1. Introduction
The progress of electrification in automotive applications relies heavily on the advance of battery technologies. Lithium-ion batteries provide longer life, better safety and higher energy/power densities than other rechargeable batteries [
1]. Both symmetrical and asymmetrical charging and discharging at higher rates are required in automotive applications. The elevated temperatures during the charging and discharging have detrimental effects on battery life and efficiency and may even lead to unwanted fire hazards [
2]. Hence, study of the thermal behavior of batteries is crucial to designing efficient thermal management systems and improve the performance and safety of lithium-ion batteries.
Heat generation in a battery is a complex process [
3]. In order to investigate the thermal behavior or heat generation of the battery, understanding fundamental battery properties, such as its specific heat and entropy change, is very important. Thus, some studies have experimentally measured such properties of certain types of lithium-ion batteries [
4,
5,
6,
7,
8]. The specific heat capacity plays an important role in determining the temperature increase in a cell with a given amount of heat generation [
3,
4,
5,
6,
7,
9]. Similarly, entropy change rate with temperature, which depends on the battery chemistry and temperature, is crucial to determining the reversible heat generation rate.
Lin et al. [
4] characterized the heat generation of a prismatic cell using an accelerated rate calorimeter. The heat generated by the cell was calculated using the measured specific heat capacity and temperature increase data. Specific heat capacity was measured using an assembly where the heater with a constant heat flux was sandwiched between two cells placed in an adiabatic chamber. The specific heat of the cell was determined to be 1067 J kg
−1 K
−1. The cell changed from an endothermic state, initially, to an exothermic state when it was charged and discharged at the current rate of 0.33 C due to the effect of the reversible heat. The trend of heat generation at 1 C and 2 C current rates during discharging also showed endothermic followed by exothermic thermal behavior. Charging at 1 C and 2 C current rates, however, did not exhibit the endothermic behavior. They concluded that, even for well-designed large batteries, Joule heating in the current collectors is higher than reversible heat. It was seen that the trends of heat generation rate during both charging and discharging were nonlinear and S-shaped. To determine the reversible heat rate, the entropy coefficient was determined by measuring the open circuit voltage (OCV) values at different temperatures. The entropy coefficients changed from negative to positive at the state of charge (SoC) of 0.4.
Bandhauer et al. [
6] also calculated the reversible heat generation using the entropy coefficient. It was determined from the slope of OCV with temperature (with the temperature range of 15–55 °C) at a required SoC. The irreversible heat generation rate was evaluated using the cell overpotential. The heat generation rate increased with an increase in current rate. The entropy coefficients were found to be within a range of ±9.93 μV K
−1 to ±13.43 μV K
−1 for the two battery samples they tested. They concluded that the heat generation rate is a strong function of ambient temperature between 15 °C and 55 °C, especially at high current rates, because both transport properties and kinetics are functions of temperature in lithium-ion batteries. At low temperature, the irreversible heat increased with the decrease of temperature due to mass transport and kinetic limitations, whereas the reversible potential is not a strong function of temperature.
Chen et al. [
10] investigated the heat generation rate as well as the efficiency of 20-Ah LiFePO
4 lithium-ion pouch cell at different temperatures (−10 to 40 °C). The battery was sandwiched between two high-density polyethylene plates 4 times thicker than the battery. A 10-W heater was used to calibrate the calorimeter. They illustrated that the heat generation increased with the increase of the discharge current rate. At low discharge current rate (0.25 C), the heat was endothermic. The heat generation was found to decrease with the increase in temperature due to the increased rates of mass transport and reduced activation loss, which reduced overpotential during discharge. They also showed that the battery capacity increased with the increase of the temperature due to the higher ionic conductivity at a higher temperature. The preferred operating temperature of the prismatic lithium-ion battery was between 30 °C and 40 °C.
Chen et al. [
11] studied the effect of several passive cooling materials on lithium-ion battery performance. They compared the performance of air with the mixture of water and ethylene glycol (50–50 vol). The battery capacity decreased by 95% when the ambient temperature was lowered from 20 °C to −10 °C due to the decrease in ionic conductivity and slowdown of electrochemical reactions of the battery. The optimum operating temperature was found to be between 20 °C and 30 °C, where the capacity of the battery was near the maximum and the performance degradation was minimized. A comparison between the battery capacity using water-ethylene glycol and air at 20 °C showed that, although the water ethylene glycol mixture helped in maintaining lower battery surface temperature, higher capacity was obtained when the battery was cooled by air because of the higher temperature of the air-cooled battery.
Alipanah et al. [
12] numerically studied passive thermal management systems of lithium-ion batteries made from pure octadecane, pure gallium and octadecane-aluminum foam composite materials at different heat fluxes and metal foam porosities. They concluded that convective heat transfer was one of the most important factors affecting the battery surface temperature uniformity.
