Evaluating Molecular Evolution of Kerogen by Raman Spectroscopy: Correlation with Optical Microscopy and Rock-Eval Pyrolysis
Abstract
1. Introduction
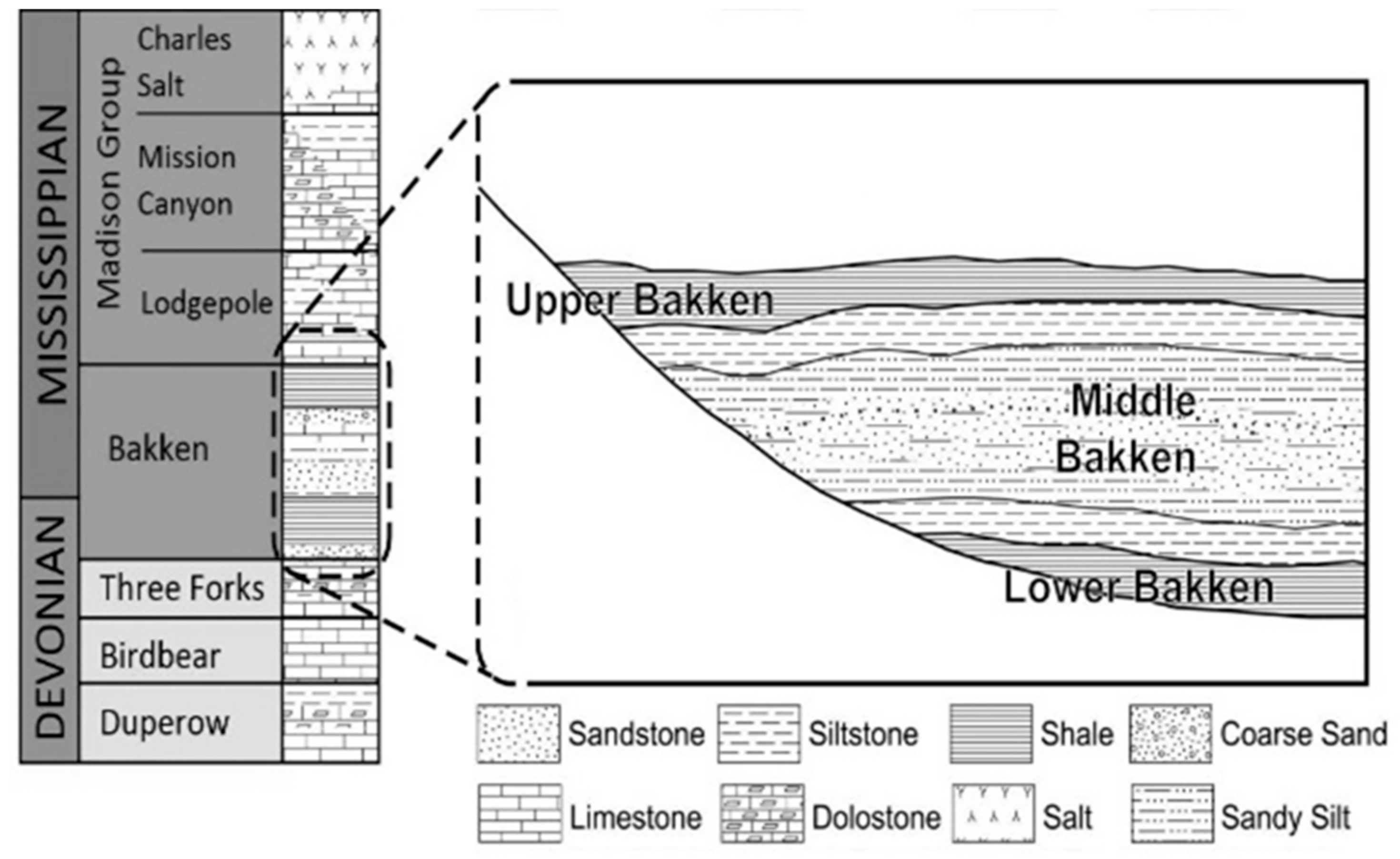
2. Geological Setting
3. Sample Preparation and Experiments
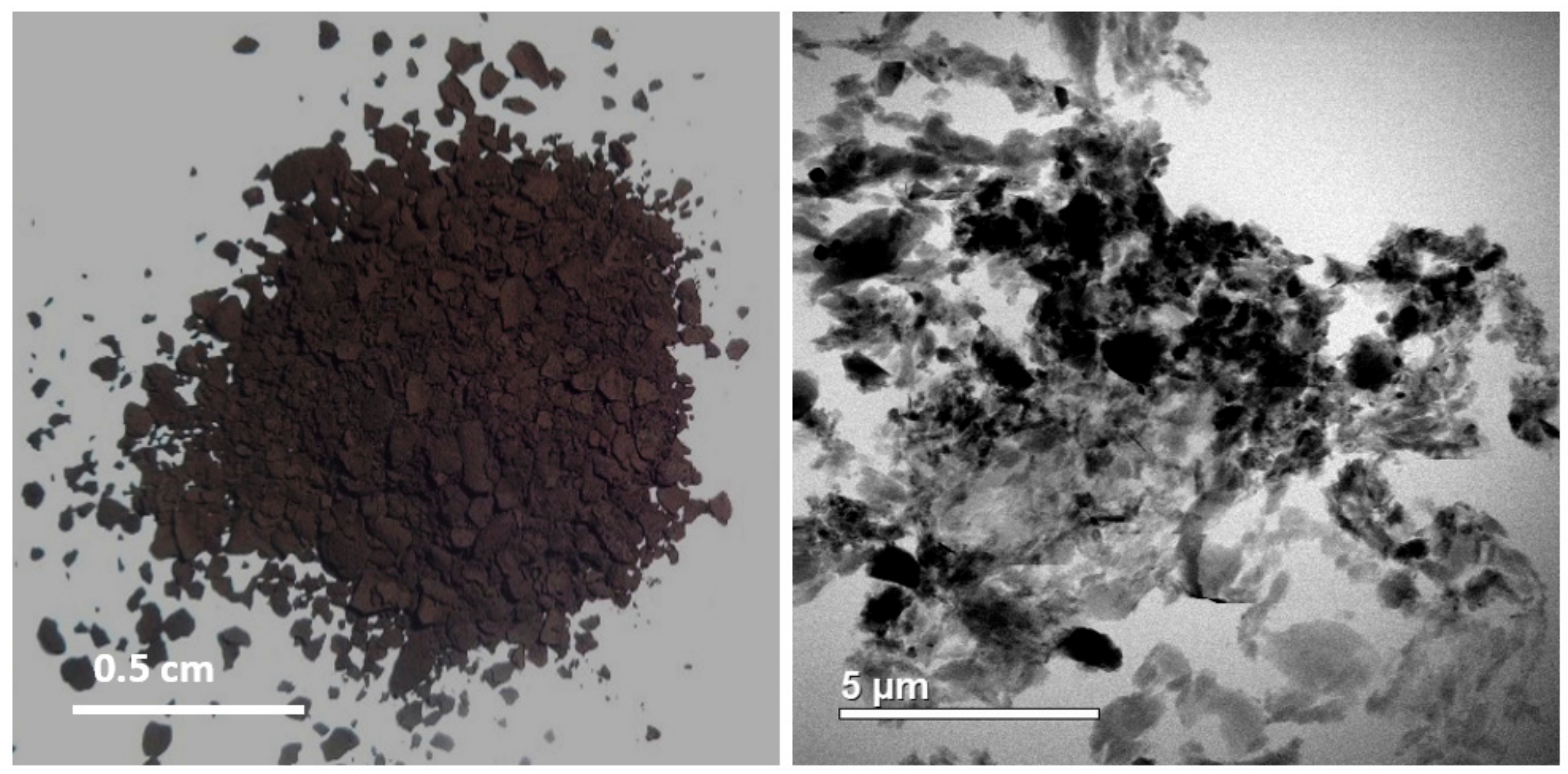
3.1. Isolation of Kerogen
- Pre-acid preparation of the samples: sample quality needs to be evaluated to ensure they are properly washed and are brought to the appropriate mesh size (crushed if necessary). Typical starting sample size is 20–30 g of material, depending on organic richness.
- The sample is then placed into concentrated 37% HCl (small increments should be added to prevent excessive foaming, while timing is dependent on sample reaction).
- Samples are placed into concentrated 48% HF (HF is for digestion of remaining silicates).
- The samples are placed into concentrated HCl for the second time.
