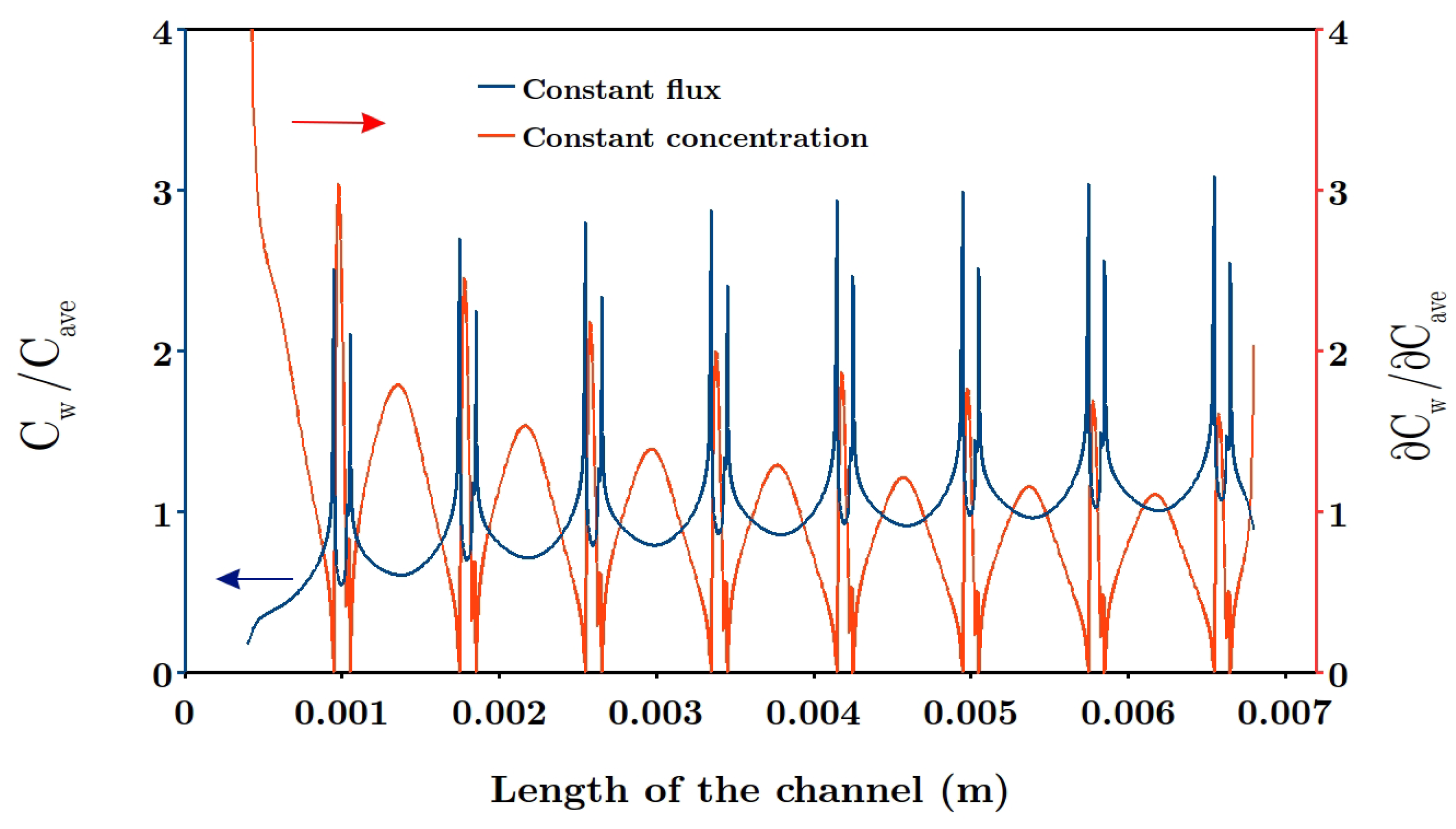
Another phenomenon which reduces the power density is known as concentration polarization, discussed by several researchers [
24,
26,
30,
31,
32,
33]. Considering this phenomenon, the generated power density becomes lower than the theoretical value, since the concentration gradient at the solution-membrane interface is smaller than the bulk concentration difference between two streams [
26,
30,
34]. In other words, concentration polarization diminishes the available theoretical power density by creating a thin diffusion boundary layer along ion exchange membranes, thus reducing the performance. Pawlowski et al. [
23] and Vermaas et al. [
35] reported that the concentration polarization can be reduced, thus increasing the power density, by enhanced mass transfer. Enhanced mass transfer could be possible by increasing the flow velocity (i.e., the Re number) [
22,
36], using eddy promoters such as spacers [
37] and increasing the active area of the membranes [
24]. Increasing the flow velocity and introducing the eddy promoters (corrugations) can mitigate the concentration polarization by disturbing the diffusive boundary layer. In fact, both active and inactive spacers create eddies and wakes downstream of corrugations, thus increasing the mixing [
36,
38].
