Methodical Aspects of Biogas Production in Small-Volume Bioreactors in Laboratory Investigations
Abstract
1. Introduction
2. Material and Methods
2.1. The First Series of Measurements
2.2. The Second Series of Measurements
2.3. The Third Series of Measurements
3. Results and Discussion
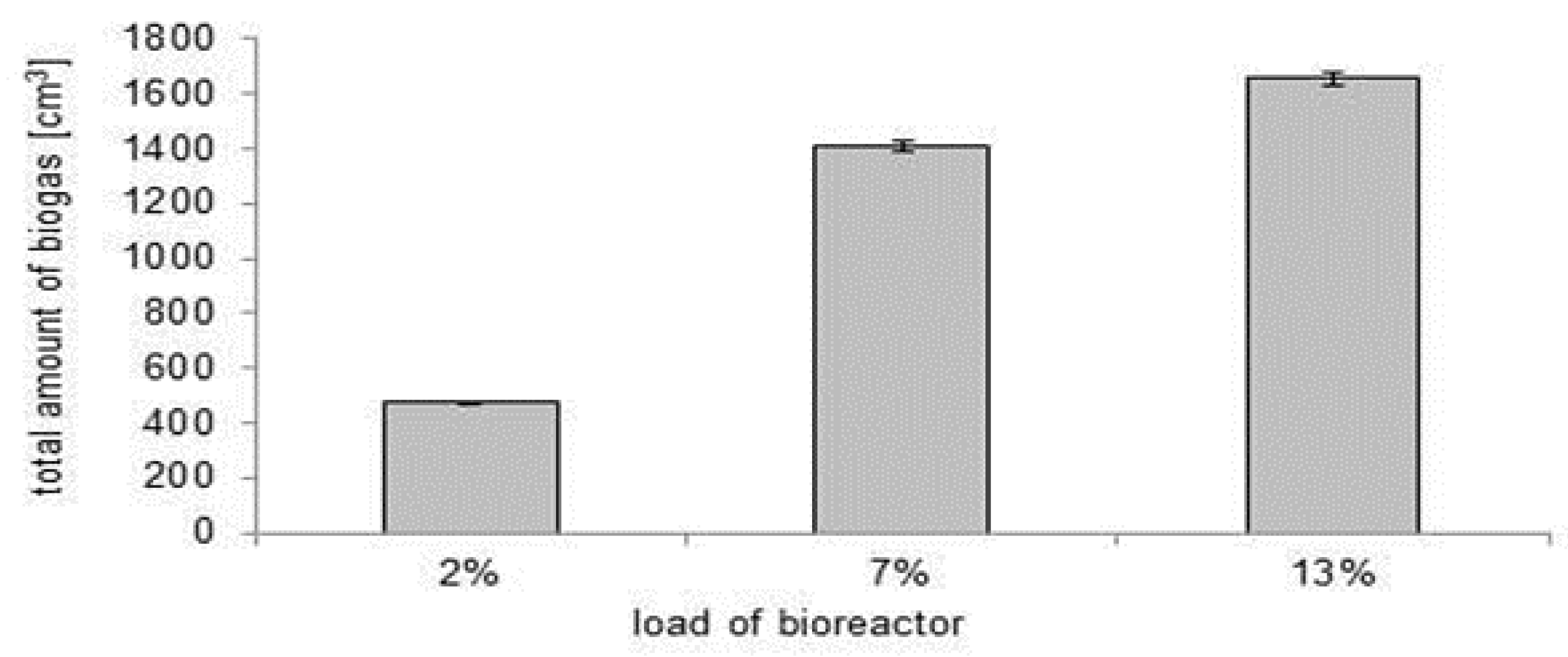
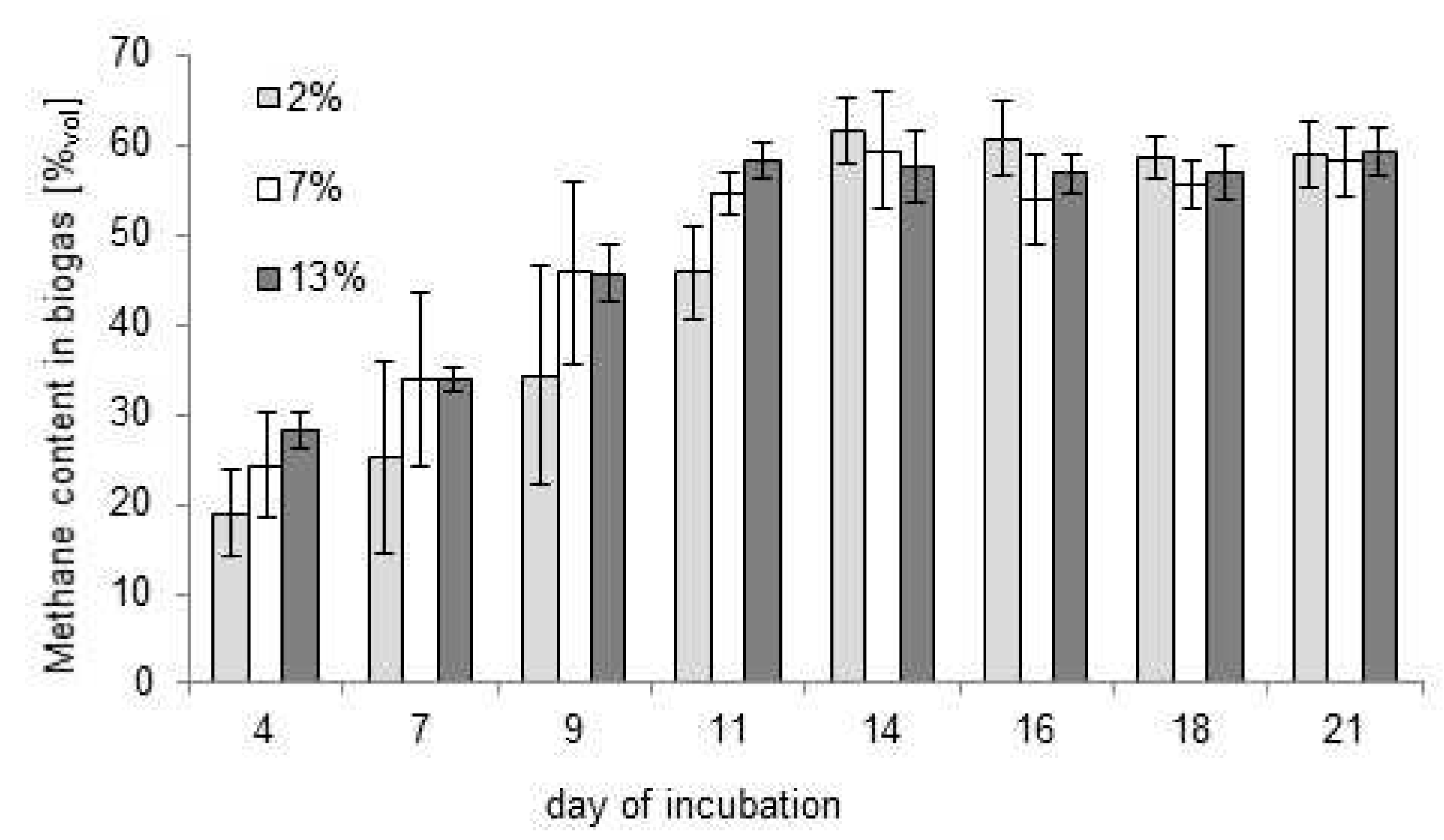
3.1. Choice of the Optimal Bioreactor Loading
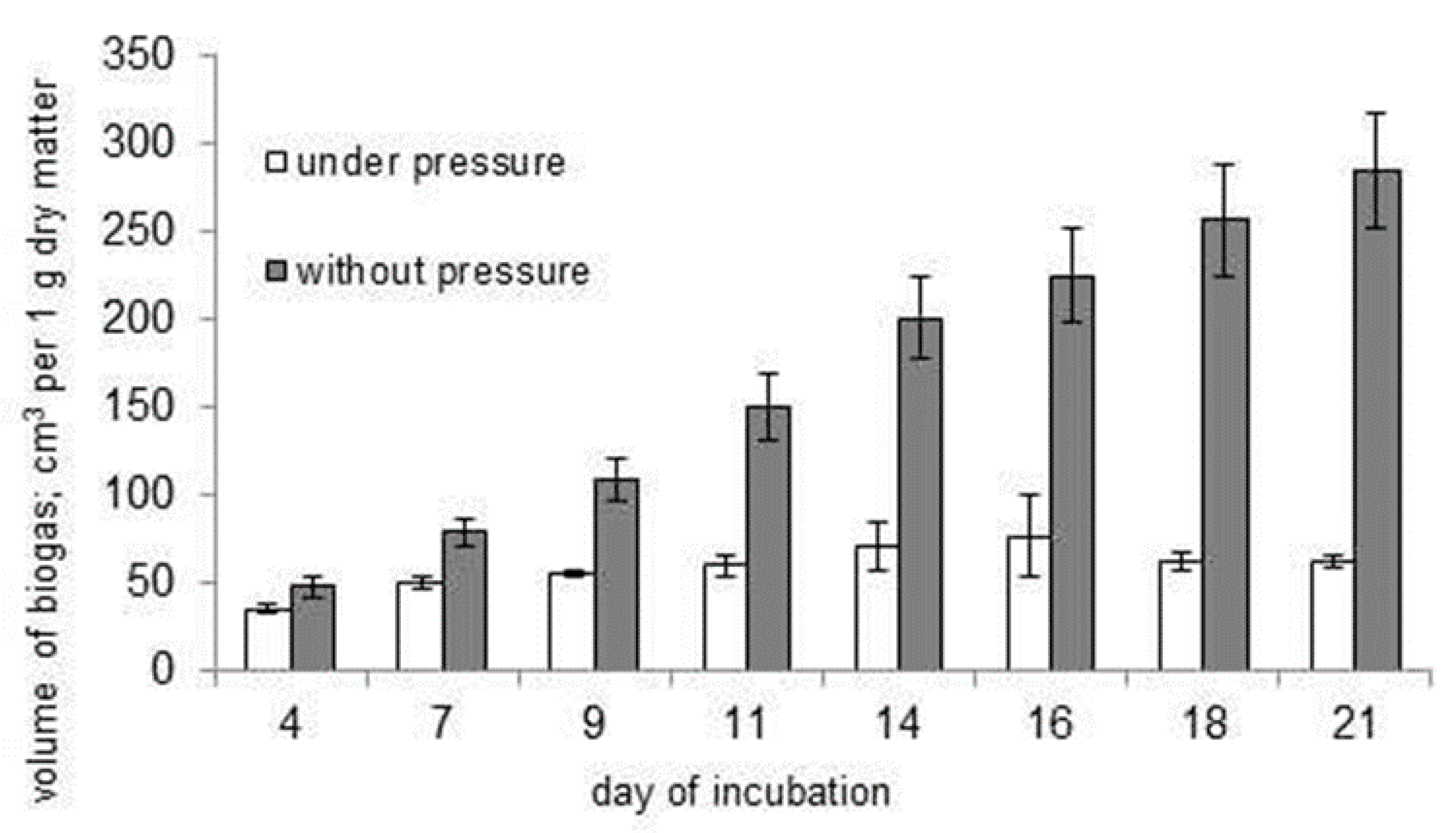
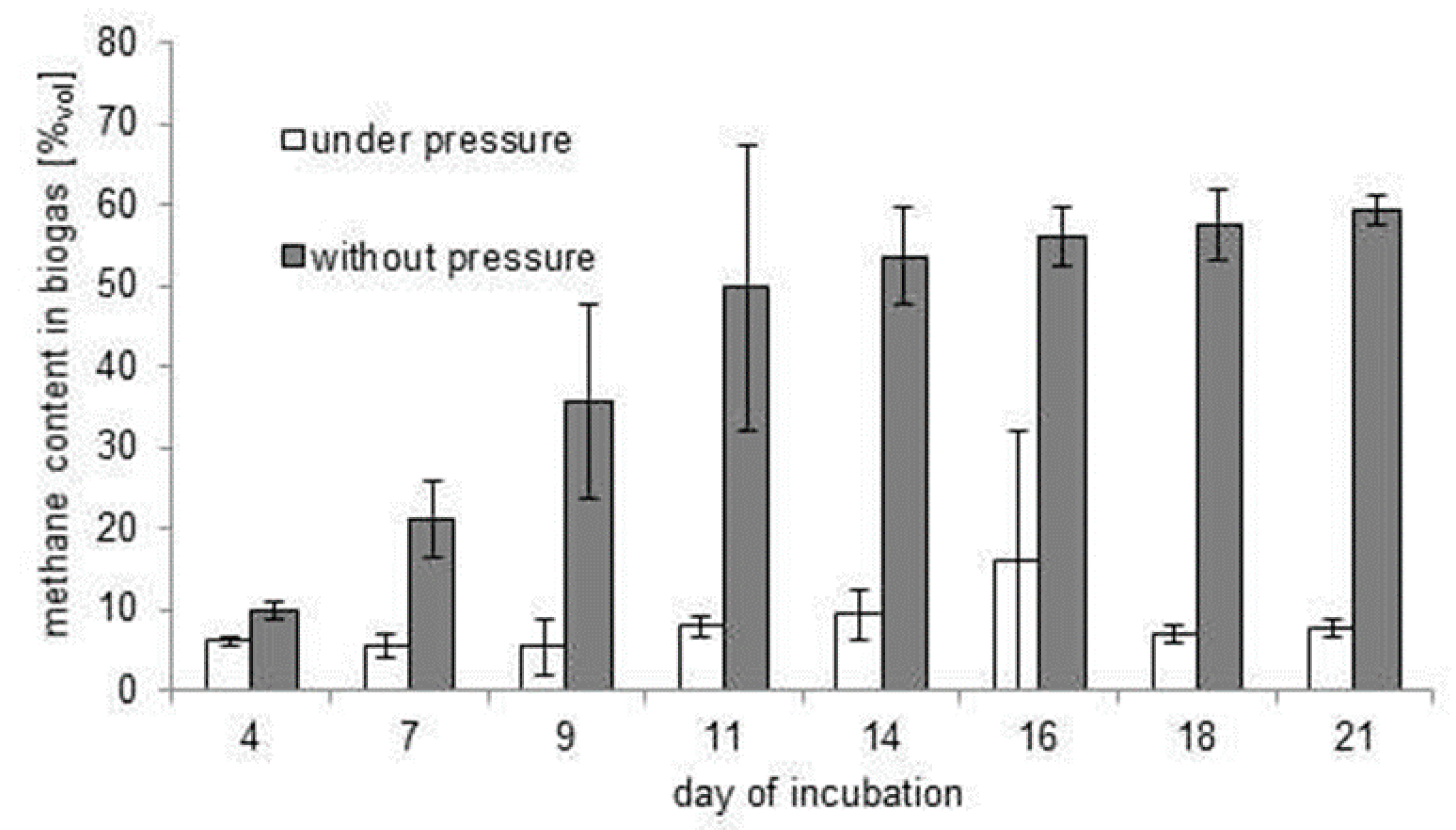
3.2. Inhibition of the Biofermentation Process by Excessive Pressure of Biogas
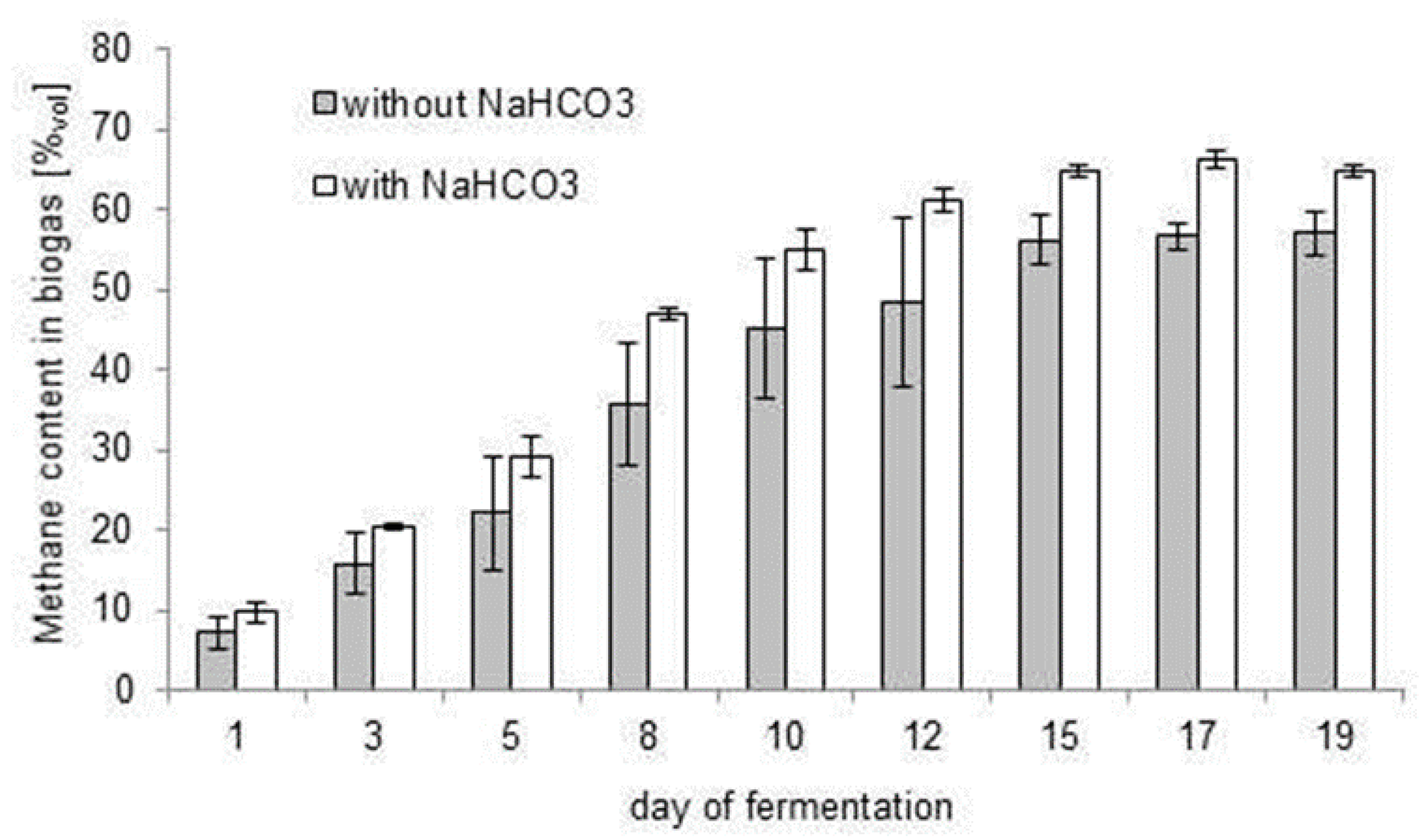
3.3. Stabilisation of the Biogas Production Process with Bicarbonate
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Adebayo, A.; Jekayinfa, S.; Linke, B. Effect of Co-Digestion on Anaerobic Digestion of Cattle Slurry with Maize Cob at Mesophilic Temperature. J. Energy Technol. Policy 2013, 3, 47–55. [Google Scholar]
- Gupta, P.; Gupta, A. Biogas production from coal via anaerobic fermentation. Fuel 2014. [Google Scholar] [CrossRef]
- Krzemińska, I.; Pawlik-Skowrońska, B.; Trzcińska, M.; Tys, J. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioproc. Biosyst. Eng. 2013. [Google Scholar] [CrossRef]
- Amon, Y.; Amon, B.; Kryvoruchko, V.; Machtmüller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Pötsch, E.; Wagentristl, H.; et al. Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotation. Bioresour. Technol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Jezierska-Tys, S.; Tys, J. Microalgae for biofuels production and environmental applications: A review. Afr. J. Biotechnol. 2010. [Google Scholar] [CrossRef]