Many studies [
3,
4,
5,
6,
7,
8,
11,
13] reported in the literature are based on cylindrical or prismatic cells. Pouch cells are more compact and have higher packing efficiency compared to other types of cells (cylindrical cell, coin cell, and prismatic cell) and are widely used in vehicular operations. Hence, large pouch cells with high capacity have been an interest of study. The compact design and high specific energy of pouch cells brings more challenges to thermal management, since there is less empty space for the thermal management system. In addition, the non-homogeneous reactions and charge transfer within the cell may result in high temperature difference. Therefore, pouch cells with large size face more challenges with regard to maintaining a uniform temperature on the surface, especially at high current rates. In the case of high-capacity cells, many experimental studies have been limited to a maximum current density of 2 C, which might not be enough for high-power applications during the acceleration and fast charging of electric vehicles.
This study first estimates, by experiments, battery thermal properties such as the entropy coefficient and heat capacity, which are important parameters for predicting battery thermal behavior and lifetime under any given operating conditions. Second, the heat generation characteristics of cells during charging and discharging cycles have been tested. There are several approaches for estimating the heat generation rate of a battery. Estimations from different approaches could vary significantly because of the accuracy of experimental approaches, errors and uncertainties of measurements, and accuracies of instruments. Thus, this study quantifies the different heat generation rates predicted by different measurement approaches. The thermal properties and heat generation characteristics of the large LiFePO
4 pouch cells measured in this study facilitate the simulation, health monitoring [
14], and design of control and management systems [
15]. Third, the temperature gradient within the battery (in both in-plane and through-plane directions) could be very high, especially at high current rates. The measurement of temperature difference caused by non-homogeneous heat generation is necessary to design effective thermal management systems. Therefore, this study measures the temperature gradient in the through-plane direction and compares the effectiveness of five different passive cooling materials (air, aluminum foam, phase change material (PCM), water, and water with aluminum foam). The experimental evidence of high temperature difference (more than 30 °C) on the battery surface highlight the urgent need for effective thermal management to remove the temperature gradient within a cell and stacks of cells.
2. Experimental Apparatus and Procedure
Commercially available LiFePO4 pouch cells with 19.5 Ah capacity provided by Smith Electric Vehicles were measured in this study. The cells have dimension of (7.25 × 160 × 227) mm3, nominal voltage of 3.3 V, and energy density of 247 Wh/L.
2.1. Measurements of the Entropy Coefficient
The OCV is required to analyze the heat generation using the overpotential of the cell, which is the difference between the measured voltage and the estimated OCV. Therefore, this study will experimentally measure the entropy coefficient in order to estimate the OCV at a given temperature and SoC. The experimental setup consists of battery cycling equipment (Arbin Instrument MSTAT4, College Station, TX, USA) and a temperature-controlled water bath (Fisher Scientific’s Isotemp 4100H7, Hampton, NH, USA). The battery cycling equipment was programmed to charge and discharge a cell at constant current rates from 1 A to 5 A. The water bath was set up to maintain the constant water temperature ranging from 5 °C to 85 °C for the desired time interval. During entropy coefficient tests, the cell was kept inside the water bath with the active area completely immersed in water and only the electrode outside the water surface to avoid short circuit. The cell temperature was brought to the uniform temperature of the bath before testing by resting the cell in the water bath for 30 min with the given set up temperature. The cell was charged and discharged with a current rate of 5 A (0.25 C) to the SoC of 0.0, 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0. The OCV of the cell, while it was resting in the water bath at the setting temperature, was measured every 10 s using the Arbin battery tester. The cell was taken out of the water bath and was allowed to rest under room temperature for at least 5 h after each temperature cycle. The temperature of the water bath was then changed to a different temperature and the same procedure was repeated. All the tests were repeated at least three times. The entropy coefficient, which is the partial derivative of OCV respect to temperature, was calculated and averaged at various temperatures (20, 25, 30, 35, 40, 45 and 50 °C).
2.2. Measurement of the Specific Heat Capacity
The specific heat capacity is a key parameter for estimating the heat generation by the cell based on the temperature increase. This study measured the specific heat capacity of the LiFePO4 pouch cells using a customized setup. The experimental setup for specific heat capacity measurement of the cell consisted of a silicon heater (Omega SRFG-608/2) sandwiched between two cells enclosed in an acrylic container with a specific heat of 1.47 kJ/kg/K and density of 1150 kg/m3 (properties are reported by the manufacturer). The rectangular enclosure, which had an inside volume of 240 × 155 × 22 mm3, was made of acrylic sheets of thickness 12.5 mm (the Makrolon polycarbonate manufactured by Bayer). A groove with a width of 3 mm and a depth of 4 mm was created on the mid-width of the side and bottom walls in order to place the cell in the middle of the container, in order to minimize the heat loss through the container. The enclosure was covered by foam rubber insulation to reduce the heat loss to the surroundings during experiments. T-type thermocouples (Omega) were used to measure temperature inside the container and on the cell surface. Three thermocouples were attached on each side of the cell. One thermocouple was inserted through a small hole drilled on each side of the container to measure the temperature and estimate the heat loss through the container. One thermocouple was also used to record the temperature of the air inside the container to account for heat absorbed by the air.