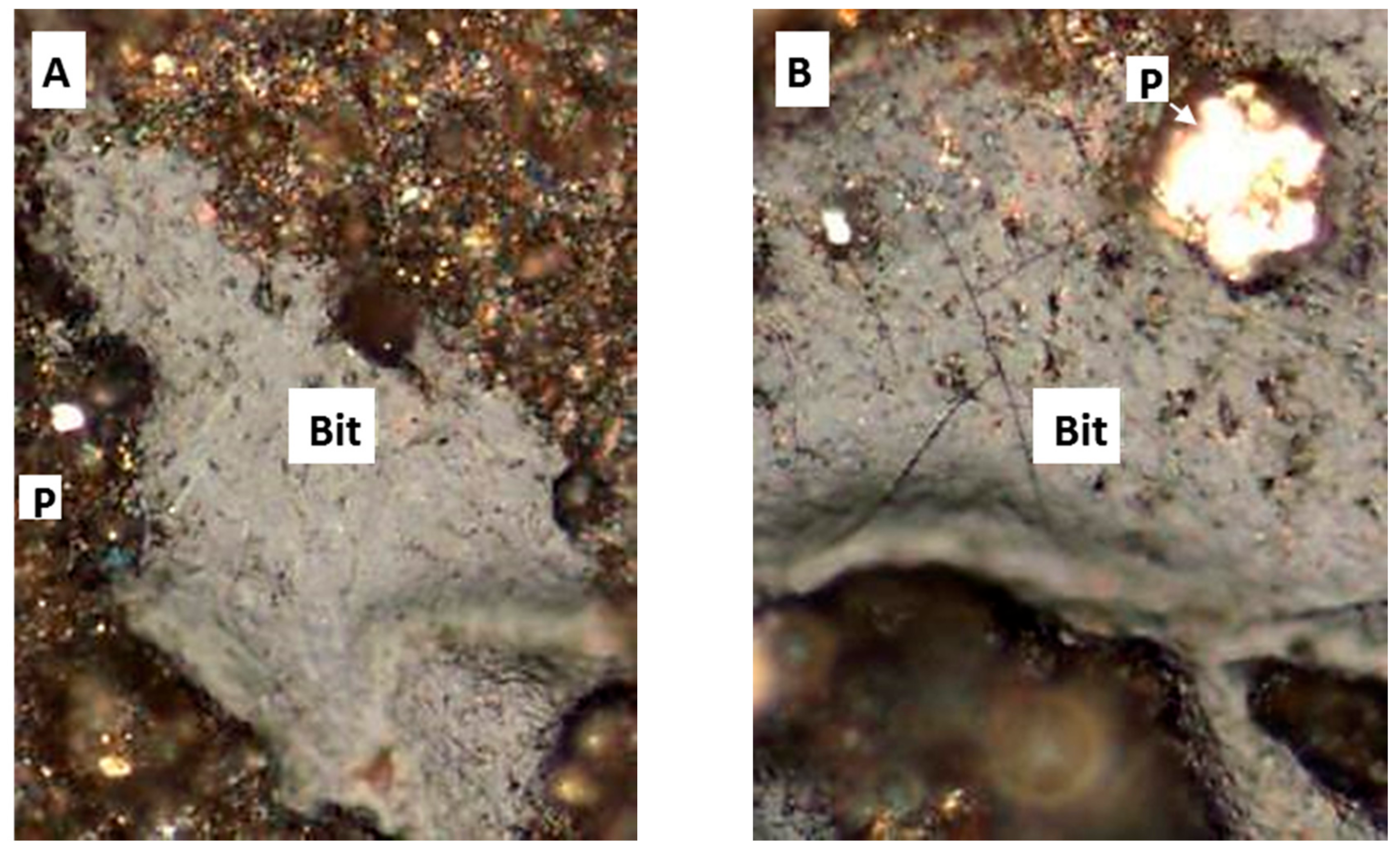
- Centrifugation: this is done essentially by taking a portion of the remnant of the sample and placing it into a centrifuge tube to create the kerogen slide. This process will separate the solid kerogen from remaining liquids, Figure 5.
3.2. Raman Spectroscopy
4. Results and Discussion
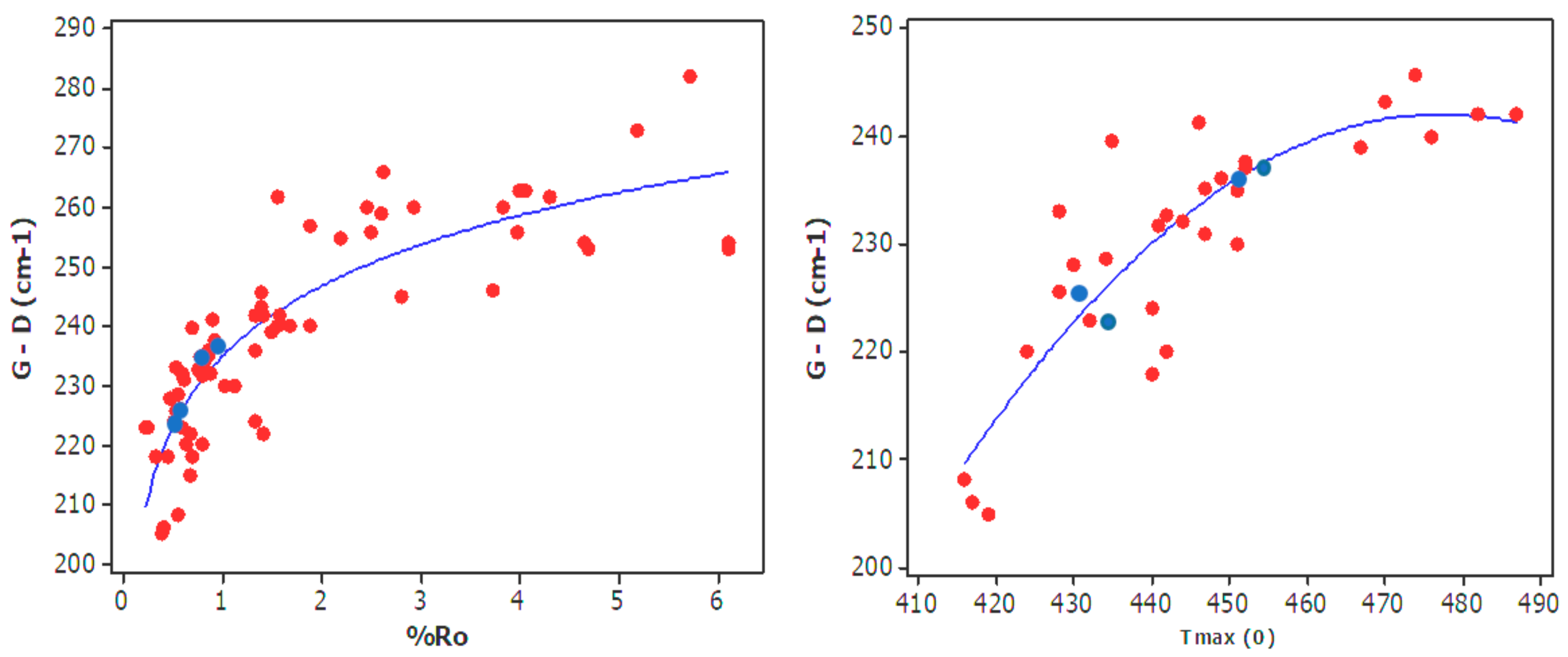
4.1. Raman Signals on Pure Kerogen
4.2. Raman Spectroscopy for Evaluating Production Potential of OM
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Hutton, A.; Bharati, S.; Robl, T. Chemical and petrographic classification of kerogen/macerals. Energy Fuels 1994, 8, 1478–1488. [Google Scholar] [CrossRef]
- McCarthy, K.; Rojas, K.; Niemann, M.; Palmowski, D.; Peters, K.; Stankiewicz, A. Basic petroleum geochemistry for source rock evaluation. Oilfield Rev. 2011, 23, 32–43. [Google Scholar]
- Wang, Z.; Li, Y.; Liu, H.; Zeng, F.; Guo, P.; Jiang, W. Study on the Adsorption, Diffusion and Permeation Selectivity of Shale Gas in Organics. Energies 2017, 10, 142. [Google Scholar] [CrossRef]
- Hackley, P.C.; Araujo, C.V.; Borrego, A.G.; Bouzinos, A.; Cardott, B.J.; Cook, A.C.; Eble, C.; Flores, D.; Gentzis, T.; Gonçalves, P.A. Standardization of reflectance measurements in dispersed organic matter: Results of an exercise to improve interlaboratory agreement. Mar. Pet. Geol. 2015, 59, 22–34. [Google Scholar] [CrossRef]
- Sauerer, B.; Craddock, P.R.; AlJohani, M.D.; Alsamadony, K.L.; Abdallah, W. Fast and accurate shale maturity determination by Raman spectroscopy measurement with minimal sample preparation. Int. J. Coal Geol. 2017, 173, 150–157. [Google Scholar] [CrossRef]
- Clementz, D.M. Effect of oil and bitumen saturation on source-rock pyrolysis: GEOLOGIC NOTES. AAPG Bull. 1979, 63, 2227–2232. [Google Scholar]
- Larter, S.; Douglas, A. A pyrolysis-gas chromatographic method for kerogen typing. Phys. Chem. Earth 1980, 12, 579–583. [Google Scholar] [CrossRef]
- Peters, K. Guidelines for evaluating petroleum source rock using programmed pyrolysis. AAPG Bull. 1986, 70, 318–329. [Google Scholar]
- Lafargue, E.; Marquis, F.; Pillot, D. Rock-Eval 6 applications in hydrocarbon exploration, production, and soil contamination studies. Rev. L'inst. Fr. Pét. 1998, 53, 421–437. [Google Scholar] [CrossRef]
- Behar, F.; Beaumont, V.; Penteado, H.D.B. Rock-Eval 6 technology: Performances and developments. Oil Gas Sci. Technol. 2001, 56, 111–134. [Google Scholar] [CrossRef]
- Espitalie, J.; Deroo, G.; Marquis, F. Rock-Eval pyrolysis and its applications. Rev. L'inst. Fr. Pét. 1985, 40, 563–579. [Google Scholar] [CrossRef]
- Tissot, B.P.; Welte, D.H. Diagenesis, Catagenesis and Metagenesis of Organic Matter, Petroleum Formation and Occurrence; Springer: Berlin, Germany, 1984; pp. 69–73. [Google Scholar]
- Waples, D.W. Organic Geochemistry for Exploration Geologists; Burgess Pub. Co.: Minneapolis, MN, USA, 1981. [Google Scholar]
- Tyson, R.V. Abundance of Organic Matter in Sediments: TOC, Hydrodynamic Equivalence, Dilution and Flux Effects, Sedimentary Organic Matter; Springer: Berlin, Germany, 1995; pp. 81–118. [Google Scholar]
- Kelemen, S.; Fang, H. Maturity trends in Raman spectra from kerogen and coal. Energy Fuels 2001, 15, 653–658. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Chopin, C.; Rouzaud, J. Raman spectra of carbonaceous material in metasediments: A new geothermometer. J. Metamorph. Geol. 2002, 20, 859–871. [Google Scholar] [CrossRef]
- Quirico, E.; Rouzaud, J.-N.; Bonal, L.; Montagnac, G. Maturation grade of coals as revealed by Raman spectroscopy: Progress and problems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Lahfid, A.; Beyssac, O.; Deville, E.; Negro, F.; Chopin, C.; Goffé, B. Evolution of the Raman spectrum of carbonaceous material in low-grade metasediments of the Glarus Alps (Switzerland). Terra Nova 2010, 22, 354–360. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.; Noronha, F. Raman spectroscopy of coal macerals and fluidized bed char morphotypes. Fuel 2012, 97, 443–449. [Google Scholar] [CrossRef]
- Hinrichs, R.; Brown, M.T.; Vasconcellos, M.A.; Abrashev, M.V.; Kalkreuth, W. Simple procedure for an estimation of the coal rank using micro-Raman spectroscopy. Int. J. Coal Geol. 2014, 136, 52–58. [Google Scholar] [CrossRef]
- Wilkins, R.W.; Boudou, R.; Sherwood, N.; Xiao, X. Thermal maturity evaluation from inertinites by Raman spectroscopy: The ‘RaMM’technique. Int. J. Coal Geol. 2014, 128, 143–152. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiao, X.; Pan, L.; Tian, H. The relationship between micro-Raman spectral parameters and reflectance of solid bitumen. Int. J. Coal Geol. 2014, 121, 19–25. [Google Scholar] [CrossRef]
- Lünsdorf, N.K. Raman spectroscopy of dispersed vitrinite—Methodical aspects and correlation with reflectance. Int. J. Coal Geol. 2016, 153, 75–86. [Google Scholar] [CrossRef]
- Mumm, A.S.; İnan, S. Microscale organic maturity determination of graptolites using Raman spectroscopy. Int. J. Coal Geol. 2016, 162, 96–107. [Google Scholar] [CrossRef]
- Ferralis, N.; Matys, E.D.; Knoll, A.H.; Hallmann, C.; Summons, R.E. Rapid, direct and non-destructive assessment of fossil organic matter via microRaman spectroscopy. Carbon 2016, 108, 440–449. [Google Scholar] [CrossRef]
- Khatibi, S.; Ostadhassan, M.; Tuschel, D.; Gentzis, T.; Bubach, B.; Carvajal-Ortiz, H. Raman spectroscopy to study thermal maturity and elastic modulus of kerogen. Int. J. Coal Geol. 2018, 185, 103–118. [Google Scholar] [CrossRef]
- Khatibi, S.; Ostadhassan, M.; Aghajanpour, A. Raman spectroscopy: An analytical tool for evaluating organic matter. J. Oil Gas Petrochem. Sci. 2018, 1, 28–33. [Google Scholar]
- Beyssac, O.; Goffé, B.; Petitet, J.-P.; Froigneux, E.; Moreau, M.; Rouzaud, J.-N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- LeFever, J.A. History of Oil Production from the Bakken Formation, North Dakota. 1991. Available online: http://archives.datapages.com/data/mgs/mt/data/0045/0003/0003.html (accessed on 30 May 2018).