- Oleszek, M.; Matyka, M.; Lalak, J.; Tys, J.; Paprota, E. Characterization of Sida hermaphrodita as a feedstock for anaerobic digestion process. J. Food Agric. Environ. 2013, 11, 1839–1841. [Google Scholar]
- Chen, Y.; Cheng, J.; Creamer, K. Inhibition of anaerobic digestion precess: A review. Bioresour. Technol. 2008. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, M.; Siepak, J. Enhancement of Methanogenesis at a Municipal Landfill Site by Addition of Sewage Sludge. Environ. Eng. Sci. 2006. [Google Scholar] [CrossRef]
- Amigun, B.; Sigamoney, R.; Von Blottnitz, H. Commercialisation of biofuel industry in Africa: A review. Renew. Sustain. Energy Rev. 2008. [Google Scholar] [CrossRef]
- Lybaek, R.; Christansen, T.; Kjaer, T. Governing innovation for sustainable development in the Danish Biogas Sector—A historical overview and analysis of innovation. Sustain. Dev. 2013. [Google Scholar] [CrossRef]
- Gaida, D.; Luis, S.B.A.; Brito, A.; Wolf, C.; Back, T.; Bongards, M.; McLoone, S.F. Optimal control of biogas plant using nonlinear model predictive control. In Proceedings of the ISSC 2011, Dublin, Ireland, 23–24 June 2011. [Google Scholar]
- Markard, J.; Stadelmann, M.; Truffer, B. Prospective analysis of technological innovation systems: Identifying technological and organizational development options for biogas in Switzerland. Res. Policy 2009. [Google Scholar] [CrossRef]
- Esposito, G.; Frunzo, L.; Liotta, F.; Panico, A.; Pirozzi, F. Bio-Methane potential test to measure the biogas production from the digestion and co-digestion of complex organic substrates. Open Environ. Eng. J. 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Opwis, K.; Mayer-Gall, T.; Gutmann, J.S.; Dammer, C.; Titscher, T.; Nickisch-Hartfiel, A.; Grün, O.; Spurk, C.; Schloderer, C.; Köppe, A.; et al. Semi-industrial production of methane from textile wastewater. Energy Sustain. Soc. 2012. [Google Scholar] [CrossRef]
