Study on Combustion and Emission Characteristics of Marine Diesel Oil and Water-In-Oil Emulsified Marine Diesel Oil
Abstract
1. Introduction
2. Experimental Apparatus and Methodology
2.1. Emulsion Oil Properties
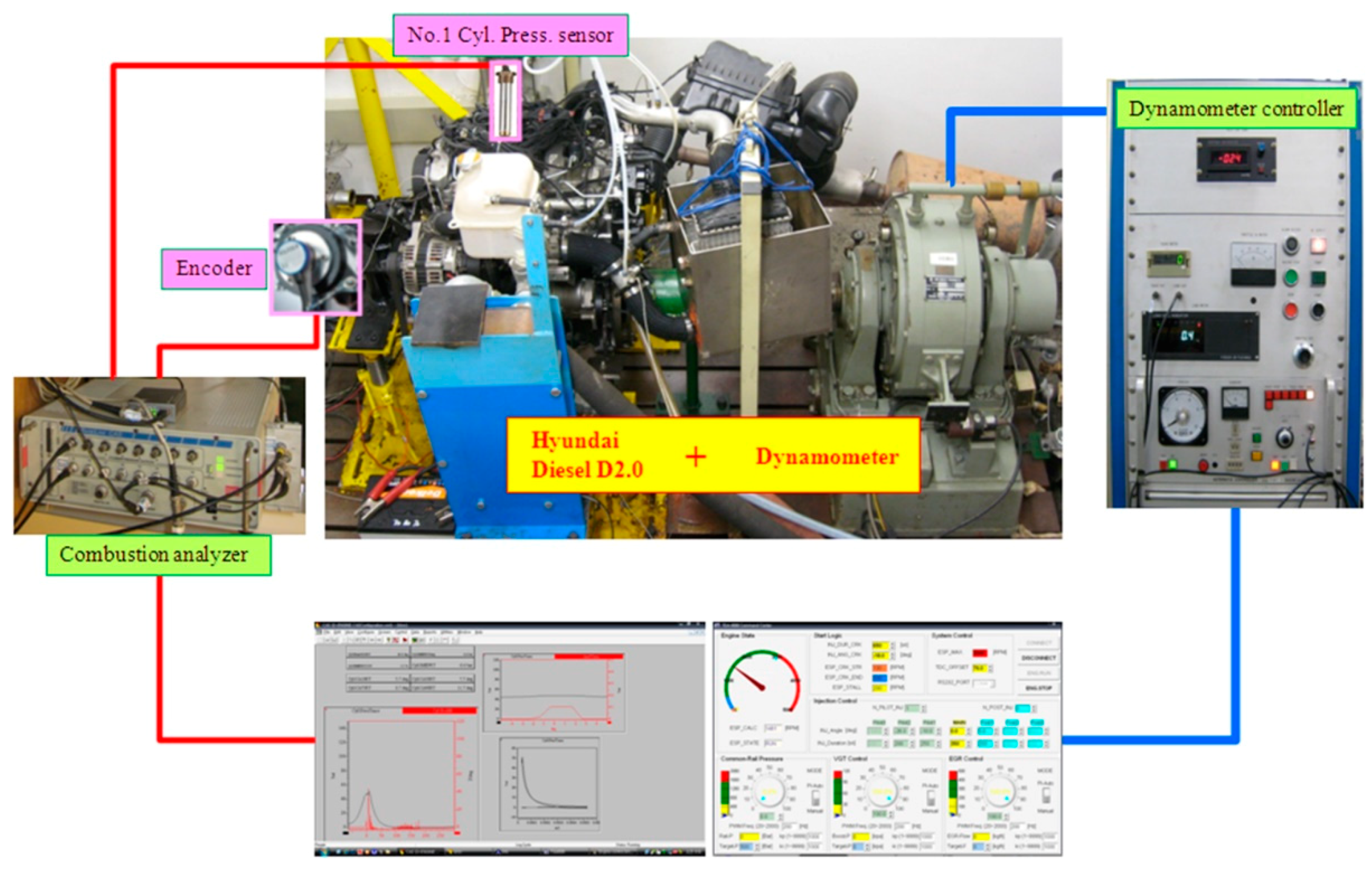
2.2. Experimental Apparatus
2.3. Experimental Conditions
3. Results and Investigations
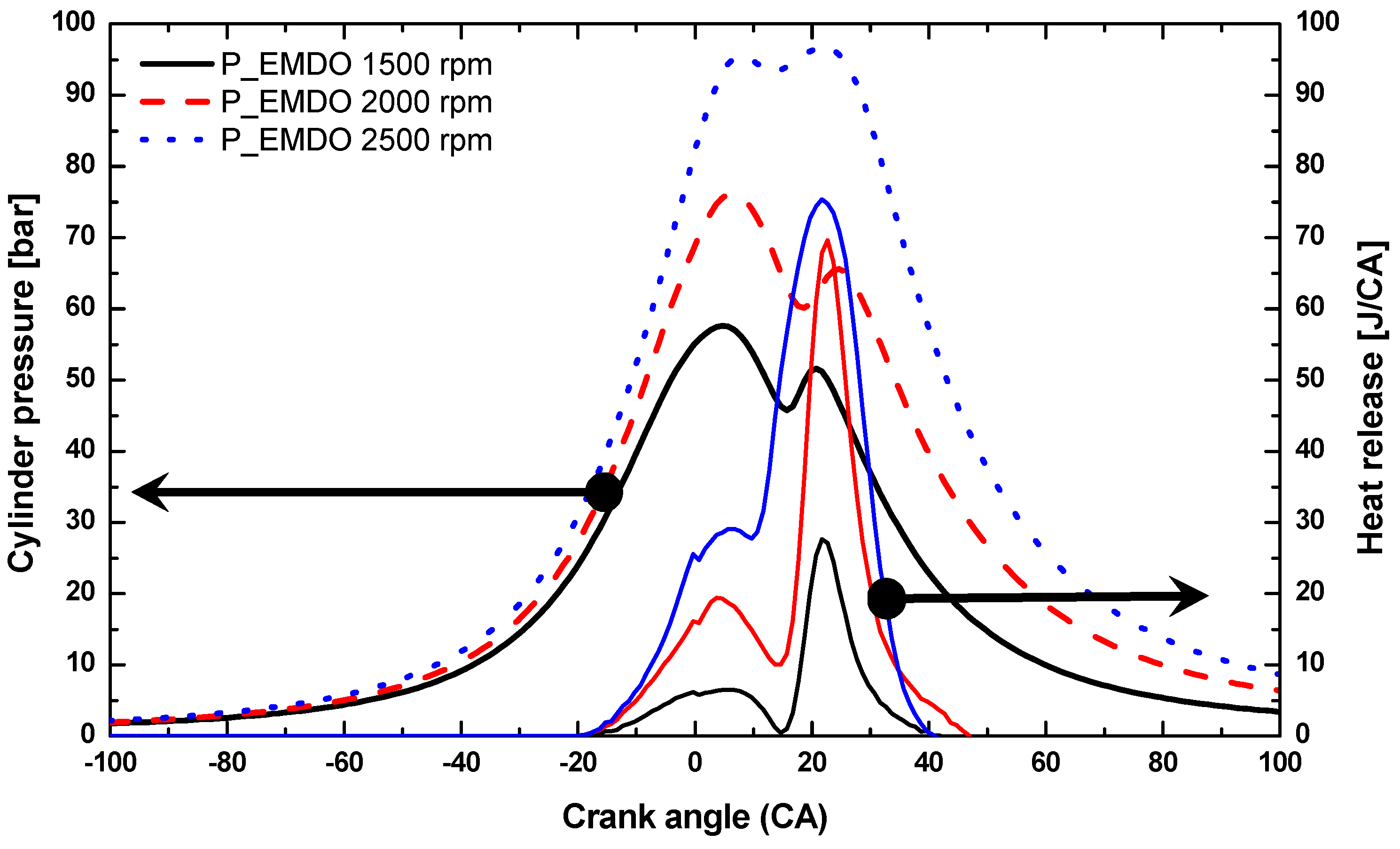
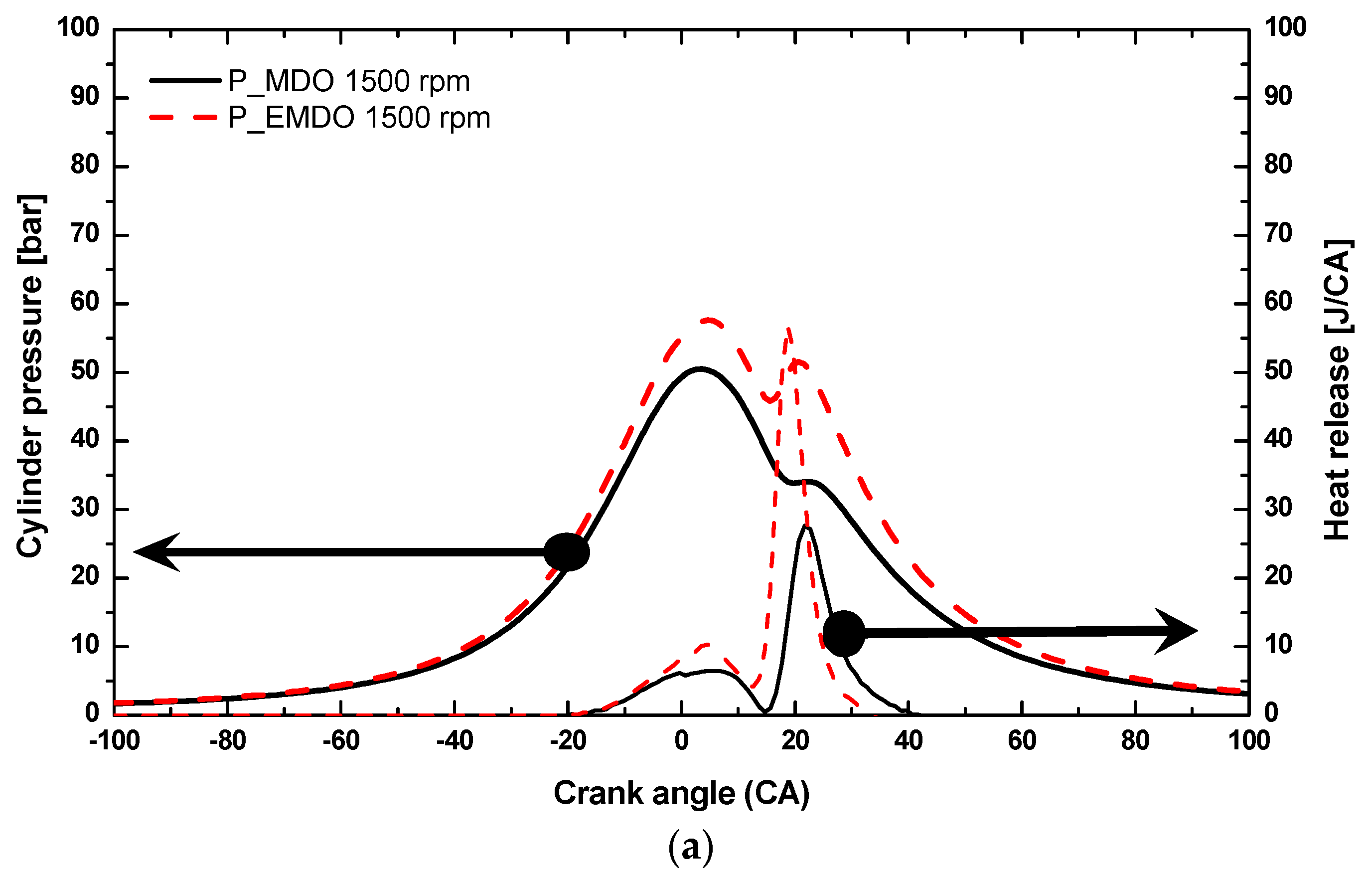
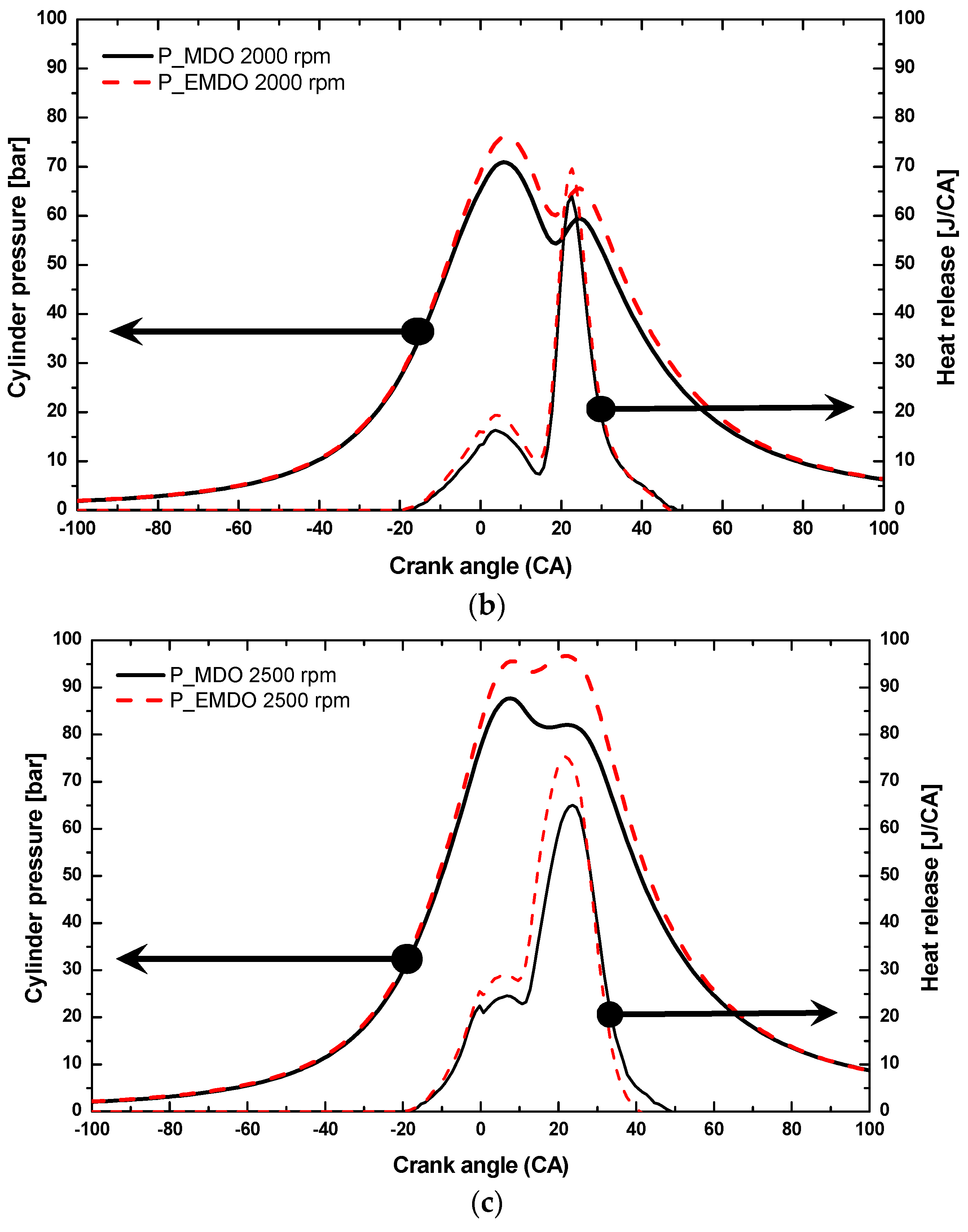