Temperature data was recorded every minute using a Data Acquisition/Switch Unit (Keysight 34972A, Santa Rosa, CA, USA). A digital weighing scale (Ohaus NV4101, Parsippany, NJ, USA) was used to measure the weight of the cell and container. A DC power supply (Gwinstek GPR-30H10D, New Taipei City, Taiwan) provided constant power to the heater to generate uniform heat flux until the temperature of the cell surface reached 60 °C. Using the temperature data at the cell surface, the temperature of the air inside the container, as well as the temperatures of the container walls at the center, the specific heat capacity of the cell is evaluated based on the energy balance:
It should be mentioned that the amount of heat absorbed/lost through the insulation was neglected due to the negligible temperature increase of the insulation material. Although the temperature distribution along the height of the wall is likely nonlinear, using the temperature at the center of the wall is a good approximation for calculating the amount of heat absorption by the container walls. The experimental error introduced by this assumption is estimated to be less than 10%. The error is estimated using the difference between the temperature at the center of the wall and the average temperature between the bottom of the container wall and the temperature in the air gap (at the top).
2.3. Heat Generation Measurement
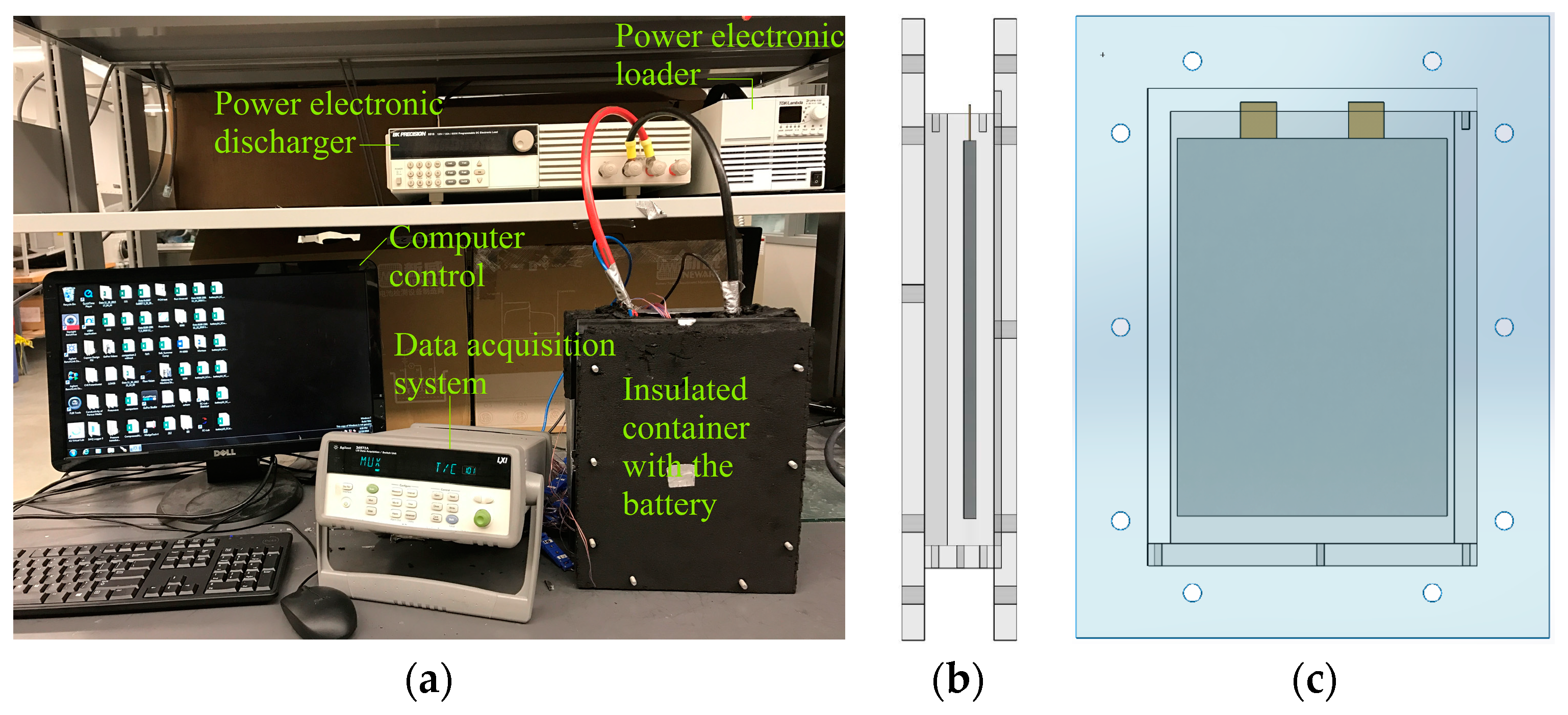
Figure 1 shows the experimental setup, as well as the side and front views of the battery in the container used to study the heat characteristic and passive cooling of lithium-ion cells using different materials. The experimental setup consisted of five parts: a rectangular enclosure with the cooling material, a data acquisitions system, a LiFePO
4 cell, a battery discharger (BK Precision-8510), and a battery charger (TDK-Lambda ZUP6-132). The thickness of the cell is 7 mm and the space between the cell and wall at each side has a width of 7.5 mm. When the cell was directly exposed to the environment, the temperature at the cell surface was measured by a FLIR
® A325sc Infrared Camera as well as thermocouples for comparison. One side of the cell surface was coated with Krylon
® Ultra-Flat Black spray paint to ensure the surface emissivity of 0.95 before being measured by the infrared camera. The temperature on the other side of the cell was measured by five T-type thermocouples (Omega Engineering Inc., Stamford, CT, USA) with an accuracy of ±0.5 °C.