- Smith, M.G.; Bustin, R.M. Late Devonian and Early Mississippian Bakken and Exshaw black shale source rocks, Western Canada Sedimentary Basin: A sequence stratigraphic interpretation. AAPG Bull. 2000, 84, 940–960. [Google Scholar]
- Parapuram, G.; Mokhtari, M.; Ben Hmida, J. An Artificially Intelligent Technique to Generate Synthetic Geomechanical Well Logs for the Bakken Formation. Energies 2018, 11, 680. [Google Scholar] [CrossRef]
- Webster, R.L. Petroleum Source Rocks and Stratigraphy of the Bakken Formation in North Dakota; AAPG/Datapages: Tulsa, OK, USA, 1984. [Google Scholar]
- Smith, M.G.; Bustin, R.M. Sedimentology of the Late Devonian and Early Mississippian Bakken Formation, Williston Basin; Williston Basin Symposium; AAPG/Datapages: Tulsa, OK, USA, 1995. [Google Scholar]
- Liu, K.; Ostadhassan, M.; Gentzis, T.; Carvajal-Ortiz, H.; Bubach, B. Characterization of geochemical properties and microstructures of the Bakken Shale in North Dakota. Int. J. Coal Geol. 2018, 190, 84–98. [Google Scholar] [CrossRef]
- Sonnenberg, S.A.; Pramudito, A. Petroleum geology of the giant Elm Coulee field, Williston Basin. AAPG Bull. 2009, 93, 1127–1153. [Google Scholar] [CrossRef]
- Jin, H.; Sonnenbergy, S.A. Characterization for source rock potential of the Bakken Shales in the Williston Basin, North Dakota and Montana. In Proceedings of the Unconventional Resources Technology Conference (URTEC), Denver, CO, USA, 12–14 August 2013. [Google Scholar]
- Gaswirth, S.B.; Marra, K.R.; Cook, T.A.; Charpentier, R.R.; Gautier, D.L.; Higley, D.K.; Klett, T.R.; Lewan, M.D.; Lillis, P.G.; Schenk, C.J. Assessment of Undiscovered Oil Resources in the Bakken and Three Forks Formations, Williston Basin Province, Montana, North Dakota, and South Dakota, 2013; US Geological Survey: Reston, VA, USA, 2013. [Google Scholar]
- Jacob, H. Classification, structure, genesis and practical importance of natural solid oil bitumen (“migrabitumen”). Int. J. Coal Geol. 1989, 11, 65–79. [Google Scholar] [CrossRef]
- Jarvie, D.; Claxton, B.; Henk, B.; Breyer, J. Oil and shale gas from Barnett shale, Ft. Worth Basin, TX. In Proceedings of the AAPG National Convention, Denver, CO, USA, 3–6 June 2001. [Google Scholar]
- Schito, A.; Romano, C.; Corrado, S.; Grigo, D.; Poe, B. Diagenetic thermal evolution of organic matter by Raman spectroscopy. Org. Geochem. 2017, 106, 57–67. [Google Scholar] [CrossRef]
- Robl, T.L.; Davis, B.H. Comparison of the HF-HCl and HF-BF3 maceration techniques and the chemistry of resultant organic concentrates. Org. Geochem. 1993, 20, 249–255. [Google Scholar] [CrossRef]
- Schimmelmann, A.; Lewan, M.D.; Wintsch, R.P. D/H isotope ratios of kerogen, bitumen, oil, and water in hydrous pyrolysis of source rocks containing kerogen types I, II, IIS, and III. Geochim. Cosmochim. Acta 1999, 63, 3751–3766. [Google Scholar] [CrossRef]
- Amer, M. Raman Spectroscopy for Soft Matter Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Reich, S.; Thomsen, C. Raman spectroscopy of graphite. Philos. Trans. R. Soc. Lond. A: Math. Phys. Eng. Sci. 2004, 362, 2271–2288. [Google Scholar] [CrossRef] [PubMed]
- Cesare, B.; Maineri, C. Fluid-present anatexis of metapelites at El Joyazo (SE Spain): Constraints from Raman spectroscopy of graphite. Contrib. Mineral. Petrol. 1999, 135, 41–52. [Google Scholar] [CrossRef]
- Marshall, C.P.; Edwards, H.G.; Jehlicka, J. Understanding the application of Raman spectroscopy to the detection of traces of life. Astrobiology 2010, 10, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Tuschel, D. Raman spectroscopy of oil shale. Spectroscopy 2013, 28, 5. [Google Scholar]
- Huang, E.-P.; Huang, E.; Yu, S.-C.; Chen, Y.-H.; Lee, J.-S.; Fang, J.-N. In situ Raman spectroscopy on kerogen at high temperatures and high pressures. Phys. Chem. Miner. 2010, 37, 593–600. [Google Scholar] [CrossRef]
- Marshall, C.P.; Love, G.D.; Snape, C.E.; Hill, A.C.; Allwood, A.C.; Walter, M.R.; Van Kranendonk, M.J.; Bowden, S.A.; Sylva, S.P.; Summons, R.E. Structural characterization of kerogen in 3.4 Ga Archaean cherts from the Pilbara Craton, Western Australia. Precambrian Res. 2007, 155, 1–23. [Google Scholar] [CrossRef]
- Lünsdorf, N.K.; Dunkl, I.; Schmidt, B.C.; Rantitsch, G.; Eynatten, H. Towards a higher comparability of geothermometric data obtained by Raman spectroscopy of carbonaceous material. Part I: Evaluation of biasing factors. Geostand. Geoanal. Res. 2014, 38, 73–94. [Google Scholar] [CrossRef]
- Spötl, C.; Houseknecht, D.W.; Jaques, R.C. Kerogen maturation and incipient graphitization of hydrocarbon source rocks in the Arkoma Basin, Oklahoma and Arkansas: A combined petrographic and Raman spectrometric study. Org. Geochem. 1998, 28, 535–542. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.; Rodrigues, S.; Noronha, F. Micro-Raman spectroscopy of collotelinite, fusinite and macrinite. Int. J. Coal Geol. 2010, 83, 415–422. [Google Scholar] [CrossRef]
- Ostadhassan, M.; Liu, K.; Li, C.; Khatibi, S. Fine Scale Characterization of Shale Reservoirs: Methods and Challenges; Springer: Berlin, Germany, 2018. [Google Scholar]
- Gao, Y.; Zou, Y.-R.; Liang, T.; Peng, P.A. Jump in the structure of Type I kerogen revealed from pyrolysis and 13C DP MAS NMR. Org. Geochem. 2017, 112, 105–118. [Google Scholar] [CrossRef]
- Baudin, F.; Disnar, J.-R.; Aboussou, A.; Savignac, F. Guidelines for Rock–Eval analysis of recent marine sediments. Org. Geochem. 2015, 86, 71–80. [Google Scholar] [CrossRef]
- Laughrey, C.; Kostelnik, J.; Harper, J.; Carter, K. The Pennsylvania Petroleum Source Rock Geochemistry Database. 2013. Available online: https://edx.netl.doe.gov/dataset/the-pennsylvania-petroleum-source-rock-geochemistry-database (accessed on 30 May 2018).