- Kaparaju, P.; Ellegaard, L.; Angelidaki, I. Optimisation of biogas production from manure through serial digestion: Lab-scale and pilot-scale studies. Bioresour. Technol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Omil, F.; Garrido, J.; Arrojo, B.; Mendez, R. Anaerobic filter reactor performance for the treatment of complex dairy wastewater at industrial scale. Water Res. 2003. [Google Scholar] [CrossRef]
- Singh, K.J.; Sooch, S.S. Comparative study of economics of different models of family size biogas plants for state of Punjab, India. Energy Convers. Manag. 2004. [Google Scholar] [CrossRef]
- Wu, S.; Ni, P.; Li, J.; Sun, H.; Wang, Y.; Luo, H.; Dach, J.; Dong, R. Integrated approach to sustain biogas production in anaerobic digestion of chicken manure under recycled utilization of liquid digestate: Dynamics of ammonium accumulation and mitigation control. Bioresour. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Montusiewicz, A.; Lebiocka, M. Co-digestion of intermediate landfill leachate and sewage sludge as a method of leachate utilization. Bioresour. Technol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.V.; Dhar, H.; Kumar, S.; Thalla, A.K.; Mukherjee, S.; Wong, J.W.C. Artificial neural network based modeling to evaluate methane yield from biogas in a laboratory-scale anaerobic bioreactor. Bioresour. Technol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ghanimeh, S.; El Fadel, M.; Saikaly, P. Mixing effect on thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Bioresour. Technol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Zinatizadeh, A.A.; Van