Pretreatment of Corn Stover Using Organosolv with Hydrogen Peroxide for Effective Enzymatic Saccharification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
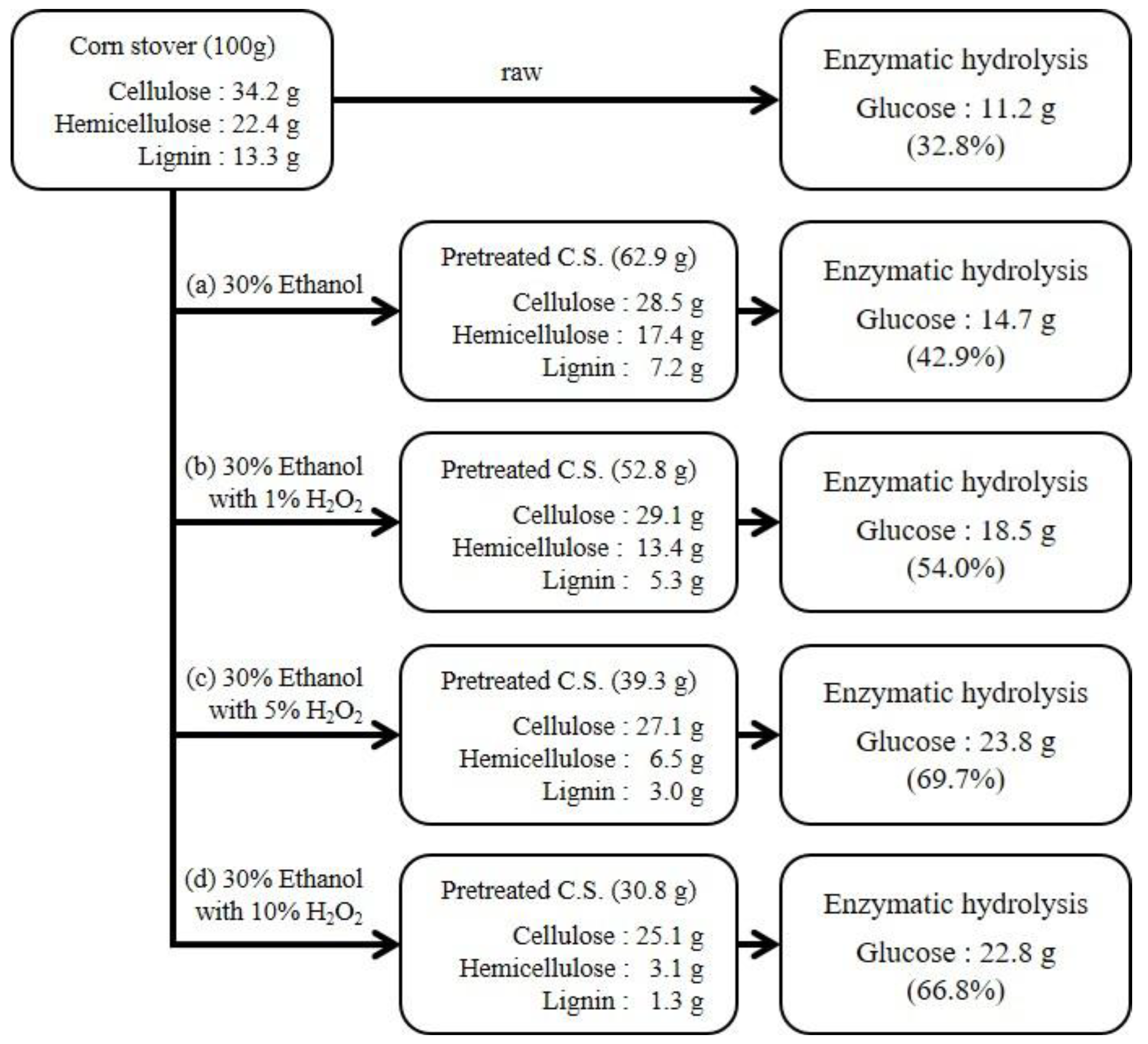
2.2. Flow-Through Pretreatment
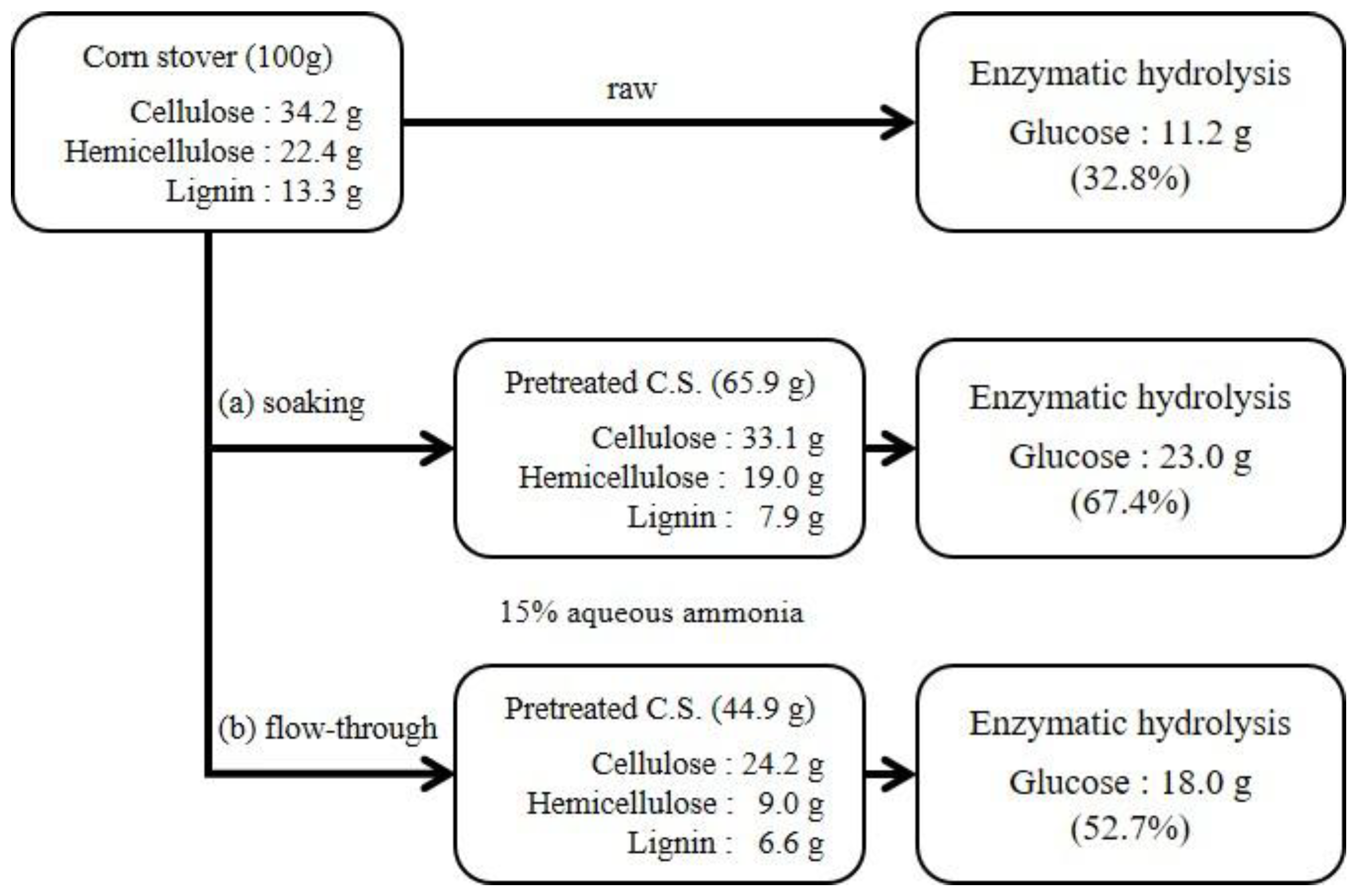
2.3. Ammonia Soaking Pretreatment
2.4. Enzymatic Digestibility Test
2.5. Analytical Methods
3. Results and Discussion
3.1. Pretreatment of Corn Stover
3.2. Enzymatic Digestibility of Pretreated Corn Stover
3.3. Mass Balance of Organosolv Pretreatment
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nitsos, C.; Rova, U.; Christakopoulos, P. Organosolv fractionation of softwood biomass for biofuel and biorefinery applications. Energies 2018, 11, 50. [Google Scholar] [CrossRef]
- Lynd, L.R.; Elander, R.T.; Wyman, C.E. Likely features and costs of mature biomass ethanol technology. Appl. Biochem. Biotechnol. 1996, 57/58, 741–761. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.M. Alkaline peroxide delignification of agricultural residues to enhance enzymatic saccharification. Biotechnol. Bioeng. 1984, 26, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Biorefin. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Martinez-Abad, A.; Giummarella, N.; Lawoko, M.; Vilaplana, F. Differences in extractability under subcritical water reveal interconnected hemicellulose and lignin recalcitrance in birch hardwoods. Green Chem. 2018. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.Q.; Wang, Z.Y.; Fan, Z.L.; Zuo, L.L. Comparison of ultrasonic and CO2 laser pretreatment methods on enzyme digestibility of corn stover. Int. J. Mol. Sci. 2012, 13, 4141–4152. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.H.; Aziz, S. Organosolv pulping: A versatile approach to wood refining. TAPPI 1985, 68, 94–97. [Google Scholar]
- Johansson, A.; Aaltonen, O.; Ylinen, P. Organosolv pulping method and pulp properties. Biomass 1987, 13, 45–65. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, T.H.; Kim, J.S. Effect of organosolv pretreatment on mechanically pretreated biomass by use of concentrated ethanol as the solvent. Biotechnol. Bioprocess Eng. 2017, 22, 431–439. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, T.H.; Kim, J.S. Flow-through pretreatment of corn stover by recycling organosolv to reduce waste solvent. Energies 2018, 11, 879. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Pettersson, I. Chemical aspects of hydrogen peroxide bleaching. Part II the bleaching of Kraft pulps. J. Wood Chem. Technol. 1982, 2, 231–250. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Park, S.C. Pretreatment of wastepaper and pulp mill sludge by aqueous ammonia and hydrogen peroxide. Appl. Biochem. Biotechnol. 2000, 84, 129–139. [Google Scholar] [CrossRef]