- Ma, Y.Z.; Holditch, S. Unconventional Oil and Gas Resources Handbook: Evaluation and Development; Gulf Professional Publishing: Houston, TX, USA, 2015. [Google Scholar]
- Wopenka, B.; Pasteris, J.D. Structural characterization of kerogens to granulite-facies graphite: Applicability of Raman microprobe spectroscopy. Am. Mineral. 1993, 78, 533–557. [Google Scholar]
- Jehlicka, J.; Urban, O.; Pokorný, J. Raman spectroscopy of carbon and solid bitumens in sedimentary and metamorphic rocks. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2341–2352. [Google Scholar] [CrossRef]
- Dennis, L.W.; Maciel, G.E.; Hatcher, P.G.; Simoneit, B.R. 13 C Nuclear magnetic resonance studies of kerogen from Cretaceous black shales thermally altered by basaltic intrusions and laboratory simulations. Geochim. Cosmochim. Acta 1982, 46, 901–907. [Google Scholar] [CrossRef]
- Saxby, J.; Stephenson, L. Effect of anigneous intrusion on oil shale at Rundle (Australia). Chem. Geol. 1987, 63, 1–16. [Google Scholar] [CrossRef]
- Kuangzong, Q.; Deyu, C.; Zhanguang, L. A new method to estimate the oil and gas potentials of coals and kerogens by solid state 13C NMR spectroscopy. Org. Geochem. 1991, 17, 865–872. [Google Scholar] [CrossRef]
- Patience, R.; Mann, A.; Poplett, I. Determination of molecular structure of kerogens using 13C NMR spectroscopy: II. The effects of thermal maturation on kerogens from marine sediments. Geochim. Cosmochim. Acta 1992, 56, 2725–2742. [Google Scholar] [CrossRef]
- Wei, Z.; Gao, X.; Zhang, D.; Da, J. Assessment of thermal evolution of kerogen geopolymers with their structural parameters measured by solid-state 13C NMR spectroscopy. Energy Fuels 2005, 19, 240–250. [Google Scholar] [CrossRef]
- Clough, A.; Sigle, J.L.; Jacobi, D.; Sheremata, J.; White, J.L. Characterization of Kerogen and Source Rock Maturation Using Solid-State NMR Spectroscopy. Energy Fuels 2015, 29, 6370–6382. [Google Scholar] [CrossRef]
- Miknis, F. NMR studies of solid fossil fuels. Magn. Reson. Rev. 1982, 7, 87–121. [Google Scholar]
- Barwise, A.; Mann, A.; Eglinton, G.; Gowar, A.; Wardroper, A.; Gutteridge, C. Kerogen characterisation by 13C NMR spectroscopy and pyrolysis-mass spectrometry. Org. Geochem. 1984, 6, 343–349. [Google Scholar] [CrossRef]
- Solli, H.; Van Graas, G.; Leplat, P.; Krane, J. Analysis of kerogens of miocene shales in a homogenous sedimentary column. A study of maturation using flash pyrolysis techniques and carbon-13 CP-MAS NMR. Org. Geochem. 1984, 6, 351–358. [Google Scholar] [CrossRef]