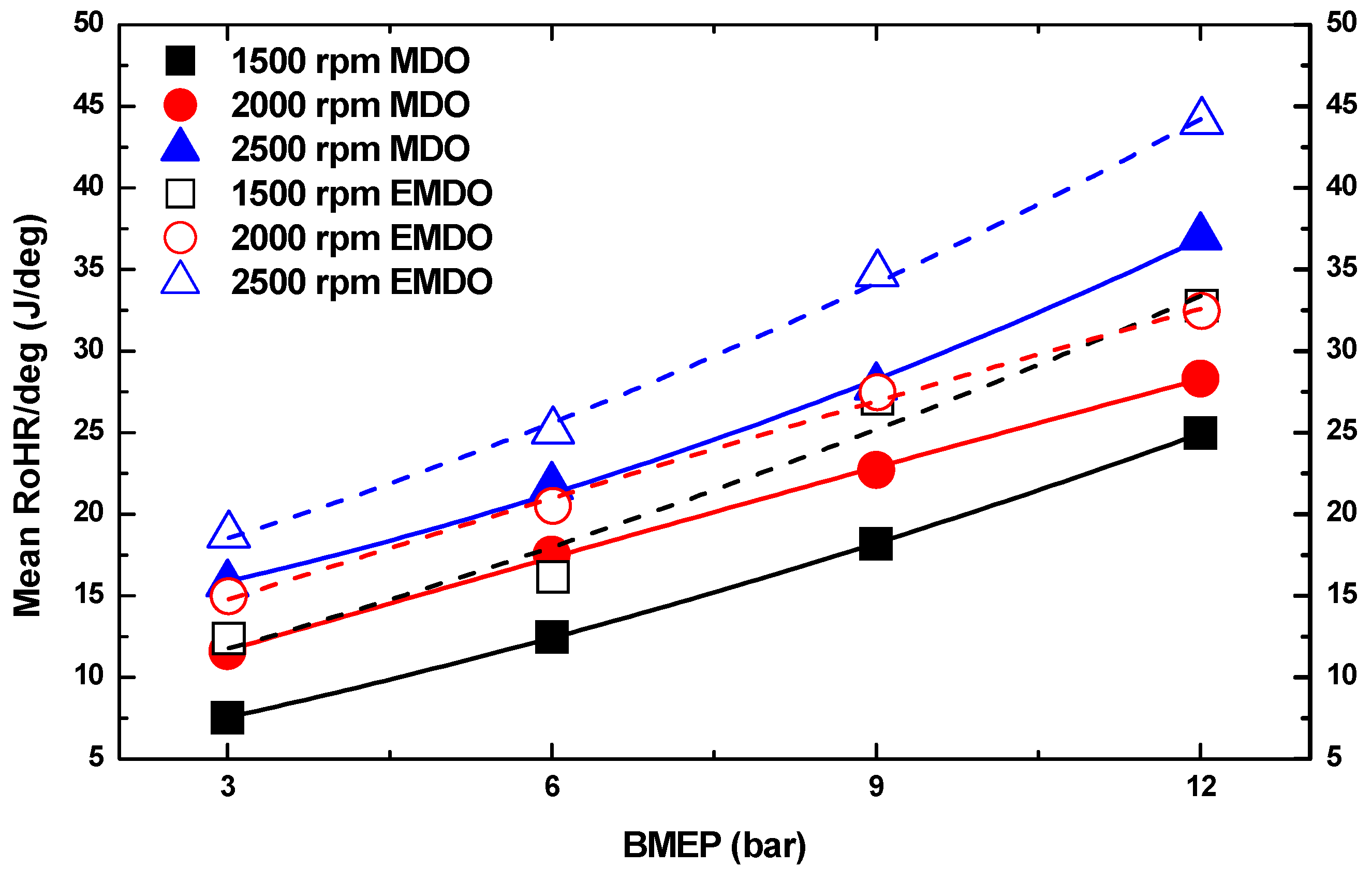
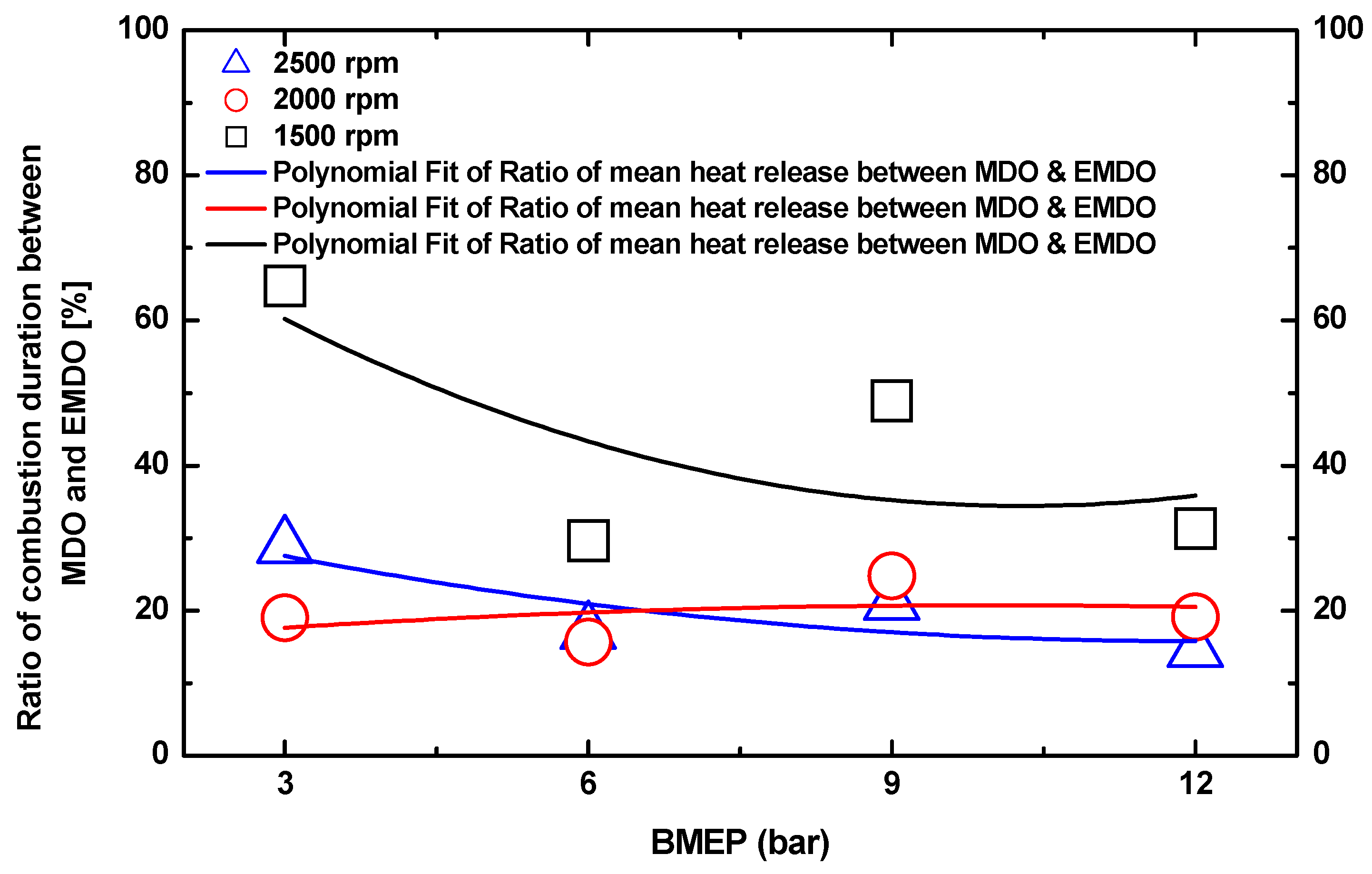
3.1. Cylinder Pressure and Heat Release Characteristics of MDO and EMDO
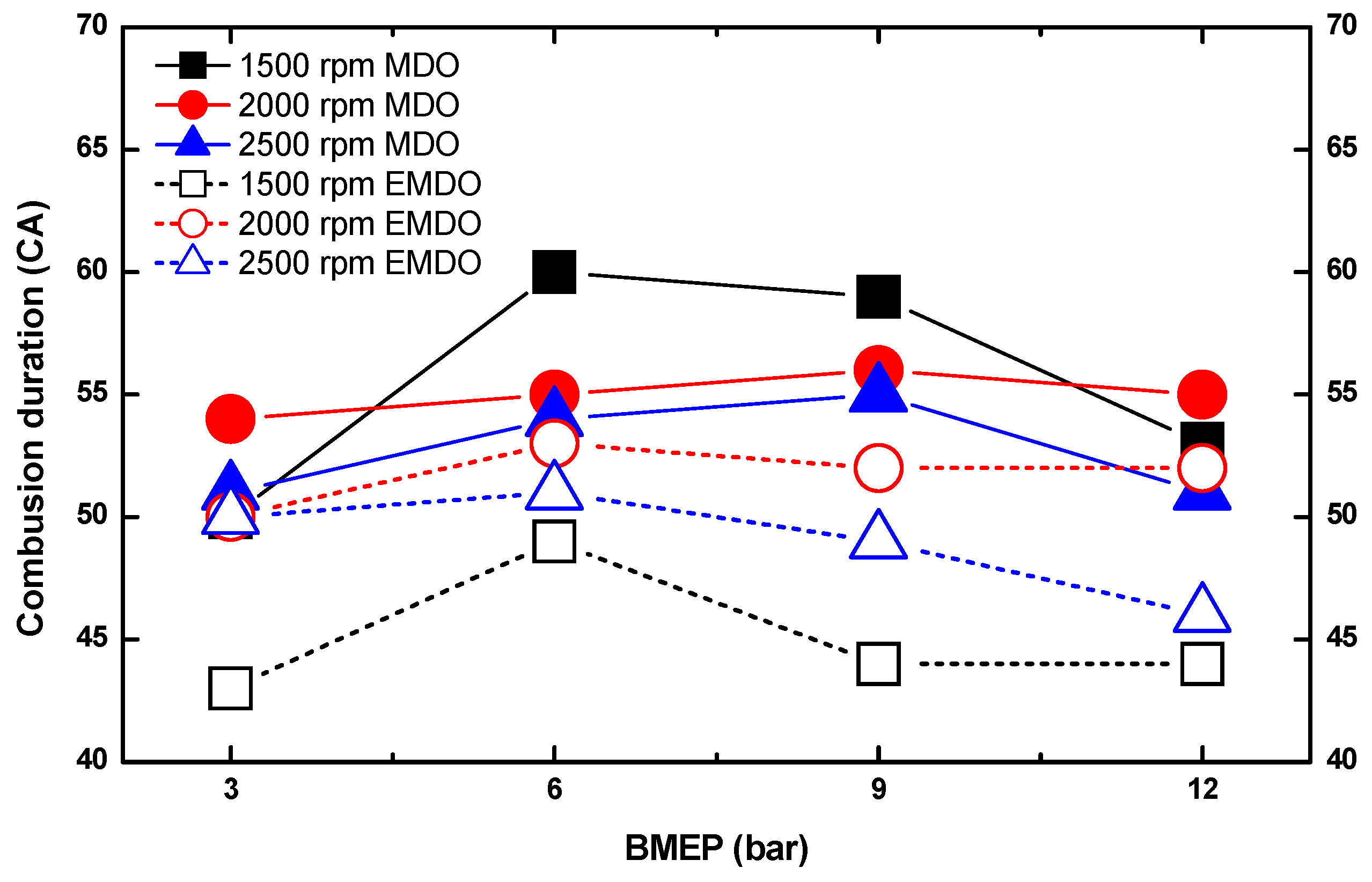
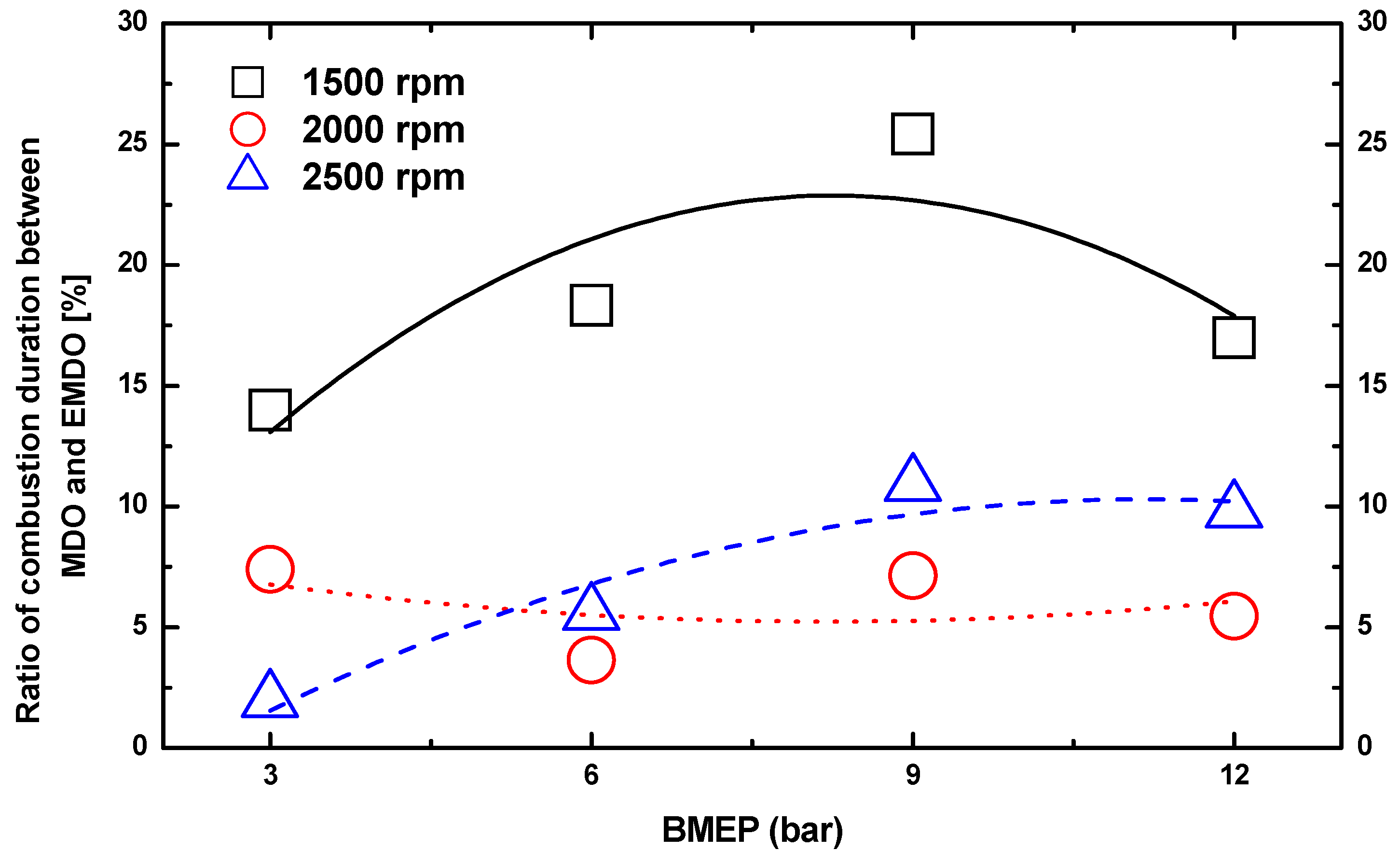
3.2. Combustion Duration Characteristics of MDO and EMDO
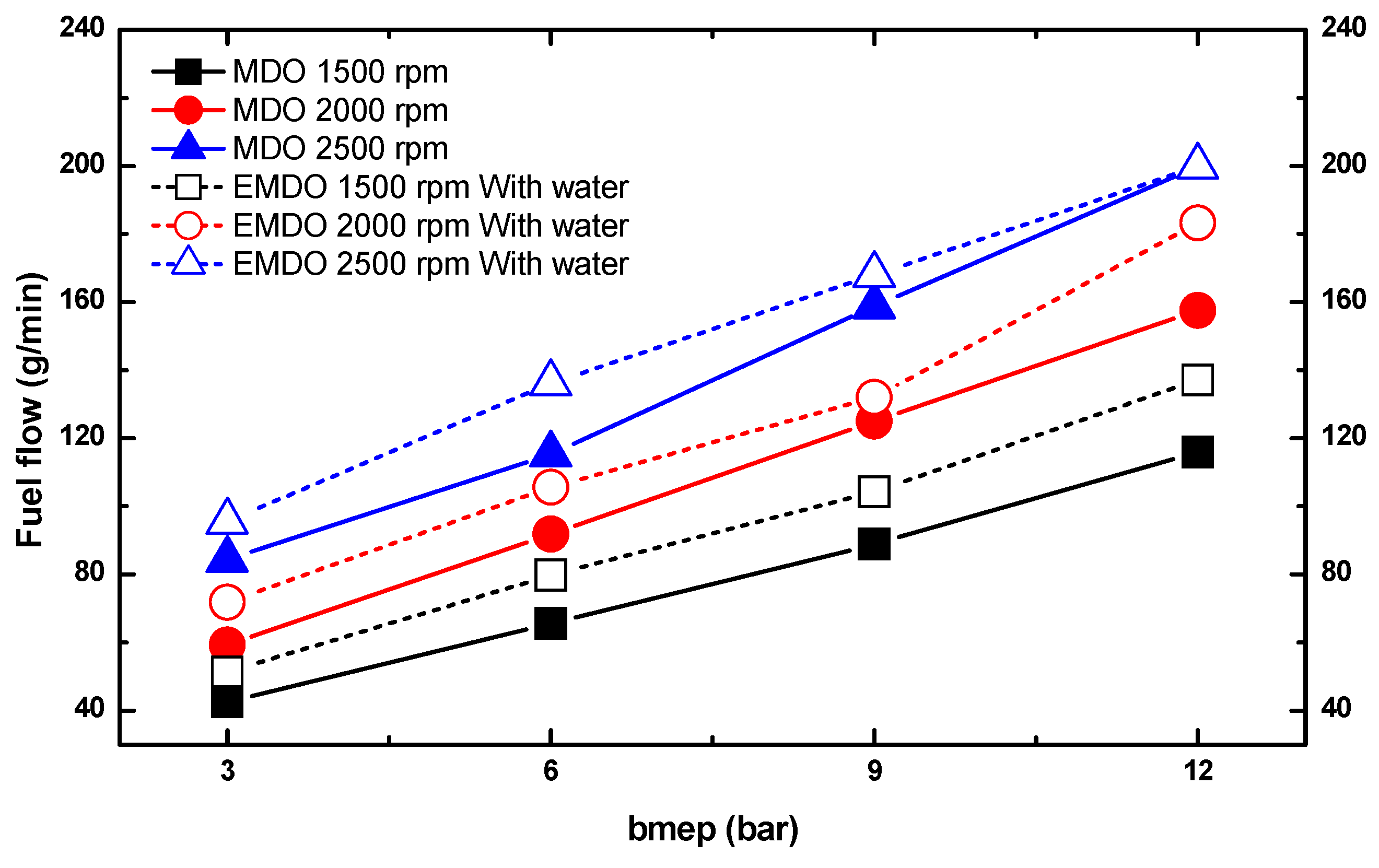
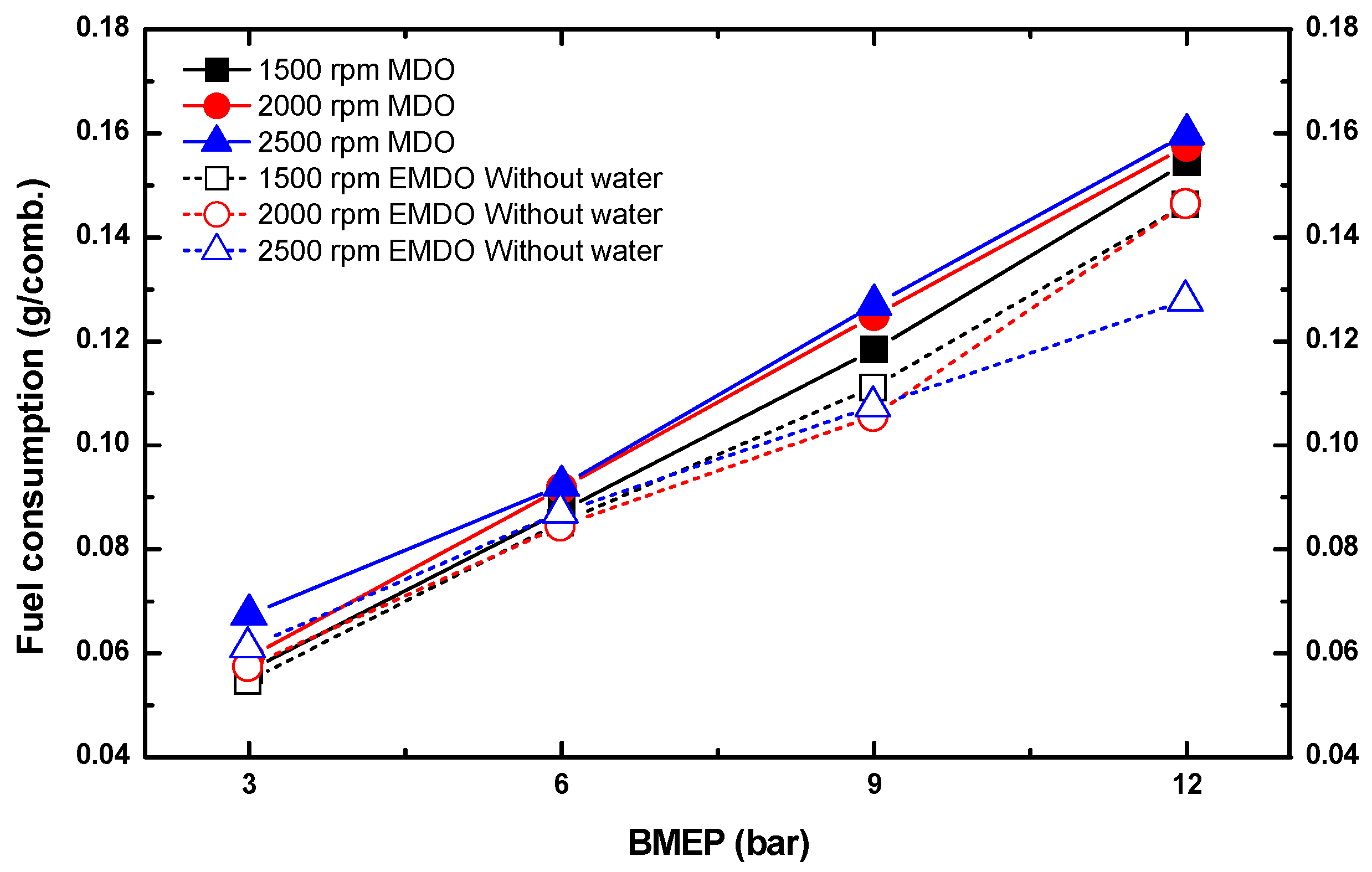
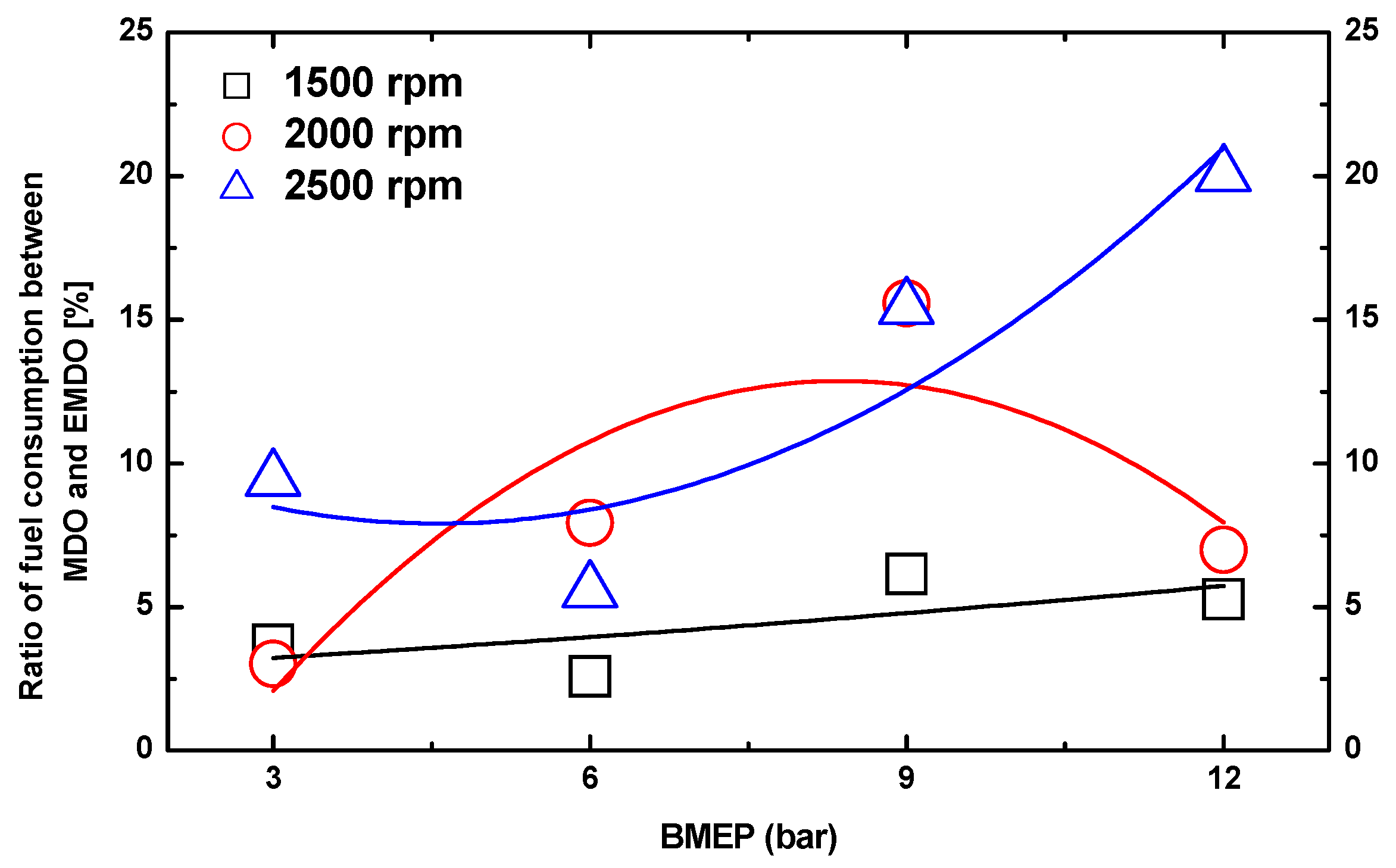
3.3. Comparison of Fuel Consumption Characteristics by Water Content between MDO and EMDO