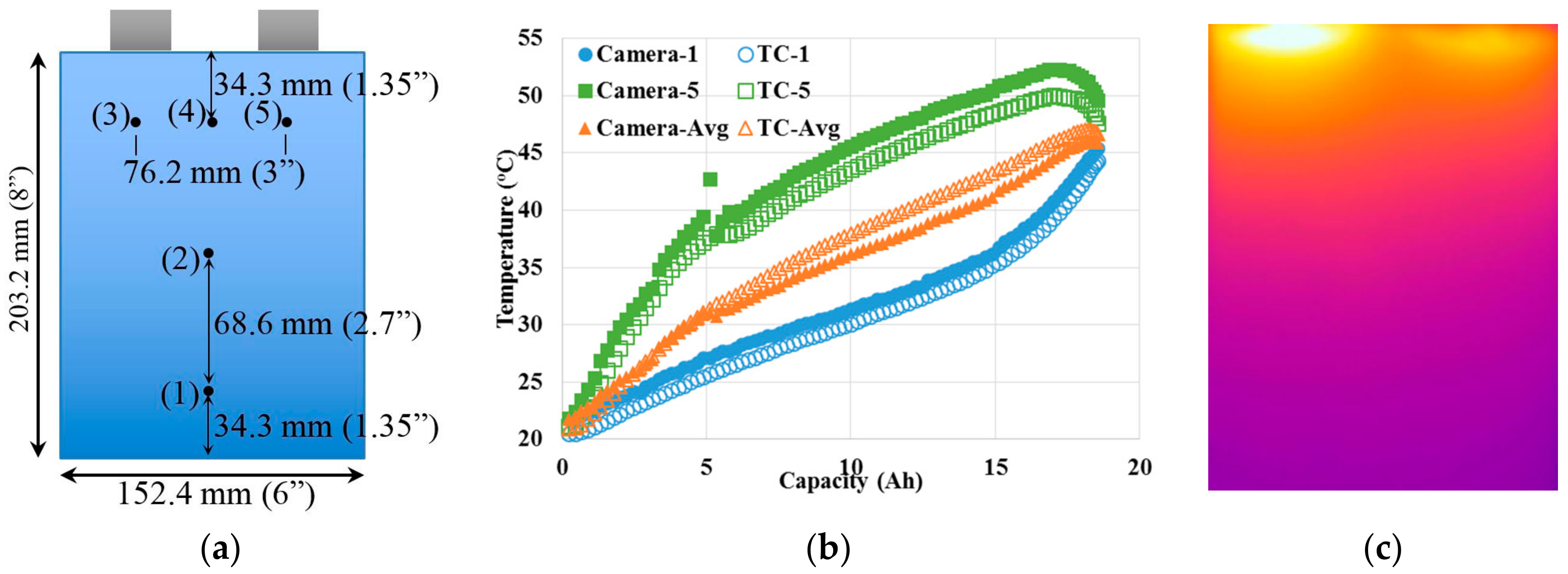
Figure 2a shows the distributions of the five thermocouples in order to measure the temperature gradient that might be present on the cell surface. The average temperature of all five thermocouples distributed along the cell surface was considered as the average cell surface temperature.
Figure 2b compares the variations of cell surface temperatures with the capacity when the cell was discharged at 4 C (78 A). The average temperature differences were 1.2 (3.5%) and 1.9 °C (4.4%) between the camera and thermocouple measurements at the respective locations of thermocouple 1 and 5. The average temperature difference of the cell surface was calculated to be 1.1 °C (3.0%) between the camera and thermocouple measurements. The experimental differences between these two measurement methods are caused by the respective accuracies of the thermocouples and thermal camera, along with the thermocouple positions identified on thermal images. The comparisons indicate that both methods are reliable techniques and both methods are used in this study. The thermal image,
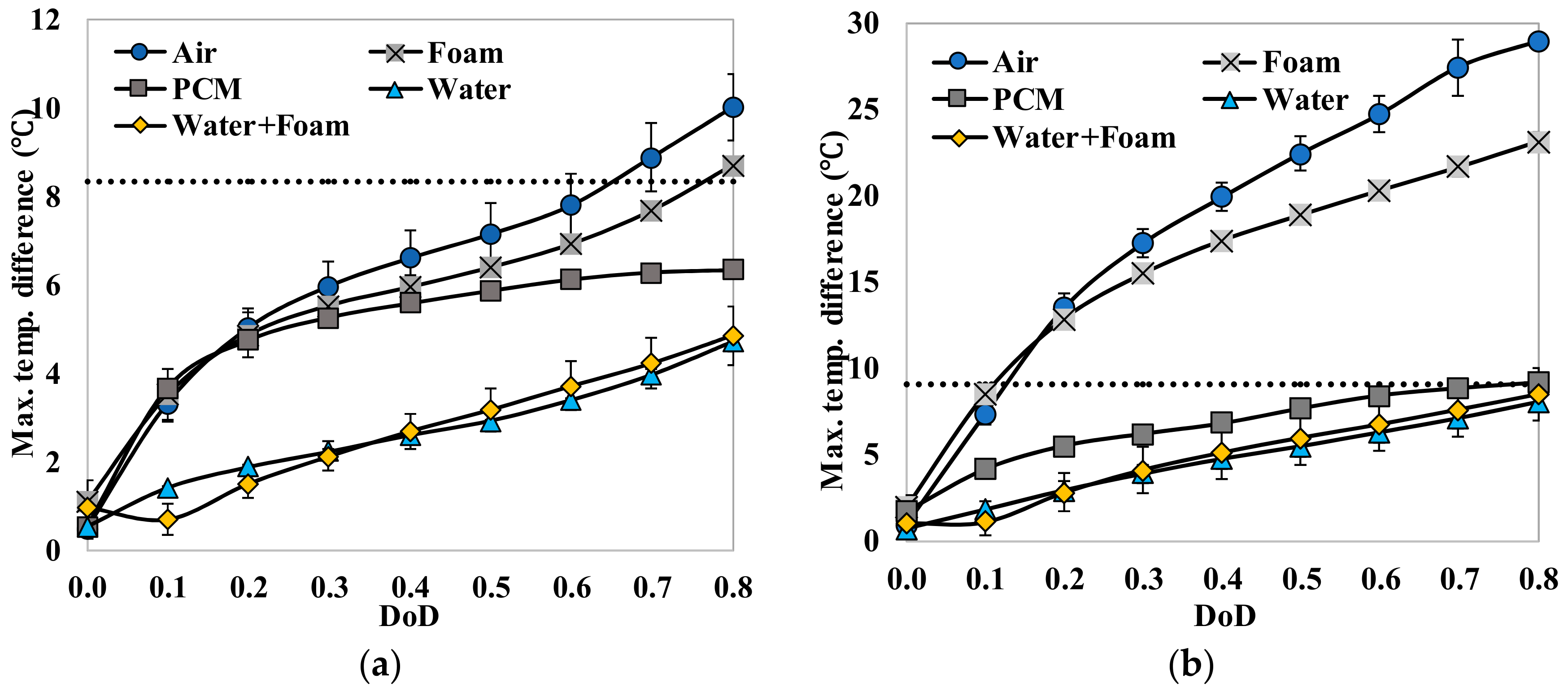
Figure 2c, at the discharge capacity of 6.5 Ah shows a very large temperature difference (>30 °C) on the cell surface. The highest temperature was observed near the terminals (TC-3 and TC-5) and the lowest temperature was observed near the bottom (TC-1). Similar results could be observed from thermocouple measurements and will be discussed in detail. The high temperature difference in the cell level indicates the importance of effective thermal management. The temperature differences are likely higher in battery modules