- Witte, E.; Schenk, H.; Müller, P.; Schwochau, K. Structural modifications of kerogen during natural evolution as derived from 13C CP/MAS NMR, IR spectroscopy and Rock-Eval pyrolysis of Toarcian shales. Org. Geochem. 1988, 13, 1039–1044. [Google Scholar] [CrossRef]
- Miknis, F.P.; Smith, J.W. An NMR survey of United States oil shales. Org. Geochem. 1984, 5, 193–201. [Google Scholar] [CrossRef]
- Hagaman, E.W.; Cronauer, D.C.; Schell, F.M. Oil-shale analysis by CP/MAS-13C NMR spectroscopy. Fuel 1984, 63, 915–919. [Google Scholar] [CrossRef]
- Schenk, H.; Witte, E.; Müller, P.; Schwochau, K. Infrared estimates of aliphatic kerogen carbon in sedimentary rocks. Org. Geochem. 1986, 10, 1099–1104. [Google Scholar] [CrossRef]
- Orr, W. Comments on pyrolytic hydrocarbon yields in source-rock evaluation. Adv. Org. Geochem. 1981, 1981, 775–787. [Google Scholar]
- Beyssac, O.; Rouzaud, J.-N.; Goffé, B.; Brunet, F.; Chopin, C. Graphitization in a high-pressure, low-temperature metamorphic gradient: A Raman microspectroscopy and HRTEM study. Contrib. Miner. Petrol. 2002, 143, 19–31. [Google Scholar] [CrossRef]
- Painter, P.C.; Snyder, R.W.; Starsinic, M.; Coleman, M.M.; Kuehn, D.W.; Davis, A. Concerning the application of FT-IR to the study of coal: A critical assessment of band assignments and the application of spectral analysis programs. Appl. Spectrosc. 1981, 35, 475–485. [Google Scholar] [CrossRef]
- Solomon, P.R.; Carangelo, R.M. FT-ir analysis of coal: 2. Aliphatic and aromatic hydrogen concentration. Fuel 1988, 67, 949–959. [Google Scholar] [CrossRef]
- Chen, J.; Luo, P.; Li, J. Using kerogen FTIR parameters for determination of organic facies. Chin. Sci. Bull. 1998, 43, 681–684. [Google Scholar] [CrossRef]
- Painter, P.; Starsinic, M.; Coleman, M. Determination of functional groups in coal by Fourier transform interferometry. Fourier Transform Infrared Spectrosc. 2012, 4, 169–240. [Google Scholar]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of micro-Fourier transform infrared spectroscopy (FTIR) in the geological sciences—A review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef] [PubMed]
- Craddock, P.R.; Le Doan, T.V.; Bake, K.; Polyakov, M.; Charsky, A.M.; Pomerantz, A.E. Evolution of kerogen and bitumen during thermal maturation via semi-open pyrolysis investigated by infrared spectroscopy. Energy Fuels 2015, 29, 2197–2210. [Google Scholar] [CrossRef]