Loosdrecht, M. A novel continuous feed and intermittent discharge airlift bioreactor (CFIDAB) for enhanced simultaneous removal of carbon and nutrients from soft drink industrial wastewater. Chem. Eng. J. 2016. [Google Scholar] [CrossRef]
- Westerholm, M.; Müller, B.; Isaksson, S.; Schnürer, A. Trace element and temperature effects on microbial communities and links to biogas digester performance at high ammonia levels. Biotechnol. Biofuel 2015. [Google Scholar] [CrossRef] [PubMed]
- Karim, K.; Hoffmann, R.; Klasson, K.T.; Al-Dahhan, M.H. Anaerobic digestion of animal waste: Effect of mode of mixing. Water Res. 2005. [Google Scholar] [CrossRef] [PubMed]
- Getahun, T.; Gebrehiwot, M.; Ambelu, A.; Van Gerven, T.; Van der Bruggen, B. The potential of biogas production from municipal solid waste in a tropical climate. Environ. Monit. Assess. 2014. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, J.; Eriksson, P.; Thorin, E. The effects of different mixing intensities during anaerobic digestion of the organic fraction of municipal solid waste. Waste Manag. 2014. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ahn, Y.H.; Speece, R.E. Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res. 2002. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Urgun-Demitras, M.; Schoene, R.P.; Snyder, S.W. Producing pipeline-quality biomethane via anaerobic digestion of sludge amended with corn stover biochar with in-situ CO2 removal. Appl. Energ. 2015. [Google Scholar] [CrossRef]
- Durán-García, M.; Ramírez, Y.; Rojas-Solórzano, L. Biogas home-production assessment using a selective sample of organic vegetable waste. A preliminary study. Interciencia 2012, 37, 128–132. [Google Scholar]