and stacks due to the limited heat dissipation rate through the surface by natural cooling. This study uses a single cell to set up a platform to develop and evaluate passive thermal management systems. The findings from this study could be easily applied to instruct the thermal management of battery modules and stacks.
Since most of the tests in this study were carried out when the cell was assembled in the container or was immersed in water, the infrared camera could not be applied and the following temperatures have been measured by thermocouples unless stated otherwise. A total of 13 T-type thermocouples with an accuracy of ±0.5 °C were used to record the temperature change. Five of those thermocouples were used to track the cell surface temperatures (
Figure 2), and the rest were used to track the temperature of cooling materials, container walls, air gap in the container and ambient temperature of the laboratory. Temperatures measured by the thermocouples were recorded every 20 s by the Keysight 34972A data acquisition/switch unit. Before starting the experiment, the setup was placed in the laboratory overnight to reach a uniform atmospheric temperature (~20 °C) throughout the cell. Discharge and charge tests of cells at given current rates (1, 2, 3, and 4 C) were conducted. Before each discharge (/charge) test, the cell was fully charged (/discharged) at 2.5 A (1/8 C) until the voltage reached 3.7 V (/2.5 V), followed by at least 5-h rest to reach thermal equilibrium with the environment. The discharge (/charge) followed the constant current procedure until the cell voltage reached 2.5 V (/3.7 V). All experiments were repeated three times.