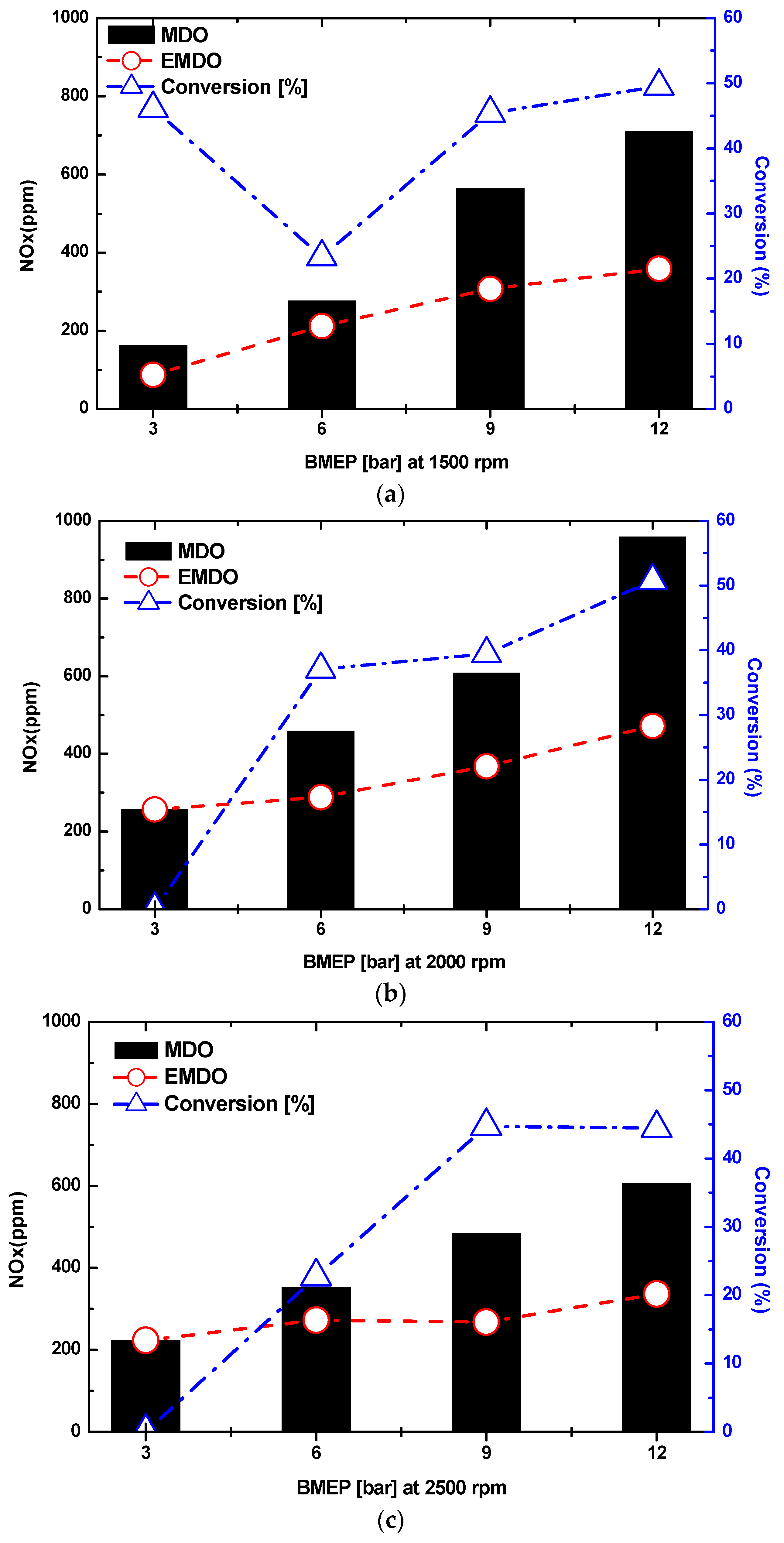
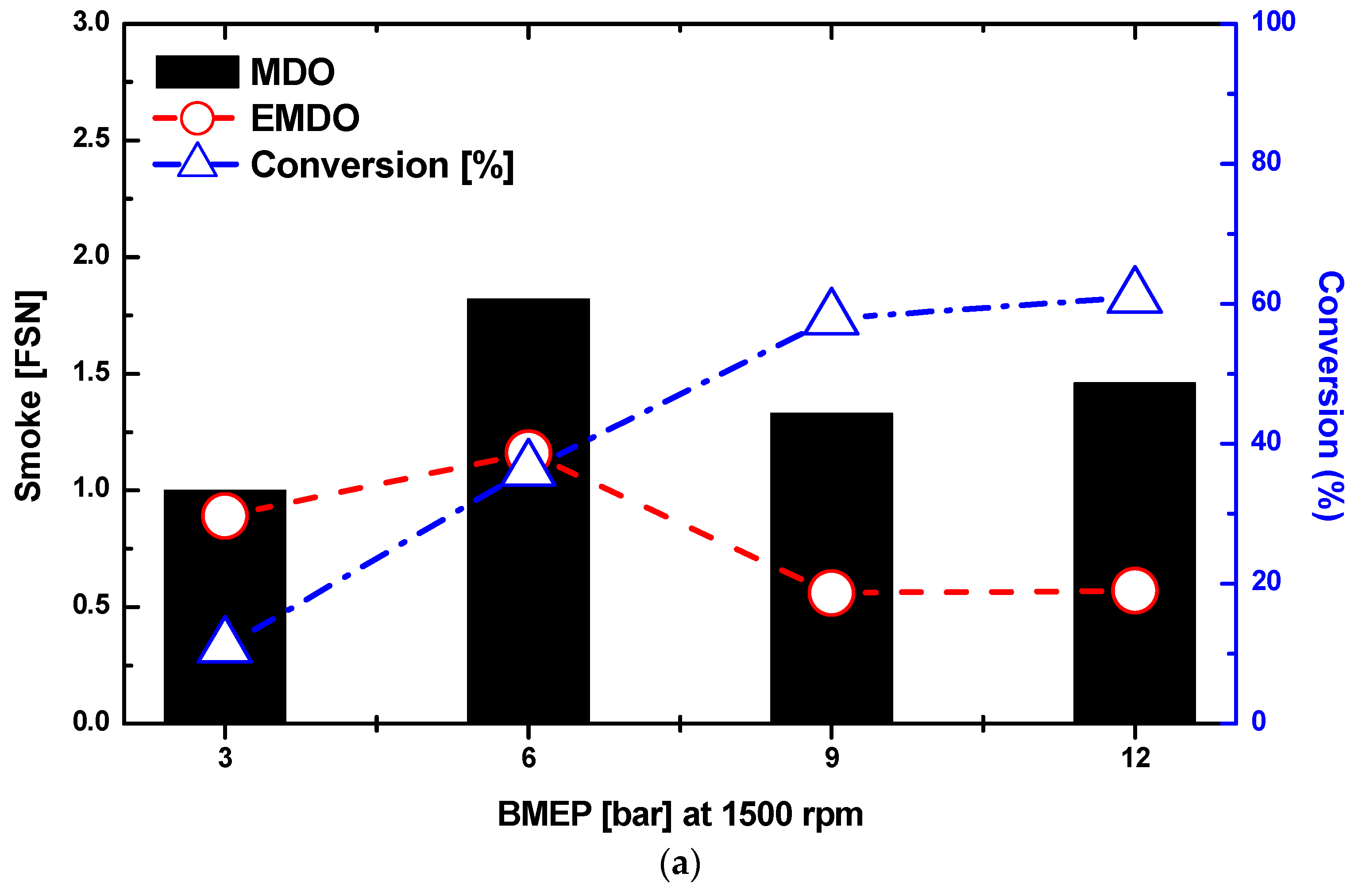
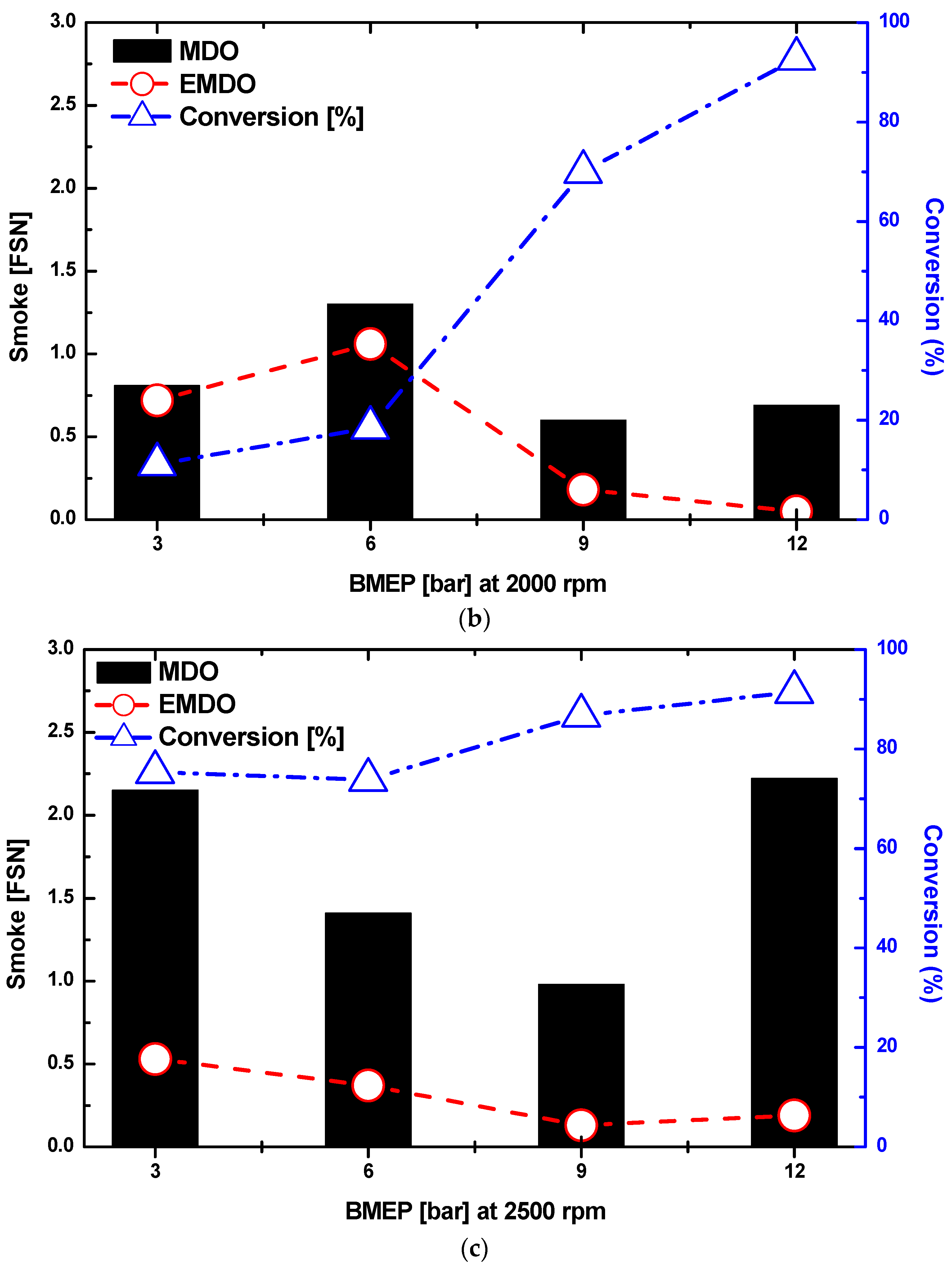
3.4. Combustion and Exhaust Characteristics by Water Content in MDO and EMDO
4. Conclusions
- (1)
- Under 3, 6, and 9 bar at 2500 rpm, EMDO exhibited higher cylinder pressure and heat release than MDO. In the case of ignition delay, EMDO was slightly faster than or similar to MDO. Rapid combustion reduced the combustion duration.