- Rojas, C.; Fang, S.; Uhlenhut, F.; Borchert, A.; Stein, I.; Schlaak, M. Stirring and biomass starter influences the anaerobic digestion of different substrates for biogas production. Eng. Life Sci. 2010. [Google Scholar] [CrossRef]
- Lo, H.M.; Chiu, H.Y.; Lo, S.W.; Lo, F.C. Effects of micro-nano and non micro-nano MSWI ashes addition on MSW anaerobic digestion. Bioresour. Technol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zhang, Z.; Luo, Y.; Qiao, W.; Xiao, M.; Zhang, M. Biogas productivity by co-digesting Taihu blue algae with corn straw as an external carbon source. Bioresour. Technol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, H.V.; Koskinen, P.E.P.; Rintala, J. Mesophilic and thermophilic anaerobic laboratory-scale digestion of Nannochloropsis microalga residues. Bioresour. Technol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Cumberbatch, J.; Wang, M.; Zhang, Q. Kinetic parameter estimation model for anaerobic co-digestion of waste activated sludge and microalgae. Bioresour. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kusch, S.; Oechsner, H.; Jungbluth, T. Biogas production with horse dung in solid-phase digestion systems. Bioresour. Technol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Kucera, L.; Kurka, O.; Bartak, P.; Bednar, P. Liquid chromatography/high resolution tandem mass spectrometry—Tool for the study of polyphenol profile changes during micro-scale biogas digestion of grape marcs. Chemosphere 2017. [Google Scholar] [CrossRef]
- Szafranek-Nakonieczna, A.; Stępniewska, Z. The influence of the aeration status (ODR, Eh) of peat soils on their ability to produce methane. Wetlands Ecol. Manag. 2015. [Google Scholar] [CrossRef]