- Park, Y.C.; Yoon, J.J.; Kim, S.H.; Kim, T.H.; Kim, J.S. Two-stage flow-through pretreatment of helianthus tuberosus residue for enzymatic production of fermentable sugar by alkaline and acidic solutions. Bioresources 2017, 12, 6504–6517. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, J.S.; Sunwoo, C.; Lee, Y.Y. Pretreatment of corn stover by aqueous ammonia. Bioresour. Technol. 2003, 90, 39–47. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S. Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 2012, 47, 31–35. [Google Scholar] [CrossRef]
- Wu, L.; Arakane, M.; Ike, M.; Wada, M.; Takai, T.; Gau, M. Low temperature alkali pretreatment for improving enzymatic digestibility of sweet sorghum bagasse for ethanol production. Bioresour. Technol. 2011, 102, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- National Renewable Energy Laboratory (NREL). Low Solids Enzymatic Saccharification of Lignocellulosic Biomass. Laboratory Analytical Procedure (LAP). NREL/TP-5100-63351. 2015. Available online: https:// www.nrel.gov/docs/fy15osti/63351.pdf (accessed on 7 May 2018).
- National Renewable Energy Laboratory (NREL). Determination of Structural Carbohydrates and Lignin in Biomass. Laboratory Analytical Procedure (LAP). NREL/TP-510-42618. 2012. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 7 May 2018).