Pressure loss is inevitable during fluid flow and depends on several factors such as channel height, spacer geometry and size, Reynolds number and fluid viscosity. The pressure drop is related to the pumping power density required for transport of the solutions through their compartments. Higher pressure drops lead to higher pumping power densities, consequently lowering the net power density [
36,
39,
40]. Accordingly, parameters such as channel height, available membrane area, mass transfer coefficients as well as corrugations have to be optimized to keep the pressure loss as low as possible with reasonably high mass transfer.
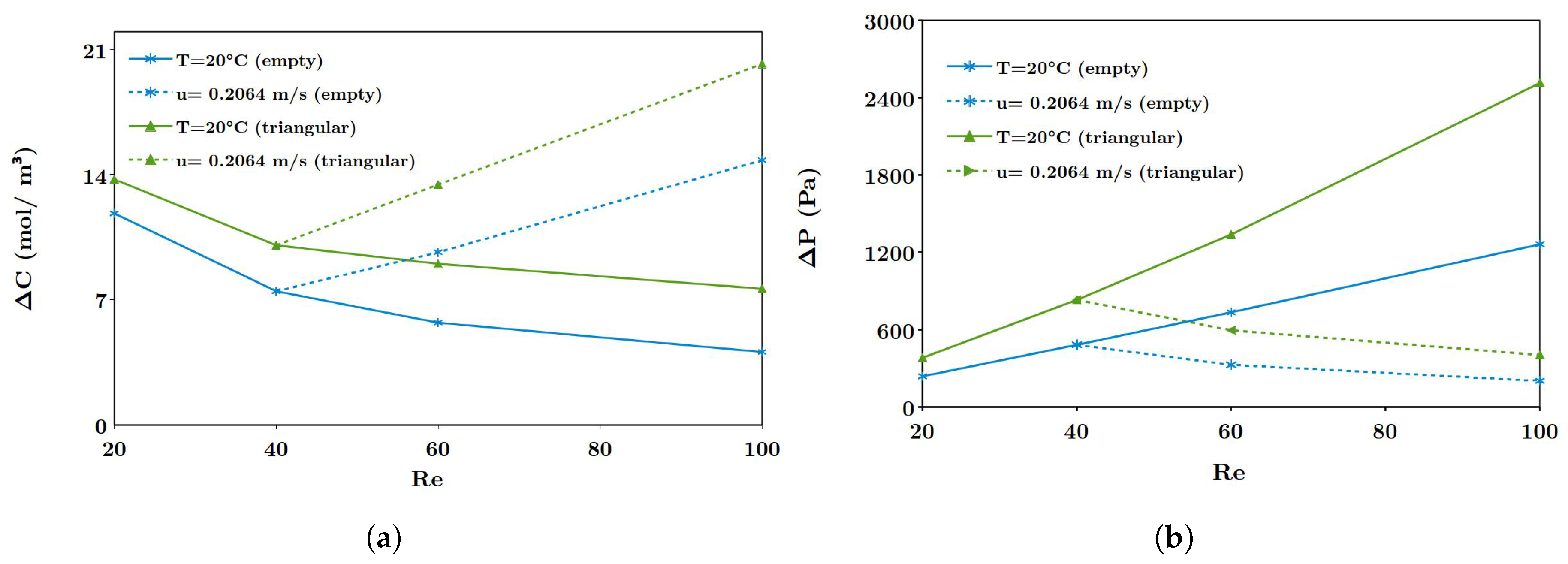
The solution temperature affects mass and momentum transport due to its influence on fluid viscosity and diffusivities. Benneker et al. [
41] investigated the utilization of waste heat for increased power density in RED systems, reporting an increase of over 25% for RED power densities when the temperature was increased from