- Kougias, G.P.; Angelidaki, I. Biogas and its opportunities—A review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Schmidt, T.; McCabe, B.; Harris, P. Process Monitoring and Control for an Anaerobic Covered Lagoon Treating Abattoir Wastewater. Chem. Eng. Technol. 2018, 41, 755–760. [Google Scholar] [CrossRef]
- Kozłowski, K.; Dach, J.; Lewicki, A.; Cieślik, M.; Czekała, W.; Janczak, D.; Brzoski, M. Laboratory Simulation of an Agricultural Biogas Plant Start-up. Chem. Eng. Technol. 2018, 41, 711–716. [Google Scholar] [CrossRef]
- Gallegos, D.; Wedwitschka, H.; Moeller, L.; Zehnsdorf, A.; Stinner, W. Effect of particle size reduction and ensiling fermentation on biogas formation and silage quality of wheat straw. Bioresour. Technol. 2017, 245, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Abdi, R.; Adl, M.; Kafashan, J. Optimization of biogas productivity in lab-scale by response surface methodology. Renew. Energy 2018, 118, 368–375. [Google Scholar] [CrossRef]
- Hilkiah, I.A.; Ayotamuno, M.J.; Eze, C.L.; Ogaji, S.O.T.; Probert, S.D. Designs of anaerobic digesters for producing biogas from municipal solid-waste. Appl. Energy 2008. [Google Scholar] [CrossRef]
- Betts, J.I.; Baganz, F. Miniature bioreactors: Current practices and future opportunities. Microb. Cell Fact. 2006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oleszek, M.; Tys, J. Lab scale measurement of biogas yield. Chem. Ind. 2013, 92, 126–130. [Google Scholar]
- Lourenço, N.D.; Lopes, J.A.; Almeida, C.F.; Sarraguça, M.C.; Pinheiro, H.M. Bioreactor monitoring with spectroscopy and chemometrics: A review. Anal. Bioanal. Chem. 2012. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Ziemiński, K. Methane fermentation process for utilization of organic waste. Int. Agrophys. 2012. [Google Scholar] [CrossRef]