- Van Krevelen, D. Coal: Typology-Physics-Chemistry-Composition; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Cheshire, S.; Craddock, P.R.R.; Xu, G.; Sauerer, B.; Pomerantz, A.E.E.; McCormick, D.; Abdallah, W. Assessing thermal maturity beyond the reaches of vitrinite reflectance and Rock-Eval pyrolysis: A case study from the Silurian Qusaiba formation. Int. J. Coal Geol. 2017, 180, 29–45. [Google Scholar] [CrossRef]
- Craddock, P.R.R.; Prange, M.; & Pomerantz, A.E. Kerogen thermal maturity and content of organic-rich mudrocks determined using stochastic linear regression models applied to diffuse reflectance IR Fourier transform spectroscopy (DRIFTS). Org. Geochem. 2017, 110, 122–133. [Google Scholar] [CrossRef]
- Oberlin, A.; Boulmier, J.; Villey, M. Electron microscopic study of kerogen microtexture. Selected criteria for determining the evolution path and evolution stage of kerogen. In Kerogen: Insoluble Organic Matter from Sedimentary Rocks; Editions Technip: Paris, France, 1980; pp. 191–241. [Google Scholar]
- Oberlin, A. High-resolution TEM studies of carbonization and graphitization. Chem. Phys. Carbon 1989, 22, 1–143. [Google Scholar]
- Rouzaud, J.; Oberlin, A. Structure, microtexture, and optical properties of anthracene and saccharose-based carbons. Carbon 1989, 27, 517–529. [Google Scholar] [CrossRef]
- Kelemen, S.; Afeworki, M.; Gorbaty, M.; Sansone, M.; Kwiatek, P.; Walters, C.; Freund, H.; Siskin, M.; Bence, A.; Curry, D. Direct characterization of kerogen by X-ray and solid-state 13C nuclear magnetic resonance methods. Energy Fuels 2007, 21, 1548–1561. [Google Scholar] [CrossRef]
- Khatibi, S.; Aghajanpour, A.; Ostadhassan, M.; Ghanbari, E.; Amirian, E.; Mohammed, R. Evaluating the Impact of Mechanical Properties of Kerogen on Hydraulic Fracturing of Organic Rich Formations. In Proceedings of the SPE Canada Unconventional Resources Conference, Calgary, AB, Canada, 13–14 March 2018; Society of Petroleum Engineers: Houston, TX, USA, 2018. [Google Scholar]
- Zhu, W.; Chang, X.; Wang, Y.; Zhai, H.; Yao, Z. Reconstruction of Hydraulic Fractures Using Passive Ultrasonic Travel-Time Tomography. Energies 2018, 11, 1–17. [Google Scholar] [CrossRef]
- He, J.; Lin, C.; Li, X.; Wan, X. Experimental investigation of crack extension patterns in hydraulic fracturing with shale, sandstone and granite cores. Energies 2016, 9, 1018. [Google Scholar] [CrossRef]