C to
C. Another important observation in their work was related to the ion selective transport at elevated temperatures, which remained unchanged over the range considered. In the study by Luo et al. [
42], utilization of waste heat was proposed in combination with ammonium bicarbonate in RED, documenting that the concentration of the dilute solution and flow rate of feed solutions have to be optimized to achieve maximum power output of RED stack.
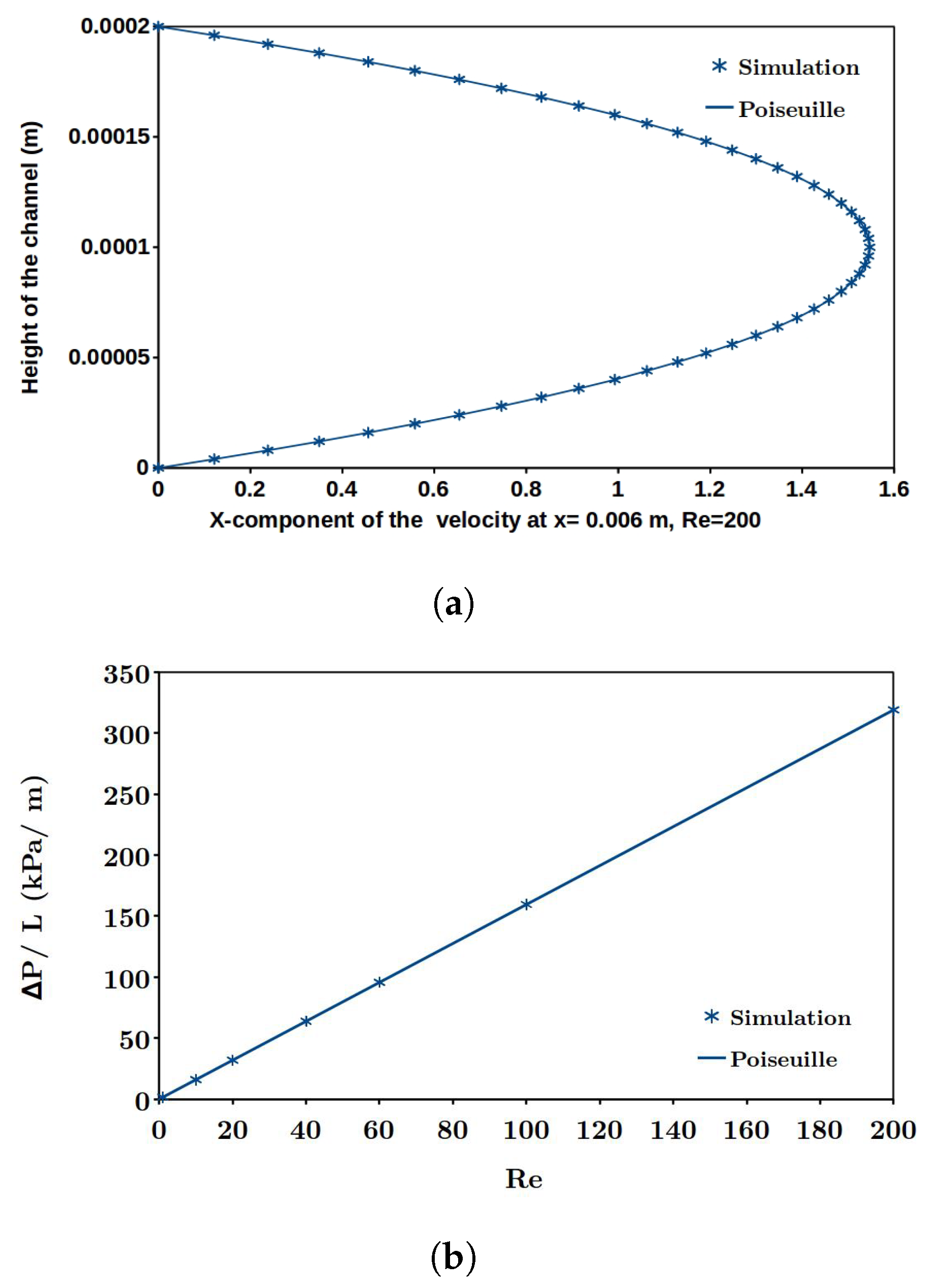
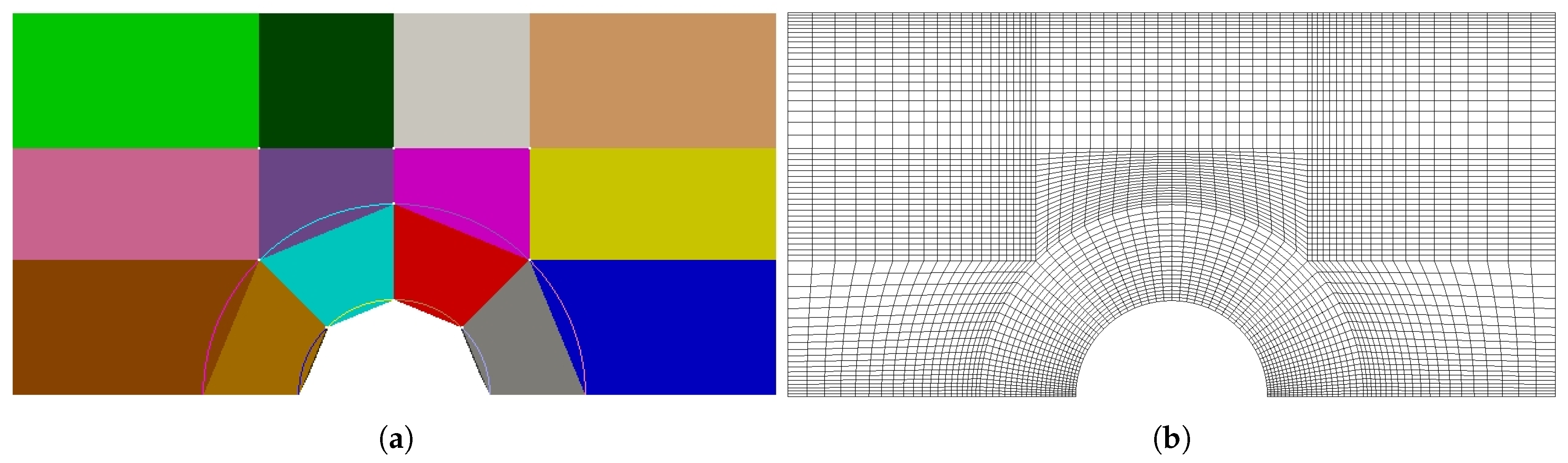
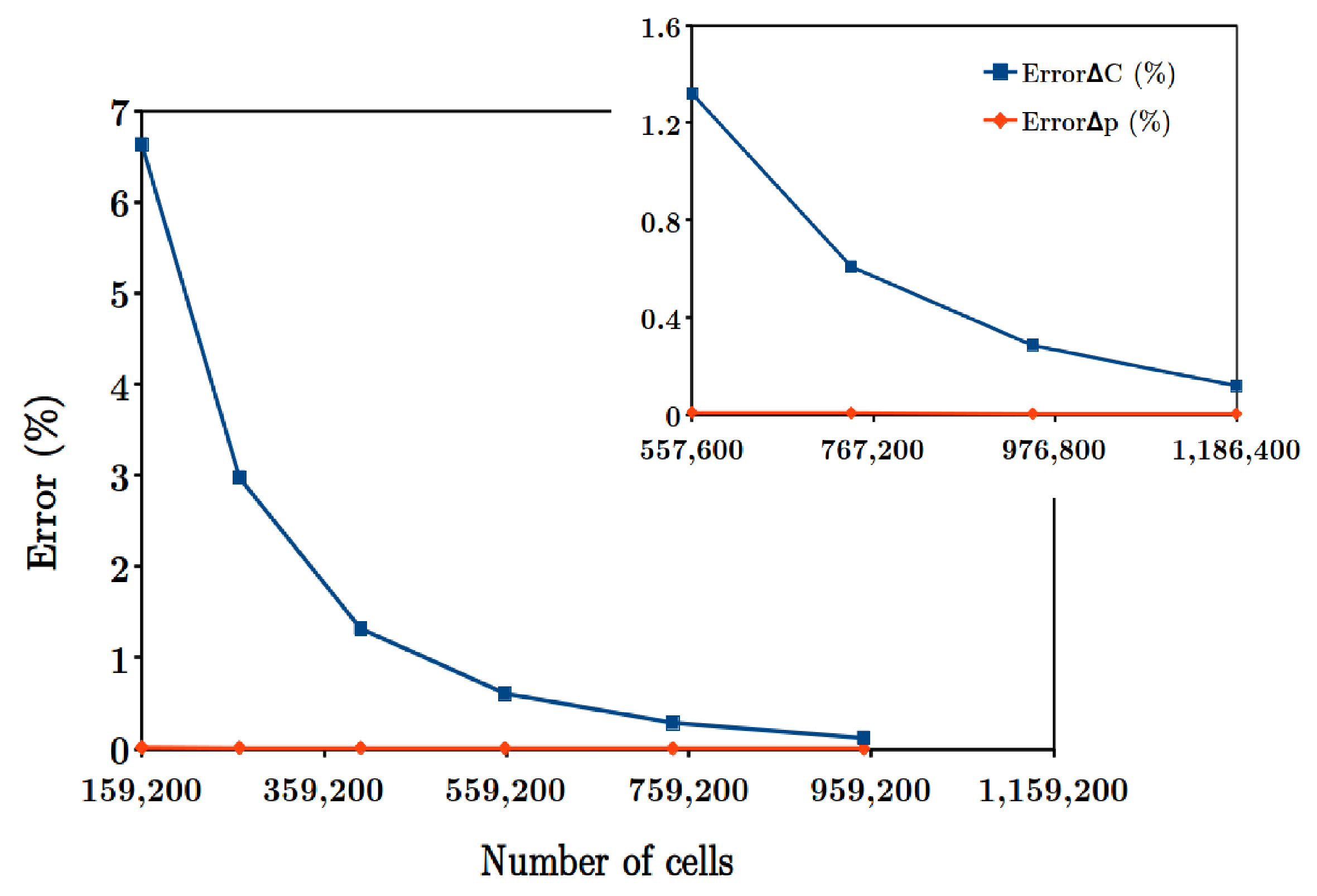
Systematic experimental screening to determine appropriate corrugation geometry and flow velocity for designing RED cell requires considerable man-hours work and cost. Robust and sophisticated computational fluid dynamics (CFD) models with appropriate local mesh refinement is a good alternative and has been adopted as a design strategy by several researchers.