- Lalak, J.; Kasprzycka, A.; Martyniak, D.; Tys, J. Effect of biological pretreatment of Agropyron elongatum ‘BAMAR’ on biogas production by anaerobic digestion. Bioresour. Technol. 2016, 200, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Sanders, W. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biol. Technol. 2014, 3, 117–129. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, P.; Zhang, G.; Fan, J.; Zhang, Y. Enhancement of anaerobic sludge digestion by high-pressure homogenization. Bioresour. Technol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, S.; Nistor, M.; Liu, J. Towards eliminating systematic errors caused by the experimental conditions in Biochemical Methane Potential (BMP) tests. Waste Manag. 2014, 34, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Piątek, M.; Lisowski, A.; Kasprzycka, A.; Lisowska, B. The dynamics of an anaerobic digestion of crop substrates with an unfavourable carbon to nitrogen ratio. Bioresour. Technol. 2016, 216, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Mittweg, G.; Oechsner, H.; Hahn, V.; Lemmer, A.; Reinhardt-Hanisch, A. Repeatability of a laboratory batch method to determine the specific biogas and methane yields. Eng Life Sci. 2016. [Google Scholar] [CrossRef]
| Group of Laboratory Bioreactors | Volume of Used Bioreactor | Publication |
|---|---|---|
| 20 dm3 semi-continuously stirred tank reactors | [19] | |
| big volume | 40 dm3 bioreactor | [20] |
| 20 dm3 bioreactor | [21] | |
| 14 dm3 digesters | [22] | |
| 5,3 dm3 bioreactor | [23] | |
| 5 dm3 bioreactor | [24] | |