- He, J.; Afolagboye, L.O.; Lin, C.; Wan, X. An Experimental Investigation of Hydraulic Fracturing in Shale Considering Anisotropy and Using Freshwater and Supercritical CO2. Energies 2018, 11, 557. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; He, J.; Zheng, B. Mechanical properties of longmaxi black organic-rich shale samples from south china under uniaxial and triaxial compression states. Energies 2016, 9, 1088. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Wang, Y.; Zheng, B.; Zhang, B. A Laboratory Study of the Effects of Interbeds on Hydraulic Fracture Propagation in Shale Formation. Energies 2016, 9, 556. [Google Scholar] [CrossRef]
- Zhai, H.; Chang, X.; Wang, Y.; Xue, Z.; Lei, X.; Zhang, Y. Sensitivity Analysis of Seismic Velocity and Attenuation Variations for Longmaxi Shale during Hydraulic Fracturing Testing in Laboratory. Energies 2017, 10, 1393. [Google Scholar] [CrossRef]
- Song, J.; Yang, Y.S.; Liu, Z.Q.; Li, X. Macro and Micro Properties of Organic Matter in Hydraulic Mud Consolidation. J. Mar. Sci. Eng. 2018, 6, 22. [Google Scholar] [CrossRef]
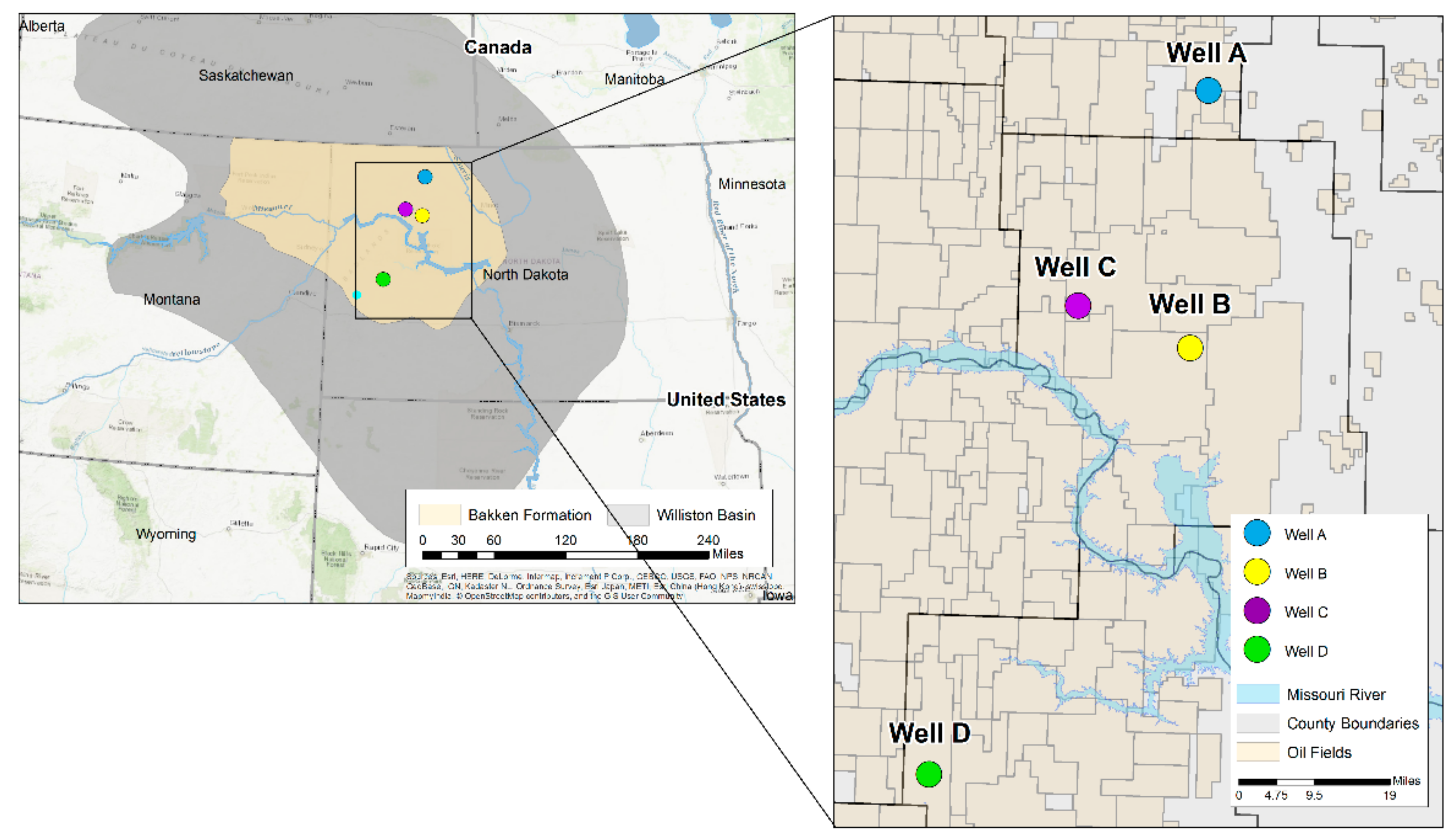


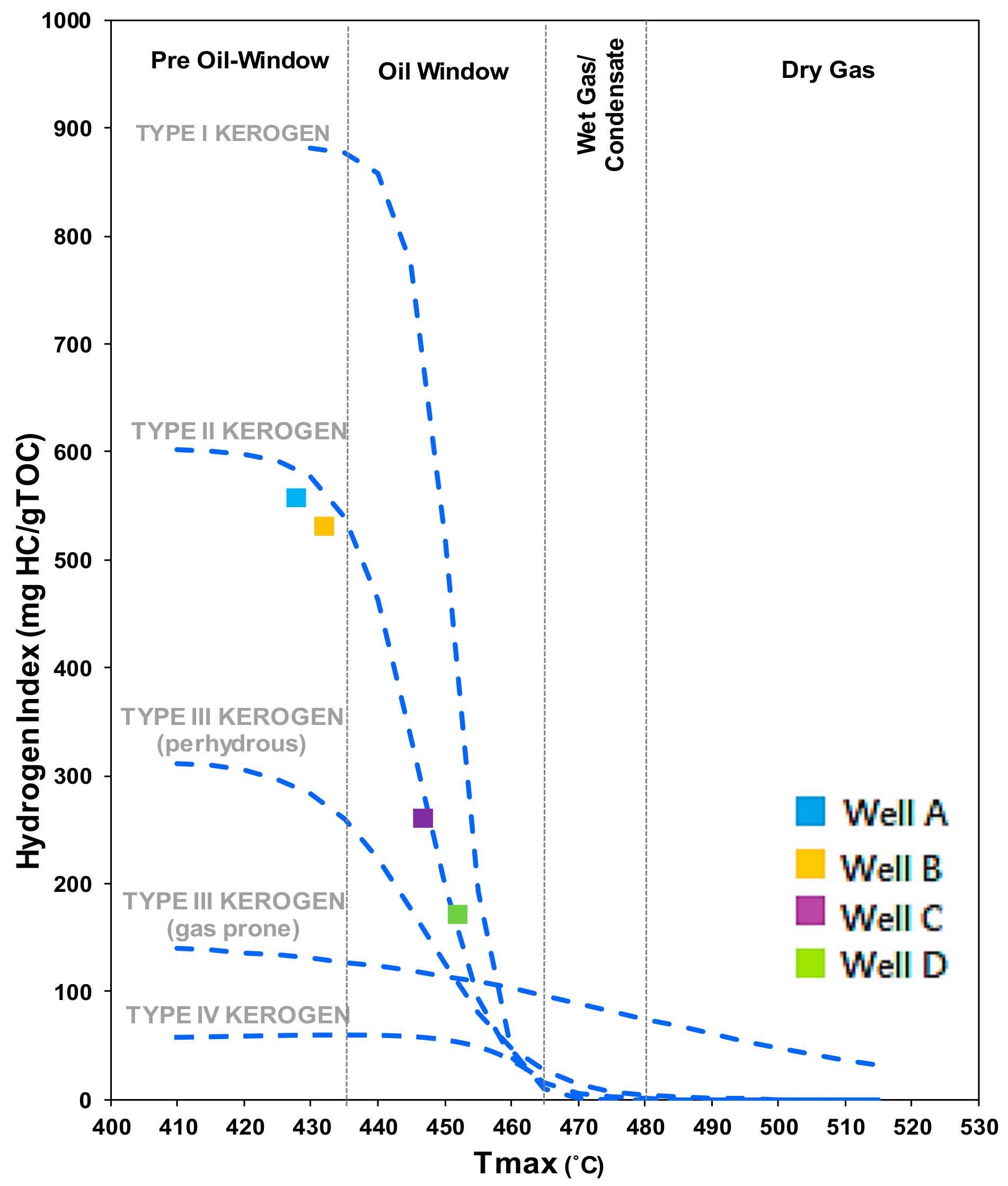

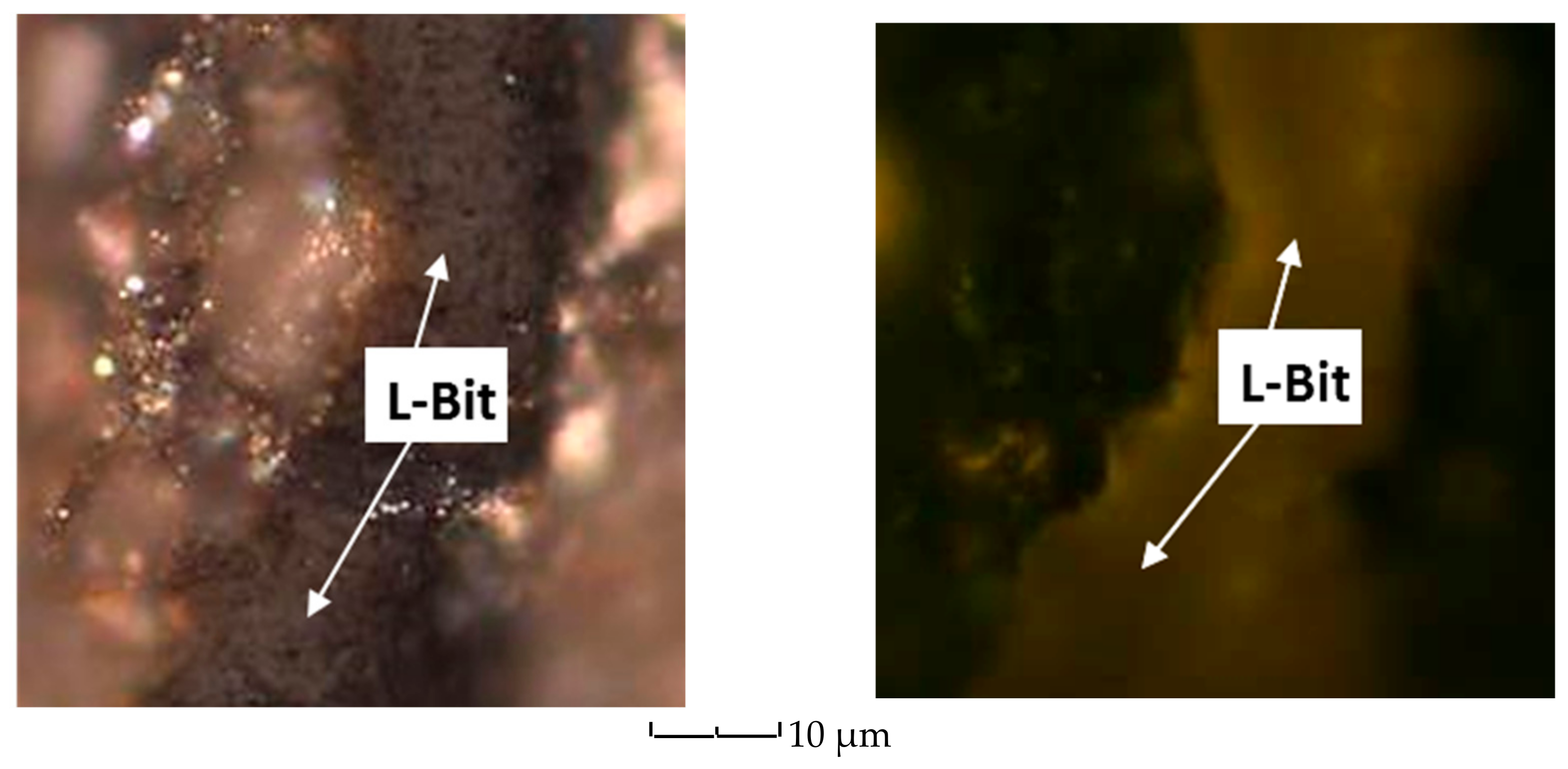
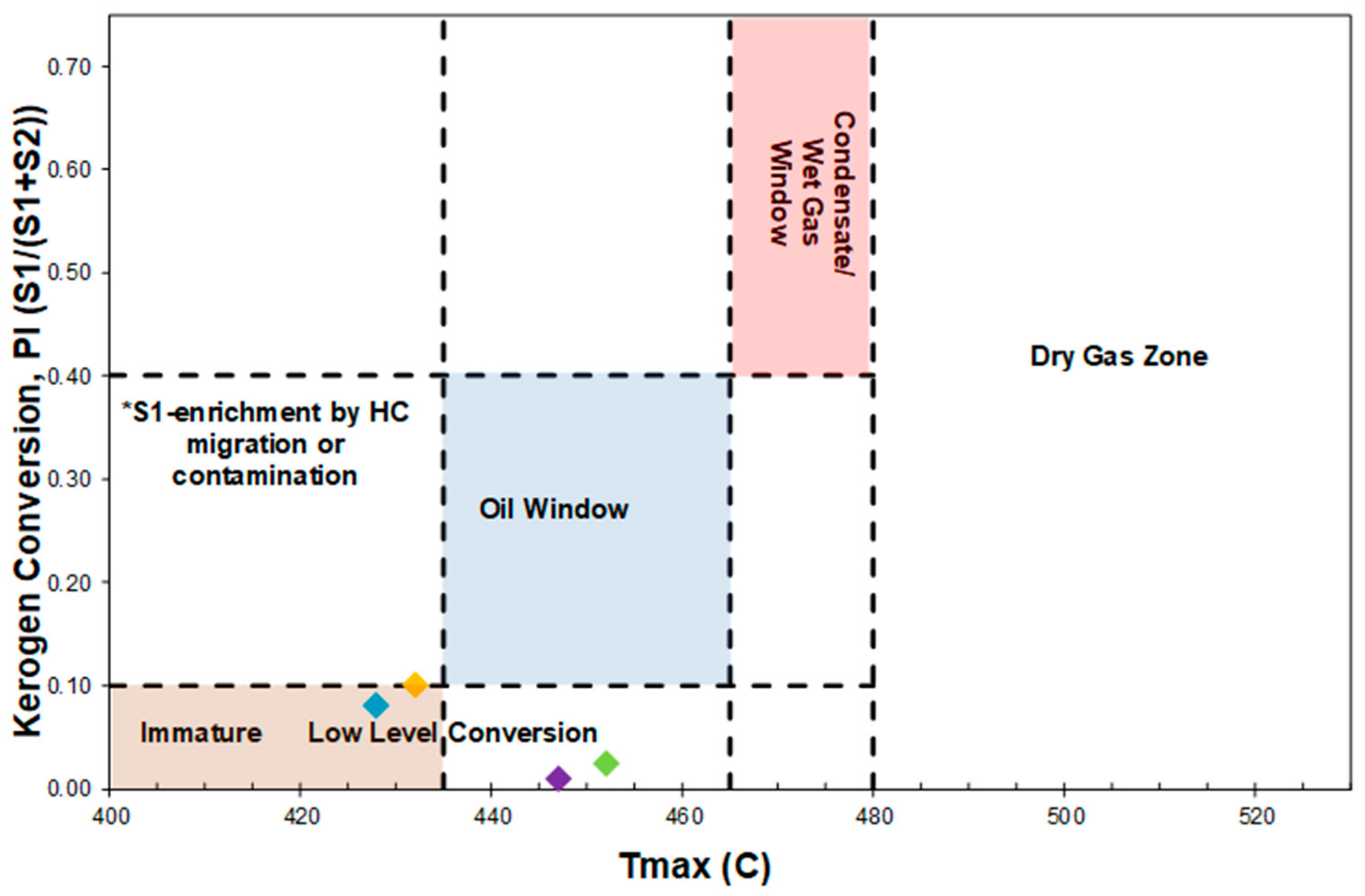
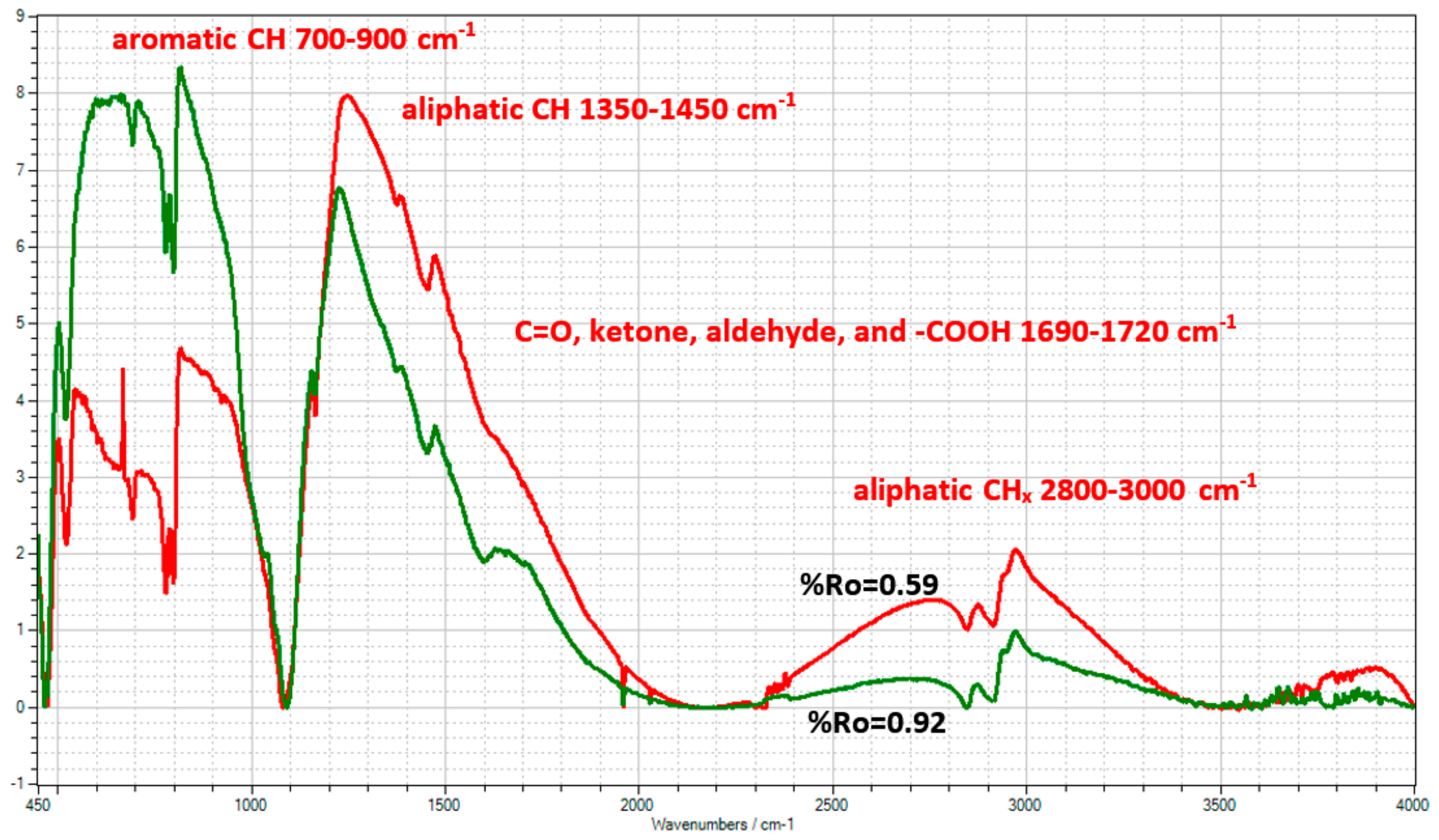
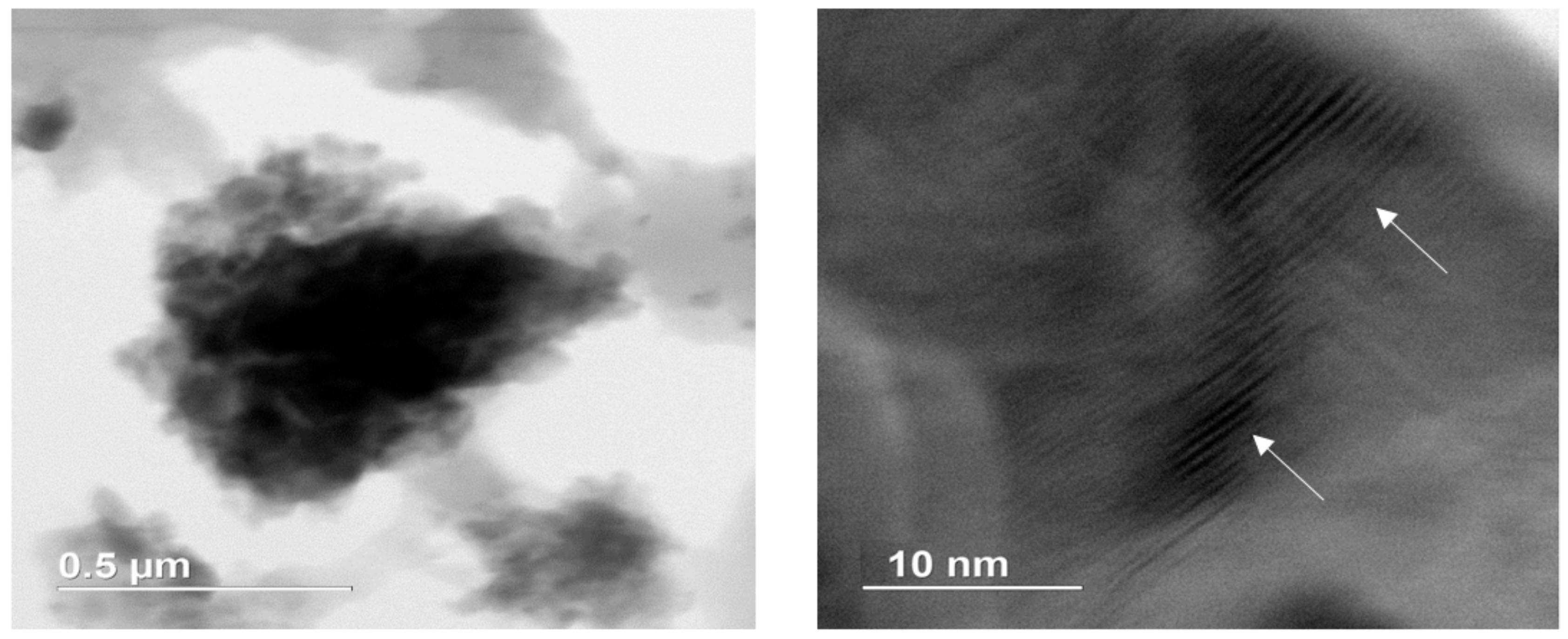
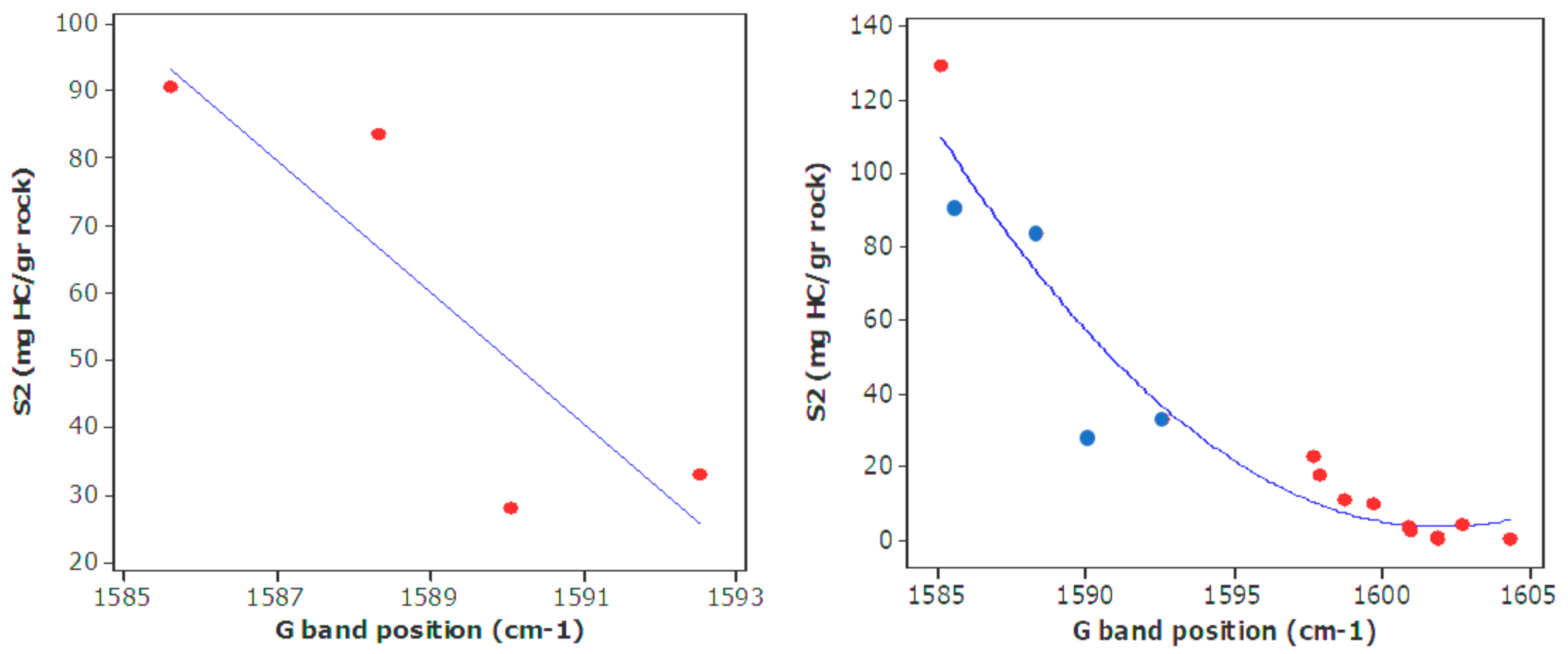
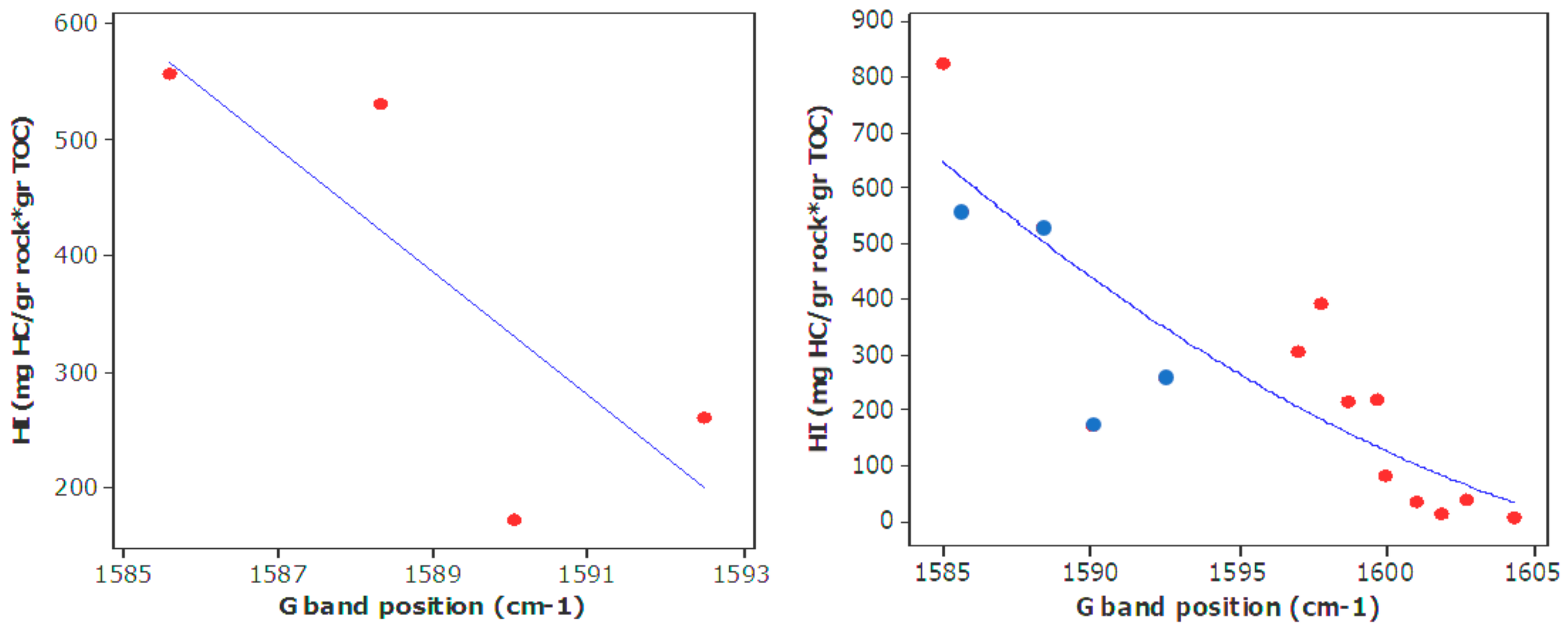
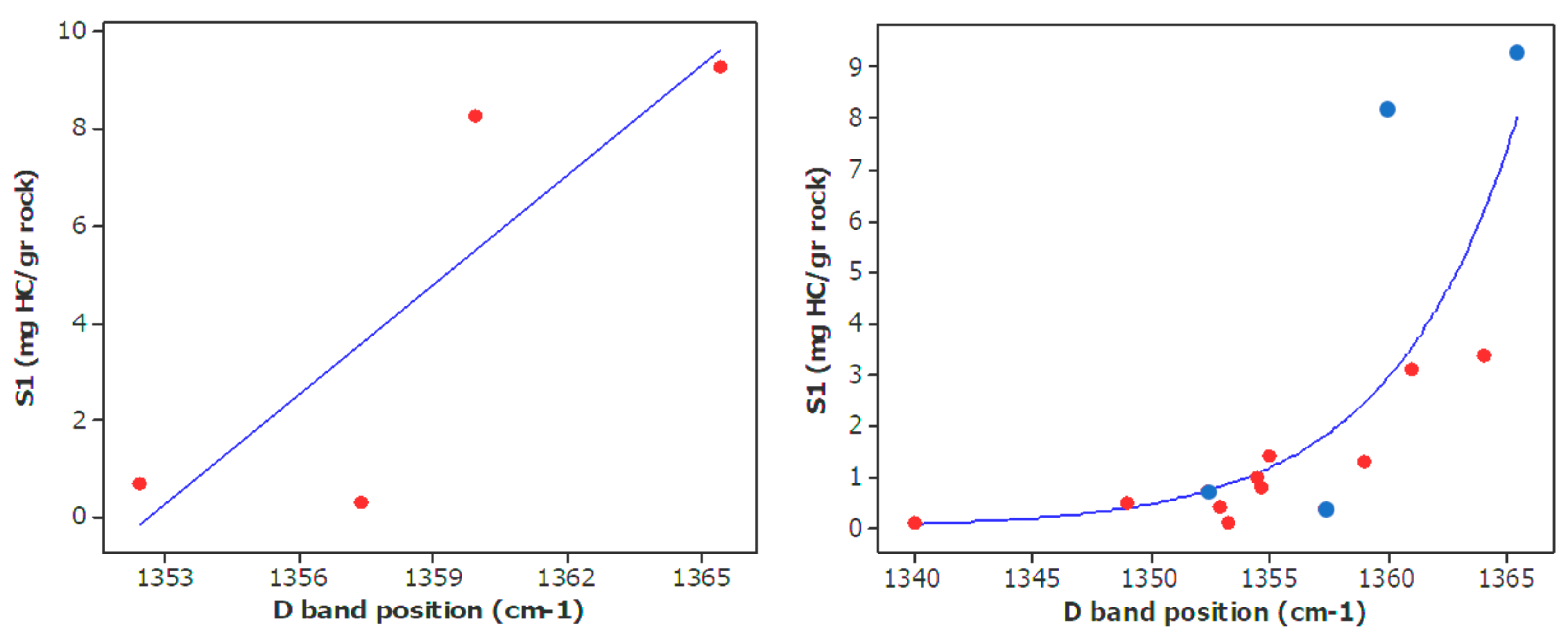
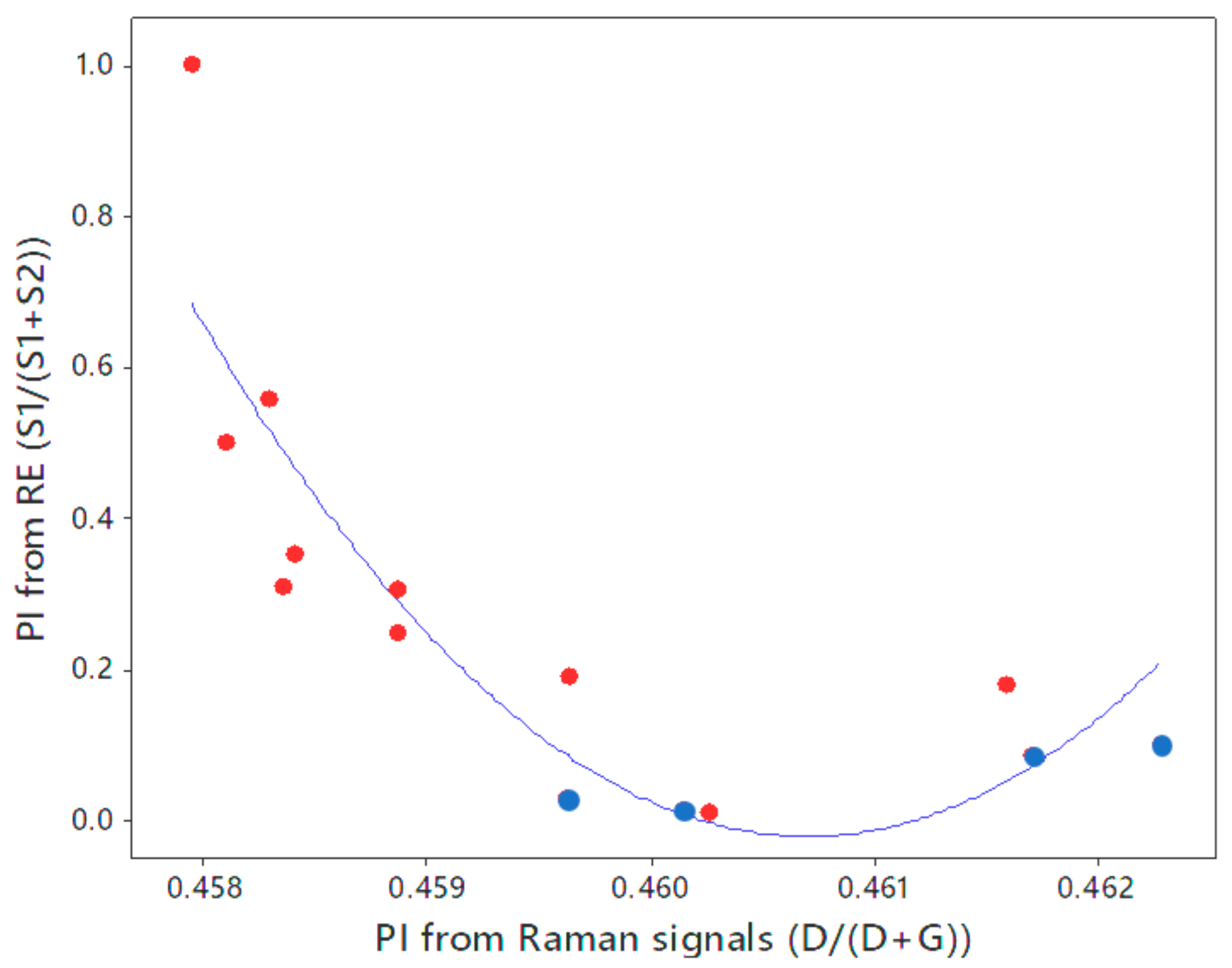
| Well No. | Depth (ft) | TOC (wt %) | S1 (mg/g) | S2 (mg/g) | HI (S2 × 100/TOC) | PI (S1/(S1 + S2)) | %BRo | %VRo-eq |
|---|---|---|---|---|---|---|---|---|
| A | 8325 | 16.27 | 8.27 | 90.69 | 557.41 | 0.08 | 0.44 | 0.67 |
| B | 9886 | 15.76 | 9.27 | 83.7 | 531.09 | 0.1 | 0.49 | 0.70 |
| C | 10,555 | 12.69 | 0.31 | 33.01 | 260.126 | 0.009 | 0.72 | 0.88 |
| D | 11,199 | 16.36 | 0.71 | 28.05 | 171.454 | 0.024 | 0.94 | 0.98 |
| Well A | Shale Sample | Kerogen Sample | Well B | Shale Sample | Kerogen Sample | ||||
| D Band | G Band | D Band | G Band | D Band | G Band | D Band | G Band | ||
| 1368.8 | 1591 | 1356.1 | 1587.7 | 1364.3 | 1587.7 | 1368.2 | 1587.8 | ||
| 1357.2 | 1589 | 1364.3 | 1581.4 | 1361 | 1590.8 | 1361 | 1590.5 | ||
| 1366.9 | 1593.3 | 1356.8 | 1587.7 | 1364.3 | 1590.8 | 1364.3 | 1590.8 | ||