- (2)
- As for the cylinder pressure and heat release, EMDO exhibited a higher cylinder pressure and shorter combustion duration than MDO under the experimental conditions. EMDO exhibited a 27% higher heat release and a 14% higher total release than MDO for the CA.
- (3)
- EMDO exhibited a 14% higher fuel consumption than MDO. Comparing their pure fuel consumptions when excluding the water content, EMDO showed approximately 5% less fuel consumption than MDO.
- (4)
- As a result of the experiment using EMDO and MDO according to the changes in load and rpm, the NOx and smoke reduction rates were 30% and 80%, respectively. Over the entire load area, drastic exhaust emission reduction performance was observed. In addition, in terms of the stability of the coefficient of variation for the indicated mean effective pressures of the two fuels, a stable combustion state was observed over the entire load area, but poor characteristics were observed over the low-load area.
- (5)
- As the water content of the emulsified fuel increased, the smoke density decreased and the smoke levels decreased. As the moisture content of the MDO increased, the smoke density decreased and the smoke levels decreased. The reduction in smoke levels as the water content increased was from (1) a reduction in combustion temperature, (2) the promotion of the mixing of air and fuel by the increasing surface area of droplets due to micro-explosions of the emulsion, (3) an increase in the water vapor concentration, and (4) the effect of the aqueous reaction of water and carbon.
Author Contributions
Funding
Conflicts of Interest
References
- U.S. Environmental Protection Agency, Assessments and Standard Division. In-Use Testing Program for Heavy-Duty Diesel Engine and Vehicles—Technical Support Document; EPA 420-R-05-006; U.S. Environmental Protection Agency: Washington, DC, USA, 2005.