Leitz and Marinčić [
43] reported a study on different corrugation geometries (or flow promoters) and addressed empirically relating the Sherwood number for each geometry as a function of the Reynolds number, testing different spacer shapes and comparing to the performance of open channel. Schwinge et al. [
32,
44] used computational fluid dynamics (CFD) to investigate the effect of different spacer geometries on the fluid flow (momentum transfer) for different Reynolds numbers, highlighting the role of CFD modeling in membrane design. The spacer filament is found to increase the re-circulation zone, which in turn increases the local mass-transfer rate. The size and shape of established wakes depend on the type, geometry and configuration of spacer as well as Reynolds number [
32].
Ahmad et al. [
45] utilized ANSYS Fluent
® to investigate concentration polarization in a flat channel under different flow velocities and transmembrane pressures, verifying their results with literature data. Increasing Re numbers and reducing transmembrane pressures would result in reduced concentration polarization and thus increased mass transport [
45]. Gurreri et al. [
30] reported that varying flow rates with velocity components perpendicular to the membranes with integrated spacers significantly affects on the mass transfer, concentration polarization and pressure drop. Pawlowski et al. [
46] performed simulation studies to estimate the net power density of RED stacks with different channel arrangements by introducing profiled membranes with corrugations perpendicular to the flow under laminar conditions. Reducing the channel height, increasing the flow velocity and introducing eddy promoters all result in higher pressure loss due to more significant hydraulic friction through the channel [
22,
23]. Li and Tung [
47] performed CFD simulations of fluid flow through a spacer-filled channel, applying suitable cell types for periodic boundary conditions. They compared the simulated pressure drop with the experimental data and reported that it depends on geometry, angle and diameter of spacer filament as well as height, width, length and channel void fraction.
CFD modeling was also successfully utilized to estimate mass transfer, pressure drop and design the profiled membrane in the studies done by Pawlowski et al. [