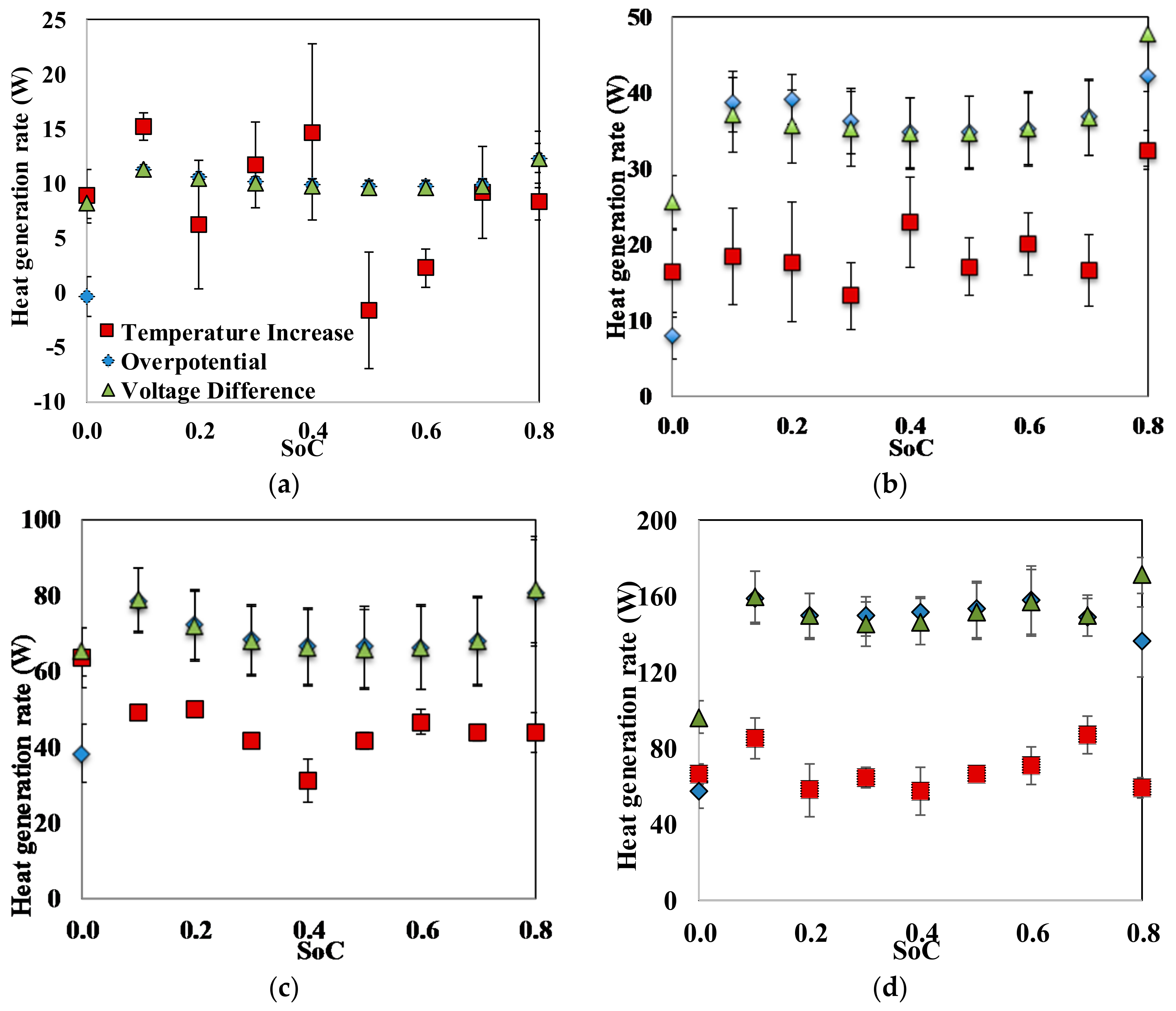
4. Conclusions
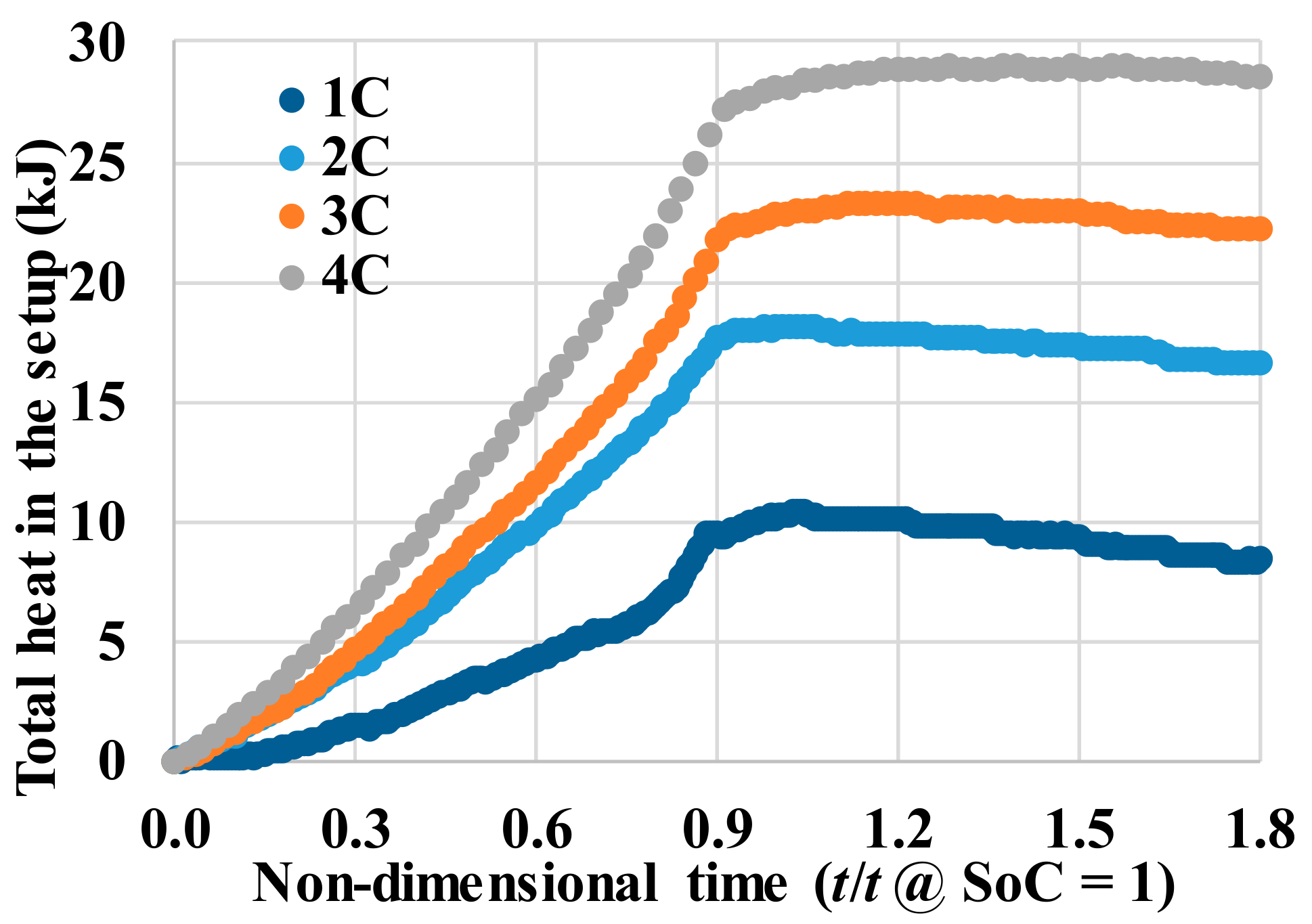
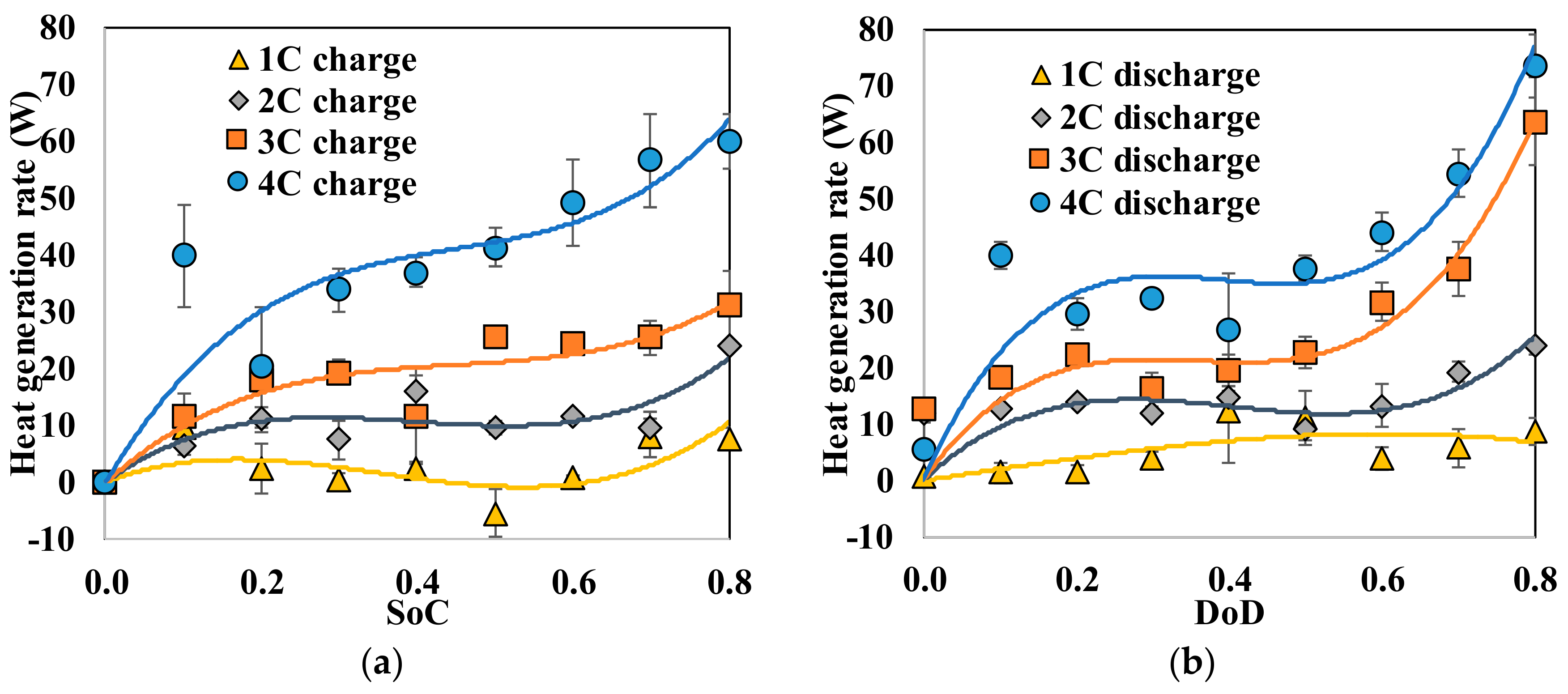
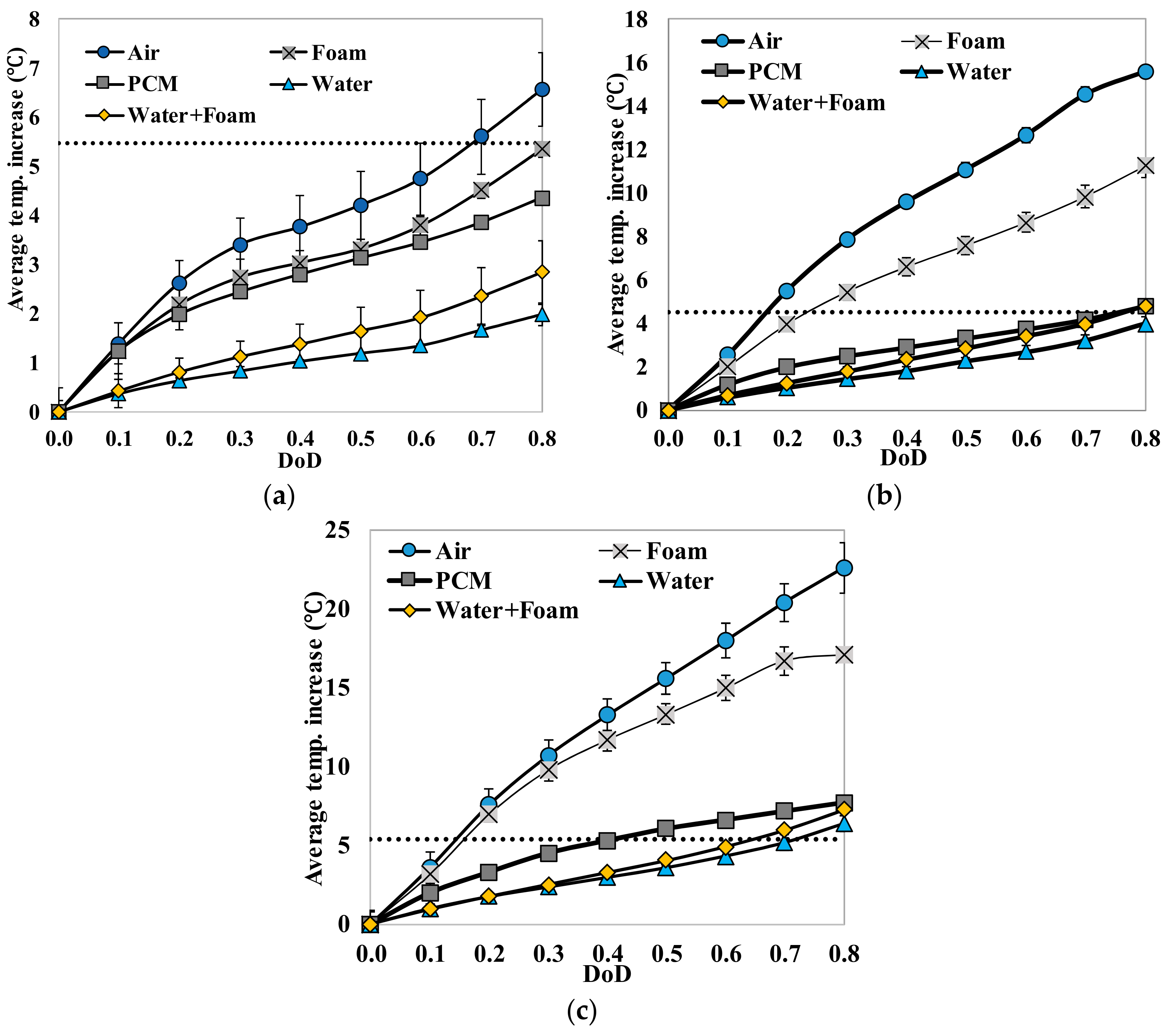
Fundamental thermal properties of the LiFePO4 cell, along with the heat generation characteristics of the cell, have been studied. The heat generation rate during the discharging process was higher than the heat generation rate during the charging process, which can be attributed to the slightly endothermic behavior observed during the charging process. The heat generation rate, as well as the total heat generated by the cell, increased with the increase in the current rate. The effectiveness of several cooling materials used in a passive thermal management system to maintain lower cell surface temperature and low surface temperature gradient has been studied. The results showed that cooling by natural convection of air might result in a large temperature increase and high temperature gradient on the cell surface (by more than 30 °C at 3 C current rate). Aluminum foam showed better cooling effects than air, while water, PCM, and water with aluminum foam are more desirable. Using PCM, water or water with aluminum foam as the cooling material, the average temperature increases at the surface are less than 8 °C and the maximum temperature differences at the surface are less than 16 °C, even at the current rate of 3 C. For such low average surface temperature increases on the cell surface using PCM, water and water with aluminum foam for cooling, the inside temperature of the cell will also stay within the safe limit. The heat generation estimation using the “Temperature Increase” method could significantly underestimate the heat generation since it can only consider the cell surface temperature.