| 1362.7 | 1582.5 | 1359.6 | 1583.4 | 1367.5 | 1584.5 | 1366.5 | 1587.5 | ||
| 1367.9 | 1593.5 | 1361.9 | 1587.7 | 1370.2 | 1587.7 | 1366.3 | 1587.7 | ||
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | ||
| 1364.7 | 1589.9 | 1359.7 | 1585.6 | 1365.4 | 1588.3 | 1365.2 | 1588.9 | ||
| St. Dev. | St. Dev. | St. Dev. | St. Dev. | St. Dev. | St. Dev. | St. Dev. | St. Dev. | ||
| 4.3 | 4.04 | 3.06 | 2.67 | 3.13 | 2.35 | 2.47 | 1.47 | ||
| Well C | Shale Sample | Kerogen Sample | Well D | Shale Sample | Kerogen Sample | ||||
| D band | G band | D band | G band | D band | G band | D band | G band | ||
| 1348.3 | 1587.7 | 1354.2 | 1591.3 | 1356.7 | 1591.3 | 1350 | 1590.1 | ||
| 1357.8 | 1590.8 | 1358.2 | 1591.3 | 1355.6 | 1591.3 | 1356.3 | 1590.8 | ||
| 1361 | 1590.8 | 1358.2 | 1594.4 | 1351.3 | 1591.3 | 1352 | 1592.4 | ||
| 1357.8 | 1594 | 1358.2 | 1594.4 | 1350.9 | 1594.4 | 1350 | 1589.3 | ||
| 1354.5 | 1597.1 | 1358.2 | 1591.3 | 1350.6 | 1597.6 | 1355 | 1587.7 | ||
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | ||
| 1355.9 | 1592.1 | 1357.4 | 1592.5 | 1353 | 1593.2 | 1352.6 | 1590 | ||
| St. Dev. | St. Dev. | St. Dev. | St. Dev. | St. Dev. | St. Dev. | St. Dev. | St. Dev. | ||
| 4.3 | 3.2 | 1.6 | 1.5 | 2.6 | 2.5 | 2.5 | 1.6 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatibi, S.; Ostadhassan, M.; Tuschel, D.; Gentzis, T.; Carvajal-Ortiz, H. Evaluating Molecular Evolution of Kerogen by Raman Spectroscopy: Correlation with Optical Microscopy and Rock-Eval Pyrolysis. Energies 2018, 11, 1406. https://doi.org/10.3390/en11061406
Khatibi S, Ostadhassan M, Tuschel D, Gentzis T, Carvajal-Ortiz H. Evaluating Molecular Evolution of Kerogen by Raman Spectroscopy: Correlation with Optical Microscopy and Rock-Eval Pyrolysis. Energies. 2018; 11(6):1406. https://doi.org/10.3390/en11061406
Chicago/Turabian StyleKhatibi, Seyedalireza, Mehdi Ostadhassan, David Tuschel, Thomas Gentzis, and Humberto Carvajal-Ortiz. 2018. "Evaluating Molecular Evolution of Kerogen by Raman Spectroscopy: Correlation with Optical Microscopy and Rock-Eval Pyrolysis" Energies 11, no. 6: 1406. https://doi.org/10.3390/en11061406
APA StyleKhatibi, S., Ostadhassan, M., Tuschel, D., Gentzis, T., & Carvajal-Ortiz, H. (2018). Evaluating Molecular Evolution of Kerogen by Raman Spectroscopy: Correlation with Optical Microscopy and Rock-Eval Pyrolysis. Energies, 11(6), 1406. https://doi.org/10.3390/en11061406