3,
40], suggesting a membrane with a chevron structure to reduce the concentration polarization. They reported that this type of membrane assures effective mass transfer at relatively low pressure loss. Ranade et al. [
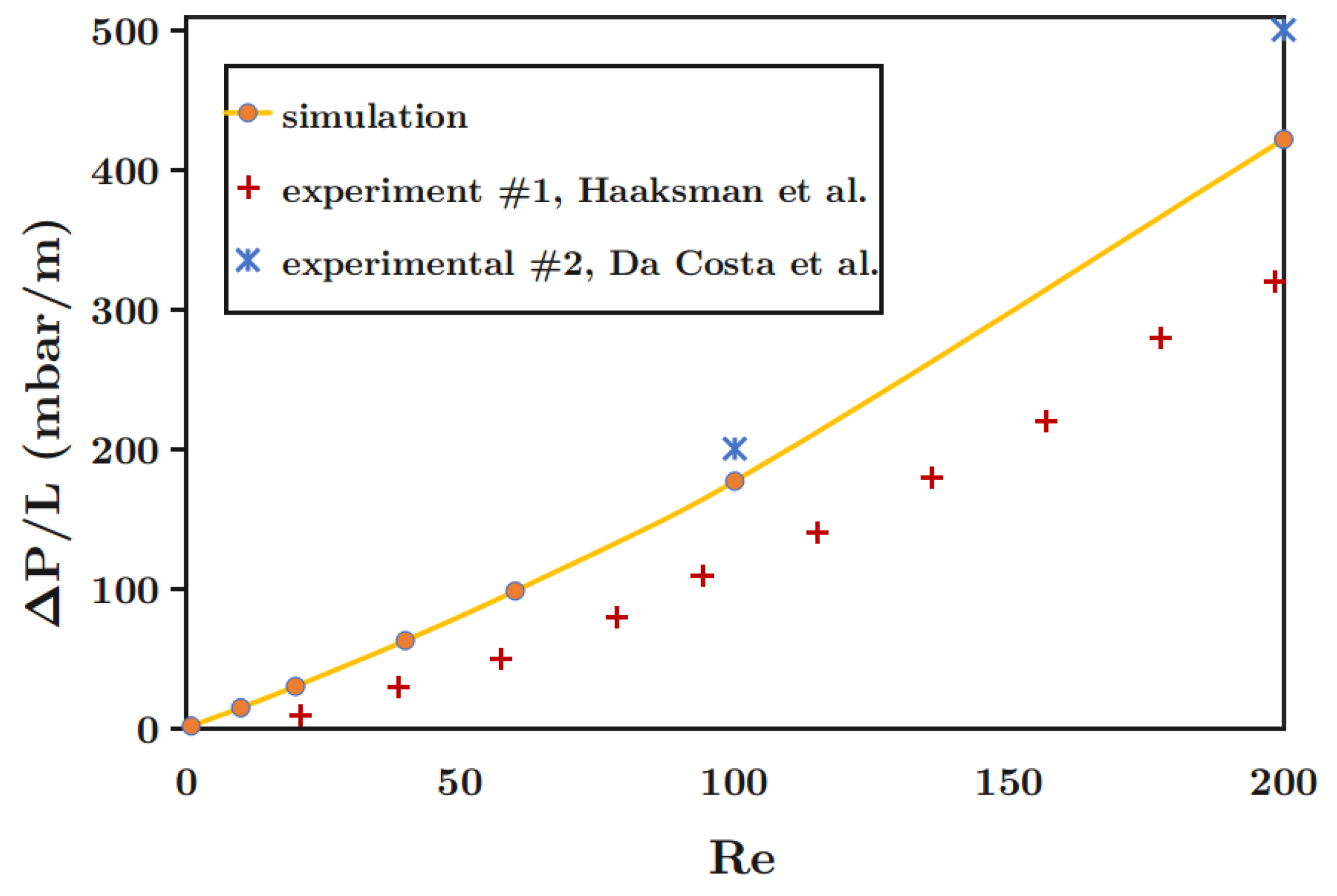
48] performed a three-dimensional simulation to predict pressure drop and flow behavior in membrane modules containing rectangular and curved spacers based on unit cell approach. They validated their results with the experimental data reported by Da Costa et al. [
49], reporting flow visualization and pressure drop measurements. CFD simulations performed by Li et al. [
50] revealed that mass transfer increases for spacer-filled channels compared to that for an empty channel, due to established transversal and longitudinal vortices. Haaksman et al. [
51] developed a pre-processing method to obtain three-dimensional geometry of a given spacer configuration measured by X-ray Computed Tomography (CT) for CFD simulations.
Coupled Navier–Stokes and Nernst–Planck simulations were presented for an ED cell by Tadimeti et al. [
52] using COMSOL Multiphysics
® [
53], performing a sensitivity analysis on channel geometry for desalination with respect to the corrugation shape, density and size. Coupled simulations for RED were performed by Jalili et al. [
54] using OpenFOAM [
55] to investigate the influence of flow velocity on the resistivity and electrical potential across flow compartments, reporting that the electrical potential obtained in RED unit cell is enhanced by decreasing the Re number and introducing flow promoters in a dilute solution channel.
Evidently, several studies have been performed to investigate the effects of flow velocities and different spacer geometries to find a trade-off between the pressure loss and mass transfer in a channel. However, to the best of our knowledge, there is limited scientific literature that compares the role of active and inactive spacers, as well as the influence of temperature on pressure drop and mass transfer. In this study, CFD modeling was performed to study the momentum and mass transfer in a dilute channel of a RED system with varying topology, velocity and solution temperature, aiming to demonstrate possible improvements for the technology, utilizing the active membranes and waste heat.