- Matsakas, L.; Nitsos, C.; Vörös, D.; Rova, U.; Christakopoulos, P. High-titer methane from organosolv-pretreated spruce and birch. Energies 2017, 10, 263. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, T.H.; Oh, K.K. Deacetylation Followed by Fractionation of Yellow Poplar Sawdust for the Production of Toxicity-Reduced Hemicellulosic Sugar for Ethanol Fermentation. Energies 2018, 11, 404. [Google Scholar] [CrossRef]
| Pretreatment Conditions | Remaining Solids (wt.%) | |||
|---|---|---|---|---|
| Solutions | Process Type | Temperature | Reaction Time | |
| 30 wt.% ethanol only | Flow-through | 150 °C | 60 min | 62.9 |
| 30 wt.% ethanol with 1 wt.% H2O2 | 52.8 | |||
| 30 wt.% ethanol with 5 wt.% H2O2 | 39.3 | |||
| 30 wt.% ethanol with 10 wt.% H2O2 | 30.8 | |||
| 15 wt.% aqueous ammonia | Soaking | 60 °C | 48 h | 65.9 |
| Flow-through | 170 °C | 60 min | 44.9 | |
| Pretreatment Conditions | Glucan | XMG | Lignin | |||||
|---|---|---|---|---|---|---|---|---|
| Process Type | Temp./Time | Solutions | Contents (wt.%) | Recovery Yield (wt.%) | Contents (wt.%) | Recovery Yield (wt.%) | Contents (wt.%) | Removal Rate (wt.%) |
| Untreated corn stover | 34.2 | - | 22.4 | - | 13.3 | - | ||
| Flow-through | 150 °C/60 min | 30 wt.% EtOH | 28.5 | 83.3 | 17.4 | 77.5 | 7.2 | 45.6 |
| 30 wt.% EtOH with 1 wt.% H2O2 | 28.1 | 82.1 | 13.4 | 59.6 | 5.3 | 60.5 | ||
| 30 wt.% EtOH with 5 wt.% H2O2 | 27.1 | 79.2 | 6.5 | 29.0 | 3.0 | 77.7 | ||
| 30 wt.% EtOH with 10 wt.% H2O2 | 25.1 | 73.5 | 3.1 | 13.6 | 1.3 | 90.3 | ||
| Pretreatment Conditions | Glucan | XMG | Lignin | |||||
|---|---|---|---|---|---|---|---|---|
| Process Type | Temperature | Reaction Time | Contents (wt.%) | Recovery (wt.%) | Contents (wt.%) | Recovery (wt.%) | Contents (wt.%) | Removal (wt.%) |
| Untreated corn stover | 34.2 | - | 22.4 | - | 13.3 | - | ||
| Soaking | 60 °C | 48 h | 33.1 | 96.9 | 19.0 | 84.9 | 7.9 | 40.4 |
| Flow-through | 170 °C | 60 min | 24.2 | 70.8 | 9.0 | 40.0 | 6.6 | 50.1 |
| Pretreatment Conditions | Enzymatic Digestibility (%) | ||||
|---|---|---|---|---|---|
| Solutions | Process Type | Temperature | Reaction Time | Glucan | XMG/Xylan |
| Untreated corn stover | 32.8 | 14.8 | |||
| 30 wt.% EtOH | Flow-through | 150 °C | 60 min | 51.5 | 28.8 |
| 30 wt.% EtOH with 1 wt.% H2O2 | 63.5 | 39.9 | |||
| 30 wt.% EtOH with 5 wt.% H2O2 | 87.9 | 59.6 | |||
| 30 wt.% EtOH with 10 wt.% H2O2 | 91.0 | 67.8 | |||
| 15 wt.% aqueous ammonia | Soaking | 60 °C | 48 h | 69.6 | 56.5 |
| Flow-through | 170 °C | 60 min | 74.5 | 77.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.C.; Kim, J.S.; Kim, T.H. Pretreatment of Corn Stover Using Organosolv with Hydrogen Peroxide for Effective Enzymatic Saccharification. Energies 2018, 11, 1301. https://doi.org/10.3390/en11051301
Park YC, Kim JS, Kim TH. Pretreatment of Corn Stover Using Organosolv with Hydrogen Peroxide for Effective Enzymatic Saccharification. Energies. 2018; 11(5):1301. https://doi.org/10.3390/en11051301
Chicago/Turabian StylePark, Yong Cheol, Jun Seok Kim, and Tae Hyun Kim. 2018. "Pretreatment of Corn Stover Using Organosolv with Hydrogen Peroxide for Effective Enzymatic Saccharification" Energies 11, no. 5: 1301. https://doi.org/10.3390/en11051301
APA StylePark, Y. C., Kim, J. S., & Kim, T. H. (2018). Pretreatment of Corn Stover Using Organosolv with Hydrogen Peroxide for Effective Enzymatic Saccharification. Energies, 11(5), 1301. https://doi.org/10.3390/en11051301