| 5 dm3 double glass cylinder | [16] | |
| 3,75 dm3 clear PVC | [25] | |
| medium volume | 2.5 dm3 batch digester | [26] |
| 2.3 dm3 and 1.3 dm3 glass bottles | [8] | |
| 1 dm3 serum flasks | [27] | |
| 1.1 dm3 glass bottles | [28] | |
| 600 cm3 digesters | [29] | |
| 600 cm3 PET bottles | [30] | |
| 500 cm3 digesters | [31] | |
| 500 cm3 plastic bottle | [32] | |
| small volume | 150 cm3 serum bottles | [33] |
| 119 cm3 glass bottles | [34] | |
| 100 cm3 serum bottles | [35] | |
| 100 cm3 glass syringe | [36] | |
| 60 cm3 vials | [37] | |
| 60 cm3 dark vials | [38] |
| Replication | Day of Incubation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 | 5 | 8 | 10 | 12 | 15 | 17 | 19 | 21 | |
| Average | 6.15 | 5.77 | 5.60 | 5.70 | 5.86 | 5.86 | 5.61 | 5.49 | 5.82 |
| Standard deviation | 0.02 | 0.09 | 0.17 | 0.08 | 0.23 | 0.62 | 0.03 | 0.14 | 0.07 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzycka, A.; Kuna, J. Methodical Aspects of Biogas Production in Small-Volume Bioreactors in Laboratory Investigations. Energies 2018, 11, 1378. https://doi.org/10.3390/en11061378
Kasprzycka A, Kuna J. Methodical Aspects of Biogas Production in Small-Volume Bioreactors in Laboratory Investigations. Energies. 2018; 11(6):1378. https://doi.org/10.3390/en11061378
Chicago/Turabian StyleKasprzycka, Agnieszka, and Jan Kuna. 2018. "Methodical Aspects of Biogas Production in Small-Volume Bioreactors in Laboratory Investigations" Energies 11, no. 6: 1378. https://doi.org/10.3390/en11061378
APA StyleKasprzycka, A., & Kuna, J. (2018). Methodical Aspects of Biogas Production in Small-Volume Bioreactors in Laboratory Investigations. Energies, 11(6), 1378. https://doi.org/10.3390/en11061378