- Herdzik, J. Emissions from marine engines versus IMO certification and requirements of Tier 3. J. KONES 2011, 18, 161–167. [Google Scholar]
- IMO. MARPOL Convention 73/78, Annex VI; IMO: London, UK, 2005. [Google Scholar]
- Azzara, A.; Rutherford, D.; Wang, H. Feasibility of IMO ANNEX VI Tier III Implementation Using Selective Catalytic Reduction; ICCT: Berlin, Germany, March 2014. [Google Scholar]
- Ithnin, A.M.; Noge, H.; Kadir, H.A.; Jazair, W. An overview of utilizing water-in-diesel emulsion fuel in diesel engine and its potential research study. J. Energy Inst. 2014, 87, 273–288. [Google Scholar] [CrossRef]
- Park, J.-K.; Oh, J.-M.; Kim, H.-I.; Lee, C.-H.; Lee, K.-H. Combustion characteristics of MDO and MDO emulsion in automotive diesel engine. Trans. Korean Soc. Mech. Eng. B 2012, 36, 945–951. [Google Scholar] [CrossRef]
- Verschaeren, R.; Schaepdryver, W.; Serruys, T.; Bastiaen, M.; Vervaeke, L.; Verhelst, S. Experimental study of NOx reduction on a medium speed heavy duty diesel engine by the application of EGR (exhaust gas recirculation) and Miller timing. Energy 2014, 76, 614–621. [Google Scholar] [CrossRef]
- Bhaskar, K.; Nagarajan, G.; Sampath, S. Optimization of FOME (fish oil methyl esters) blend and EGR (exhaust gas recirculation) for simultaneous control of NOx and particulate matter emissions in diesel engines. Energy 2013, 62, 224–234. [Google Scholar] [CrossRef]
- Jafarmadar, S. Multidimensional modeling of the effect of EGR (exhaust gas recirculation) mass fraction on energy terms in an indirect injection diesel engine. Energy 2014, 66, 305–313. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Tortorelli, M. Low temperature SCR on supported MnOx catalysts for marine exhaust gas cleaning: Effect of KCl poisoning. Chem. Eng. J. 2016, 283, 223–230. [Google Scholar] [CrossRef]
- Guo, M.; Fu, Z.; Ma, D.; Ji, N.; Song, C.; Liu, Q. A Short Review of Treatment Methods of Marine Diesel Engine Exhaust Gases. Procedia Eng. 2015, 121, 938–943. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, K.H. An experimental study of the combustion characteristics in SCCI and CAI based on direct-injection gasoline engine. Exp. Therm. Fluid Sci. 2007, 31, 1121–1132. [Google Scholar] [CrossRef]
- Lee, K.; Lee, C. An Experimental Study of the Extent of the Operating Region and Emission Characteristics of Stratified Combustion Using the Controlled Autoignition Method. Energy Fuels 2006, 20, 1862–1869. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, K.H. An Experimental Study on the Combustion and Emission Characteristics of a Stratified Charge Compression Ignition (SCCI) Engine. Energy Fuels 2007, 21, 1901–1907. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–346. [Google Scholar] [CrossRef]
- Birkhold, F.; Meingast, U.; Wassermann, P.; Deutschmann, O. Analysis of the Injection of Urea-Water-Solution for Automotive SCR DeNOx-System; Modeling of Two-Phase Flow and Spray/Wall-Interaction; SAE Technical Paper 2006-01-0643; SAE International: Warrendale, PA, USA, 2006. [Google Scholar]
- Birkhold, F.; Meingast, U.; Wassermann, P.; Deutschmann, O. Modeling and simulation of the injection of urea-water-solution for automotive SCR DeNOx-system. Appl. Catal. B 2007, 70, 119–127. [Google Scholar] [CrossRef]
- Lif, A.; Holmberg, K. Water-in-diesel emulsions and related systems. Adv. Colloid Interface Sci. 2006, 123–126, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.B.; Hou, I.Y.; Wang, L.; Ma, F.H. A unified model for the micro-explosion of emulsified droplets of oil and water. Fuel Process. Technol. 2002, 79, 107–119. [Google Scholar] [CrossRef]
- Watanabe, H.; Matsushita, Y.; Aoki, H.; Miura, T. Numerical simulation of emulsified fuel spray combustion with puffing and micro-explosion. Combust. Flame 2010, 157, 839–852. [Google Scholar] [CrossRef]
- Ogunkoya, D.; Li, S.; Rojas, O.J.; Fang, T. Performance combustion and emissions in a diesel engine operated with fuel-in-water emulsions based on lignin. Appl. Energy 2015, 154, 851–861. [Google Scholar] [CrossRef]
- Rashid, M.A.A.; Ithnin, A.M.; Yahya, W.J.; Ramlan, N.A.; Mazlan, N.A.; Sugeng, D.A. Integration of real-time non-surfactant emulsion fuel system on light duty lorry. IOP Conf. Ser. Mater. Sci. Eng. 2017, 257, 012051. [Google Scholar] [CrossRef]
- Ithnin, A.M.; Yahya, W.J.; Ahmad, M.A.; Ramlan, N.A.; Kadir, H.A.; Sidik, N.A.C.; Koga, T. Emulsifier-free Water-in-Diesel emulsion fuel: Its stability behaviour engine performance and exhaust emission. Fuel 2018, 215, 454–462. [Google Scholar] [CrossRef]
- Mwangi, J.K.; Lee, W.-J.; Chang, Y.-C.; Chen, C.-Y.; Wang, L.C. An overview: Energy saving and pollution reduction by using green fuel blends in diesel engines. Appl. Energy 2015, 159, 214–236. [Google Scholar] [CrossRef]


| Tier | Effective Date | NOX Limit (g/kWh) | ||
|---|---|---|---|---|
| N < 130 | 130 ≤ N < 2000 | N > 2000 | ||
| Tier I | 2000 | 17 | 45 × n−0.2 | 9.8 |
| Tier II | 2011 | 14.4 | 44 × n−0.2 | 7.7 |
| Tier III | 2016 | 3.4 | 9 × n−0.2 | 1.96 |
| Item/Classification | Marine Diesel Oil (MDO) | Emulsified Marine Diesel Oil (EMDO) |
|---|---|---|
| Lower calorific value (J/g) | 41,060 | 32,990 |
| Gross calorific value (J/g) | 43,670 | 36,050 |
| Sulphur content (wt %) | 0.15 | 0.1 |
| Density at 15 °C (kg/m3) | 923.6 | 929.7 |
| Viscosity at 15 °C (cP) | 21.7 | 34.3 |
| Moisture (vol %) | 0.5 | 16.8 |
| Flash point (°C) | 104 | 86 |
| Item/Description | Specifications |
|---|---|
| Engine type | Four-stroke turbo-charged direct injection diesel engine |
| Number of cylinders | 4 |
| Bore × stroke (mm) | 83 × 92 |
| Displacement (cc) | 1991 |
| Fuel injection system | Common rail (max: 1600 bar) |
| Max power (ps/rpm) | 146/4000 |
| Max torque (kg m/rpm) | 32/1800–2500 |
| Item | Specifications |
|---|---|
| Dynamometer | HUSHINO ESF-H-150; eddy current type; 110 kW at 10,000 rpm |
| Exhaust gas analyser | Horiba, MEXA 7100 |
| Smoke meter | AVL 415 |
| Fuel | MDO/EMDO |
|---|---|
| Engine speed (rpm) | 1500, 2000, 2500 |
| Brake mean effective pressure (BMEP) | 3, 6, 9, 12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Oh, J.; Lee, C. Study on Combustion and Emission Characteristics of Marine Diesel Oil and Water-In-Oil Emulsified Marine Diesel Oil. Energies 2018, 11, 1830. https://doi.org/10.3390/en11071830
Kim M, Oh J, Lee C. Study on Combustion and Emission Characteristics of Marine Diesel Oil and Water-In-Oil Emulsified Marine Diesel Oil. Energies. 2018; 11(7):1830. https://doi.org/10.3390/en11071830
Chicago/Turabian StyleKim, Moonchan, Jungmo Oh, and Changhee Lee. 2018. "Study on Combustion and Emission Characteristics of Marine Diesel Oil and Water-In-Oil Emulsified Marine Diesel Oil" Energies 11, no. 7: 1830. https://doi.org/10.3390/en11071830
APA StyleKim, M., Oh, J., & Lee, C. (2018). Study on Combustion and Emission Characteristics of Marine Diesel Oil and Water-In-Oil Emulsified Marine Diesel Oil. Energies, 11(7), 1830. https://doi.org/10.3390/en11071830





